Abstract
Background
Low-dose aspirin has been shown to reduce the incidence of cancer, but its role in the treatment of cancer is uncertain.
Objectives
We conducted a systematic search of the scientific literature on aspirin taken by patients following a diagnosis of cancer, together with appropriate meta-analyses.
Methods
Searches were completed in Medline and Embase in December 2015 using a pre-defined search strategy. References and abstracts of all the selected papers were scanned and expert colleagues were contacted for additional studies. Two reviewers applied pre-determined eligibility criteria (cross-sectional, cohort and controlled studies, and aspirin taken after a diagnosis of cancer), assessed study quality and extracted data on cancer cause-specific deaths, overall mortality and incidence of metastases. Random effects meta-analyses and planned sub-group analyses were completed separately for observational and experimental studies. Heterogeneity and publication bias were assessed in sensitivity analyses and appropriate omissions made. Papers were examined for any reference to bleeding and authors of the papers were contacted and questioned.
Results
Five reports of randomised trials were identified, together with forty two observational studies: sixteen on colorectal cancer, ten on breast and ten on prostate cancer mortality. Pooling of eleven observational reports of the effect of aspirin on cause-specific mortality from colon cancer, after the omission of one report identified on the basis of sensitivity analyses, gave a hazard ratio (HR) of 0.76 (95% CI 0.66, 0.88) with reduced heterogeneity (P = 0.04). The cause specific mortality in five reports of patients with breast cancer showed significant heterogeneity (P<0.0005) but the omission of one outlying study reduced heterogeneity (P = 0.19) and led to an HR = 0.87 (95% CI 0.69, 1.09). Heterogeneity between nine studies of prostate cancer was significant, but again, the omission of one study led to acceptable homogeneity (P = 0.26) and an overall HR = 0.89 (95% CI 0.79–0.99). Six single studies of other cancers suggested reductions in cause specific mortality by aspirin, and in five the effect is statistically significant. There were no significant differences between the pooled HRs for the three main cancers and after the omission of three reports already identified in sensitivity analyses heterogeneity was removed and revealed an overall HR of 0.83 (95% CI 0.76–0.90). A mutation of PIK3CA was present in about 20% of patients, and appeared to explain most of the reduction in colon cancer mortality by aspirin. Data were not adequate to examine the importance of this or any other marker in the effect of aspirin in the other cancers. On bleeding attributable to aspirin two reports stated that there had been no side effect or bleeding attributable to aspirin. Authors on the other reports were written to and 21 replied stating that no data on bleeding were available.
Conclusions and Implications
The study highlights the need for randomised trials of aspirin treatment in a variety of cancers. While these are awaited there is an urgent need for evidence from observational studies of aspirin and the less common cancers, and for more evidence of the relevance of possible bio-markers of the aspirin effect on a wide variety of cancers. In the meantime it is urged that patients in whom a cancer is diagnosed should be given details of this research, together with its limitations, to enable each to make an informed decision as to whether or not to take low-dose aspirin.
Systematic Review Protocol Number
CRD42015014145
Introduction
Despite significant advances in diagnosis and treatment in recent decades, cancer is still one of the main causes of morbidity and mortality worldwide. It claims more than 81,000 males and 74,000 females every year in the UK alone, the crude annual mortality rate being about 250 deaths in every 100,000 people [1]. Much effort is now being focused on ‘targeted cancer therapies’, that is, drugs that interfere with specific molecules involved in cancer cell growth and cell survival, but as yet there have been few successes.
There is convincing evidence that regular low-dose aspirin not only reduces vascular disease incidence and mortality [2,3], but also reduces the incidence and mortality of colorectal and other cancers [4–7]. Furthermore, there is growing evidence which suggests that aspirin, used as an adjuvant treatment following a diagnosis of cancer, may reduce metastatic spread and may increase the survival of patients with cancer.
Chan et al (2009) [8], Langley (2011) [9,10] and others have pointed out that effects of aspirin on certain biological mechanisms justify an expectation of benefit from aspirin used as an adjunct treatment of patients with cancer. These effects include an interruption of tumour growth, a retardation of metastatic spread, an inhibition of angiogenesis, enhancements of both DNA mismatch repair and cellular apoptosis and an abrogation of invasiveness. Benefit from treatment with aspirin is therefore not unexpected.
Our aim in what follows is to provide a comprehensive systematic review and meta-analysis of the available evidence on the effects of aspirin used as an adjunct treatment of cancer in the reduction of mortality and metastatic spread.
Methods
The review protocol was registered in PROSPERO (registration number CRD42015014145). In reporting we have followed the PRISMA guidelines [11].
In December 2015 observational and interventional studies in Medline and Embase were searched using a pre-defined strategy with indexed descriptors and keywords including “aspirin”, “acetylsalicylic acid”, “cancer” “tumour”, “neoplasm”, “mortality”, “death”, “adverse effect”, “bleed”. The search was limited to human studies in peer-reviewed journals and conference abstracts. Reference lists of the included studies were also searched and recent conference proceedings scanned and topic experts contacted for additional studies.
Studies were selected for inclusion in meta-analyses if (a) the studied population comprised patients diagnosed with cancer; (b) aspirin was taken regularly after cancer diagnosis independently of whether it had been taken before diagnosis; (c) they were case-control studies, cohort studies or controlled trials; and (d) cause-specific mortality was available. All-cause cancer mortality, incidence of metastases and adverse effects were noted but were not criteria for selection.
Two reviewers independently excluded reports that did not meet inclusion criteria based on title and abstract. Full published reports were obtained for the remainder, and inclusion criteria were applied.
The origin of the patient group and other details in each report were examined, and if there appeared to be two reports based on the same patients, if the evidence required for the meta-analyses was not clear, or if important items were missing, the author(s) was contacted and asked for clarification. Authors of all the papers were also asked whether they had data on gastrointestinal or other bleeding, and if this had been a concern at any time. All these processes were conducted by one of the authors and were checked as appropriate by another author.
The methodological quality of the included studies was assessed and graded independently by two authors using the Newcastle-Ottawa Scale [12]. Differences in grading of reports on a nine point scale, were discussed and agreed.
Meta-analyses were conducted grouping the studies according to study design: intervention and observational studies. Subgroup analyses were conducted according to cancer types, key mutations and whether or not patients had taken aspirin only after diagnosis.
The summary statistics derived in the meta-analyses were either a hazard ratio or a risk ratio each with 95% confidence intervals. The analyses were carried out using the statistical package STATA. The inverse-variance method was used to weight the individual studies and provide the pooled estimate of effects. A 'random effects' model was used throughout to incorporate an estimate of between-study variation into the calculation of common effects. Funnel plots were created to highlight outlying studies and look at publication bias. Publication bias was assessed using Egger's test [13]. Sensitivity analyses were performed to assess the influence of individual studies on the combined hazard ratios. Heterogeneity was assessed using the Q statistic and investigated by repeating the meta-analyses excluding, first low scoring studies and then, if substantial heterogeneity was still present, outlying studies, identified by the sensitivity analyses were omitted.
Results
The literature search identified 373 reports and following omissions of duplicates and irrelevant reports, 42 were found to be relevant and gave sufficient data to be included in meta-analyses. We present a summary diagram in Fig 1 showing the selection process.
Fig 1. Prisma flow diagram.
Table 1 summarises a few basic features of the papers included in the meta-analyses together with those upon which further investigations are based. A final column gives an assessment of quality of the studies, judged according to the Newcastle-Ottawa Scale [12].
Table 1. Details of the studies.
| Authors | Source | Design | Number of aspirin users and non-users. Duration of follow-up | Deaths in aspirin users and all-cause deaths | Comment | Grade |
|---|---|---|---|---|---|---|
| Randomised controlled trials | ||||||
| Rothwell et al. [14] | Five early vascular trials | Randomised for vascular reduction | 17,285 subjects randomised | 385 deaths on aspirin, 402 deaths on placebo | RCT | |
| Lipton et al. [15] | Series of patients | Ad hoc randomisation | 57 patients randomised, F-U 24 months | Life table analysis, Numbers N.A. | RCT | |
| LeBeau et al. [16] | Series of patients | Ad hoc randomisation | 303 patients randomised, F-U 18 months | 152 deaths on aspirin, 147 deaths on placebo | RCT | |
| Cregan et al. [17] | Series of patients | Ad-hoc randomisation of patients with renal cancer | 176 patients randomised, F-U 8.8 months | 52 total deaths on aspirin, 56 deaths on placebo | RCT | |
| Liu et al. [18] | Sequence of patients | Randomised by admission to ward | 445 users, 1153 non-users, F-U 5 years | 217 deaths in users, 685 deaths in non-users | RCT | |
| Reports of colorectal cancer | ||||||
| Bastiaannet et al. [19] | Eindhoven Cancer Registry | Cohort of 4481 patients with cancer | 3305 users, 1176 non-users, F-U N.A. | 114 CRC deaths in users, 610 deaths in non-users | Most appear to have had aspirin, not other NSAIDs | 9 |
| Bains et al [20] | Cancer Registry of Norway | Cohort of 25644 patients with cancer | 6109 users, Non users N.A. | 1172 CRC deaths in users, 6356 CRC deaths in non-users, 2088 total deaths in users, 7595 total deaths in non-users | Conference presentation | 7 |
| Cardwell et al. [21] | UK Clinical Practice Research Datalink | Nested case-control in a cohort of 4794 patients with cancer | Numbers N.A. Mean F-U 7.2 years | 395 CRC deaths in users, 1164 deaths in non-users, 700 total deaths in users, 1514 total deaths in non-users | 9 | |
