Abstract
Background
Multiple viruses are often detected in children with respiratory infection but the significance of co-infection in pathogenesis, severity and outcome is unclear.
Objectives
To correlate the presence of viral co-infection with clinical phenotype in children admitted with acute respiratory infections (ARI).
Methods
We collected detailed clinical information on severity for children admitted with ARI as part of a Spanish prospective multicenter study (GENDRES network) between 2011–2013. A nested polymerase chain reaction (PCR) approach was used to detect respiratory viruses in respiratory secretions. Findings were compared to an independent cohort collected in the UK.
Results
204 children were recruited in the main cohort and 97 in the replication cohort. The number of detected viruses did not correlate with any markers of severity. However, bacterial superinfection was associated with increased severity (OR: 4.356; P-value = 0.005), PICU admission (OR: 3.342; P-value = 0.006), higher clinical score (1.988; P-value = 0.002) respiratory support requirement (OR: 7.484; P-value < 0.001) and longer hospital length of stay (OR: 1.468; P-value < 0.001). In addition, pneumococcal vaccination was found to be a protective factor in terms of degree of respiratory distress (OR: 2.917; P-value = 0.035), PICU admission (OR: 0.301; P-value = 0.011), lower clinical score (-1.499; P-value = 0.021) respiratory support requirement (OR: 0.324; P-value = 0.016) and oxygen necessity (OR: 0.328; P-value = 0.001). All these findings were replicated in the UK cohort.
Conclusion
The presence of more than one virus in hospitalized children with ARI is very frequent but it does not seem to have a major clinical impact in terms of severity. However bacterial superinfection increases the severity of the disease course. On the contrary, pneumococcal vaccination plays a protective role.
Introduction
Molecular techniques including polymerase chain reaction (PCR) have increased the sensitivity of detection for common and emerging respiratory viruses, and often reveal the presence of more than one pathogen in respiratory patients.[1],[2] The importance of viral co-infections in the pathogenesis, severity or course of respiratory infections is not well established. The high sensitivity of molecular techniques raises questions about the clinical relevance of positive test results. The presence of a virus does not necessarily indicate causation of clinical symptoms or disease [3]. On the contrary, bacterial co-infection is usually associated with a more severe diseases course and worse prognosis, despite the precise interaction between bacteria and viruses is not always clear.
Our study aims to analyze the relationship between viral or bacterial co-infection detected by molecular methods, and the clinical phenotype of children admitted to hospital with lower tract acute respiratory infections (LT-ARI).
Materials and Methods
Study design and recruitment criteria
Two independent observational prospective patient groups were enrolled in Spain (main group) and in the United Kingdom (replication group). Spanish children were recruited between January 2011 and January 2013 through a national hospital based research network: GENDRES (Genetic, vitamin D and Respiratory infections research network– www.gendres.org), which includes 13 Spanish tertiary hospitals. UK children were recruited between October 2009 and May 2010 at St Mary’s hospital (UK). In both cohorts, eligible study participants were children under 14 years of age with a lower respiratory tract illness of sufficient severity to warrant admission to hospital, and a virus identified on nasopharyngeal swab or aspirate sample. All types of LT-ARI were included, from bronchiolitis to pneumonia, with or without wheezing, fever, rhinorrhea and respiratory distress.
A nasopharyngeal sample (aspirate or swab) was obtained during admission for viral pathogen detection. Besides the diagnostic procedures performed at origin in the referring hospital, viral detection was performed in all recruited subjects using a panel of 19 viruses, by nested PCR: respiratory syncytial virus; influenza virus (A, B); parainfluenza virus types (1–4); adenovirus (A-F); rhinovirus; metapneumovirus; coronavirus (NL63, 229E, OC43) and bocavirus (Methods previous described in Cebey-López et al. [4]). No difference was found on the molecular diagnosis yield in our series when using aspirate or swab as sample source.
All investigators were trained in the study protocol for patient recruitment, sample processing and sample storage. The study was performed according to Good Clinical Practice. Written informed consent was obtained from a parent or legal guardian for each subject before study inclusion. The study was approved by the Ethical Committee of Clinical Investigation of Galicia (CEIC ref. 2010/015). The UK cohort study was approved by the St Mary’s Research Ethics Committee (REC 09/H0712/58).
Clinical data collection
Detailed clinical data on each patient were collected using a secured web-based platform. This included risk factors for LT-ARI (prematurity, immunization status, obesity, diabetes, asthma and previous admissions to hospital), current medications, and family history of asthma or other respiratory diseases. The severity of each respiratory case was ranked as follows: 1) physician criteria (mild, moderate or severe); 2) Wood-Downes scale (0 to 10 points; mild < 3, moderate 4–7, severe > 8); and 3) a newly developed scale -named GENVIP score- (0 to 20 points) that assesses food tolerance, degree of medical intervention needed, respiratory distress, respiratory frequency, apnea, malaise and fever (see S1 Table for further details on this scale). Supplemental oxygen and / or mechanical ventilation requirement during admission were also recorded. Bacterial superinfection diagnosis was established according to the referring physician criteria, based on clinical data, inflammatory markers, and radiological findings, and/or appropriate cultures in sterile sites (e.g blood or cerebrospinal fluid). For the study purposes, cases classified as bacterial co-infections included both cases with and without microbiological confirmation as in our series, cultures were not carried out systematically, but only when a bacterial superinfection was suspected by the referring physician.
