Abstract
Human-modified habitats are expanding rapidly; many tropical countries have highly fragmented and degraded forests. Preserving biodiversity in these areas involves protecting species–like frugivorous bats–that are important to forest regeneration. Fruit bats provide critical ecosystem services including seed dispersal, but studies of how their diets are affected by habitat change have often been rather localized. This study used stable isotope analyses (δ15N and δ13C measurement) to examine how two fruit bat species in Madagascar, Pteropus rufus (n = 138) and Eidolon dupreanum (n = 52) are impacted by habitat change across a large spatial scale. Limited data for Rousettus madagascariensis are also presented. Our results indicated that the three species had broadly overlapping diets. Differences in diet were nonetheless detectable between P. rufus and E. dupreanum, and these diets shifted when they co-occurred, suggesting resource partitioning across habitats and vertical strata within the canopy to avoid competition. Changes in diet were correlated with a decrease in forest cover, though at a larger spatial scale in P. rufus than in E. dupreanum. These results suggest fruit bat species exhibit differing responses to habitat change, highlight the threats fruit bats face from habitat change, and clarify the spatial scales at which conservation efforts could be implemented.
Introduction
Anthropogenic changes to tropical forests have been extensive. More than 350 million hectares have been removed globally [1,2], changing the availability of food resources, and modifying foraging behavior in mammals [3]. In fruit bats, modifications of the foraging behavior in response to anthropogenic habitat change can have particularly important ecological consequences in and near tropical forests. Because of the key roles of fruit bats as pollinators and seed dispersers of tropical trees [4], and because some fruit bat species can use degraded forests [5, 6] and shift from native to non-native food sources [7], foraging behavior responses by fruit bats to habitat change may have a disproportionate influence on tree reproduction and regeneration in tropical landscapes [4,8,9].
Bat foraging studies typically use a standard set of methods: cafeteria trials on captive bats [10], pollen/seed sampling from captured bats [11], field examination of feeding refuse [7], guano analyses [12], direct observations of free-ranging bats [13], radio telemetry [5], and stable isotope analyses [14]. Of these methods, stable isotope analyses provides several advantages for long-term and large-scale examination of fruit bat diets and the factors that affect them. First, many fruit bats are sequential specialists, intensively focusing on one or a few resources at a time, then rapidly shifting to new resources in accordance with tree phenology [15]. While direct observational methods require a long-term data set to understand the complete annual diet of sequential specialists, stable isotope analyses can provide insight into foraging over time periods encompassing such temporal shifts (data can represent diets over several weeks or years, depending on the sample [16,17]). In addition, fruit bats travel quickly, forage over tens of kilometers, and forage in diverse habitats [18], which can be difficult to track with standard methods, yet stable isotope analyses can provide insights into bat foraging across its entire range, not only in a single location. The use of stable isotopes does have some disadvantages, including the need to obtain physical samples from bats and to assume baseline homogeneity of stable isotopes [19]. Nevertheless, when combined with a sampling regime that accounts for multiple individuals in different populations across large geographic areas, stable isotope analyses can provide new avenues for understanding how dietary composition, vertical stratification, and diet breadth shift at the population level in response to large-scale habitat change.
Previous work has demonstrated the versatility of stable isotopes for investigating shifts in fruit bat foraging behavior resulting from habitat change [14]. Stable isotopes (δ15N and δ13C) have been used in nectar-feeding bats to identify when diets switch from C3 to C4 plants in a nitrogen-limited diet (δ15N values are lower in C3 than C4 plants [20]), to hypothesize the presence of commercially grown fruits in diets (δ15N may be higher in agricultural soils due to nitrogen fertilizers [21]), and to determine the direction of seeds dispersed by bats (δ13C is higher in seeds in successional sites than the primary forest [22]). In addition, δ13C values have been used in bats to assess vertical stratification–the tendency of different species to feed at different canopy heights–among species that co-occur (δ13C values decrease at higher canopy strata [23]). Finally, stable isotopes can be used to examine diet breadth (a wider range of stable isotope values in a sample of bats indicates a wider diet breadth at the population level [24]).
The potential for stable isotope analyses to increase understanding of how fruit bat foraging behavior changes in response to habitat change could prove particularly beneficial in Madagascar, where more than 80% of the forest cover has been lost and most of the remaining forests are fragmented or degraded [25,26]. Madagascar’s fruit bat community is comprised of three species–Pteropus rufus, Eidolon dupreanum, and Rousettus madagascariensis–that are all important seed dispersers and pollinators [12,27], but that also have declining populations of conservation concern (P. rufus and E. dupreanum are Vulnerable and R. madagascariensis is Near Threatened [28]). Methods other than the use of stable isotope analyses to evaluate foraging behavior in these species have some limitations due to the diversity of habitats used by these species (including both intact and degraded forest [29,30,31,32] across broad, overlapping ranges [33]) and the large areas covered by individual bats during foraging (although these species do not migrate seasonally [34,35], they can travel up to 30 km in one night [36]). Perhaps in part because of these limitations, these species remain poorly understood [37], and no studies have compared their foraging habits across large spatial scales.