| Chan et al. [8] | US Nurses and Health Professionals Cohorts | Cohort of patients with cancer | 549 users, 730 non-users. Median F-U 11.8 years | 81 CRC deaths in users, 141 CRC deaths in non-users, 193 total deaths in users, 287 total deaths in non-users | Varied dose of aspirin judged by frequency | 8 |
| Coghill et al. [22] | Seattle Cancer Family Register | Cohort of patients with cancer | 234 users, 293 non-users. Mean F-U 8 years | 37 events in users, 72 events in non-users | 9 | |
| Din et al [23] | Series of cases of cancer | Case-control selected patients from a trial cohort | 354 users, 526 non-users. F-U 1 years | 125 deaths in users, 761 in non-users | NSAIDS, but data for aspirin given | 6 |
| Domingo et al. [24] | Series of patients | Cohort study | 125 users, 771 non-users. F-U N.A. | 22 deaths in users, 174 in non-users | Incidence and all-cause mortality in relation to PIK3CA state | 8 |
| Fuchs et al. [25] | Series of patients | Cohort study | 72 users, 830 users. Mean F-U 2.4 years | Numbers of deaths N.A. | 5 | |
| Goh et al. [26] | Series of patients | Cohort study | 92 users, 634 non-users. F-U ‘long term’ | 21 CRC deaths in users, 160 CRC deaths in non-users | 9 | |
| Liao et al. [27] | Nurses HS and Health Professionals Cohorts | Selected cohort of patients | 337 users, 627 non-users. Mean F-U 5 years | 68 CRC deaths in users, 122 CRC deaths in non-users | 7 | |
| McCowan et al. [28] | Database of residents | Cohort of selected new patients | 1340 users, 1650 non-users, F-U 11 years | 420 CRC deaths in users, 601 CRC deaths in non-users, 897 total deaths in users, 1101 total deaths in non-users | 9 | |
| Ng et al. [29] | Series of patients | Cohort study | 75 users, 725 non-users. F-U 5 years | 19 CRC recurrence in users, 21, CRC recurrence in non-users., 14 total deaths in users, 146 total deaths in non-users | 7 | |
| Reimers et al [30] | Cohort of study of cancer patients | 178 users, 784 non-users. F-U N.A. | 68 deaths in users, 380 deaths in non-users | HLA class 1 antigen groups amalgamated | 9 | |
| Sun et al. [31] | US Nurses and Health Professionals cohorts | cohort of selected cancer patients | 931 subjects. Other details N.A. F-U 28 years | 931 incident cases. Detailed numbers N.A. | 3 | |
| Walker et al. [32] | UK GP Research Database | Cohort of selected patients | 476 users, 10141 non-users. Median F-U 1.7 years | 192 total deaths in users, 3910 total deaths in non-users | 9 | |
| Zanders et al [33] | Eindhoven Cancer Registry | Cohort of selected patients with diabetes | 490 users, 156 non-users. F-U 1.5 years | Numbers N.A. | Diabetic patients | 9 |
| Reports of breast cancer | ||||||
| Barron et al [34] | Ireland National Cancer Registry | Cohort of 12507 patients with cancer | 764users, 4540 non-users. F-U 7.4 years | 50 breast cancer deaths in users, 311 breast cancer deaths in non-users, 311 total deaths in users, 459 total deaths in non-users | 7 | |
| Blair et al [35] | Iowa Women’s Health Study | Cohort of 591 women with cancer | 472 users, 120 non-users, F-U 15 years | 26 breast cancer deaths in users, 22 breast cancer deaths in non-users, 57 total deaths in users, 44 total deaths in non-users | 8 | |
| Bowers et al [36] | A Centre for Cancer Care | Cohort of 440 women with cancer | 159 users, 281 non-users. F-U N.A. | Number of deaths not available | NSAIDs. 81% were aspirin | 7 |
| Cronon-Fenton [37] | Population based cohort in Denmark | Cohort study of 34188 patients | Median F-U 7.1 years | Numbers N.A. | Conference report | 6 |
| Frazer et al. [38] | Database of residents | Cohort of 4627 women | 1244 users, 3383 non-users. F-U 16 years | 252 breast cancer deaths in users, 563 breast cancer deaths in non-users, 577 total cancer deaths in users, 1225 total cancer in non-users | 8 | |
| Holmes et al. [39] | US Nurses Health Study | Cohort of 4164 women | Number of users N.A. 5521 non-users. F-U n.a. | 109 breast cancer deaths in users, 56 breast cancer deaths in non-users | 8 | |
| Holmes et al. [40] | National Cancer Registry | Nested case-control within 27426 women | 1661 users, 3322 non-users. F-U up to 5 years | 395 breast cancer deaths in users, 750 breast cancer deaths in non-users | 9 | |
| Kwan et al. [41] | Cohort of cancer patients | Cohort of 2292 women | Total 2292 women. Mean F-U 2.5 years | 41 recurrent cancers in users, 209 recurrent cancers in non-users | NSAIDs | 8 |
| Murray et al. [42] | UK Clinical Practice Research Datalink | Nested case-control study | 1173 users, 1173 non-users. Mean F-U 6.9 years | 262/1435 cancer deaths in users, 1056/5697 cancer deaths in non-users | 9 | |
| Wernli et al. [43] | Cohort of cancer survivors | Cohort of 3058 selected patients with breast cancer | 541 users of NSAIDs, 2517 non-users of NSAIDs. F-U 6 years approx. | 7 breast cancer deaths in users, 141 breast cancer deaths in non-users, 37 total deaths in users, 383 total deaths in non-users | NSAIDs | 7 |
| Reports of prostate cancer | ||||||
| Assayaq et al [44] | UK National Cancer Data Repository | Cohort of 11779 newly diagnosed patients | Numbers in users N.A. F-U 5.4 years | 801 cancer deaths in users, 992 cancer deaths in non-users, 1816 total deaths in users, 1686 deaths in non-users | 9 | |
| Caon et al. [45] | Patient series | Cohort of newly 3851 diagnosed patients | 509 users, 2428 non-users, F-U 7 years | 194 cancer deaths in users, 904 cancer deaths in non-users | 8 | |
| Choe et al. [46] | Patient registry | Cohort of 5955 patients | 1817 users, 1736 no relevant drugs. Medium F-U 70 months | 36 cancer deaths in users, 298 cancer ca deaths in non-users | 7 | |
| Daugherty et al. [47] | Screened cohort | Cohort of patients with cancer | Numbers N.A. Medium F-U 5 years | 136 cancer deaths | 8 | |
| Dhillon et al. [48] | US Health Professionals cohort | Cohort study | 1579 users, 1926 non-users, F-U up to 18 years | 177 cancer deaths. Details N.A. | 8 | |
| Flahavan [49] | Irish National Cancer Registry | Cohort study of 2936 | 1131 users, 1805 non-users. Median F-U 5.5 years | Numbers of deaths N.A. | 8 | |
| Grytli et al. [50] | Cancer Registry of Norway | Cohort of selected patients | 1279 users, 3515 non-users. Mean F-U 39 months | 504 cancer deaths in users | 9 | |
| Jacobs, Chun et al. [51] | Series of patients | Cohort study | 45 users, 29 non-users. Mean F-U 56.6 months | 6 cancer deaths in users, 8 cancer deaths in non-users | 6 | |
| Jacobs, Newton et al. [52] | Prospective cohort of subjects | New cancer patients. Also ‘High-risk’ patients | 3600 users, 3058 non-users, F-U up to 9 years | 134 cancer deaths in users, 112 cancer deaths in non-users | 8 | |
| Stock et al. [53] | Cancer Registry | cohort of selected cancer patients | 419 users, 1200 non-users. Maximum F-U 120 months | 115 cancer deaths in users, 338 cancer deaths in non-users | NSAIDs | 9 |
| Reports of other cancers | ||||||
| Nagle et al [54] | Series of women with ovarian cancer | Cohort study of 1305 women with ovarian cancer | Numbers N.A., F-U 4.9 years | 834 deaths | 6 | |
| Fontaine et al. [55] | Series of patients with lung cancer | Cohort study of women with lung cancer | 412 users, 1353 non-users, F-U 7.5 years | Numbers of deaths N.A. | 7 | |
| Pastore et al. [56] | Series of patients with bladder cancer | Cohort of 574 patients with bladder cancer | 98 users, 56 non users F-U 2 years | Numbers of deaths N.A. | 8 | |
| Chae et al. [57] | 536 patients with mixed cancers | Cohort of 536 women with mixed cancers | 54 users, 482 non-users. Median F-U 8.8 months | Numbers of deaths N.A. | 4 | |
| Chae et al [58] | Patients with relapsed/refractory chronic lymphocytic leukaemia | Retrospective study of 280 patients with chronic lymphocytic leukaemia | 37 users, 17 non-users. Median F-U 4 years | Numbers of deaths N.A. | 5 | |
| MacFarlane et al [59] | Series of 2392 patients with head and neck and oesophageal cancers | Cohort study of 2392 patients with oesophagus cancer; 1195 with head & neck cancer | 1197 oesophagus, F-U 9 months, 1195 head & neck, F-U 35 months | 965 oesophagus cancer deaths, 509 head & neck deaths cancer. Details N.A. | 7 | |
| Reports included in other tables | ||||||
| Algra & Rothwell [4] | Based on a literature search | Overviews of 6 RCTs; 150 case-control studies, and 45 cohort studies | In case-control studies. Followed for up to 20 years | 245 in RCTs; 141,577 in case-control studies, 41,575 in cohort studies | Details on metastatic spread in RCTs and in 5 observational studies | 9 |
| Ljung et al. [60] | National Cancer Registry | Selected patient cohort | 3424users, 23104 non-users, F-U 5 years | Numbers with lymph node metastases. Numbers N.A. | 8 | |
| Kothari et al. [61] | Two cancer centres | Series of selected 999 patients with colon cancer | 49 users, 136 non-users. Mean F-U 54 months | Detail of deaths N.A. | 7 | |
CI: confidence interval; CRC: Colorectal cancer;; F-U: Follow-up; N.A.: not available; NSAID: non-steroidal anti-inflammatory drug; RCT: randomised controlled trial; RR: risk ratio.
In the tables that follow we summarise the individual papers and report the results of meta-analyses and when available we give data for both cause-specific mortality and all-cause mortality.
Aspirin, specific and overall mortality
Our search identified four reports of randomised trials, together with a report of pooled trials [14] (Table 2). The ad hoc trials [15–18] were small and the results did not achieve significance. The report by Rothwell et al (2012) [14] describes a 6.5 year follow-up of five early vascular trials, during which time 987 new cancers developed. In these, aspirin was associated with a reduction in cancer deaths (HR 0.71; 95% confidence limits (CI) 0.57, 0.90). The effect of aspirin in all five trials together is homogeneous (P = 0.30), but this result should be taken with caution as clinical heterogeneity such as differences between the design of the studies, the patient populations etc. may be too great to justify the pooling of results.
Table 2. Mortality in randomized trial patients with cancer who took aspirin versus placebo/no-aspirin.
| Study | Design | Cancer | Aspirin/none | Outcome | Numbers of outcome events aspirin/placebo | Effect of aspirin (95% CI) |
|---|---|---|---|---|---|---|
| Rothwell Wilson [14] | Pooled analysis of five RCTs | All solid cancers | 385.402 | Cancer deaths | 385,402 | HR 0.71 (0.57,0.90) |
| All deaths | N.A. | HR 0.81 (0.65, 1.00) | ||||
| Lipton [15] | RCT | Colorectal | 35,22 | Cause-specific mortality | N.A. | HR 0.65 (0.02–18.06) a |
| Lebeau [16] | RCT | Lung | 153/150 | Cause-specific mortality | 152,147 | HR 1.01 (0.81–1.27) a |
| Cregan [17] | RCT | Renal | 89/87 | Cause-specific mortality | 56,57 | HR 0.91 (0.63–1.31) a |
| Liu et al [18] | RCT b | Oesophagus | 445/658 | Cause-specific mortality | 217,388 | HR 0.83 (0.68, 1.01) |
| Cause specific mortality: HR 0.85 (0.74–0.97) heterogeneity p = 0.30 | ||||||
| All-cause mortality: HR 0.81 (0.65–1.00) Rothwell et al [14] alone | ||||||
CI: confidence interval; HR Hazard Ratio; HR: hazard ratio; RCT: randomised controlled trial.
aHazard ratios taken from Langley [20]
bRandomisation was achieved by admitting patients to two different wards in which aspirin and placebo were given.