Data analysis
In this analysis, we compared clinical data to the results of pathogen identification in respiratory samples. We performed all the analysis using the R Software, Version 3.0.2 (www.r-project.org). General data were presented as means with 95% confidence intervals (CI). Different statistical models were used to assess the bivariate association between the variables depending on the dependent variable. The relationship between demographic and clinical variables with mono-infection and co-infection was analysed using simple logistic regression. A binary logistic model was used for the binary variables (co-infection status, oxygen requirements, respiratory support needed and PICU admission), linear model for continuous variables (Wood-Downes Score and GENVIP Score), negative binomial regression model for counted data (hospital stay length) and logistic multinomial model for the multinomial variable (respiratory distress status). Multiple regression models were considered using the significant risk factors obtained in the bivariate analysis and sex and age variables. In order to reduce the likelihood of false significant results due to too many statistical comparisons, the Bonferroni multiple test correction were considered. A χ2 test was performed to evaluate the correlation between bacterial superinfection and pneumococcal vaccine. Level of statistical significance was set at 0.05.
Results
The GENDRES cohort included nasopharyngeal samples from 204 patients with a median age of 16.7 (95% CI: 12.7–20.6) months and a male-to-female sex ratio of 1.7. One patient was excluded due to incomplete clinical data. In the UK a cohort of 97 patients’ data was analyzed, with a median of age of 36.6 (95% CI: 27.6–45.6) months and a male-to-female ratio of 0.94 (S1 Fig). Comparison between both cohorts is shown in Table 1.
Table 1. Description of the characteristics of the two cohorts analyzed: the GENDRES cohort and the UK cohort.
P-value results from the comparison between both cohorts. A P-value < 0.05 was considered significant.
| Variable | GENDRES cohort n (%) | UK cohort n (%) | P-value |
|---|---|---|---|
| Sex (female proportion) 1 | 75 (36.9) | 50 (51.5) | 0.018 |
| Age (months) 1 | <0.001β | ||
| 0–12 | 136 (66.7) | 39 (40.2) | |
| 13–24 | 25 (12.3) | 17 (17.5) | |
| 25–48 | 26 (12.8) | 17 (17.5) | |
| <48 | 16 (7.9) | 24 (24.7) | |
| Pneumoccocal vaccine1 | 110 (53.9) | 57 (64.0) | 0.124 |
| Bacterial superinfection1 | 56 (29.5) | 53 (54.6) | <0.001β |
| Mono-infection1 | 95 (46.6) | 56 (57.7) | 0.084 |
| RSV | 53 (28.3) | 21 (24.7) | |
| Rhinovirus | 17 (9.1) | 12 (14.1) | |
| Co-infection1 | 92 (49.2) | 29 (34.1) | 0.012 |
| RSV + rhinovirus | 23 (12.3) | 3 (3.5) | |
| RSV+ bocavirus | 10 (5.3) | 5 (5.9) | |
| PICU admission1 | 38 (29.0) | 43 (44.3) | 0.024 |
| Respiratory support1 | 30 (14.8) | 36 (38.3) | <0.001β |
| Oxygen needed1 | 56 (29.5) | 55 (57.9) | <0.001β |
| Hospital stay length2* | 6 (4, 9) | 4 (2, 9) | <0.001β |
1Fisher Exact Test.
2Wilcoxon test.
*Median and interquartile range in days.
βSignificant under Bonferroni correction.
Viral mono-infection versus viral multi-infection
The PCR results were preciously described in Cebey-López et al. [4]. Clinical data, family or past medical history, need of PICU admission or hospital length of stay, and oxygen or respiratory support need, were equivalent in the GENDRES and the UK cohort (Table 2).
Table 2. Relationship between demographic and clinical variables with mono-infection and co-infection is shown for both GENDRES and UK cohort.
The correlation was analysed using simple logistic regression. Data are presented as OR (95% confidence interval) and P-value. The characteristics of the two cohorts analyzed were compared and when P-value results were significant when different: the GENDRES cohort and the UK cohort. P-value results from the comparison between both cohorts. A P-value < 0.005 was considered significant.