This study aimed to compare the feeding ecology of Malagasy fruit bats in light of the large-scale habitat changes occurring in Madagascar. The objectives were to examine how fruit bat diets varied (1) by species and (2) across space as a result of changes in forest cover. Due to small sample sizes, R. madagascariensis is only included in some analyses; most analyses include only P. rufus and E. dupreanum. For objective one, we hypothesized that, although all three species are largely frugivorous [12,31,38,39] their diets would differ. Specifically, in accordance with previous evidence of differences in the composition and abundance of food items in the diet of the three species [13,33], we expected among-species differences in diet would be stronger than within-species differences. Similarly, we hypothesized that diet breadth would differ between species; we expected that, in accordance with previous studies on dietary diversity, P. rufus would have the most largest diet breadth [12,31], R. madagascariensis would have an intermediate diet breadth [38], and E. dupreanum would have the smallest diet breadth [39]. We further hypothesized that the bats would exhibit vertical stratification (a type of resource partitioning) when multiple species were present in the same area [14]. Based on previous field observations [33], we expected that stable isotope values would be consistent with P. rufus feeding at higher vertical strata than E. dupreanum.
For objective two, we hypothesized that the bat diets would differ spatially. Because the extent of anthropogenic degradation of forest habitat (but not forest type) varied across our study region, we expected their diets would vary regionally. Specifically, because of the different types of fruit resources consumed by bat species in degraded areas [14,29,31], and because of the long nightly distances they can fly [18], we hypothesized that diets would change as the percent of forest cover decreased at spatial scales close to the maximum nightly flight distances.
Methods
Ethical Research Statement
Research design, including the recruitment of hunters and meat sellers into the study, was approved by an ethical review board (Temple University Institutional Review Board, protocol number 21414), as was the collection of hair samples from wild animals (exempted study because no live animals were involved in the research, Temple University Institutional Animal Care and Use Committee). No animals were killed specifically for this study (see Methods). Data were not collected on how animals were killed; the actual capture and killing of the animals was outside the scope of the researchers’ interactions with hunters and meat sellers. Samples were collected from carcasses at the point of sale in urban towns (e.g., where hunters sold carcasses to market vendors or other individuals; at market stands directly to the public; in restaurants); these locations were typically at least 5 km distant from the rural locations where the bats were hunted. To avoid either creating incentives promoting wild meat hunting or imposing burdens on those participating in the study, sampling was undertaken so as to provide neither cost nor benefit to meat sellers. Meat sellers were not paid for their participation, but were reimbursed for any telephone credit used for the study. One exception was for one market vendor in Antsiranana, who was reimbursed a nominal amount (USD$0.50–1.00 per sample for 17 samples; market price of one bat was USD$1.00–2.50) to compensate for a reduction in sale price of the bat meat due to removal of hair samples. In Antsiranana, Anivorano Nord, Andriba, and Ansiafabositra, hair samples were also collected from bats via hunters. These hunters were recruited through a related research project [40] and were not compensated. Research was conducted under the authorization of the Madagascar Ministry of Water and Forests. Permission to conduct research was also requested and obtained from the highest ranking, locally elected official of each town. Hair samples were exported from Madagascar and were declared to the U.S. Fish and Wildlife Service at the New York City port of entry.
Study Site
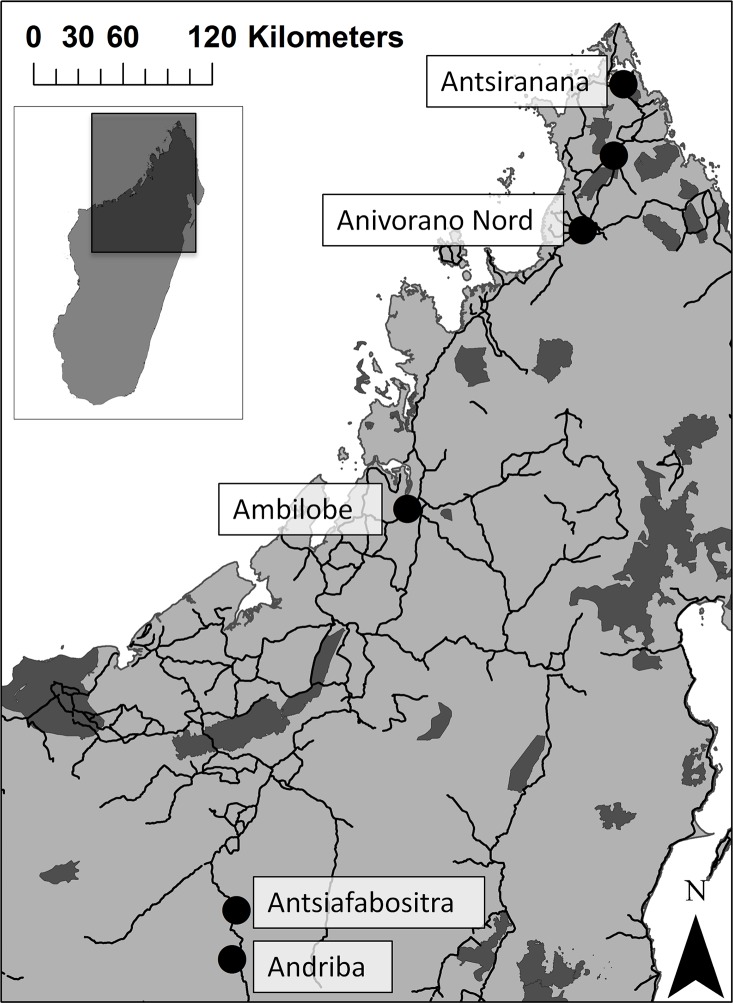
Samples were collected (June-August 2013) in six towns in Madagascar where there were formal bat traders (i.e., organized, persistent, publicly known, entities involved in the regular trade of bats) (Table 1, Fig 1). The towns were 45 to 425 km apart, and varied in distance from the coast and from protected areas (Fig 1). Four of the towns were near known roosting sites for at least one of the species [32]. Given that samples were collected from formal traders in towns, they were not systematically collected across the landscape, though the traders always sourced bats from a variety of hunting locations around each town.