In Table 3 data from observational studies are listed within three main groups: 16 on colorectal cancer [8,19–32], 10 on breast [34–43] and ten on prostate cancers [44–53], and then data relating to six other cancers [54–59]. A column contains comments of possible relevance on some of the reports of possible relevance.
Table 3. Results of aspirin treatment of cancer in observational studies.
| Study | Aspirin/none | Mortality | Deaths (aspirin, no aspirin) | Results (95% CI) | Comment |
|---|---|---|---|---|---|
| Colorectal cancer | |||||
| Bastiaannet et al [19] | 275/ 1176 | All-cause | 114, 610 | HR 0.77 (0.63, 0.95) | Frequent use HR 0.70 (0.57, 0.88) |
| Bains et al [20] | 6109/19535 | Specific | 1172, 6356 | HR 0.53 (0.50, 0.57) | |
| All-cause | 2088, 7595 | HR 0.71 (0.68, 0.75) | |||
| Cardwell et al [21] | 1005/ 2365 | Specific | 395, 1164 | HR 0.99 (0.86, 1.15) | |
| Chan et al [8] | 549/1279 | Specific mort | 81, 141 | HR 0.71 (0.53, 0.95) | Specific: Only post diagnosis: RR 0.53 (0.33, 0.86). Pre and post: RR 0.89 (0.59, 1.35) |
| All-cause | 193, 287 | HR 0.79 (0.65, 0.97) | All-cause: Only post diagnosis RR 0.68 (0.61, 0.92). Pre and post RR 0.95 (0.71, 1.28) | ||
| Coghill et al [22] | 56/346 | Cause specific | 37, 72 | HR 0.76 (0.61, 0.95) | |
| Din et al [23] | 354/526 | Cause specific | 125, 761 | OR 0.78 (0.65, 0.92) | Aspirin result; also data on NSAIDs a |
| Domingo et al [24] | 125/761 | Recurrence | 22, 174 | HR 0.86 (0.55–1.35) | Wild and mutated combined |
| All-cause | HR 0.88 (0.53, 1.47) | ||||
| Fuchs et al [25] | 72/830 | Recurrence or death | N.A. | HR 0.48 (0.24, 0.99) | Compared with non-consistent use. Consistent users: HR 0.45 (0.21, 0.97) for disease recurrence |
| All-cause | HR 0.52 (0.19, 1.46) | ||||
| Goh et al [26] | 92/726 | Specific | 21, 160 | HR 0.71 (0.43, 1.16) | Death or recurrence 0.38 (0.17, 0.84). Benefit only after 5 years) |
| Liao et al [27] | 155/395 | Specific | 68, 122 | HR 0.83(0.61–1.23) | Wild and mutated combined |
| 403/964 | All-cause | HR 0.87 (0.71, 1.06) | |||
| McCowan et al [28] | 894/2980 | Specific | 420, 601 | HR 0.58 (0.45, 0.75) | |
| All-cause | 897, 1101 | HR 0.67 (0.57, 0.79) | |||
| Ng et al [29] | 75/724 | Recurrence or death | 19, 214 | HR0.68 (0.42, 1.11) | Consistent aspirin HR 0.51 (0.28, 0.95) |
| Overall mortality | 14, 146 | HR 0.63 (0.35, 1.12) | |||
| Reimers et al [30] | 178/784 | Overall mortality | 68, 380 | RR 0.67 (0.52, 0.88) c | HLA class 1 antigen groups amalgamated |
| Sun et al [31] | ?/931 | Cancer specific survival | 931 total events | HR 0.77 (0.52, 1.14) | CTNNBI mutated and non- mutated groups combined |
| Walker et al [32] | 2619/13,994 | All-cause | 192, 3910 | HR 0.91 (0.82, 1.00) | No aspirin pre diagnosis: HR 0.99 (0.84, 1.16); aspirin pre diagnosis: HR 0.86 (0.76, 0.98) |
| Zanders et al [33] | 490/ | All-cause | N.A. | HR 0.98 (0.93, 1.03) | Diabetic patients |
| Colorectal specific mortality: HR 0.71 (0.58, 0.87) heterogeneity p = 0.0005 | |||||
| With Bains omitted b: HR 0.76 (0.66, 0.88) heterogeneity p = 0.035 | |||||
| All-cause mortality: HR 0.80 (0.70, 0.92) heterogeneity p = 0.0005; no omission removes significant heterogeneity | |||||
| Breast cancer | |||||
| Barron et al [34] | 764/4540 | Specific | 50, 311 | HR 0.98 (0.74, 1.30) | Selected de-novo aspirin users |
| Total | 311,495 | HR 1.11 (0.83, 1.50) | |||
| Blair et al [35] | 254/591 | Specific | 26, 22 | HR 0.53 (0.30,-0.93) | Selected overweight women |
| All-cause | 57, 44 | HR 0.53 (0.36–0.79) | |||
| Bowers et al [36] | 159/440 | Recurrence | N.A. | OR 0.48 (0.22, 0.98) | NSAIDs, 81% of which are stated to be aspirin a |
| Cronin-Fenton et al [37] | Recurrence | N.A. | HR 1.0 (0.90, 1.1) | Conference report | |
| Frazer et al [38] | 815/1802 | Specific | 252, 563 | HR 0.42 (0.31–0.55) | |
| All-cause | 577, 1225 | HR 0.53 (0.45–0.63) | |||
| Holmes et al. [39] | ?4164/11416 person/years | Specific | 109, 56 | RR 0.36 (0.24–0.54) | |
| All-cause | RR 0.54 (0.41–0.70) | ||||
| Holmes et al [40] | 1661 cases 3322 controls | Specific | 395, 750 | HR 0.96 (0.80, 1.16) | |
| Kwan et al [41] | 270/2292 | Recurrence | 41, 209 | RR 1.09 (0.74, 1.61) | |
| Murray et al [42] | 262/1435 | Specific | 262, 1435 | OR 1.00 (0.71, 1.41) | ‘High’ dose aspirin 0.94 (0.48, 1.84), but dose imprecise |
| Wernli et al [43] | 7 breast cancer deaths | Specific | 7, 141 | HR 0.64 (0.27, 1.37) | |
| 37 total deaths | All-cause | 37, 383 | HR 0.91 (0.65, 1.29) | ||
| Breast specific mortality: HR 0.69 (0.46, 1.02), heterogeneity p<0.0005 | |||||
| With Frazer omitted b: HR 0.87 (0.69, 1.09), heterogeneity p = 0.186 | |||||
| All-cause mortality: HR 0.73 (0.49, 1.08), heterogeneity p<0.0005; no omission removes significant heterogeneity | |||||
| Prostate cancer | |||||
| Assayag et al [44] | 801/1793 | Specific | 801, 992 | 1.46 (1.29, 1.65) | Aspirin only after diagnosis: HR 1.84 (1.59, 2.12) cause specific; HR 1.70 (1.53, 1.88) all-cause. Aspirin also before diagnosis: HR 0.97 (0.81. 1.16) cause specific; HR 0.98 (0.87, 1.18) all-cause |
| 1686/3502 | All-cause | 1816, 1686 | 1.37 (1.26, 1.50) | ||
| Caon et al [45] | 917/3851 | Cause specific | 194, 904 | HR 0.91 (0.65, 1.28) | |
| Choe et al [46] | 1817/5552 | Cause-specific | 36, 298 | HR 0.43 (0.21–0.87) | |
| Daugherty et al [47] | 136 | Cause specific | 136 total | HR 0.77 (0.48, 1.25) | Advanced disease: HR 0.37 (0.15, 0.92). Localised disease: HR 0.86 (0.47, 1.58) |
| Dhillon et al [48] | N.A. | Cause specific | 177 total | HR 1.08 (0.76–1.54) | |
| Flahavan et al [49] | 1131/2936 | Cause-specific | N.A. | HR 0.88 (0.67, 1.15) | High aspirin: HR 0.73 (0.51, 1.05) |
| Gryll et al [50] | 504/3165 | Cause-specific | 504, N.A. | HR 0.94 (0.78, 1.14) | |
| Jacobs Chun et al [51] | 41/74 | All-cause | 6, 8 | HR 0.44 (0.15–1.28) | High risk patients selected |
| Jacobs, Newton et al [52] | 301/7118 | Cause specific | 134, 112 | HR 0.98 (0.74, 1.29) | In high risk patients: HR 0.60 (0.37, 0.97) |
| Stock et al [53] | 453/1,619 | Cause specific | 115, 338 | HR 1.03 (0.79, 1.34) | Survival after 5yrs of NSAIDs: HR 0.54 (0.26, 1.13) |
| Prostate specific mortality: HR 0.94 (0.76, 1.17), heterogeneity p<0.0005 | |||||
| With Assayag omitted b: HR 0.89 (0.79, 0.99) heterogeneity p = 0.261 | |||||
| All-cause mortality: HR 0.89 (0.30, 2.61), heterogeneity p = 0.04, Only two studies | |||||
| Other cancers | |||||
| Nagle et al [54] | N.A. (Ovarian) | Overall survival | 115,338 | HR 0.92 (0.81. 1.06) | Aspirin plus NSAIDs |
| Fontaine et al [55] | 412/1,765 (Lung) | Survival | N.A. | HR 0.84 | |
| Pastore et al [56] | 574 (Bladder) | Recurrence | 42,98 | OR 0.75 (0.45, 1.24) | Effect of aspirin negated by statins |
| Chae et al [57] | 536 (Mix of female cancers) | Survival | N.A. | HR 0.82 (0.57, 1.18) | PIK mutation: HR 0.59 (0.35, 0.98).Wild type: HR 1.80 (1.01, 3.23) |
| Chae et al [58] | 280 (chronic lymphocytic leukaemia) | Survival | N.A. | HR 0.40 (0.21, 0.79). | Aspirin + statins together |
| MacFarlane et al [59] | 416/779 (head & neck) | Survival | 178/331 all-cause deaths | HR 0.56 (0.44, 0.71) | Post-diagnostic aspirin |
| 387/810 (Oesophagus) | Survival | 209/756 all-cause deaths | HR 0.54 (0.45. 0.64) | ||
CI: confidence interval; HR: hazard Ratio; N.A.: not available; NSAID: non-steroidal anti-inflammatory drug; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio.
aThe inclusion of NSAIDs other than aspirin posed difficulties but we assumed that aspirin was the major drug used, and evidence for this is given in one of the studies [36].
bThe examination of heterogeneity by the omission of papers was based on sensitivity analyses. The Newcastle-Ottawa grade of Frazer et al [38] and Assayaq et al [44] were both 9/10 and correspondence with the authors revealed no likely reason the heterogeneity. The data for Bains et al (Grade 7/10) is taken from a poster presentation and details of adjustments for confounding appear to be limited. Attempts to correspond with the author failed.
cReimers [30] reported a risk ratio and could not be included in the meta-analysis.