| Variable | GENDRES cohort (n = 203) | UK-cohort (n = 97) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GENDRES% (95% CI) | OR (95% CI) | P-value | Multiple OR (95% CI) | P -value | UK-cohort % (95% CI) | OR (95% CI) | P -value | Multiple OR (95% CI) | P -value | |
| Demographic characteristics | ||||||||||
| Sex. Female | 36.9 | 1.287 | 0.405 | 1.069 | 0.838 | 51.5 | 1.228 | 0.657 | 1.14 | 0.802 |
| (30.3, 43.6) | (0.711, 2.340) | (0.564, 2.014) | (41.1, 62.0) | (0.498, 3.086) | (0.41, 3.18) | |||||
| Age | β | |||||||||
| 12–24 months | 12.3 | 3.723 | 0.010 | 3.173 | 0.028 | 17.5 | 8.937 | 0.002 | 8.73 | 0.002 |
| (7.8 16.8) | (1.428, 10.955) | (1.177, 9.569) | (9.4, 25.6) | (2.377, 40.192) | (2.3, 39.66) | |||||
| 24–48 months | 12.8 | 4.189 | 0.005 | 3.463 | 0.018 | 17.5 | 1.773 | 0.378 | 1.79 | 0.372 |
| (8.2, 17.4) | (1.635, 12.201) | (1.290, 10.447) | (9.4, 25.6) | (0.484, 6.373) | (0.49, 6.44) | |||||
| > 48 months | 7.9 | 0.621 | 0.447 | 0.544 | 0.339 | 24.7 | 0.867 | 0.836 | 0.84 | 0.802 |
| (4.2, 11.6) | (0.161, 2.015) | (0.139, 1.800) | (15.6, 33.9) | (0.203, 3.261) | (0.19, 3.24) | |||||
| Family history | ||||||||||
| Asthma | 39.9 | 0.753 | 0.343 | n.a. | ||||||
| (33.2, 46.6) | (0.417, 1.352) | |||||||||
| Respiratory conditions | 15.8 | 0.824 | 0.623 | n.a. | ||||||
| (10.8, 20.9) | (0.375, 1.785) | |||||||||
| Patient medical history | ||||||||||
| Premature birth | 8.5 | 0.450 | 0.199 | n.a. | ||||||
| (4.5, 12.5) | (0.118, 1.443) | |||||||||
| Pneumococcal vaccine | 53.9 | 2.055 | 0.016 | 1.550 | 0.176 | 64.0 | 1.990 | 0.189 | ||
| (47.1, 60.8) | (1.151, 3.709) | (0.821,2.932) | (54.1, 74.0) | (0.734, 5.849) | ||||||
| Pulmonary conditions | 3.5 | 0.517 | 0.453 | n.a. | ||||||
| (0.9, 6.0) | (0.070, 2.718) | |||||||||
| Asthma | 11.8 | 0.943 | 0.899 | n.a | ||||||
| (7.4, 16.3) | (0.374, 2.354) | |||||||||
| Clinical data | ||||||||||
| Bacterial superinfection | 29.5 | 1.396 | 0.308 | 54.6 | 1.319 | 0.549 | ||||
| (23.0, 36.0) | (0.736, 2.667) | (44.2, 65.1) | (0.536, 3.315) | |||||||
βSignificant under Bonferroni correction.
In the GENDRES cohort the presence of rhinovirus as co-pathogen was associated with a significantly increased Wood-Downes score by 1.289 points (95% CI: 0.387, 2.192); P-value = 0.006. RSV infection was associated with increased oxygen requirements [OR (95% CI): 3.154 (1.302, 7.966); P-value = 0.012] (Table 3). These isolated findings were not replicated in the UK cohort (Table 3).
Table 3. Comparison of virus and disease severity of the main cohort considering the virus as single pathogen or as co-infection in the sample.
Different statistical models were considered to study the bivariate association between the variables depending on the dependent variable. A binary logistic model was used for the binary variables oxygen needed and respiratory support needed, and a negative binomial regression model for counted data (hospital stay length). Data are presented as OR (confidence interval 95%) and the level of statistical significance was set at 0.05.