Table 1. Towns where hair samples were collected, with hunting sites for each town.
| Town (hunting sites listed under each town)a | Number of meat sellers enrolled in study | Town populationb | Number of individuals sampled | ||
|---|---|---|---|---|---|
| P. rufus | E. dupreanum | R. madagascariensis | |||
| Andriba | 1 | 32,000 | |||
| Mangasoavina | - | 1 | - | ||
| Antsiafabositrac | 2 | 8,328 | 4 | - | - |
| Antsohihy | 1 | 105,317 | |||
| Ambaliha | 12 | - | - | ||
| Ambodimanany | 1 | - | - | ||
| Amboroho | 2 | - | - | ||
| Ambilobe | 1 | 56,427 | |||
| Ambakiarano | - | 11 | - | ||
| Amborondolo | 30 | - | 1 | ||
| Beramanja | 15 | - | - | ||
| Isesy | 15 | 2 | - | ||
| Mahivoragno | - | 16 | - | ||
| Mamoro | 3 | - | - | ||
| Anivorano Nordd | 6 | 15,000 | 6 | 20 | - |
| Antsiranana | 4 | 87,569 | |||
| Akonokono | 15 | 2 | - | ||
| Andranofanjava | 16 | - | - | ||
| Daraina | 7 | - | 5 | ||
| French Mountain | 7 | - | - | ||
| Mangoaka | 5 | - | 1 | ||
| Total: | 15 | - | 138 | 52 | 7 |
Towns in which data were collected along with the number of meat sellers enrolled in the study, the human population of the towns in which data were collected, and the number of hair samples collected in each town by species.
aHunting sites are those reported by hunters and meat sellers in each town; this may not be an exhaustive list of hunting sites for each town.
bTown population estimates were taken from the Ilo database [44].
cIn Antsiafabositra the hunting site or sites were unknown, so we do not differentiate by hunting site.
dIn Anivorano Nord, bats were hunted only at one hunting site but the name of this hunting site is unknown.
Fig 1. Locations of cities, indicated by black circles, where wild meat samples were collected.
Dark gray regions denote protected habitat, black lines indicate roads, and the inset indicates the location of the study region on the island of Madagascar. Reprinted under a CC BY license, with permission from Reuter, original copyright 2015 [41].
Bat Hair Samples
We used bat hair for stable isotope analyses (as opposed to tissues) because the slow turnover rate of hair integrates diet information over a long period (several months [16]). No bats were captured. Samples were obtained from bat carcasses from hunters or wild meat vendors including restaurants and food market stands during the legal hunting season (i.e. when bats are legal to hunt and sell in Madagascar, Table 1 [29]). The bat carcasses was always intact (e.g., not cut into pieces) with no hair burned off or otherwise damaged, though all carcasses generally showed signs of small injuries (e.g., the causes of death). All three species are sold through the wild meat trade [42]. Sampling carcasses from the wild meat trade facilitated collection of samples across a large spatial scale and has been used to collect hair and tissue samples for ecological studies in other areas of Africa [43]. This sampling procedure could have introduced some bias if hunters hunted in areas not representative of typical foraging areas or if hunters prioritized some bat species over others. Such bias is considered minimal for this study since hunters have an economic incentive to capture bats where they are most abundant, because hunters often use indiscriminate hunting techniques such as large nets that capture any species present [42], and because our study focused on the regional scale and hunter preferences for particular types of hunting grounds or species may not be consistent across large spatial scales.
Samples of ventral hair were collected from each bat carcass by a trained research team member within 24 h of bat capture. Hair was cut with sterilized scissors, then stored in labeled 1.5-mL plastic, centrifuge tubes. Care was taken to avoid contamination by blood. Hair samples were also collected opportunistically from other mammals being sold by the same meat sellers to provide the first, or some of the first, reported values of stable isotopes for those species (Table 2). The data from hair samples of these other non-bat mammal species are not analyzed further due to small sample sizes; they are presented for informational purposes only.
Table 2. Table of stable isotope values by mammal species, with means ± SD.
| Bat Species (number of hunting sites) | δ13C (‰) | δ15N (‰) |
|---|---|---|
| E. dupreanum (n = 6) | ||
| Average | -21.33 ± 0.79 | 7.25 ± 0.84 |
| Median | -21.53 | 7.39 |
| Minimum Value | -19.86 | 5.95 |
| Maximum Value | -21.99 | 8.43 |
| P. rufus (n = 14) | ||
| Average | -22.44 ± 0.75 | 6.69 ± 0.86 |
| Median | -22.70 | 6.48 |
| Minimum Value | -23.35 | 5.42 |
| Maximum Value | -21.85 | 8.74 |
| R. madagascariensis (n = 3) | ||
| Average | -21.57 ± 0.90 | 8.21 ± 1.44 |
| Median | -21.45 | 7.99 |
| Minimum Value | -22.53 | 6.90 |
| Maximum Value | -20.74 | 9.76 |
| Other Mammal Species | ||
| Felis silvestris (Wild Cat, n = 1) | -20.39 | 11.52 |
| Potamochoerus larvatus (Wild Pig/Bushpig, n = 3) | -22.77 ± 0.61 | 8.81 ± 0.62 |
| Setifer setosus (Greater Tenrec, n = 1) | -21.81 | 10.70 |
| Tenrec ecaudatus (Common Tenrec, n = 2) | -19.12 ± 2.73 | 7.14 ± 1.91 |
| Viverricula indica (Small Indian Civet, n = 4) | -18.96 ± 0.95 | 9.09 ± 0.78 |
Means and standard deviations are calculated using hunting sites are replicates for bats and individuals are replicates for other species. Minimum and maximum values depict the hunting site with the lowest mean value and the highest mean value.