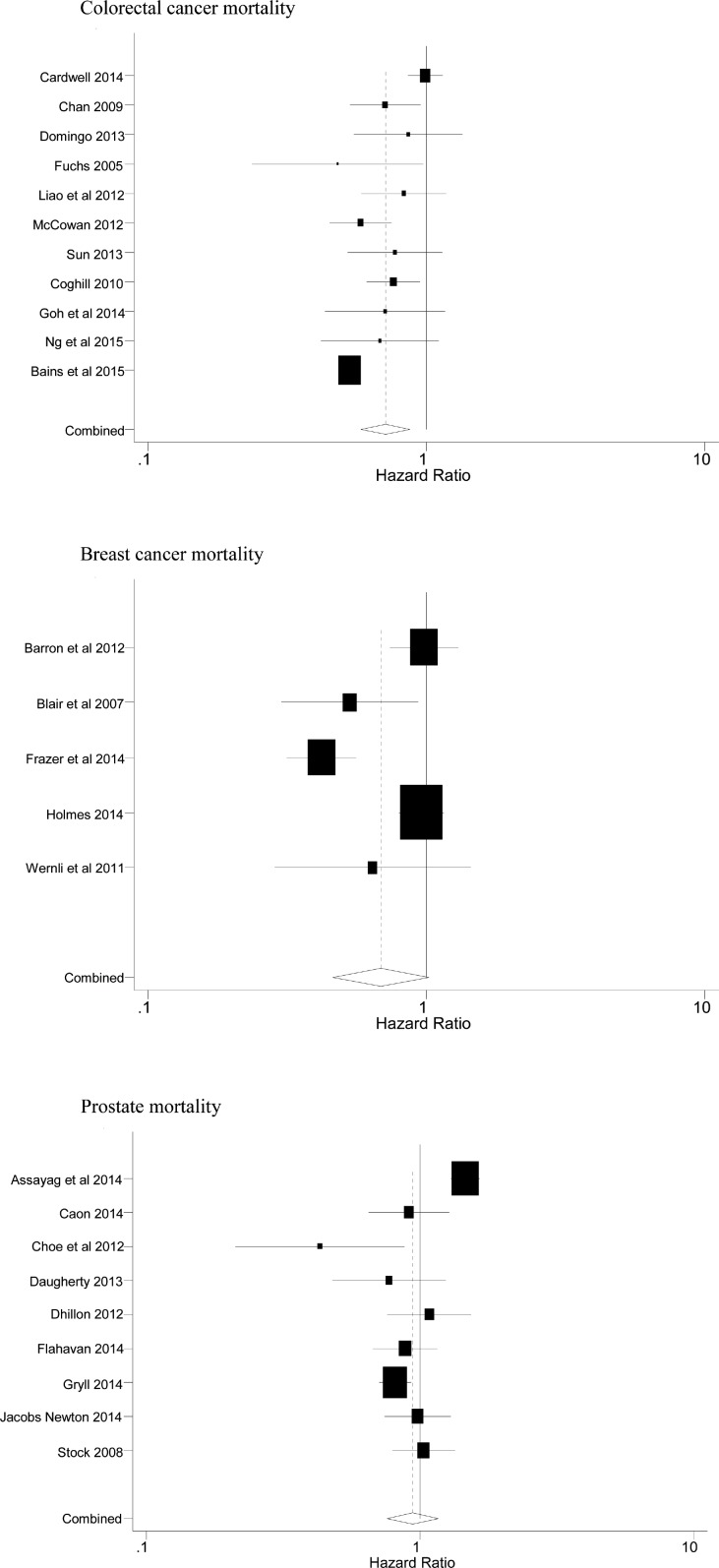
The sixteen reports of patients with colorectal cancer [8,19–33] give a pooled hazard ratio (HR) of 0.71 (95% CI 0.58, 0.87) for cause specific mortality (11 reports) and there is marked heterogeneity. Sensitivity analyses identified Bains [20] and when omitted there is reduced heterogeneity, and the HR is 0.76 (95% CI 0.66, 0.88). Three of these studies give data for the effect of aspirin in the proximal and the distal colon separately. Two of them [21,22] are homogeneous and combining them gives: HR 0.74 (95% CI 0.52, 1.04) for proximal colon and HR 1.03 (95% CI 0.78, 1.35) for distal colon. The third study [26] shows that compared with the effect in the distal colon, aspirin was associated with an HR of 0.84 (95% CI 0.56, 1.24) for cancer in the proximal colon. Pooled data for all-cause mortality are shown in the Table.
Data for ten breast cancer studies [34–43] are shown in Table 3, and a pooled HR on the effect of aspirin on cause-specific deaths cancer mortality in five studies is 0.69 (95% CI 0.46, 1.02). There is significant heterogeneity (P<0.0005), but on omitting a paper identified in sensitivity analysis [38] the heterogeneity is reduced (P = 0.19) and the HR becomes (0.87; 95% CI 0.69, 1.09). Data on all-cause mortality is given in the Table.
Amongst ten studies of aspirin and prostate cancer [44–53] nine give a cause specific mortality of 0.94 (95% CI 0.76, 1.17) with significant heterogeneity, Sensitivity analyses indicated that one study is responsible [44] and its omission led to an HR of 0.89 (95% CI 0.79, 0.99) and heterogeneity p = 0.26.
Six studies of other cancers [54–59] are included in Table 3. Benefit from aspirin is suggested in all six, but they are too diverse to justify meta-analysis.
A few of the reports give details of grade or stage of the cancer, but these were too few to enable any relevant analyses in relation to the effect of aspirin. Later however we quote a few comments on aspirin and ‘advanced’ cancer. Figs 2 and 3 respectively show the Forest plots of the cause specific mortality and the all-cause mortality (although only two reports [44,52] stated all-cause mortality in patients with prostate cancer).
Fig 2. Forest plots of the cause specific mortality summarised in Table 3.
Fig 3. Forest plots of the all-cause mortality summarised in Table 3.
It is possible to examine the pooling of the HRs for the three main cancers, and it seems not unreasonable to do this because the various pairs of HRs do not differ significantly (thus: for colon and breast cancer P = 0.90; for colon and prostate cancer P = 0.06 and for breast and prostate cancers P = 0.18). An overall meta-analysis for cause-specific mortality from these three cancers is 0.78 (95% CI 0.66, 0.92). Naturally, this has to be accepted with great caution, particularly as there is significant heterogeneity (P<0.0005), even though the omission of the three papers already identified as ‘outliers’ by sensitivity analysis [20,38,40] reduces the heterogeneity (P = 0.03) and gives an overall HR of 0.83 (95% CI 0.76, 0.90). Egger’s test for publication bias [13] is not significant (P = 0.30).
Aspirin and metastatic spread
An effect of aspirin on metastatic spread is clearly an evidence of treatment and the data in Table 4, although sparse, are therefore of considerable importance. Two studies of breast cancer [14,46], two of prostate [23,38] and one of both cancers together with colon [4], give evidence of a reduction in spread by aspirin. A combined estimate gives a relative risk for aspirin of 0.77 (95% CI 0.65, 0.92), though there is significant heterogeneity between the studies.
Table 4. Aspirin and metastatic spread in observational studies.
| Study | Cohort | Numbers (aspirin, no aspirin) | Cancer | Reduction (95% CI) | Comment |
|---|---|---|---|---|---|
| Algra & Rothwell [4] | 150 Case-control and 45 cohort studies | 141577 in case-control 41575 in cohorts | All cancers | RR 0.71 (0.60, 0.84) | No reduction in localised spread: OR 0.98 (0.88–1.09) |
| Choe et al [46] | Selected patients from a cancer centre | 2175, 3780 | Prostate | RR 0.50 (0.37–0.68) | |
| Jacobs, Chun et al [51] | Series of patients | 45, 29 | Prostate | RR 0.42 (0.12, 1.45) | Reported as 12.2% vs. 26.7%, P = 0.039 at 5 years |
| Barron et al [62] | Ireland National Cancer Register | 740, 2056 | Breast | RR 0.89 (0.81. 0.97) | Spread to lymph nodes: RR 0.81 (0.68, 0.96) in quarter women with highest aspirin dose |
| Ljung et al [60] | Nationwide Swedish cohort | N.A. | Breast | RR 0.94 (0.87–1.03) | Anticoagulants; 96% were aspirin |
| RR 0.80, (0.50–1.29) | In younger women | ||||
| HR 0.84 (0.64, 1.11) | Reduced spread to lymph nodes | ||||
| RR 0.77 (0.65–0.92), heterogeneity p<0.0005, Algra 2012 [4] omitted | |||||
CI: confidence interval; HR: hazard Ratio; N.A.: not available; OR: odds ratio; RR: risk ratio.
Aspirin and mutations
A mutation in PIK3CA, a gene which produces a protein that increases Cox-2 and prostaglandin activity, has been shown to enhance the response of the tumour to aspirin. The prevalence of this mutation is stated in several of the present studies as around 15–20% [24,27,61]. Table 5 summarises the relevant data and confirms a marked reduction in mortality in tumours with the mutation (HR 0.45; 95% CI 0.28, 0.71), while it is uncertain if there is benefit from aspirin in patients without the mutation (HR 0.94; 95% CI 0.67, 1.32). This last statement is based on comparison between pairs of HRs using the normal approximation of the difference between log HRs.