| Risk Factor | Hospital stay length | Oxygen needed | Respiratory support | Wood Downes score | GENVIP score | PICU admission | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 163) | (n = 186) | (n = 186) | (n = 177) | (n = 125) | (n = 119) | |||||||
| OR (95% CI) | P -value | OR (95% CI) | P -value | OR (95% CI) | P–value | Coefficient (95% CI) | P -value | Coefficient (95% CI) | P -value | OR (95% CI) | P -value | |
| Mono-infected | ||||||||||||
| RSV | 1.092 | 0.450 | 3.154 | 0.012 | 3.556 | 0.122 | 0.571 | 0.188 | 1.384 | 0.107 | 1.121 | 0.846 |
| (0.868, 1.374) | (1.302, 7.966) | (0.832, 24.487) | (-0.284,1.425) | (-0.307, 3.075) | (0.362, 3.728) | |||||||
| Rhinovirus | 0.785 | 0.154 | 0.327 | 0.042 | 1.167 | 0.854 | -0.823 | 0.144 | -1.134 | 0.447 | 1.410 | 0.623 |
| (0.562, 1.094) | (0.109, 0.962) | (0.165, 5.257) | (-1.932, 0.285) | (-4.095, 1.826) | (0.330, 5.410) | |||||||
| Bocavirus | 0.999 | 0.997 | 0.435 | 0.327 | -0.187 | 0.859 | -2.610 | 0.111 | ||||
| (0.646, 1.548) | (0.076, 2.482) | (-2.270, 1.897) | (-5.840, 0.619) | |||||||||
| Adenovirus | 1.211 | 0.456 | 0.859 | 0.413 | 1.581 | 0.624 | ||||||
| (0.733, 2.019) | (-1.217, 2.935) | (-4.841, 8.002) | ||||||||||
| Co-infected | ||||||||||||
| RSV | 1.150 | 0.281 | 0.938 | 0.892 | 1.550 | 0.456 | -0.222 | 0.646 | 0.243 | 0.815 | 1.426 | 0.597 |
| (0.892, 1.481) | (0.361, 2.364) | (0.509, 5.328) | (-1.217, 2.935) | (-1.829, 2.316) | (0.402, 5.887) | |||||||
| Rhinovirus | 1.443 | 0.003 | 1.642 | 0.288 | 2.921 | 0.085 | 1.289 | 0.006 | 0.920 | 0.379 | 2.169 | 0.146 |
| (1.135, 1.836) | (0.658, 4.158) | (0.923, 11.199) | (0.387, 2.192) | (-1.157, 2.997) | (0.104, 1.398) | |||||||
| Bocavirus | 0.697 | 0.003 | 1.243 | 0.642 | 0.889 | 0.832 | -0.654 | 0.168 | -0.551 | 0.589 | 1.174 | 0.794 |
| (0.551, 0.883) | (0.499, 3.168) | (0.290, 2.632) | (-1.590, 0.282) | (-2.583, 1.481) | (0.352, 3.910) | |||||||
| Adenovirus | 0.938 | 0.613 | 1.000 | 1.000 | 0.682 | 0.515 | 0.290 | 0.553 | -0.848 | 0.422 | 1.426 | 0.597 |
| (0.730, 1.204) | (0.396, 2.604) | (0.198, 22.080) | (-0.677, 1.257) | (-2.947, 1.252) | (0.383, 5.305) | |||||||
Bacterial superinfection
Children presenting a bacterial superinfection had more severe respiratory distress [OR (95% CI): 4.356 (1.564, 12.128); P-value = 0.005] and a higher severity score [2.124 (95% CI: 0.864, 3.385); P-value = 0.001]. They were more likely to be admitted to PICU in the GENDRES cohort [OR (95% CI): 2.851 (1.300, 6.252); P-value = 0.009] and the UK cohort [5.357 (2.081, 15.085); P-value = 0.001]. Children with bacterial co-infection required significantly more respiratory support in both cohorts: discovery cohort [OR (95% CI): 6.368 (2.724, 14.886); P-value < 0.001] and replication cohort [OR (95% CI): 3.432 (1.402, 8.404); P-value = 0.007], and they had a longer hospital stay in both cohorts: 1.48 days (P-value = 0.025) longer stay in GENDRES cohort and 1.87 days (P-value = 0.005) in UK cohort, respectively (Fig 1; S3–S8 Tables). In addition, 34.0% of the patients with bacterial infection in the GENDRES cohort received the pneumococcal vaccine and 24.7% did not receive it (P-value = 0.213).
Fig 1. Influence of bacterial superinfection, pneumococcal vaccine and the presence of viral co-infection on disease severity of children with ARI, according to oxygen and respiratory support requirement, clinical scales, hospital stay length and PICU admission.
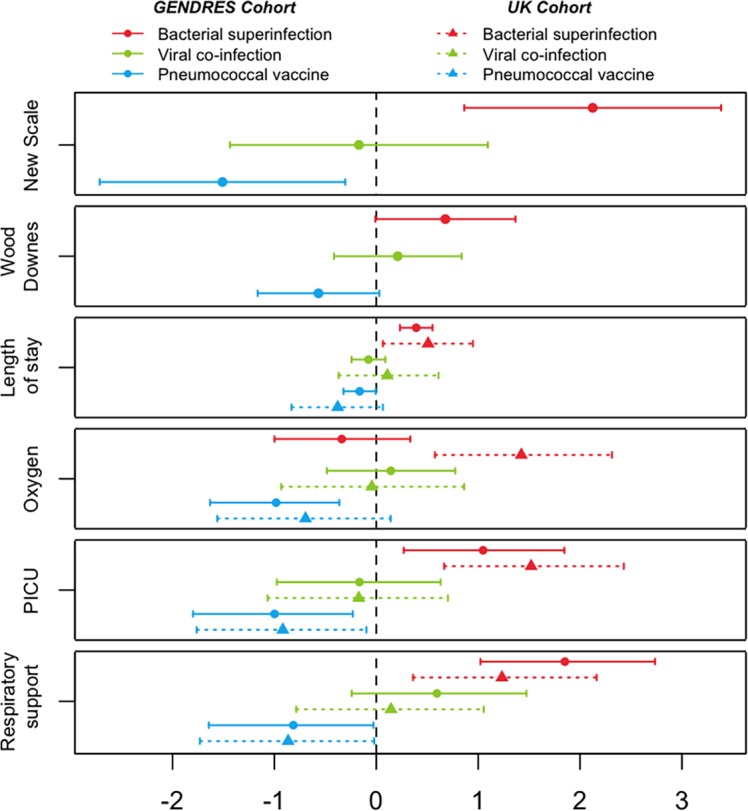
Data are shown as OR (95% CI) for both main cohort and replication cohort. A binary logistic model was used for the binary variables (co-infection status, oxygen requirements, respiratory support needed and PICU admission), linear model for continuous variables (Wood-Downes Score and the GENVIP score) and negative binomial regression model for counted data (number of days since admission).