Stable Isotope Analyses
We used both δ13C and δ15N stable isotopes in this study, because of the known relationship of these isotopes with foragers’ diet [14]. While δ13C may also reflect the photosynthetic pathway of food plants or vertical differences in foraging, most plants used for food by fruit bats in the region we studied likely use the C3 pathway, and in the seasonally dry forest region we studied, tree height is restricted (and thus vertical differences in canopy height differences are limited). Further, while δ15N may also indicate the trophic level of the forager, the presence of legumes, or the agricultural application of fertilizers to food crops, the focal species all forage on plants at the same trophic level (i.e., plants [18]), and fertilizer application to fruiting trees on typical small-scale farms in Madagascar is limited (BJS pers. obs.). Finally, although δ15N isotopes can vary considerably in dry deciduous forests [45], we argue that, as described in the ‘Statistical Analysis’ section below, geography and vegetation type both likely play a limited role in influencing isotope values in this dataset. We therefore interpret variation in δ13C and δ15N isotopes as primarily indicating dietary differences, and only secondarily vertical stratification (δ13C differences) or foraging in human-modified landscapes (δ15N). The use of δ13C and δ15N isotopes also facilitated comparison against previous studies on the same species [14].
Hair samples (0.3–0.7 mg) were washed in ethanol and packaged in tin capsules for mass spectrometry [46]. Samples were analyzed using a Costech elemental analyzer interfaced with a continuous flow Micromass (Manchester, UK) Isoprime isotope ratio mass spectrometer (EA-IRMS) for 15N/14N and 13C/12C ratios [46]. Measurements are reported in δ notation and were calculated using the following formulae:
And:
Ovalbumin was used as a routine standard (2 standards for each set of 10–12 samples). Precision for δ13C and δ15N was generally ± 0.2‰ and ± 0.4‰ (standard deviation of 10 laboratory standards). The stable isotope values (δ13C and δ15N) for each hair sample can be found in S1 Appendix.
Social Surveys
Hunters and meat sellers provided information about the location each bat was hunted (‘hunting site’). Meat sellers sometimes sourced bats from more than one hunting site (Table 1). GPS coordinates for hunting sites were recorded during visits to hunting sites with hunters, or by extracting coordinates from maps or satellite images on the basis of landmarks indicated by the hunter or meat seller. GPS coordinates can be found in S1 Appendix.
Statistical Analyses
Unless otherwise noted, tests were completed with JMP statistical software (JMP®, Version 10. SAS Institute Inc., Cary, NC, 1989–2007). Summary data are the mean ± SD. Sample sizes for R. madagascariensis were too low to include in most statistical analyses however we include data for this species when possible throughout the results because relatively little is known about this species. Because variation may be greater among than within hunting sites, hunting locations were used as replicates or random effects whenever possible. When this was not possible, we have noted the type of replicate used next to each statistical test.
Dietary differences among species were analyzed using a Permutational ANOVA (individuals as replicates) and pairwise post-hoc tests (Primer statistical software [47]) of Bray-Curtis similarity, a measure of similarity that can examines relationships among replicates in combined δ13C and δ15N values (as opposed to analyzing δ13C and δ15N values separately). This statistical test and software are frequently used to test for changes or differences in isotopic signatures (δ13C and δ15N [48,49,50]). Dietary differences and overlap were also visualized and diet breadth calculated using standard Bayesian ellipses adjusted for small sample size (SEAc) using the SIBER package for R statistical software [51].
Differences in dietary breadth were examined by using the Bayesian approach (105 posterior draws) for comparing the standard ellipse areas of the different species [51].
We tested for vertical stratification in two ways. First, we examined whether P. rufus and E. dupreanum differed in their δ13C and δ15N values. We used mixed effects models with ‘species’ as the independent variable and a dependent variable of either δ13C values or δ15N values. A random effect [52] for ‘hunting site’ was included in each model to account for the non-independence of the isotopic signatures of bats within hunting sites. Second, we examined whether vertical stratification was evident when P. rufus and E. dupreanum were captured together (on the same night by the same hunter) versus when they were captured separately. We used a Wilcoxon Test with hunting sites as replicates to compare stable isotope values when P. rufus and E. dupreanum were captured together or separately. Four Wilcoxon tests were performed: 1) comparison of P. rufus δ13C values at hunting sites where no E. dupreanum were caught (n = 11) versus sites where E. dupreanum were also caught (n = 3); 2) comparison of P. rufus δ15N values at hunting sites where no E. dupreanum were caught (n = 11) versus sites where E. dupreanum were also caught (n = 3); 3) comparison of E. dupreanum δ13C values at hunting sites where no P. rufus were caught (n = 3) versus sites where P. rufus were also caught (n = 3); and 4) comparison of E. dupreanum δ15N values at hunting sites where no P. rufus were caught (n = 3) versus sites where P. rufus were also caught (n = 3);
We tested for regional difference in diet using Permutational ANOVA tests [47], by evaluating whether δ13C and δ15N values changed for P. rufus or E. dupreanum across hunting sites. Individuals were replicates.