Table 5. Effect of aspirin: relevance of PIK3CA mutation.
| Authors | Cancer | Wild (95% CI) | Mutation/overexposure (95% CI) |
|---|---|---|---|
| Cause specific mortality | |||
| Chan et al [8] | Colorectal | HR 1.22 (0.36, 4.18) | HR 0.39 (0.20, 0.76) |
| Domingo et al [24] | Colorectal | HR 0.94 (0.59, 1.49) | HR 0.11 (0.01, 0.83) |
| Liao et al [27] | Colorectal | HR 0.90 (0.53, 1.54) | HR 0.28 (0.04, 2.10) |
| Kothari et al [61] | Colorectal | No patients | HR 0.66 (0.31, 1,38) |
| ‘wild’ cancers: HR 0.94 (0.67–1.32), heterogeneity P = 0.91 | |||
| ‘mutant’ cancers, HR 0.45 (0.28–0.71), heterogeneity P = 0.40 | |||
| All-cause mortality | |||
| Chan et al [8] | Colorectal | HR 1.05 (0.55, 2.02) | HR 0.62 (0.42, 0.93) |
| Domingo et al [24] | Colorectal | HR 0.95 (0.56, 1.61) | HR 0.29 (0.04, 2.33) |
| Liao et al [27] | Colorectal | HR 0.97 (0.68, 1.37) | HR 0.59 (0.24, 1.41) |
| Kothari et al [61] | Colorectal | No patients | HR 0.95 (0.55, 1.63) |
| Chae et al [57] | Several cancers | HR 1.80 (1.01, 3.23) | HR 0.75 (0.17, 3.20) |
| ‘wild’ cancers: HR 1.10 (0.84–1.44), heterogeneity P = 0.31 | |||
| ‘mutant’ cancers, HR 0.69 (0.52–0.93), heterogeneity P = 0.66 | |||
CI: confidence interval; HR: hazard ratio.
Evidence on these or other mutations appears not to be available in the present studies of breast or prostate cancers.
Aspirin taken before a cancer diagnosis
Does aspirin treatment affect cancers which have developed while aspirin has been taken? It may be that cancers which develop while aspirin is being taken are less responsive to the effect of aspirin. Table 6 summarises relevant data and shows that aspirin taken before the diagnosis of cancer is of little or no relevance to the treatment effect.
Table 6. Aspirin also taken prior to diagnosis.
| Author | Cancer | Aspirin only after diagnosis, not before (95% CI) | Aspirin after and before diagnosis (95% CI) |
|---|---|---|---|
| Cause specific mortality | |||
| Chan et al [8] | Colorectal | HR 0.53 (0.33, 0.86) | HR 0.89 (0.59, 1.35) |
| Coghill et al [22] | Colorectal | HR 0.77 (0.58, 1.00) | HR 0.75 (0.56, 1.00) |
| Goh et al [36] | Colorectal | HR 0.81 (0.51, 1.28) | HR 1.06 (0.71, 1.58) |
| Cardwell et al [21] | Colorectal | HR 1.08 (0.71, 1.63) | HR 0.75 (0.43, 1.29) |
| Liao et al [27] | Colorectal | HR 0.83 (0.50–1.39) | HR 0.79 (0.49–1.27) |
| Barron et al [62] | Breast | HR 0.99 (0.68, 1.46) | HR 0.80 (0.62, 1.04) |
| Kwan et al [41] | Breast | RR 1.23 (0.72, 2.11) | RR 0.99 (0.60, 1.64) |
| Assayag et al [44] | Prostate | HR 1.84 (1.59, 2.12) | HR 0.97 (0.81. 1.16) |
| Jacobs/Newton et al [52] | Prostate | HR 0.97 (0.65, 1.45) | HR 1.04 (0.73, 1.47) |
| Aspirin before & after diagnosis: HR 0.84 (0.70–1.00). Heterogeneity: p = 0.70 (colorectal cancer studies only) | |||
| Aspirin only after diagnosis: HR 0.79 (0.65–0.97). Heterogeneity: p = 0.30 (colorectal cancer studies only) | |||
| All-cause mortality | |||
| Bastiaannet et al [19] | Colorectal | HR 0.70 (0.57, 0.88) | HR 0.88 (0.83, 0.94) |
| Chan et al [8] | Colorectal | HR 0.68 (0.51, 0.92) | HR 0.96 (0.71, 1.28) |
| Walker et al [32] | Colorectal | HR 0.99 (0.84, 1.16) | HR 0.86 (0.76, 0.98) |
| Goh et al [26] | Colorectal | HR 0.86 (0.58, 1.27) | HR 1.04 (0.72, 1.48) |
| Liao et al [27] | Colorectal | HR 0.91 (0.66–1.26) | HR 0.81 (0.58–1.12) |
| Barron et al [62] | Breast | HR 1.11 (0.83, 1.50) | HR 0.81 (0.66, 0.99) |
| Assayag et al [44] | Prostate | HR 1.69 (1.53, 1.88) | HR 0.99 (0.87, 1.18) |
| Macfarlane et al [59] | Oesophagus | HR 0.84 (0.97, 1.26) | HR 1.11(0.97, 1.26) |
| Aspirin before and after diagnosis: HR 0.88 (0.83–0.93). Heterogeneity: p = 0.82 (colorectal cancer studies only) | |||
| Aspirin only after diagnosis: HR 0.83 (0.69–0.98) Heterogeneity: p = 0.06 (colorectal cancer studies only) | |||
CI: confidence interval; HR: hazard ratio.
Advanced disease and high risk groups
Data in three reports of prostate cancer suggest that the effect of aspirin may be greater in advanced disease. Thus Daugherty et al [47] describe an effect of aspirin in ‘advanced’ prostate cancer (HR 0.37; 95% CI 0.15, 0.92) which is greater, than in localized disease (HR 0.86; 95% CI 0.47, 1.58) Similarly Jacobs, Newton et al [52] report an HR of 0.60 (95% CI 0.37, 0.97) in ‘high-risk’ patients, contrasted with the effect of aspirin in the total cohort (HR 0.98; 95% CI 0.74, 1.29) in the total series of patients. Neither of these differences are however significant, nor is a result reported by Jacobs, Chun et al [51] who selected ‘high risk’ patients and reported a reduction by aspirin HR 0.44; (95% CI 0.15, 1.28).
The dose and consistency of aspirin taking
Many of the reports state, or imply that aspirin at a dose appropriate for vascular protection had been used and only a very few reports comment further. Several studies report greater effects with ‘high’ dose aspirin [49,63] though no difference is significant.
Several authors refer to the consistency of aspirin taking. Chan et al [8] give evidence consistent with a gradient (P < 0.04), the maximum benefit being with more than five aspirin tablets per week. Baastinnet et al [19] reported that the benefit of aspirin in all who took the drug as HR 0.77 (95% CI 0.63, 0.95), whereas ‘frequent’ use was associated with a possible slight increase in protection (HR 0.70; 95% CI 0.57. 0.88). Ng et al [29] reported HR 0.51 (95% CI 0.28, 0.95) for consistent use, compared with HR 0.68 (95% CI 0.42, 1.11) in the total cohort. Fuchs et al [25] state that compared with non-consistent use, ‘consistent’ users had a much greater reduction (HR 0.45; 95% CI 0.21, 0.97).
Several authors state that an effect of aspirin because apparent only after 3–5 years of therapy. Goh et al [26] state that they found evidence of benefit only after 5 years, Stock et al [53] who reported no benefit to prostate cancer overall (HR 1.03; 95% CI 0.79, 1.34) states that after five years of aspirin taking there was benefit (HR 0.54; 95% CI 0.26, 1.13) and an effect of aspirin taking in the study of lung cancer [55] became significant only after three years (HR = 0.84; CIs not stated).
Aspirin and bleeding
An excess of bleeding attributable to aspirin has been well studied in short-term vascular trials [64,65]. It is however appropriate to ask whether or not the risk of bleeding attributable to aspirin is similar in patients with cancer to that reported from the vascular trials. A few reports in the present series give a measure of reassurance on this. Din et al [23] who examined NSAID use state that there were no major bleeding complications. Liu et al [18] state that no side effects caused by aspirin were noted in any patient in the study. Curigliano et al [66] examined short-term aspirin taking by patients with breast cancer and stated that no major bleeding complication occurred.
The corresponding authors of the other reports in this series were written to. Replies received from twenty-one authors stated that no data on bleeding had been recorded.
Discussion
The evidence we present from a systematic overview of the literature gives support to the use of aspirin as an additional treatment of cancer. The evidence is limited, and while it is encouraging in the case of bowel cancer, there is insufficient evidence to dismiss a role for aspirin as an adjunct treatment of cancers other than colorectal. In fact, its use can be justified on the basis of its likely benefit on outcomes other than death, including its probable reduction in metastatic spread and its reduction in vascular disease events, including venous thromboembolism.
Differences between individual studies leading to significant heterogeneity is to be expected in any collection of observational studies such as those we present, and it does limit confidence in the results. However, if, for each of the three cancers, an out-lying study identified by detailed sensitivity analyses is omitted, heterogeneity is reduced to an acceptable level and for each cancer there is evidence suggestive of reductions in mortality and in metastatic spread.
In colon cancer there is evidence of a reduction in colorectal deaths of about 25%, and perhaps about 20% in All-cause mortality. If one report [13] is omitted the evidence of benefit in breast cancer is of a possible 13% reduction in cause-specific deaths, and for prostate cancer a possible reduction of perhaps about 11%.
With the present level of evidence, the pooling of data for the three cancers would seem to be not unreasonable and following omissions of three outliers, unacceptable heterogeneity is resolved. A meta-analysis then suggests a possible overall reduction by aspirin of about 15% (pooled HR 0.83; 95% CI 0.76–0.90). The evidence of a reduction in metastatic spread (RR 0.77; 95% CI 0.65–0.92) gives further encouragement to the use of therapeutic aspirin in cancer while awaiting evidence from ad hoc randomised trials.
It would be unreasonable to attempt to draw firm conclusions from the single studies on lung cancer [31], on oesophageal cancer [45], and on lymphatic leukaemia [22]. However the suggestive benefit in these studies, together with that reported for a mix of colon and women’s cancer [21] indicates an urgent need for more observational studies in the less common cancers, some of which may never be subjected to evaluation in randomised trials. It would also be helpful if in the reporting of new studies information on the stage and grade etc. of the cancers could be indicated.
The evidence we present on the biomarker PIK3CA has been confirmed in an overviews by other authors [67], and similar data on other markers have been shown by other authors [68]. Thus Sun et al [31] who examined CTNNB1, a gene associated with cell adhesion and of relevant to familial polyposis, reported a marked enhancement in the effect of aspirin (HR 0,53; 95% CI 0.30, 0.95) compared with the effect in patients with the wild gene (HR 1.06; 95% CI 0.62, 1.83). A different approach to this issue was adopted by Chan et al [8] who used an overexpression of COX-2 in the primary tumour as an indication of a relevant mutation, and showed that overexpression was associated with reductions in colon cancer mortality (HR 0.39; 95% CI 0.20, 0.76) compared with the effect in other patients (HR 1.22; 95% CI 0.36, 4.18).
