Abstract
Although the incidence of cancer rises with age, tumor growth is often slowed in older hosts. The B16/F10 melanoma cell line is commonly used in murine models of age-related tumor growth suppression. We wished to determine if the growth pattern and gene expression of B16/10 tumors grown in aged mice could be simulated in 3D collagen matrices derived from aged mice. Outcome measures were tumor size in vitro and gene expression of the key growth regulatory molecules: growth hormone receptor (GHR), IL-10Rβ, IL-4Rα, and IL-6. B16/F10 tumors were grown in 20–25-mo-old C57/BL6 male mice. Tumor sizes ranged from 30 to 4,910 mg in vivo. Tumors from a subset of mice were removed after euthanasia, and equivalent amounts of each tumor were placed in aged 3D collagen and grown for 5 d. Tumor sizes in aged 3D collagen correlated highly with their original tumor size in vivo. Gene expression changes noted in vivo were also maintained during tumor growth in aged 3D collagen in vitro. The relative expression of GHR was increased, IL-10Rβ was unchanged, and IL-4Rα and IL-6 were decreased in the larger tumors relative to the smaller tumors in vitro, in a pattern similar to that noted in vivo. We propose that 3D matrices from aged mice provide an in vitro model of tumor growth that correlates highly with tumor size and expression of key regulatory molecules in vivo.
Keywords: Aged, Mice, Gene expression, B16/F10 melanoma tumors, 3D collagen
Introduction
Increased susceptibility to malignancies is part of the aging process, but tumor incidence, growth, and metastases are reduced in the very old (Suen et al. 1974; Ershler 1986; Pavlidis et al. 2012). These observations are complemented by studies of age-related changes in the milieu of tissues that suggest aging may provide a favorable environment for the induction of new neoplasms, but an age-related “protective effect” against tumor growth and invasiveness (Sprenger et al. 2010). Murine tumors transplanted in aged mice often exhibit a growth pattern mimicking that of older humans, thereby providing an animal model of age-related tumor suppression. Several tumors that grow rapidly in young mice, for example, Lewis lung carcinoma, fibrosarcoma, and AKR lymphoma, grow slowly in aged mice (Anisimov 2006). One of the most commonly used models is the B16/F10 melanoma line, which is often cited for highlighting mechanisms that explain delayed growth in aged C57Bl6 mice (Ershler et al. 1984; Pili et al. 1994). 3D collagen matrices derived from aged mice retain many of the features of aged collagen in vivo. The 3D collagen maintains the loose, disorganized structure of aged collagen in tissues, but is free of cells and circulating factors (Bartling et al. 2009; Damodarasamy et al. 2010). Accordingly, 3D matrices are increasingly used to better study cell behavior in vitro that mimics that found in vivo (Yamada and Cukierman 2007). The aim of the present study was to determine if B16/F10 tumor cells, derived from tumors of aged mice and placed in aged 3D collagen matrices, retain their growth patterns and expression of key regulatory molecules.