For objective two, we used a model selection approach to understand how forest cover and climate affected stable isotope values. For these models, the primary predictor variable related to forest cover, which was calculated from satellite imagery [53], using images taken by the Landsat 8 satellite during August-October 2013. These dates were selected to match the time frame of hair samples collection, and because images showed low cloud cover. To evaluate the influence of the spatial scale of local habitat change on bat diets, satellite images were clipped to three different radii (5, 15, and 30 km) using ArcGIS [54]; 30 km has been estimated as the maximum foraging radius for P. rufus which has the largest nightly flight distance of the three species [18,36]. Clipped images were processed using CLASlite (version 3.1 [55]), a program used to classify tropical landscapes into forest and non-forest cover [56]. Forest cover was conservatively defined as a pixel with no more than 5% bare ground and no less than 85% live vegetation (30 meter spatial resolution [55]). Post-processing, raster files were analyzed for their percent forest cover using ArcGIS [54].
To evaluate whether changing stable isotope values could result from habitat or geographic differences across the study area, we evaluated whether dominant vegetation near hunting sites varied. Countrywide vegetation maps developed between 2003 and 2006 [57] showed that one forest type—Western Dry Forest—was the dominant non-degraded forest type within 30 km at all hunting sites (Table 3), suggesting that the role of habitat in isotope variation may be limited. To evaluate variation in stable isotope values across the study area, stable isotope values from leaves of a fruiting tree species that occurs across this region (Mangifera indica) were compared. While it would have been preferable to present stable isotope data from additional sites and species, difficulties in reaching hunting sites and time and resource limitations on sampling precluded broader collection of reference samples. Sample sizes were too small, however, to determine whether δ13C and δ15N stable isotope values in M. indica changed across the study area (but the values are presented in Table 4 for reference purposes). However, because all of the bats came from the same habitat type (Western Dry Forest), we suggest that the role of habitat in isotope variation is limited. Thus, in this study, we suggest that the observed differences in isotope values are primarily indicative of dietary differences. We acknowledge the limitations of this assumption.
Table 3. Annual Temperature Range and forest cover characteristics of each hunting site.
| Town (hunting sites listed under each town) | Annual Temperature Range (Mean)a | Percent forest cover (radius)b in 2013 | Percent of native remnant primary vegetation by forest type (30 km radius) in 2003e | |||||
|---|---|---|---|---|---|---|---|---|
| 5km | 15km | 30km | WDF | W | HF | M | ||
| Andriba | ||||||||
| Mangasoavina | 190 | 13% | 18% | 27% | 59% | 0% | 41% | 0% |
| Antsiafabositrac | 183 | 11% | 11% | 4% | 90% | 0% | <1% | 0% |
| Antsohihy | ||||||||
| Ambaliha | 146 | 25% | 24% | 11% | 68% | 4% | 6% | 23% |
| Amboroho | 172 | 6% | 2% | 5% | 69% | 3% | 5% | 24% |
| Ambilobe | ||||||||
| Ambakiarano | 148 | 19% | 1% | 5% | 96% | 2% | 2% | <1% |
| Amborondolo | 155 | 53% | 56% | 24% | 74% | 5% | 1% | 19% |
| Beramanja | 149 | 33% | 30% | 35% | 55% | 10% | <1% | 35% |
| Isesy | 146 | 6% | 14% | 5% | 67% | 12% | <1% | 21% |
| Mahivoragno | 135 | 2% | 3% | 5% | 75% | 10% | 1% | 14% |
| Mamoro | 144 | 23% | 25% | 40% | 66% | 5% | 10% | 20% |
| Anivorano Nordd | 132 | 48% | 7% | 11% | 70% | <1% | 29% | 0% |
| Antsiranana | ||||||||
| Akonokono | 119 | 19% | 6% | 6% | 73% | 10% | 10% | 6% |
| Andranofanjava | 125 | 53% | 5% | 11% | 48% | 5% | 44% | 2% |
| Daraina | 122 | 51% | 54% | 27% | 55% | 4% | 34% | 7% |
| Mangoaka | 113 | 5% | 13% | 7% | 60% | 10% | 26% | 4% |
Characteristics of the hunting sites, including: annual temperature range (mean), percent of remaining forest cover within 5, 15, and 30 km of the hunting side, and percent of native vegetation by forest type.
WDF = Western Dry Forest/Deciduous Seasonally Dry Forest. W = Wetlands/Marshlands. HF = Humid Forest/Evergreen Forests. M = Mangroves.
aData derived from WorldClim database; this database expresses temperature as degrees Celsius multiplied by 10 [58].
bData extracted from satellite images downloaded from the USGS Earth Explorer database [53].
cIt was not possible to determine where the bats were hunted so the center of the town was used as the ‘hunting site’.
dBats were hunted only at one hunting site, but the name of this hunting site is unknown.
eData derived from Moat and Smith [57].