All this suggests that the reduction by aspirin may be restricted to patients whose tumours show mutation in PIK3CA, HLA class I antigen, or show COX-2 over-expression. In colorectal cancer these subgroups represent approximately 17%, 54% and 50% of all patients, and our data suggest a reduction of about 50% in colorectal mortality, though another overview [67] suggested a reduction of only about 30%, while neither overview showed any reduction in those without the mutation. The scarcity of evidence on mutation in cancers other than colon is most unfortunate [69].
And yet any selection of patients for treatment with aspirin on the basis of a mutation or any other marker of cancer risk would be totally unwarranted on present evidence. Metastases are a major source of pain and other undesirable effects in solid cancers [70,71] and perhaps 90% of cancer deaths are at least in part due to metastases [72] and the withholding of aspirin would deny these possible benefits. Furthermore, the risk factors for vascular disease overlap with those for cancer and the withholding of aspirin would also deny patients the vascular benefits of aspirin, including the possible reduction of the excess risk of venous thromboembolism during chemotherapy. In fact, the marked increase in the risk of venous thrombosis in patients with cancer [66,73], has been shown to be reduced by low-dose aspirin [74], and it has therefore been recommended that prophylactic anticoagulants should be considered in all patients with cancer [75].
A major uncertainty in what we report arises from the possible omission of relevant reports, together with publication bias, and the test we performed suggests that this last may have occurred (Egger's test [13] P = 0.037). Furthermore, underlying all observational studies is the issue of residual confounding, and while this cannot be dismissed, it seems unlikely to have operated to any important degree as all the studies reviewed included multivariate adjustments.
On the other hand certain time biases could be present in some of the studies, and especially in retrospective case-control studies [76]. Patients not taking aspirin at the time of diagnosis can be defined, used as ‘controls’ and followed thereafter. Patients who start taking aspirin after receiving a diagnosis of cancer cannot be identified as ‘cases’ until they start taking the drug. It is possible that these will be identified later than the ‘control’ patients, and they will therefore be observed and deaths identified during a shorter time that that during which the patients not taking aspirin are observed. This has been called an ‘immortal’ time bias and Assayag & Azolay [44] include a detailed discussion of it.
All the reports in the present series were examined and while immortal time bias cannot be dismissed with certainty, an important effect upon the overall estimates of the effect of aspirin seems most unlikely. In fact, there is little difference in the overall mortality of the patients who had taken aspirin before diagnosis, and (presumably) continued to take it after diagnosis (HR 0.92), and the patients who had not taken aspirin before diagnosis (HR 0.90), in whom there could have been a time lag and thus, an ‘immortal time bias’ (see Table 6).
While a serious limitation in the present evidence is that little comes from randomised trials, yet evidence from further observational studies is urgently needed to evaluate more fully how patients likely to benefit from aspirin can be identified. In particular evidence on PIK3CA, other mutations and other possible markers should be collected as a matter of urgency in cancers of breast, prostate and other organs. The results of such studies should be made available for the encouragement and guidance of colleagues setting up randomised trials, and, in fact, the further question arises whether or not these mutations are of relevance to aspirin used in prophylaxis.
The possible benefits of aspirin must of course be evaluated against it side effects. Shortly after aspirin taking commences the risk of a gastrointestinal (GI) bleed is high but the risk falls rapidly thereafter [77,78], and in short-term trials the additional risk of a bleed from low-dose aspirin amounts to perhaps one or perhaps two patients in every 1,000 on low-dose aspirin [64,65]. After about three years of aspirin taking however, there appears to be no evidence of any excess GI bleeds attributable to the drug [78]. Moreover, the incidence of GI bleeding is highly sensitive to the presence of gastric pathology [78,79], and careful enquiries should therefore be made about current or past gastric symptoms, and about a high alcohol consumption [80]. The use of a gastroprotective drug together with the aspirin should be carefully considered if pathology is suspected.
The most serious bleeds are those that lead to death, and despite frequent references to fatal bleeds attributed to aspirin, there appears to be no valid evidence that deaths from GI bleeds are increased by low-dose aspirin [81]. In a recent study of patients admitted to hospital with ‘gross’ GI bleeding [82] the hospital stay of patients who had been taking aspirin was significantly shorter than that of patients who had not been on aspirin, and no patient whose bleed had been attributed to aspirin experienced an uncontrolled haemorrhage or died due to excessive bleeding.
Cerebral bleeds attributable to aspirin are rare, about one or two per 10,000 patient-years. Hypertension is the major factor in such bleeds [2] and in a randomised trial of aspirin based upon patients with hypertensive disease all of whom were adequately treated with anti-hypertensive drugs, there was the same number of cerebral bleeds in ten thousand patients on aspirin (19 patients) as in ten thousand on placebo (20 patients) [83]. A reduction in the risk of a cerebral bleed is therefore likely if the blood pressure of every person starting aspirin is checked, and adequately treated if raised [84].
Other overviews
An early overview of studies of aspirin and cancer was based on three small randomised trials and two observational trials, and this led to the conclusion: ‘aspirin may have a role in the adjuvant setting… and should not be overlooked [9]. A further overview of three studies of colorectal, one of breast, two of prostate and one of oesophageal cancers judged that aspirin decreases the development and spread of metastases [10].
Li et al [85] reported a systematic review and meta-analysis of seven studies of colorectal cancer. Aspirin use after a diagnosis of colon cancer was reported to reduce overall all-cause mortality (HR 0.84; 95% CI 0.75, 0.94), but if aspirin had been taken before cancer benefit was uncertain (HR 0.77; 95% CI 0.52, 1.14). Ye et al [86] conducted a meta-analysis of seven of the studies of colon cancer in the present review, and reported effects of aspirin on both colon cancer mortality and overall mortality very close to what we report.
Huang et al [87] identified 16 studies in which a NSAID or aspirin had been used in patients with breast cancer. On the basis of meta-analyses they reported that aspirin taken after diagnosis, but not before, was associated with improved breast cancer survival (HR 0.69; 95% CI 0.50, 0.96). Zong et al [88] also reported a systematic review and meta-analysis of breast cancer patients in eight cohort studies and two nested case-control studies, and judged that post-diagnostic aspirin was associated with a significant reduction in the relative risk of death from breast cancer (RR 0.84; 95% CI 0.63, 1.12). Liu et al [89] conducted a systematic review and identified 39 studies of NSAIDs, including aspirin. A meta-analysis of prostate specific mortality in seven studies of aspirin gave an HR = 0.86 (95% CI 0.78, 0.96) and the effect of aspirin in these studies were judged to be more consistent than those for other NSAIDs.
Conclusions
It appears likely that low-dose aspirin has a beneficial role as an adjunct treatment of cancer. Reductions in mortality are shown in colon cancer, probably in prostate cancer and possibly in breast and individual studies of several other cancers also suggest benefit. Aspirin benefit in colorectal cancers, and possible other cancers, may be restricted to patients with tumours expressing certain genetic mutations. However, other benefits of low-dose aspirin, including reductions in metastatic spread and in vascular events, including venous thromboembolism appear to be independent of these biomarkers, and so information on aspirin should be given to patients whatever the state of the possible biomarkers.
The heterogeneity within the currently available studies–both between different cancers, and within the different studies of each cancer, together with evidence suggesting some publication bias, are such that further evidence from a number of adequately powered randomised, placebo controlled trials is urgently required, including trials of less common cancers. Evidence on the possible role of aspirin in uncommon cancers, and the possible enhancement of its effect if mutation and other markers of increased sensitivity to the actions of aspirin, are also urgently needed. Much of this evidence could some from further observational studies.
Nevertheless, despite the need for randomised trials, we believe the evidence of benefit from aspirin is sufficiently persuasive that physicians should engage with patients in a presentation and discussion of aspirin as an additional treatment. Furthermore, we hold that patients should be given this evidence within the context of a healthy lifestyle [90], they should be allowed to make their own decision about aspirin therapy, and should then be supported in whatever decision they make [91].
Supporting Information
(DOC)
Acknowledgments
We are grateful to Ruth Langley who reviewed an early draft.
Data Availability
All data are contained in the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Office for National Statistics. Cancer Incidence and Mortality in the United Kingdom 2009–10. 6th December 2012.