Pneumococcal vaccine
In the GENDRES cohort the pneumococcal vaccine was given to 53.9% (46.9, 61.1) of the patients of whom 43.8% (33.3, 54.2) were mono-infected and 62.7% (52.2, 73.1) were viral co-infected patients [OR (95% CI): 1.550 (0.821, 2.932); P-value = 0.176]. Vaccinated patients had lower risk of being admitted to PICU in GENDRES cohort [OR (95% CI): 0.301 (0.116, 0.735); P-value = 0.011] and in the UK cohort [OR (95% CI): 0.208 (0.046, 0.776); P-value = 0.027] and had less risk of respiratory support requirement in the main cohort [OR (95% CI): 0.324 (0.124, 0.790); P-value = 0.016] and in the replication one [OR (95% CI): 0.267 (0.070, 0.901); P-value = 0.040]. In the Spanish cohort, patients who received the pneumococcal vaccine received less oxygen support [OR (95% CI): 0.328 (0.162, 0.639); P-value = 0.001], and had a lower clinical severity score [-1.499 (95% CI: -2.768, -0.231) points; P-value = 0.021] and a lower respiratory distress score [OR (95% CI): 2.917 (1.078, 7.889); P-value = 0.035]. These findings were not replicated in the UK cohort (Fig 1; S1–S7 Tables).
Discussion
Our study revealed that even though multiple viral detection is frequent in hospitalized children with LT-ARI, this association is not related to either disease severity or to any other clinical features studied. PICU admission, disease severity according to different scales, need for respiratory support, and length of hospital stay followed a similar pattern in viral mono- versus co-infected children. Contrariwise, bacterial superinfection increased the severity of the disease course, while pneumococcal vaccination played a protective role.
The detection of multiple coincident viruses in clinical settings is becoming more common since the introduction of molecular based multiplex tests, but the clinical significance of these findings remains unclear and seems to have no impact in disease severity [5]. Both an increase in disease severity in relation to dual infections [6–9] and the absence of this association [10–19] have been reported. Richard et al. [8] found that co-infected children were almost three times more likely to be admitted to the PICU than those with single viral infections. Compared to our study Richard et al. developed a retrospective and monocentric study in which they only considered dual infections, infants and bronchiolitis.
There is contradictory evidence linking disease severity with specific respiratory viruses. A shorter hospital stay has been reported in children with rhinovirus bronchiolitis than with RSV [20]. Rhinovirus and RSV co-infection is reported to increase the risk of severe disease [8] or the bronchiolitis relapse [21, 22]. Other studies did not find significant differences in severity between co-infection and single infection [12, 18, 23, 24]. In our study we did not find increased severity of illness in children with RSV-rhinovirus dual infection. In our series, only RSV as mono-infection increased oxygen requirements, and rhinovirus as a co-infecting pathogen increased the Wood-Downes score in the Spanish cohort, but these isolated findings arising from the multivariate analysis could not be replicated in the UK cohort.
Several studies have reported increased severity with bocavirus (hBoV) co-infections [13, 25–27]; this was not the case in our series (also in agreement with Pen et al. [15]). hBoV was commonly detected in our patients, with no impact in the severity of the illness. As hBoV was detected in alongside other respiratory viruses with an established pathogenic potential, it is possible that hBoV detection reflects asymptomatic persistence or prolonged viral shedding [28].
Bacterial superinfection was the only factor consistently linked to greater severity. Studies of the pandemic influenza indicate that respiratory viruses predispose to bacterial complication and interaction between viruses and bacteria in respiratory infections has been extensively reported in the literature [29], but the underlying mechanisms between viral and bacterial synergism are complex and remain unclear [30]. Common respiratory viral infections, such as influenza or respiratory syncytial virus have been linked to seasonal increases in Streptococcus pneumoniae disease [31]. The relationship between bacterial and viral infection is clouded by the low sensitivity of bacterial detection in sterile-site samples by traditional culture methods, and the reliance on non-specific clinical data for the for diagnosis of bacterial co-infection, including inflammatory markers, radiological findings and / or appropriate cultures, resulting in 30% of the cases in the GENDRES cohort and 55% in the UK cohort. Bacterial superinfection increased most measures of severity in both cohorts (PICU admission, respiratory support requirement, GENVIP score, hospital stay length and respiratory distress).