Table 4. Table of stable isotope values for the leaves of Mangifera indica (Common name: Mango).
| Town | δ13C (‰) | δ15N (‰) |
|---|---|---|
| Antsiranana (n = 2) | ||
| Mean ± SD | -29.64 ± 0.72 | 3.92 ± 1.12 |
| Median | -28.61 | 4.48 |
| IQR | 1.43 | 2.23 |
| Anivorano Nord (n = 3) | ||
| Mean ± SD | -30.11 ± 0.71 | 3.63 ± 1.53 |
| Median | -30.41 | 3.50 |
| IQR | 1.32 | 3.07 |
| Ambilobe (n = 3) | ||
| Mean ± SD | 28.61 ± 0.09 | 4.47 ± 1.66 |
| Median | -29.76 | 4.13 |
| IQR | 1.43 | 2.23 |
| Antsohihy (n = 5) | ||
| Mean ± SD | 30.10 ± 0.88 | 3.78 ± 2.56 |
| Median | -29.81 | 2.87 |
| IQR | 1.37 | 4.32 |
Stable isotope values (individuals are replicates) at sites spread across a distance of 440 km. Samples were collected within 30 km of the towns; leaves were collected directly from trees and were dried prior to storage in sealed plastic containers. Samples (100 mg per tree) were analyzed using the same procedures as for the hair analysis. IQR = Interquartile range.
To control for the effect of climate on stable isotope values [59], we also examined the annual temperature range of each site. This variable was used as a proxy for local climate due to its strong correlation with other climate variables (annual precipitation levels, precipitation seasonality, temperature seasonality; P < 0.05 and |r| > 0.8 for all pairwise comparisons at hunting sites). Annual temperature range was calculated by clipping the WorldClim [58] raster file of temperature data from 1950–2000 to a 30 km radius of each hunting site, and averaging across all pixels included within the clipped radius. Forest cover and climate data are presented in Table 3.
The importance of forest cover on bat diets was analyzed using an information theoretical approach employing model estimation and selection [60,61]. For P. rufus, a set of candidate mixed effects models was established a priori and all met the assumptions of linear mixed models. Candidate models included forest cover in %, annual temperature range, and interactions between these two variables (S1 Table). Models were validated by calculating the R2 of the best-fit models and these values are reported in the results. Large sample sizes that allowed for the use of individual bats as replicates, and hunting site was used as a random effect. However, because of small sample sizes in E. dupreanum, only regression models with one predictor variable were considered, and hunting sites were treated as replicates. To facilitate comparison against E. dupreanum, regression models for P. rufus were also evaluated separately using hunting sites as replicates. The full set of these candidate regression models for E. dupreanum and P. rufus are also in S1 Table. Models were ranked separately for each species on the basis of the corrected Akaike’s Information Criterion (AICc), which is adjusted for small sample size [60]. Delta AICc (ΔAICc) and Akaike weights (wi) were calculated for comparison purposes [61]. The best model was the model with the lowest AICc; given the conceptual similarity of candidate models only others with substantial support were considered (ΔAICc < 2). The mixed effects models in P. rufus and regression models in P. rufus and E. dupreanum with the most support (the lowest ΔAICc) were further examined to determine the relative influence of the forest cover in % and annual temperature range variables on the δ13C and δ15N values.
Results
Sampling Effort
Hair samples from fruit bats (n = 138 for P. rufus, n = 52 for E. dupreanum, and n = 7 for R. madagascariensis) were taken from six towns and linked via social surveys to 17 hunting sites (Table 1). Stable isotope data from opportunistic samples of wild cat (Felis silvestris), wild pig (Potamochoerus larvatus), greater tenrec (Setifer setosus), common tenrec (Tenrec ecaudatus), and small Indian civet (Viverricula indica) were collected opportunistically; results are provided in Table 2.
Species Differences in Diet, Diet Breadth, and Resource Partitioning
Diets
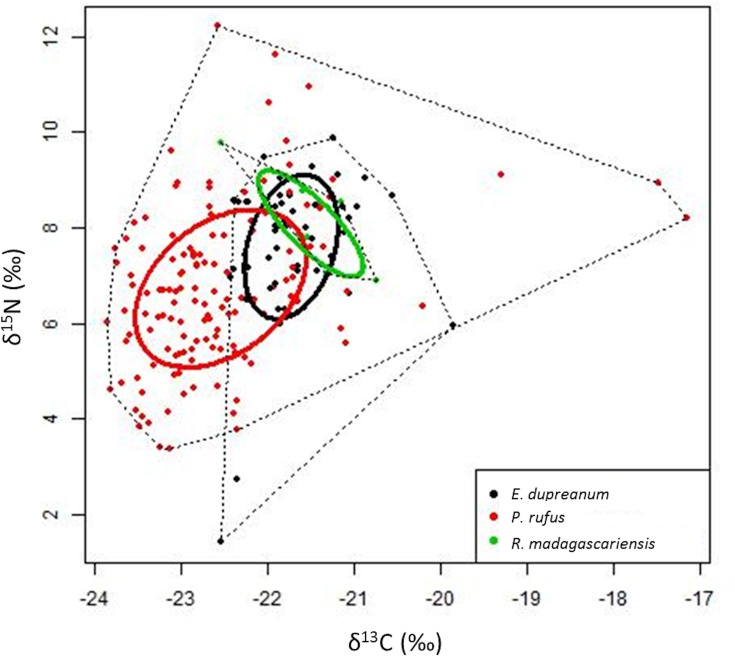
In accordance with our hypothesis, diets (Bray-Curtis similarity of δ13C and δ15N values) differed among species (PERMANOVA, Pseudo-F = 12.99, df = 2, p = 0.001; individuals as replicates), with P. rufus having different stable isotope values than both E. dupreanum (Pairwise PERMANOVA, t = 4.63, p = 0.001) and R. madagascariensis (Pairwise PERMANOVA, t = 2.43, p = 0.015). E. dupreanum had the highest δ13C values while R. madagascariensis had the highest δ15N values (Fig 2).