Methods, Results, and Discussion
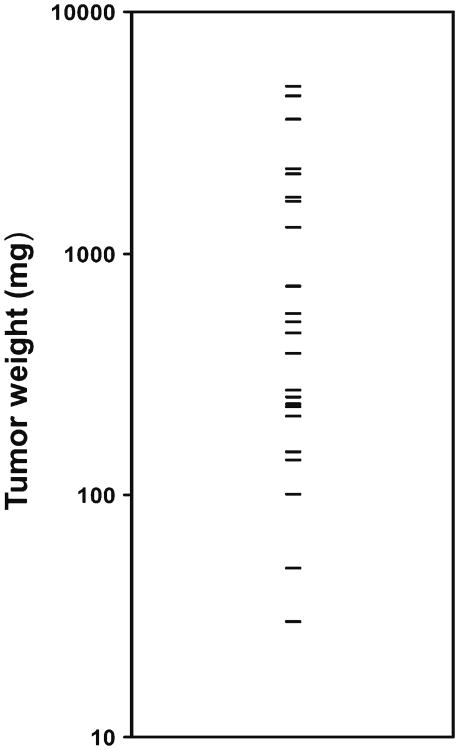
Host age affects the growth of many tumors (Pavlidis et al. 2012). Young (4–6 mo) and aged (20–25 mo) male mice of the C57/Bl6 strain were obtained from the NIA Rodent Colony at Charles River Laboratories (Wilmington, MA). Animals were housed in the SPF facility at the Harborview Research and Training Building at the Univ of WA, and the Univ of WA Animal Welfare Committee approved all procedures. B16/F10 melanoma cells were obtained from the ATCC (ATCC® number CRL-6475 Manassas, VA), and phenotype was confirmed by morphology and the presence of melanin. Cell lines were grown to 80% confluence in Dulbecco's modified Eagle's medium (DMEM) with 5% fetal bovine serum (FBS), trypsinized, washed, and resuspended in PBS at a concentration of 1×105 (B16/F10 cells)/100 μl. After the animals had acclimated for 5 d, B16/F10 cells (100 μl) were injected subcutaneously into the left flank of 25 aged mice in an identical fashion. Tumor volume was monitored as previously described (Reed et al. 2007). Mice were followed for 30 d, and measurements of tumor mass (in milligram) were obtained at the time of euthanasia. Although historically used as a model of age-related tumor growth suppression (Ershler et al. 1984; Reed et al. 2007), we found that growth of B16/F10 tumors was highly variable in aged mice and resulted in a large range of tumor sizes. Final tumor weights ranged from a low of 30 mg to a maximum of 4,910 mg (Fig. 1).
Figure 1.
Tumor weights in vivo are highly variable in aged mice. B16/F10 cells (1×105 cells/100 μl) were injected subcutaneously into the left flank of 25 aged (20–25 mo) male mice in an identical fashion. Tumor volume was monitored as previously described (Reed et al. 2007). Mice were followed for 30 d, and measurements of tumor mass (in milligram) were obtained at the time of euthanasia. The scatter plot shows that tumor sizes were highly variable and ranged from 30 to 4,910 mg.
We chose a subset of aged mice (n=6) with tumors representing sizes in the midrange (165–900 mg) to approximate typical variations in growth in vivo. After euthanasia, equivalent amounts of tumor from each mouse were obtained by weighing. Tumors were then minced, and equivalent size and weights of tumor fragments were placed in 35 μl of collagen that had been polymerized into 3D gels. To make the gels, collagen was extracted from tail tendons of young (4–6 mo) and aged (20–25 mo) C57Bl/6 male mice (n=6 each), pooled, and processed as previously described (Damodarasamy et al. 2010). Total protein content was quantified by a BCA assay (Thermo Fisher, Rockford, IL), and specific collagen content was quantified by the Sircol assay (Accurate Chemical and Scientific, Westbury, NY). Gels were prepared by combining 1 vol of collagen extract, 1/9 vol of 10-strength NaHCO3-saturated medium 199 (Invitrogen, Grand Island, NY), DMEM, and FBS to yield a solution with final collagen and FBS concentrations of 0.6 mg/ml and 1%, respectively. Polymerization of the collagen was carried out by 60-min incubation at 37°C. After polymerization, gels were treated with hyaluronidase (45 U/ml of collagen, Sigma, St. Louis, MO) to eliminate hyaluronan (HA). HA content can vary greatly in aged dermal collagen (Stern and Maibach 2008) and can affect cell proliferation in 3D collagen gels (Kawasaki et al. 1999). After 5 d, tumors were measured for growth in 3D collagen in vitro and then processed for RNA extraction.