Interestingly, pneumococcal vaccination was revealed as an independent protective factor of disease severity in our patients. Pneumococcal vaccine reduced the severity of viral LT-ARIs through a reduction in oxygen requirement, invasive and non-invasive ventilation, admission to PICU, respiratory distress, and GENVIP score. A reduced incidence of viral alveolar pneumonia has been previously reported after pneumococcal vaccination [31, 32], although there was no demonstrable reduction in the number of confirmed pneumococcal infections. This is likely to reflect the limited sensitivity of culture-proven pneumococcal disease in pneumonia. The protective effect of pneumococcal vaccines found in our study might reinforce the importance of the paradigm of viral-penumococci interaction at nasopharynx level in the pathogenesis and clinical course of the disease [33] Current pneumococcal conjugate vaccines significantly decrease nasopharyngeal carriage of pneumococci [34] and thus reduces the possibility of this viral-pneumococci direct interaction.
One of the limitations of the present study is that our samples were not tested for viral load by quantitative PCR and the viral load of certain viruses–like RSV- has been associated with the co-infection status and the severity [3]. Also, the study did not consider milder or asymptomatic children. In addition, bacterial superinfection rate in our series might be overestimated as diagnosis was accepted as true even without microbiological confirmation, just based on referring physicians’ criteria.
Several studies had shown that viruses can be found in children with no respiratory infections [6, 35], and further research is needed to understand the natural history of respiratory viral carriage and infection. However, our findings were consistent in both independent cohorts very different between them, so this makes the outcomes more robust.
In summary, the severity and course of an acute respiratory episode requiring hospitalization in children did not correlate with the presence of one or more viruses. In contrast, bacterial co-infection was associated with more severe disease, whereas pneumococcal vaccination decreased severity. Future studies are needed to investigate whether particular viruses, or combinations of viruses, influence the risk of bacterial co-infection.
Supporting Information
(JPG)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We greatly thank all the patients that kindly contributed to this study. The authors thank the study investigators of GENDRES network (www.gendres.org). Miriam Cebey López, Antonio Salas Ellacuriaga, Ana Vega Gliemmo, José Peña Guitián, Alexa Regueiro, Antonio Justicia Grande, Leticia Pías Peleteiro, María López Sousa, María Jose de Castro, Carmen Curros Novo, Elena Rodrigo, Miriam Puente Puig, Rosaura Leis Trabazo, Nazareth Martinón Torres, Alberto Gómez Carballa, Jacobo Pardo Seco, Antonio Justicia Grande, José María Martinón Sánchez, Belén Mosquera Pérez, Isabel Villanueva González, Lorenzo Redondo Collazo, Carmen Rodríguez-Tenreiro, María del Sol Porto Silva and Federico Martinón Torres (Área Asistencial Integrada de Pediatría and GENVIP, Hospital Clínico Universitario, Santiago de Compostela); Máximo Francisco Fraga Rodríguez, Orlando Fernández Lago, José Ramón Antúnez (Biobank, Servicio Anatomía Patológica, Hospital Clínico Universitario, Santiago de Compostela); Enrique Bernaola Iturbe, Laura Moreno Galarraga, Jorge Álvarez (Hospital Materno Infantil Virgen del Camino, Pamplona); Teresa González López, Delfina Suarez Vázquez, Ángela Vázquez Vázquez, Susana Rey García (Complejo Hospitalario Universitario de Orense), Francisco Giménez Sánchez, Miguel Sánchez Forte (Hospital Torrecárdenas, Almería); Cristina Calvo Rey, María Luz García García (Hospital Severo Ochoa de Madrid); Ignacio Oulego Erroz, David Naranjo Vivas, Santiago Lapeña, Paula Alonso Quintela, Jorge Martínez Sáenz de Jubera, Estibaliz Garrido García (Hospital de León); Cristina Calvo Monge, Eider Oñate Vergara (Hospital de Donostia, San Sebastián); Jesús de la Cruz Moreno, Mª Carmen Martínez Padilla, Eugenia Villanueva Martínez, Ana González Espín (Complejo Hospitalario de Jaén); Manuel Baca Cots (Hospital Quirón, Málaga), David Moreno Pérez, Ana Cordón Martínez, Antonio Urda Cardona, José Miguel Ramos Fernández, Esmeralda Núñez Cuadros (Hospital Carlos Haya, Málaga); Susana Beatriz Reyes, María Cruz León León (Hospital Virgen de la Arrixaca, Murcia). Further details may be consulted at http://www.gendres.org or www.genvip.eu
We thank Stuart Gormley and the clinical team at St Mary’s hospital for assistance in recruiting the UK cohort.