- 2.Antithrombotic Trialists’ Collaboration.(2009) Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 373: 1849–1860. 10.1016/S0140-6736(09)60503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battistoni A, Mastomarino V, Volpe M.(2015) Reducing cardiovascular and cáncer risk: how to address global prevention in clinical practice. Clin Cardiol. 10.1002/cic.22394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Algra AM, Rothwell PM. (2012) Effects of regular aspirin on long-term cancer incidence and metastases: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncology 13: 518–527 10.1016/S1470-2045(12)70112-2 [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Price JF, Fowkes FGR, Zanchetti A, Roncaglioni MC, Tognoni MC et al. (2012) Short-term effects of daily aspirin on cancer incidence, mortality and non-vascular death: analysis of the time course of risks and benefits in 51 randomised trials. Lancet 379: 1602–12. 10.1016/S0140-6736(11)61720-0 [DOI] [PubMed] [Google Scholar]
- 6.Mills EJ, Wu P, Alberton M,.Kanters, Lanas A, Lester R. (2012) Low-dose aspirin and cancer mortality: a meta-analyisi of randomised trials. Amer J Med. 125: 560–567 10.1016/j.amjmed.2012.01.017 [DOI] [PubMed] [Google Scholar]
- 7.Burn J, Gerdes A-M, Macrae F, Mecklin J-P, Moeslein G, Olschwang S et al. (2011) Long-term effect of aspirin on cancer in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised trial. Lancet 10.1016/S0140-6736(11)61049-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan AT, Ogino S, Fuchs CS, Meklin GP, Moeslein G, Olschwang et al. (2009) Aspirin use and survival after diagnosis of colorectal cancer. JAMA 302: 649–659. 10.1001/jama.2009.1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langley RW, Burdett S, Tierney JF, Caferty F, Parmar MK, Venning G. (2011) Aspirin and cancer: has aspirin been overlooked as an adjuvant therapy. Brit J Cancer 105: 1107–1113. 10.1038/bjc.2011.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langley RE. (2013) Clinical evidence for the use of aspirin in the treatment of cancer. ecancer 7 297 / 10.3332/ecancer.2013.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O’Connell D, Peterson JEA, Welch V, Losos M, et al. (2000). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- 13.Systematic Reviews in Health Care, Meta-analysis in context Edited by Egger Matthias, Smith George Davey, Altman Douglas G. BMJ Books; 2009 [Google Scholar]
- 14.Rothwell PM, Wilson M, Elwin C-E, Norring B, Algra A, Warlow CP et al. (2010) Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 376: 1741–1750. 10.1016/S0140-6736(10)61543-7 [DOI] [PubMed] [Google Scholar]
- 15.Lipton A, Scialla S, Harvey H, Dixon R, Gordon R, Hamilton R et al. (1982) Adjuvant antiplatelet therapy with aspirin in colorectal cancer. J Med 13: 419–429. [PubMed] [Google Scholar]
- 16.Lebeau B, Chastang C, Muir JF, Vincent J, Massin F, Fabre C et al. (1993) No effect of an antiaggregant treatment with aspirin in small cell lung cancer treated with CCAVP16 chemotherapy. Results from a randomised clinical trial of 303 patients. Cancer 71: 1741–1745. [DOI] [PubMed] [Google Scholar]
- 17.Cregan ET, Twito DI, Johansson SL Schoid DJ Johnson S, Flaum MA et al. (1991) A randomised prospective assessment of recombinant leukocyte A interferon with or without aspirin in advanced renal adenocarcinoma. J clin Oncol 9: 2104–2109. [DOI] [PubMed] [Google Scholar]
- 18.Liu J- F, Jamieson GG, Wu T-C, Zhu G-J Drew PA. (2009) A preliminary study on the postoperative survival of patients given aspirin after resection for squamous cell carcinoma of the esophagus or adenocarcinoma of the cardia. Ann Surg Oncol. 16:1397–1402. 10.1245/s10434-009-0382-z [DOI] [PubMed] [Google Scholar]
- 19.Bastiaannet E, Sampieri K, Dekkers OM, de Craen AJ, van Herk-Sukel MP, Lemmens V. (2012) Use of aspirin postdiagnosis improves survival for colon cancer patients. Brit J Cancer 106: 1564–1570. 10.1038/bjc.2012.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bains S, Mahic M, Cvancarova M, Yaqub S, Dorum LM, Bjornbeth BA, et al. (2015) Impact of aspirin as secondary prevention in an unselected cohort of 25,644 patients with colorectal cancer: A population-based study. Journal of Clinical Oncology.Conference: 2015 Annual Meeting of the American Society of Clinical Oncology, ASCO Chicago, IL United States.Conference Start: 20150529 Conference End: 20150602.Conference Publication: (var.pagings) [Google Scholar]
- 21.Cardwell CR, Kunzmann AT, Cantwell MM, Hughes C, Baron JJ, Powe DG, et al. (2014) Low-dose aspirin after diagnosis of colorectalm cancer does not increase survival: a case-control analysis of a population-based cohort. Gastroentero 146:700–708. [DOI] [PubMed] [Google Scholar]
- 22.Coghill AE, Newcomb PA, Campbell PT Burnett-Hartman AN, Adams SV, Poole EM, et al. (2011) Prediagnostic NSAIDs use and survival after diagnosis of colorectal cancer. Gut 60: 491–498 10.1136/gut.2010.221143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Din FVN, Theodoratou E, Farrington SM, Tenesa A, Bannetson RA, Cetnarsky R et al. (2010) Effect of aspirin and NSIADs on risk and survival from colorectal cancer. Gut 59: 1670–1679. 10.1136/gut.2009.203000 [DOI] [PubMed] [Google Scholar]
- 24.Domingo E, Church DN, Sieber O, Ramamoorthy R, Yanagisawa Y, Johnstone E, et al. (2013) Evaluation of PIK3CA mutation as a predictor of benefit from NSAID drug therapy in colorectal cancer. J clin Oncol 10.1200/JCO.2013.50.0322 [DOI] [PubMed] [Google Scholar]
- 25.Fuchs C, Meyerhardt JA, Heseltine DL, Holis D, Chan LB, Saltz RL, et al. (2005) Influence of regular aspirin on survival for patients with stage III colon cancer. Findings from Intergroup trial CALGB 89803. J Clin Oncology 23: suppl 3530. [Google Scholar]
- 26.Goh HH, Leong WQ, Chew MH, Pan YS, Tony LKH, Chew L, et al. (2014) Post-operative aspirin use and colorectal cancer-specific survival in patients with stage I-III colorectal cancer. Anticancer Research 34: 7407–7414. [PubMed] [Google Scholar]
- 27.Liao X, Lockhead P, Nishara R, Morikawa T, Kuchiba A, Yamauchi M, et al. (2012) Aspirin use, tumour PIK3CA mutation and colorectalm cancer survival. NEJM 367: 1597–15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCowan C, Munro AJ, Donnan PT, Steele RJC. (2013) Use of aspirin post-diagnosis in a cohort of patients with colorectal cancer and its association with all-cause and colorectal specific mortality. Eur J Cancer. 49: 1049–1057. 10.1016/j.ejca.2012.10.024 [DOI] [PubMed] [Google Scholar]
- 29.Ng K, Meyerhardt JA, Chan AT, Sato K, Chan JA, Niedzwiecki D, Salyz LB, et al. (2015) Aspirin ad COX-2 inhibitor use in patients with stage III colon cancer. J Natl Cancer Inst 2015; 10.1093/jmci/dju345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reimers MS, Bastiaannet E, Langley RE, van Eijk R, van Vlierberghe RL, Lemmens VE, et al. (2014) Expression of HLA class 1 antigen, aspirin use and survival after a diagnosis of colon cancer. JAMA Intern Med. 174(5):732–9 10.1001/jamainternmed.2014.511 [DOI] [PubMed] [Google Scholar]
- 31.Sun RR, Nishihara QR, Chan AT, Ogino S (2013). "Aspirin and colorectal cancer incidence and mortality by ctnnb1 expression: A molecular pathological epidemiology (MPE) study." Cancer Epidemiol Biomarkers Prev 22: 472–473. [Google Scholar]
- 32.Walker AJ, Grainge, Card TR (2012) Aspirin and other NSAID drug use and colorectal cancer survival: a cohort study. Brit J Cancer;107:1602–7. 10.1038/bjc.2012.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanders MM, van Herk-Sukel MP, Vissers PA, Herings RM, Haak HR, van der Pol-Franse LV. (2015) Are metformin, statin and aspirin use still associated with overall mortality among colorectal cancer patients with diabetes if adjusted for one another? Br J Cancer;113(3):401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barron TI, Murphy LM, Brown C, Bennet K, Visvanthan K, Sharp L. (2015) De-novo post-diagnostic aspirin use and mortality in women with stage I-III breast cancer. Cancer Epidemiol Biomarkers Prev. 24: 898–904. 10.1158/1055-9965.EPI-14-1415 [DOI] [PubMed] [Google Scholar]
- 35.Blair CK, Sweeney C, Anderson KE, Folson AR. (2007) NSAID use and survival after breast cancer diagnosis in post-menopausal women. Breast cancer Res Treat 101: 191–197. [DOI] [PubMed] [Google Scholar]
- 36.Bowers LW, Maximo LXF, Brenner AJ et al. (2014) NSAID use reduces breast cancer recurrence in overweight and obese women: role of prostaglandin-aromatase interactions. Cancer Res 74:4446–4457 10.1158/0008-5472.CAN-13-3603 [DOI] [PubMed] [Google Scholar]
- 37.Cronin-Fenton D, Heide-Jorgensen U, Ahern TP, Lash TL, Christiansen P, Ejlertsen B et al. Low-dose non-steroidal antiinflammatory drugs, selective COX-2 inhibitor prescriptions and breast cancer recurrence. A danish population based cohort study. Pharmacoepidemiology and drug safety Conference. Boston USA.
- 38.Fraser DM, Sullivan FM, Thompson AM, McCowan C. (2014) Aspirin use and survival after the diagnosis of breast cancer: a population-based cohort study. Br J Cancer 111: 623–627. 10.1038/bjc.2014.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. (2014) Aspirin intake and survival after breast cancer. J Clin Oncol. 28: 1467–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmes MD, Olsson H, Pawitan Y, Holm J, Lundholm C, Andersson TM-L. et al. (2014) Aspirin intake and breast cancer survival–a nationwide study using prospectively recorded data in Sweden. BMC Cancer 14: 391–398. 10.1186/1471-2407-14-391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwan ML, Habel LA, Slattery ML, Caan B. (2007) NSAIDs and breast cancer recurrence in a prospective cohort study. Cancer Causes Control 18:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray LJ, Cooper JA, Hughes CM, Powe DG, Cardwell CR. (2014) Post-diagnostic prescriptions for low-dose aspirin and breast cancer-specific survival: a nested case-control study in a breast cancer cohort from the UK Clinical Practice Research Datalink. Breast Cancer Res 16(2) R34 2014 Apr 4 10.1186/bcr3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wernli KJ, Hampton JM, Trentham-Dietz A, Newcomb PA. (2011).Use of antidepressants and NSAIDs in relation to mortality in long-term breast cancer survivors. Pharmacoepidemiol Drug Safety 20: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Assayaq J, Pollak MN, Azoulay L. (2014) The use of aspirin and the rosk of mortality in patients with prostate cancer J Urol 193: 1220–1225. 10.1016/j.juro.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 45.Caon J, Paquette M, Hamm J, Pickles T. (2014). Does statin or ASA affect survival when prostate cancer is treated with external beam radiation therapy? Prostate Cancer 184297. 10.1155/2014/184297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choe KS, Cowan JE, Chan JM, Carroll PR, D’Amico AV, Liauw SL. (2012) Aspirin use and the risk of prostate cancer mortality in men treated with prostatectomy or radiotherapy. J Clin Oncology 30: 3540–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daugherty SER, Pfeiffer R, Ghazarian A, Izmirlian G, Ororok P,McGlyn KM et al. (2013). Frequency of aspirin use and prostate cancer mortality among prostate cancer cases in the control arm of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Amer Assoc for Cancer Res.pOSTER LB-15. [Google Scholar]