Tumor size in 3D collagen was determined by area calculations from digital images. Tumor growth in the aged 3D collagen matrix in vitro correlated highly with the size the original tumor had attained in aged mice in vivo (Fig. 2). This suggests that cells retained their original growth patterns in the aged 3D collagen matrix (R2 correlation coefficient=0.77; an R2 near 1 indicates that a regression line fits the data well; p value of aged in vivo versus aged 3D collagen was not significant by paired t test). This result was not found when the tumors grown in aged mice were placed in young 3D collagen matrix (R2=0.48; p value of aged in vivo versus young 3D collagen was <0.05 by paired t test). Insets show that a tumor that grew large in aged mice in vivo had similar growth in aged 3D collagen in vitro (upper panels), and a tumor that was small in aged mice in vivo grew poorly in aged 3D collagen in vitro (lower panels).
Figure 2.
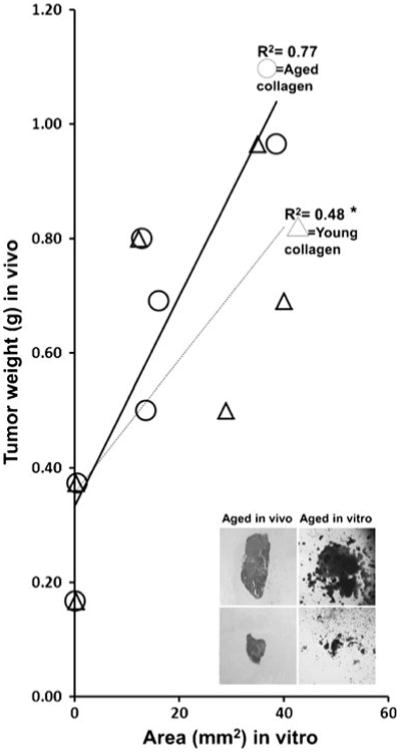
Tumor weights in aged mice in vivo correlate with tumor areas in aged 3D collagen in vitro. Collagen was extracted from tail tendons of aged or young C57Bl/6 male mice (n=6 in each age group), pooled, and polymerized into 3D collagen as previously described (Damodarasamy et al. 2010). Tumors grown in aged mice in vivo and that ranged from 165 to 900 mg in size were minced into fragments. Equivalent size and weights of tumor fragments were then placed in 35 μl of 3D collagen gels derived from aged or young mice tail tendons. Tumors were allowed to grow for 5 d, and then, tumor size was determined by area calculations from digital images. The line graph shows that tumor sizes in aged 3D collagen correlated highly with their original tumor size in aged mice in vivo (R2=0.77; circles; p=not significant). In contrast, tumor sizes in young 3D collagen did not correlate with their original tumor size in aged mice in vivo (R2=0.48; triangles; *p<0.05). Representative insets show that a tumor that grew large in aged mice in vivo had robust growth in aged 3D collagen in vitro (upper panels), and a tumor that was small in aged mice in vivo grew poorly in aged 3D collagen in vitro (lower panels).
Aging is associated with numerous changes in cytokine-mediated immune responses (Kovacs et al. 2009). Cytokines are secreted by activated immune cells as well as cancer cells. The latter recruit additional inflammatory cells, which both stimulate tumor progression and the production of cytokines that can limit tumor growth (Trinchieri 2012). 3D collagen matrices do not contain nontumor cells or circulatory mediators, thereby permitting analyses of cytokines that are produced exclusively by resident tumor cells. We wished to examine the gene expression of key cytokines and/or their receptors associated with B16/F10 tumor growth in aged tissues in vivo and aged 3D collagen in vitro. We chose four genes that regulate growth pathways, such as JAK-STAT, and are implicated in melanoma tumor promotion and progression: growth hormone receptor (GHR) (Lincoln et al. 1999), interleukin-10 receptor beta (IL-10Rβ) (Seftor et al. 2002), interleukin-4 