We also extend our gratitude to the nursery and laboratory service from the Hospital Clínico Universitario de Santiago de Compostela for their effort.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Spanish Government (Research Program Health Research Fund (FIS; PI10/00540) National Plan I + D + I and FEDER funds), by Regional Galician funds (Promotion of Research Project 10 PXIB 918 184 PR) (FMT), by Ministerio de Ciencia e Innovación (SAF2011-26983), and by the Plan Galego IDT, Xunta de Galicia (EM 2012/045) (AS). A grant was received from the Sistema Universitario Gallego - Modalidad REDES (2014-PG139) from the Xunta de Galicia (to AS and FMT). MCL research activities have been supported by grants from Instituto de Investigación Sanitaria de Santiago de Compostela. FMT research activities have been supported by grants from Instituto Carlos III (Intensificación de la actividad investigadora). Investigators received funding from the European Union’s seventh Framework program under ECGA no. 279185 (EUCLIDS) during the production of this paper. JH was supported by the Imperial College Comprehensive Biomedical Research Centre and the Wellcome Trust Centre for Respiratory Infection at Imperial College (DMPED P26077 to JH). Micropathology Ltd. provided support in the form of salaries for authors ES and CF, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Kahn JS. Newly discovered respiratory viruses: significance and implications. Current opinion in pharmacology. 2007;7(5):478–83. Epub 2007/08/11. 10.1016/j.coph.2007.07.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freymuth F, Vabret A, Cuvillon-Nimal D, Simon S, Dina J, Legrand L, et al. Comparison of multiplex PCR assays and conventional techniques for the diagnostic of respiratory virus infections in children admitted to hospital with an acute respiratory illness. Journal of medical virology. 2006;78(11):1498–504. Epub 2006/09/26. 10.1002/jmv.20725 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhedin S, Lindstrand A, Rotzen-Ostlund M, Tolfvenstam T, Ohrmalm L, Rinder MR, et al. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics. 2014;133(3):e538–45. Epub 2014/02/26. 10.1542/peds.2013-3042 . [DOI] [PubMed] [Google Scholar]
- 4.Cebey-Lopez M, Herberg J, Pardo-Seco J, Gomez-Carballa A, Martinon-Torres N, Salas A, et al. Viral Co-Infections in Pediatric Patients Hospitalized with Lower Tract Acute Respiratory Infections. PloS one. 2015;10(9):e0136526 10.1371/journal.pone.0136526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asner SA, Science ME, Tran D, Smieja M, Merglen A, Mertz D. Clinical disease severity of respiratory viral co-infection versus single viral infection: a systematic review and meta-analysis. PloS one. 2014;9(6):e99392 Epub 2014/06/17. 10.1371/journal.pone.0099392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Zalm MM, van Ewijk BE, Wilbrink B, Uiterwaal CS, Wolfs TF, van der Ent CK. Respiratory pathogens in children with and without respiratory symptoms. J Pediatr. 2009;154(3):396–400,.e1. 10.1016/j.jpeds.2008.08.036 Epub Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonzel L, Tenenbaum T, Schroten H, Schildgen O, Schweitzer-Krantz S, Adams O . Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. Pediatr Infect Dis J. 2008;27(7):589–94. Epub 2008/06/04. 10.1097/INF.0b013e3181694fb9 . [DOI] [PubMed] [Google Scholar]
- 8.Richard N, Komurian-Pradel F, Javouhey E, Perret M, Rajoharison A, Bagnaud A, et al. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr Infect Dis J. 2008;27(3):213–7. Epub 2008/02/19. 10.1097/INF.0b013e31815b4935 . [DOI] [PubMed] [Google Scholar]
- 9.Semple MG, Cowell A, Dove W, Greensill J, McNamara PS, Halfhide C, et al. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191(3):382–6. Epub 2004 Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suryadevara M, Cummings E, Bonville CA, Bartholoma N, Riddell S, Kiska D, et al. Viral etiology of acute febrile respiratory illnesses in hospitalized children younger than 24 months. Clinical pediatrics. 2011;50(6):513–7. Epub 2011/01/26. 10.1177/0009922810394834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huijskens EG, Biesmans RC, Buiting AG, Obihara CC, Rossen JW. Diagnostic value of respiratory virus detection in symptomatic children using real-time PCR. Virol J. 2012;9:276 10.1186/743-422X-9-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand HK, de Groot R, Galama JM, Brouwer ML, Teuwen K, Hermans PW, et al. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr Pulmonol. 2012;47(4):393–400. 10.1002/ppul.21552 Epub 2011 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi EH, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis. 2006;43(5):585–92. Epub 2006 Jul 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf DG, Greenberg D, Kalkstein D, Shemer-Avni Y, Givon-Lavi N, Saleh N, et al. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr Infect Dis J. 2006;25(4):320–4. Epub 2006/03/29. 10.1097/01.inf.0000207395.80657.cf . [DOI] [PubMed] [Google Scholar]