Fig 2. Dietary differences and overlap by species.
δ13C and δ15N values of individual bats with species differences and overlaps in diet visualized using standard ellipses adjusted for small sample size (colored circles) and convex hulls (dotted lines) [51]. Standard ellipses calculated to include 40% of the data points within a species. Convex hulls capture all data points within a species.
Diet breadth
The diet breadth (calculated as the standard ellipse area or SEAc) was 4.75 for P. rufus, 2.45 for E. dupreanum, and 1.26 for R. madagascariensis (Fig 2). In accordance with our hypothesis, the diet breadth of P. rufus was larger than the diet breadths of E. dupreanum (comparison of 105 posterior draws, p < 0.01) and R. madagascariensis (p = 0.027). There was, however, no difference in the diet breadths of E. dupreanum and R. madagascariensis (p = 0.32; S1 Fig).
Vertical stratification
Vertical stratification was apparent in two lines of evidence. First, when compared across all hunting sites, P. rufus had lower δ13C values than E. dupreanum (Mixed Effects Model, Estimate = 0.39, Std. Error = 0.12, F-Ratio = 11.84, P = 0.0008, hunting site as a random effect). However, the two species did not differ in their δ15N values (Mixed Effects Model, Estimate = 0.38, Std. Error = 0.20, F-Ratio = 3.60, P = 0.06, hunting site as a random effect).
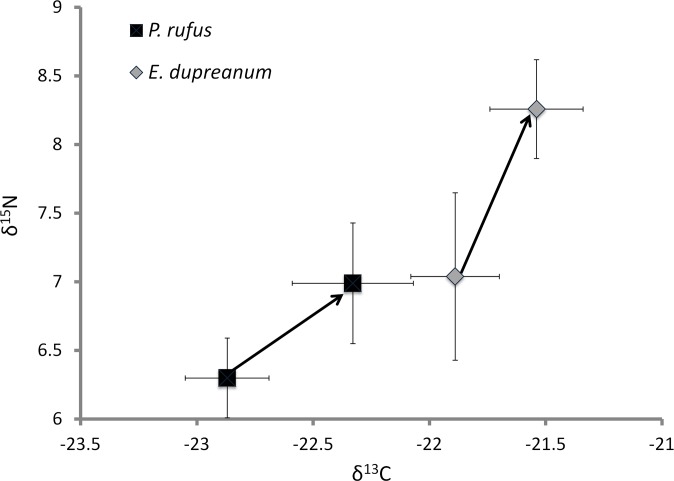
Second, at hunting sites where P. rufus was caught alongside E. dupreanum, δ13C values (Wilcoxon Test, Chi-square = 15.99, p < 0.0001; hunting sites as replicates) and δ15N values (Wilcoxon Test, Chi-square = 5.89, p = 0.0152) were higher than in areas where they were caught alone (n = 11 hunting sites where P. rufus was caught alone, n = 3 hunting sites where P. rufus was caught alongside E. dupreanum). Likewise, E. dupreanum had higher δ13C and δ15N values when caught alongside P. rufus than when caught alone (n = 3 hunting sites where E. dupreanum caught alongside P. rufus, n = 3 hunting sites were E. dupreanum caught alone, Wilcoxon Test, Chi-square = 7.28, p = 0.007 and Chi-square = 10.68, p = 0.0011, respectively; Fig 3). R. madagascariensis was always caught alongside P. rufus; it was not possible to determine whether it modified its resource use in the presence of other species.
Fig 3. Dietary differences in P. rufus and E. dupreanum when caught alone or alongside each other.
Differences in diet (δ15N and δ13C values) when P. rufus and E. dupreanum were caught at hunting sites alone (start of arrow) and when they were caught alongside each other (end of arrow). When caught in the presence of another species, both P. rufus (diamonds) and E. dupreanum (squares) significantly shifted in their δ15N and δ13C values. Hunting sites are replicates in this analysis, and the two species were caught alongside each other at three hunting sites (n = 36 P. rufus individuals and n = 24 E. dupreanum individuals), E. dupreanum was caught alone at a further three sites (n = 28 individuals) and P. rufus was caught alone at 11 hunting sites (n = 102 P. rufus individuals). Data show mean ± SD.
Regional Differences and Impact of Forest Cover and Annual Temperature Range on Diets
Regional changes in diet
In accordance with our hypothesis, both P. rufus and E. dupreanum diets (measured as Bray-Curtis similarity of δ13C and δ15N values) differed by hunting site (PERMANOVA, P. rufus, Pseudo-F = 5.41, df = 13, p = 0.001; E. dupreanum, Pseudo-F = 3.88, df = 5, p = 0.037; individuals as replicates).
Influence of forest cover on diet
There was some support for our hypothesis, as the diets of both P. rufus and E. dupreanum were affected by forest cover, albeit at different scales. After controlling for annual temperature range, the diet of P. rufus was best explained by forest cover at the largest spatial scale examined. Specifically, δ13C and δ15N values for P. rufus were best explained by a combination of the annual temperature range (average, annual temperature range during 1950–2000) and remnant forest cover within 30 km of their hunting site; the best model for δ13C also contained the temperature-forest cover interaction (Table 5). For δ15N, models with annual temperature range and different forest cover radii (5 and 15 km) also had some support. Further examination of the best models for P. rufus revealed that the influence of the forest cover-temperature interaction was strong for δ13C values (mixed effects model; forest cover within 30 km = 0.01, Std. Error = 0.02, p = 0.42; annual temperature range = -0.02, Std. Error = 0.01, p = 0.15; interaction = -0.004, Std. Error = 0.001, p = 0.012; model R2 = 0.41), but that the forest cover did not influence δ15N values (mixed effects model; forest cover within 30 km = -0.03, Std. Error = 0.02, p = 0.32; annual temperature range = -0.01, Std. Error = 0.02, p = 0.68; model R2 = 0.27). When these models were analyzed using hunting sites as replicates (n = 14 for P. rufus), δ13C and δ15N values were best explained by models with a single predictor variable. Models with the single variable of annual temperature range explained stable isotope variables the best, although models containing the single predictor of forest cover at all three radii also received some support (ΔAICc < ~2; Table 5).