receptor alpha (IL-4Rα) (Obiri et al. 1994), and interleukin-6 (IL-6) (Colombo et al. 1992; Hoejberg et al. 2012). Six tumors grown in vivo were removed and a portion immediately processed for RNA extraction using Trizol (Ambion, Grand Island, NY). The other portion of the tumor was grown in aged or young 3D collagen in vitro as described above, and cells from these tumors were isolated and processed for RNA extraction in a manner identical to that from the primary tumors. RNA purity was assessed by spectrophotomeric analyses, and 1 μg of RNA was reverse transcribed using the iScript cDNA Synthesis System (BioRad, Hercules, CA). Real-time quantitative PCR was performed with iTaq SYBR Green supermix (BioRad) using the following primers:
Growth hormone receptor (F: AAACGGTGAATA ACGGCTTG R: GGTGTTTAAGGTTCGCCTTG)
IL-4Rα (F: CCTCACACTCCACACACCAATG R: AGCCTGGGTTCCTTGTAGGT)
IL-10Rβ (F: GTCGTGCTGTGGCTCATTTA R: AGCAGAAACGTGCTGTGATG))
IL-6 (F: CCGGAGAGGAGACTTCACAG R: TCCACGATTTCCCAGAGAAC)
GAPDH (F: CCACCCAGAAGACTGTGGAT R: GGATGCAGGGATGATGTTCT)
All experiments were performed in duplicate and normalized to glyceraldehyde-3-phosphate dehydrogenase mRNA. Fluorescent signals were analyzed, and relative quantitation was calculated using the comparative threshold cycle method. The mean fold changes (log 2) in gene expression of the three largest tumors relative to the three smallest tumors in vivo and in vitro were calculated.
Growth hormone receptor activates pathways that increase mitosis and cell motility, thereby promoting tumor progression, invasion, metastasis, and angiogenesis (Herrington and Carter-Su 2001). Others have shown that growth hormone receptor expression in malignant melanoma cells is substantially higher than in adjacent basal melanocytes (Lincoln et al. 1999). As expected, we found that growth hormone receptor is increased in the large tumors, relative to small tumors, both in aged tissues in vivo and in aged 3D collagen in vitro (Fig. 3). IL-4Rα, an immunomodulatory cytokine receptor that regulates phosphorylation of insulin receptor substrate molecules in addition to inhibition of JAK signaling, is highly expressed in human malignancies and can inhibit the growth of tumor cells (Jiang et al. 2000; Puri et al. 2009). IL-6 is a pleiotropic inflammatory cytokine that has been extensively studied in many cancers, including melanoma (Hoejberg et al. 2012). Interestingly, although IL-6 and IL-4Rα have opposing effects on JAK signaling (IL-6 stimulates while IL-4Rα inhibits), transcription of both was reduced in the larger tumors in aged hosts in vivo and in aged 3D collagen in vitro (Fig. 3). The effects of IL-10 are often the opposite of that of IL-6, resulting in unpredictable expression patterns during tumor growth. Notably, IL-10Rβ transcription was similar in large and small tumors both in aged hosts in vivo and in aged 3D collagen vitro (Fig. 3). As expected from the growth patterns, there was no correlation between gene expression in vivo and gene expression in vitro when the tumors from aged mice were grown in young 3D collagen (Fig. 3, inset table).
Figure 3.