- 15.Peng D, Zhao D, Liu J, Wang X, Yang K, Xicheng H, et al. Multipathogen infections in hospitalized children with acute respiratory infections. Virology journal. 2009;6:155 Epub 2009/10/01. 10.1186/1743-422X-6-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chorazy ML, Lebeck MG, McCarthy TA, Richter SS, Torner JC, Gray GC. Polymicrobial Acute Respiratory Infections in a Hospital-Based Pediatric Population. Pediatr Infect Dis J. 2013. Epub 2013/01/26. 10.1097/INF.0b013e31828683ce . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Garcia ML, Calvo C, Perez-Brena P, De Cea JM, Acosta B, Casas I. Prevalence and clinical characteristics of human metapneumovirus infections in hospitalized infants in Spain. Pediatric pulmonology. 2006;41(9):863–71. Epub 2006/07/20. 10.1002/ppul.20456 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aberle JH, Aberle SW, Pracher E, Hutter HP, Kundi M, Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr Infect Dis J. 2005;24(7):605–10. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida LM, Suzuki M, Nguyen HA, Le MN, Vu TD, Yoshino H, et al. Respiratory syncytial virus, its co-infection and paediatric lower respiratory infections. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2013. Epub 2013/05/07. 10.1183/09031936.00101812 . [DOI] [PubMed] [Google Scholar]
- 20.Jartti T, Aakula M, Mansbach JM, Piedra PA, Bergroth E, Koponen P, et al. Hospital Length-of-stay Is Associated With Rhinovirus Etiology of Bronchiolitis. Pediatr Infect Dis J. 2014;33(8):829–34. 10.1097/inf.0000000000000313 [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa K, Mansbach JM, Teach SJ, Fisher ES, Hershey D, Koh JY, et al. Multicenter Study of Viral Etiology and Relapse in Hospitalized Children with Bronchiolitis. Pediatr Infect Dis J. 2014. 10.1097/INF.0000000000000293 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadopoulos NG, Moustaki M, Tsolia M, Bossios A, Astra E, Prezerakou A, et al. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. American journal of respiratory and critical care medicine. 2002;165(9):1285–9. Epub 2002/05/07. 10.1164/rccm.200112-118BC . [DOI] [PubMed] [Google Scholar]
- 23.Mansbach JM, McAdam AJ, Clark S, Hain PD, Flood RG, Acholonu U, et al. Prospective multicenter study of the viral etiology of bronchiolitis in the emergency department. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2008;15(2):111–8. Epub 2008/02/16. 10.1111/j.1553-2712.2007.00034.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansbach JM, Piedra PA, Teach SJ, Sullivan AF, Forgey T, Clark S, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Archives of pediatrics & adolescent medicine. 2012;166(8):700–6. Epub 2012/04/05. 10.1001/archpediatrics.2011.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(36):12891–6. Epub 2005/08/25. 10.1073/pnas.0504666102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan BH, Lim EA, Seah SG, Loo LH, Tee NW, Lin RT, et al. The incidence of human bocavirus infection among children admitted to hospital in Singapore. Journal of medical virology. 2009;81(1):82–9. Epub 2008/11/26. 10.1002/jmv.21361 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung JY, Han TH, Kim CK, Kim SW. Bocavirus infection in hospitalized children, South Korea. Emerg Infect Dis. 2006;12(8):1254–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schildgen O, Muller A, Allander T, Mackay IM, Volz S, Kupfer B, et al. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clinical microbiology reviews. 2008;21(2):291–304, table of contents. Epub 2008/04/11. 10.1128/CMR.00030-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosch AA, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS pathogens. 2013;9(1):e1003057 Epub 2013/01/18. 10.1371/journal.ppat.1003057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicoli EJ, Trotter CL, Turner KM, Colijn C, Waight P, Miller E. Influenza and RSV make a modest contribution to invasive pneumococcal disease incidence in the UK. The Journal of infection. 2013;66(6):512–20. Epub 2013/03/12. 10.1016/j.jinf.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberger DM, Givon-Lavi N, Shemer-Avni Y, Bar-Ziv J, Alonso WJ, Greenberg D, et al. Influence of pneumococcal vaccines and respiratory syncytial virus on alveolar pneumonia, Israel. Emerging infectious diseases. 2013;19(7):1084–91. Epub 2013/06/15. 10.3201/eid1907.121625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madhi SA, Klugman KP, Vaccine Trialist G. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nature medicine. 2004;10(8):811–3. 10.1038/nm1077 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith CM, Sandrini S, Datta S, Freestone P, Shafeeq S, Radhakrishnan P, et al. Respiratory syncytial virus increases the virulence of Streptococcus pneumoniae by binding to penicillin binding protein 1a. A new paradigm in respiratory infection. American journal of respiratory and critical care medicine. 2014;190(2):196–207. Epub 2014/06/19. 10.1164/rccm.201311-2110OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azzari C, Martinon-Torres F, Schmitt HJ, Dagan R. Evolving role of 13-valent pneumococcal conjugate vaccine in clinical practice. Pediatr Infect Dis J. 2014;33(8):858–64. Epub 2014/03/13. 10.1097/inf.0000000000000328 . [DOI] [PubMed] [Google Scholar]
- 35.Advani S, Sengupta A, Forman M, Valsamakis A, Milstone AM. Detecting respiratory viruses in asymptomatic children. Pediatr Infect Dis J. 2012;31(12):1221–6. Epub 2012/06/29. 10.1097/INF.0b013e318265a804 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(JPG)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