- 48.Dhillon PK, Kenfield SA, Stampfer MJ, Giovannucci EL, Chan JM. (2012) Aspirin use after a prostate cancer diagnosis and cancer survival in a prospective cohort. Cancer Prev Res 5:1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flahavan EM, Bennett K, Sharp L, Barron TI. (2014) A cohort study investigating aspirin use and survival in men with prostate cancer. Ann oncol 25: 154–159. 10.1093/annonc/mdt428 [DOI] [PubMed] [Google Scholar]
- 50.Grytli HH, Fagerland MW, Fossa SD, Tasken KA. (2014) Associatopj between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high risk or metastatic disease. Eur Urol 65: 635–641. 10.1016/j.eururo.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 51.Jacobs CD, Chun SG, Yan J, Xie X, Pistenmas DA, Hannan R, Loyan Y et al. (2014) Aspirin improves outcome in high risk prostate cancer patients treated with radiation therapy. Cancer Biol Ther. 15: 699–706. 10.4161/cbt.28554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacobs EJ, Newton CC, Stevens VJ, Campbell PT, Freedland SJ et al. (2014). Daily aspirin use and prostate cancer-specific mortality in a large cohort of men with nonmetastatic prostate cancer. J Clin Oncology 32: 3716–3722. [DOI] [PubMed] [Google Scholar]
- 53.Stock DC, Groome PA, Siemens DR, Rohland SL, Song Z. (2008) Effects of non-selective NSAIDs ib the 2016)aggressiveness of prostate cancer. Prostate 68:1655–65. 10.1002/pros.20834 [DOI] [PubMed] [Google Scholar]
- 54.Nagle CM, Ibiebele TI, DeFazio A, Protani MM, Webb PM. Australian Ovarian Cancer Study Group. (2015) Aspirin, nonaspirin non-steroidal anti-inflammatory drugs, acetaminophen and ovarian cancer survival. Cancer Epidemiol. 39(2):196–9. 10.1016/j.canep.2014.12.010 Epub 2015 Feb 7. [DOI] [PubMed] [Google Scholar]
- 55.Fontaine E, McShane J, Page R, Shackcloth M, Mediratta N, Carr M et al. (2010) Aspirin and non-small cell lung cancer resections: effect on long-term survival. Eur J Cardiothorac Surg. 38: 21–26. 10.1016/j.ejcts.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 56.Pastore AL, Palleschi G, Fuschi A, Silvestri L, Salhi Y, Costantini E, et al. (2015) Can daily intake of aspirin and /or statins influence the behaviour of non-muscle invasive bladder cancer? A retrospective study on a cohort of patients undergoing transurethral bladder resection. BMC Cancer 2015;15:120 10.1186/s12885-015-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chae Y, Hong DS, Kim KH, Falchook GS, Piha-Paul SA, Subbiah V et al. (2013) PIK3CA mutations, aspirin use and mortality in patients with women’s cancers or colorectal cancers treated in early-phase clinical trials. POSTER Epidemiology, Primary and Secondary Prevention. Abstract No. 1454 [Google Scholar]
- 58.Chae YK, Trinh L, Jain P, Wang X, Rozovski U et al. , (2014) Statin and aspirin use is associated with improved outcome of FCR therapy in relapsed/refractory chronic lymphocytic leukaemia. Blood 22: 1424–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Macfarlane TV, Murchie P, Watson MC. (2015) Aspirin and other non-steroidal anti-inflammatory drug prescriptions and survival after the diagnosis of head and neck and oesophageal cancer. Cancer Epidemiol. 2015 December;39(6):1015–22. 10.1016/j.canep.2015.10.030 Epub 2015 Nov 18. [DOI] [PubMed] [Google Scholar]
- 60.Ljung R, Sennerstam R, Mattsson F, Auer G, Lagergren J. (2013) Anticoagulant medication at time of needle biopsy for breast cancer in relation to risk of lymph node metastasis. Int J Cancer 135: 238–241. 10.1002/ijc.28671 [DOI] [PubMed] [Google Scholar]
- 61.Kothari N, Kim R, Jorissen RN, Desai J, Tie J, Wong H-L et al. (2014) Impact of regular aspirin use on overall and cancer-specific survival in patients with colorectal cancer harbouring a PIK3CA mutation. Acta Oncologica 10.3109/0284186X.2014.990158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barron TI, Flahavan EM, Sharp L,Bennett K, Visvanathan K. (2014) Recent prediagnostic aspirin use, lymph node involvement, and 5-year mortality in women with stage I-III breast cancer: A nationwide population-based cohort study. Cancer Research 74: 4065–7 10.1158/0008-5472.CAN-13-2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barron TI, Sharp L, Bennett K, Visvanathan K. Aspirin use and mortality in women with stage I-III breast cancer: A population-based study. Poster at ASCO Meeting. J Clin Oncol 2012. (Suppl. Abstract 521). [Google Scholar]
- 64.Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. (2005) Low-dose aspirin for the prevention of atherosclerosis. NEJM 353: 2373–2383. [DOI] [PubMed] [Google Scholar]
- 65.Valkhoff VE, Sturkenboom MCJM, Hill C, van Zanten SV, Kuioers EJ. (2013) Low-dose acetylsalicylic acid use and the risk of upper gastrointestinal bleeding: a meta-analysis of randomised clinical trials and observational studies. Can J Gastroenterol 27: 159–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Curigliano G, Balduzzi A, Cardillo A, Ghisini R, Peruzzotti G, Orlando et al. (2007). Low-dose aspirin for the prevention of venous thromboembolism in breast cancer patients treated with infusional chemotherapy after insertion of central vein catheter. Supportive Care in Cancer 15: 1213–1217. [DOI] [PubMed] [Google Scholar]
- 67.Paleari L,Pumtoni M, Clavarezza M, DeCensi M, Cusick J, DeCensi A. (2015) PIK3CA mutation, aspirin use after diagnosis and survival of colorectal cancer. A systematic review and meta-analysis of epidemiological studies. Clin Oncol. Available: 10.1016/k.clon.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 68.Basu GD, Xiu J, Arguello D, Feldman RA, Millis SZ, Bender RP et al. (2014) Prevalence of KRAS, BRAF, NRAS, PIK3CA and PTEN alterations in colorectal cancer: analysis of a large international cohort of 5,900 patients. J Clin Oncol 32, 2014 (suppl 3; abstr 399) [Google Scholar]
- 69.Cizkova M, Susini A, Vacher S Cizeron G, Andrieu C, Driouch K et al. (2012) PIK3CA mutation impact on survival in breast cancer patients and in ERα, PR and ERBB2-based subgroups. Breast Cancer Research 14:R28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Talmadge JE, Fidler Isaiah J.. (2010) The Biology of Cancer Metastasis: Historical Perspective. Cancer Res 70; 5649–5669 10.1158/0008-5472.CAN-10-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mehlen P, Puisieux A. (2006) Metastasis: a question of life or death. Nature Rev Cancer 6: 449–458 [DOI] [PubMed] [Google Scholar]
- 72.Wang Y. (2010) Breast cancer metastasis driven by ErbB2 and 14-3-3ξ. Cell Adh Migr. 4(1): 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baron JA, Gridley G, Weiderpass E. Nyren O, Linet M. (2000) Venous thromboembolism and cancer. Lancet 361: 1077–1080. [DOI] [PubMed] [Google Scholar]
- 74.Anon. (2000) Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 355: 1295–1302. [PubMed] [Google Scholar]
- 75.Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI et al. (2007) American Society of Clinical Oncology Guideline: Recommendations for Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer. J Clin Oncology 25: 5490–5505. [DOI] [PubMed] [Google Scholar]
- 76.Suissa S, Azoulay L. (2012) Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 35:2665–2673. 10.2337/dc12-0788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia Rodriguez LA, Hermandez S, de Abajo J. (2001) Association between aspirin and upper gastrointestinal complications: systematic review of epidemiologic studies. Br J Clin Pharmacol 52: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rothwell PM, Wilson M, Price JF, Belch JFF, Meade TW, Mehta Z. (2012) Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 379: 1591–1601. 10.1016/S0140-6736(12)60209-8 [DOI] [PubMed] [Google Scholar]
- 79.Hernandez-Diaz S, Garcia Rodriguez LA. (2006) Cardioprotective aspirin users and their excess risk of upper GI complications. BMC Med 4:22 10.1186/1741-7015-4-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kraufman DW, Kelly JP, Wiholm BE, Laszlo A, Sheehan JE, Koff RS et al. (1999) The risk of acute major upper gastrointestinal bleeding among users of aspirin and ibuprofen at various levels of alcohol consumption. Am J Gastroenterol 94: 3189–3196. [DOI] [PubMed] [Google Scholar]
- 81.Elwood P, Morgan G. (2014) Aspirin propylaxis: Putting gut bleeds into perspective. Editorial in Gastroenterology and Hepatology 10: 61–63. [PMC free article] [PubMed] [Google Scholar]
- 82.Wehbeh A, Tamim HM, Abu Daya H, Abou Mrad R, Badreddine RJ, Eloubeidi MA. et al. (2015) Aspirin has a protective effect against adverse outcomes in patients with nonvarical upper gastrointestinal bleeding. Dig Dis Sci 60, 2077–2087. 10.1007/s10620-015-3604-1 [DOI] [PubMed] [Google Scholar]
- 83.Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S et al. (1998) Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) trial. HOT study group. Lancet 351, 1755–1762. [DOI] [PubMed] [Google Scholar]
- 84.Gorelick PB, Weisman SM. (2005) Risk of Hemorrhagic Stroke With Aspirin Use. An Update. Stroke 36: 1801–1807. [DOI] [PubMed] [Google Scholar]
- 85.Li P, Wu H, Zang H, Shi Y, Xu J, Ye Y et al. (2014) Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut 10.1136/gutjnl-2014-308260 [DOI] [PubMed] [Google Scholar]
- 86.Ye X-F, Wang J, Shi W-T, He J. (2014) Relationship between aspirin use after diagnosis of colorectal cancer and patient survival: a meta-analysis of observational studies. Brit J Cancer 11: 2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang XZi, Gao P, Sun JX, Song YX, TSAI Cc, Liu J et al. (2015) Aspirin and non-steroidal anti-inflammatory drugs after but not before diagnosis are associated with improved breast cancer survival: a meta-analysis. Cancer Causes Control 26: 589–600. 10.1007/s10552-015-0539-y [DOI] [PubMed] [Google Scholar]
- 88.Zhong S, Zhang X, Chen L Ma T, Tang J, Zhao J. (2015) Association between aspirin use and mortality in breast cáncer patients: a meta-analysis of observational studies. Breast Cancer Res Treat 150: 199–207. 10.1007/s10549-015-3300-z [DOI] [PubMed] [Google Scholar]
- 89.Liu Y, Chen j-Q, Xie L, Wang J, Li T, He Y et al. (2014) Effect of aspirin and other non-steroidal anti-inflammatory drugs on prostate cancer incidence and mortality: a systematic review and meta-analysis. BMC Med BMC Medicine 2014, 12:55 10.1186/1741-7015-12-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elwood P, Galante J, Pickering J Palmer S, Bayer A, Ben-Shlomo Y et al. (2013) Healthy Lifestyles Reduce the Incidence of Chronic Diseases and Dementia: Evidence from the Caerphilly Cohort Study. 2013: PLOS ONE: December 09, 2013 10.1371/journal.pone.0081877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kassirer JP. (1994) Incorporating patients’ preferences into medical decisions. New Eng J Med 330:1895–1896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All data are contained in the paper and its Supporting Information files.