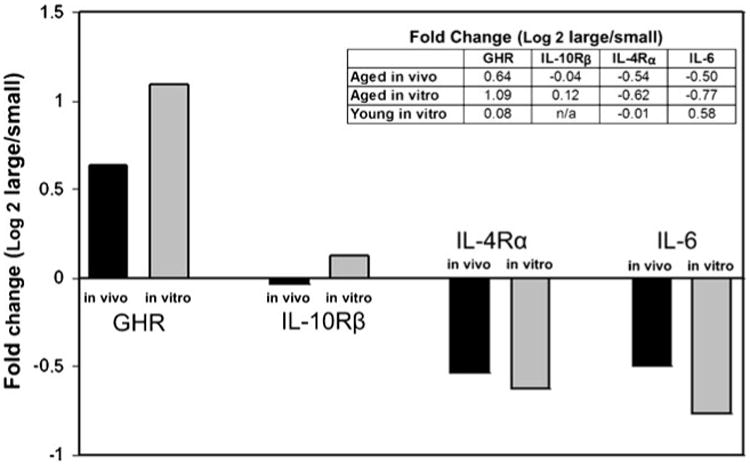
Gene expression in aged mice in vivo correlates with expression in aged 3D collagen in vitro. Tumors grown in aged mice in vivo were removed and a portion immediately processed for RNA extraction using Trizol (Ambion). The other portions of the tumor were grown in aged 3D collagen or young 3D collagen in vitro, and after 5 d, cells from these tumors were isolated and processed for RNA extraction in a manner identical to that from the primary tumors. The mean fold changes (log 2) in gene expression of the three largest tumors relative to the three smallest tumors in vivo and in vitro were calculated. The bar graph shows that gene expression changes in aged mice in vivo were maintained during tumor growth in aged 3D collagen in vitro: relative expression of GHR was increased, IL-10Rβ was unchanged, and IL-4Rα and IL-6 were decreased in the larger tumors relative to the smaller tumors. The inset table shows fold change (large versus small) when comparing gene expression in tumors grown in aged mice in vivo and gene expression when the same tumors were placed in aged 3D collagen in vitro and young 3D collagen in vitro. Note that gene expression patterns were not maintained when the tumors grown in aged mice were placed in young 3D collagen (n/a=not detected).
Our report is limited by the time the tumor cells could remain in the 3D matrix without degradation and the focus on only four key regulatory cytokines. Nonetheless, we propose that 3D collagen matrices derived from aged mice provide an in vitro model that correlates highly with tumor size and expression of key regulatory cytokines in aged hosts in vivo. These observations might be useful in developing therapeutic modalities that directly test tumor cells, in the absence of other cell types and circulating mediators, in a 3D milieu that simulates intact aged extracellular matrix.
Acknowledgments
The authors thank Nathan Karres, Margaret Eugenio, and Matthew NR Johnson for assistance with the tumor samples. This work was supported by U54 CA126540, R01 AG015837, and R21 AG033391.
Contributor Information
Itay Bentov, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, USA.
Mamatha Damodarasamy, Division of Gerontology and Geriatric Medicine, Department of Medicine, University of Washington, Seattle, USA.
Stephen Plymate, Division of Gerontology and Geriatric Medicine, Department of Medicine, University of Washington, Seattle, USA; Veterans Affairs Puget Sound Health Care System, Seattle, WA, USA.
May J. Reed, Email: mjr@uw.edu, Division of Gerontology and Geriatric Medicine, Department of Medicine, University of Washington, Seattle, USA; Department of Medicine, Harborview Medical Center, University of Washington, Box 359625, 325 Ninth Avenue, Seattle, WA 98104, USA.
References
- Anisimov VN. Effect of host age on tumor growth rate in rodents. Front Biosci. 2006;11:412–422. doi: 10.2741/1808. [DOI] [PubMed] [Google Scholar]
- Bartling B, Desole M, Rohrbach S, Silber RE, Simm A. Age-associated changes of extracellular matrix collagen impair lung cancer cell migration. FASEB J. 2009;23:1510–1520. doi: 10.1096/fj.08-122648. [DOI] [PubMed] [Google Scholar]
- Colombo MP, Maccalli C, Mattei S, Melani C, Radrizzani M, Parmiani G. Expression of cytokine genes, including IL-6, in human malignant melanoma cell lines. Melanoma Res. 1992;2:181–189. doi: 10.1097/00008390-199209000-00006. [DOI] [PubMed] [Google Scholar]
- Damodarasamy M, Vernon RB, Karres N, Chang CH, BianchiFrias D, Nelson PS, Reed MJ. Collagen extracts derived from young and aged mice demonstrate different structural properties and cellular effects in three-dimensional gels. J Gerontol A Biol Sci Med Sci. 2010;65:209–218. doi: 10.1093/gerona/glp202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershler WB. Why tumors grow more slowly in old people. J Natl Cancer Inst. 1986;77:83783–83789. [PubMed] [Google Scholar]
- Ershler WB, Stewart JA, Hacker MP, Moore AL, Tindle BH. B16 murine melanoma and aging: slower growth and longer survival in old mice. J Natl Cancer Inst. 1984;72:161–164. doi: 10.1093/jnci/72.1.161. [DOI] [PubMed] [Google Scholar]
- Herrington J, Carter-Su C. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol Metab. 2001;12:252–257. doi: 10.1016/s1043-2760(01)00423-4. [DOI] [PubMed] [Google Scholar]
- Hoejberg L, Bastholt L, Schmidt H. Interleukin-6 and melanoma. Melanoma Res. 2012;22:327–33. doi: 10.1097/CMR.0b013e3283543d72. [DOI] [PubMed] [Google Scholar]
- Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol. 2000;105:1063–1070. doi: 10.1067/mai.2000.107604. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Ochi M, Uchio Y, Adachi N, Matsusaki M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J Cell Physiol. 1999;179:142–148. doi: 10.1002/(SICI)1097-4652(199905)179:2<142::AID-JCP4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Kovacs EJ, Palmer JL, Fortin CF, Fülöp T, Jr, Goldstein DR, Linton PJ. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol. 2009;30:319–24. doi: 10.1016/j.it.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln DT, Sinowatz F, Kölle S, Takahashi H, Parsons P, Waters M. Up-regulation of growth hormone receptor immunoreactivity in human melanoma. Anticancer Res. 1999;19:1919–1931. [PubMed] [Google Scholar]
- Obiri NI, Siegel JP, Varricchio F, Puri RK. Expression of high-affinity IL-4 receptors on human melanoma, ovarian and breast carcinoma cells. Clin Exp Immunol. 1994;95:148–155. doi: 10.1111/j.1365-2249.1994.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis N, Stanta G, Audisio RA. Cancer prevalence and mortality in centenarians: a systematic review. Crit Rev Oncol Hematol. 2012;83:145–152. doi: 10.1016/j.critrevonc.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Pili P, Guo Y, Chang J, Nakanishi H, Martin GR, Passaniti A. Altered angiogenesis underlying age-dependent changes in tumor growth. J Natl Cancer Inst. 1994;86:1303–1314. doi: 10.1093/jnci/86.17.1303. [DOI] [PubMed] [Google Scholar]
- Puri S, Mahapatra AK, Hussain E, Sarkar C, Sinha S, Joshi BH. A review of studies on targeting interleukin 4 receptor for central nervous system malignancy. Curr Mol Med. 2009;9:732–739. doi: 10.2174/156652409788970661. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Karres N, Eyman D, Cruz A, Brekken RA, Plymate S. The effects of aging on tumor growth and angiogenesis are tumor-cell dependent. Int J Cancer. 2007;120:753–60. doi: 10.1002/ijc.22351. [DOI] [PubMed] [Google Scholar]
- Seftor EA, Meltzer PS, Schatteman GC, Gruman LM, Hess AR, Kirschmann DA, Seftor RE, Hendrix MJ. Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: role in vasculogenic mimicry. Crit Rev Oncol Hematol. 2002;44:17–27. doi: 10.1016/s1040-8428(01)00199-8. [DOI] [PubMed] [Google Scholar]
- Sprenger CC, Plymate SR, Reed MJ. Aging-related alterations in the extracellular matrix modulate the microenvironment and influence tumor progression. Int J Cancer. 2010;127:2739–2748. doi: 10.1002/ijc.25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R, Maibach HI. Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clin Dermatol. 2008;26:106–122. doi: 10.1016/j.clindermatol.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Suen KC, Lau LL, Yermakov V. Cancer and old age. An autopsy study of 3,535 patients over 65 years old. Cancer. 1974;33:1164–1168. doi: 10.1002/1097-0142(197404)33:4<1164::aid-cncr2820330440>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]