Table 5. Results of model selection (ΔAICc < 2), with corrected Akaike’s information criterion (AICc) and Akaike weights (wi).
| Fixed Effects | AICc | ΔAICc | wi |
|---|---|---|---|
| P. rufus– δ13C values (individuals as replicates, ‘hunting site’ as random effect) | |||
| T + FC30 + T * FC30 | 355.58 | 0 | 0.45 |
| P. rufus– δ15N values (individuals as replicates, ‘hunting site’ as random effect) | |||
| T + FC30 | 506.71 | 0 | 0.36 |
| T + FC5 | 508.18 | 1.47 | 0.17 |
| T + FC30 + T * FC30 | 508.32 | 1.61 | 0.16 |
| T + FC15 | 508.54 | 1.83 | 0.14 |
| P. rufus– δ13C values (hunting sites as replicates, no random effects) | |||
| T | 36.39 | 0 | 0.28 |
| FC15 | 37.10 | 0.71 | 0.20 |
| FC30 | 37.86 | 1.47 | 0.14 |
| FC5 | 37.91 | 1.52 | 0.13 |
| T + FC30 + T * FC30 | 38.17 | 1.78 | 0.12 |
| P. rufus– δ15N values (hunting sites as replicates, no random effects) | |||
| T | 39.03 | 0 | 0.35 |
| FC30 | 39.79 | 0.77 | 0.24 |
| FC5 | 40.91 | 1.87 | 0.14 |
| E. dupreanum– δ13C values (hunting sites as replicates, no random effects) | |||
| FC15 | 18.49 | 0 | 0.75 |
| E. dupreanum– δ15N values (hunting sites as replicates, no random effects) | |||
| FC5 | 27.76 | 0 | 0.40 |
| T | 28.26 | 0.50 | 0.31 |
| FC30 | 29.28 | 1.52 | 0.19 |
Complete list of models tested with results can be found in S1 Table. The predictor variables included: T = annual temperature range; FC5 = forest cover within 5 km radius of hunting site; FC15 = forest cover within 15 km radius of hunting site; and FC30 = forest cover within 30 km radius of hunting site.
Annual temperature range was a poor predictor of δ13C values in E. dupreanum. The diet of E. dupreanum was correlated with forest cover, though at smaller spatial scales than P. rufus. E. dupreanum diets were best explained by forest cover within 15 km (δ13C values; Table 5) or 5 km (δ15N values) of the hunting site. For δ15N, alternate models with larger forest cover radii or annual temperature range also had some support. Further examination of the best models for E. dupreanum indicated forest cover had a significant influence on δ13C values (Regression; forest cover within 15 km, p = 0.006; model R2 = 0.88) but not on δ15N values (Regression; forest cover within 5 km, p = 0.12; model R2 = 0.50). Sample size was too low to analyze the impacts of annual temperature range and forest cover on R. madagascariensis diet.
Discussion
Diets and Diet Breadth of Fruit Bats in Madagascar
The three species rely on similar plant resources and have overlapping ranges [32,33]. Therefore, we expected the bats to show evidence of trophic niche differentiation due to resource competition; this was supported by our results. While the species differed in their diets, there was overlap in their diets, and (in accordance with Dammhahn & Goodman [14]) the differences among the three species in mean δ13C and δ15N values were not large in magnitude, suggesting relatively little separation in their diets. For example, mean δ13C values for the three species differed by only ~1.1‰ (range: -21.2 to -22.29‰), whereas a dry season study of fruit bats in South America found a difference of 6.8‰ (range: -21.5 to -28.3‰ [23]). The small species-level differences in stable isotope values cannot be explained by distinct and highly specialized diets in the three species; our results suggest substantial dietary overlap (Fig 2), in line with prior studies [32,33]. Another possible reason for dietary overlap is a decrease in resource availability over the dry season in seasonal habitats [13]. Any such seasonal restriction in diet composition, however, would only be captured partially in our results (the timing of our data collection and the turnover rate of hair samples [16] means that the results encompass both wet and dry seasons). Thus, we hypothesize that other causes of limited fruit availability, such as the replacement of native forests that provide diverse food resources with human-dominated landscapes that provide less diverse food resources [29,31], could be driving the diet overlap we observed.
P. rufus had a larger diet breadth than the two other species. This accords with prior studies, which have recorded P. rufus consuming a larger number of plant species [12,29] than the other two species [38,39]. It also suggests that P. rufus may more commonly use native habitats, where food sources are more diverse than in degraded habitats or orchards.
In terms of vertical stratification, P. rufus had lower δ13C values than E. dupreanum, which could indicate that P. rufus feeds higher in the forest canopy than E. dupreanum [20,23]. Though absolute differences in the stable isotope values were small, even small differences in δ13C values can indicate differences in foraging height of a half-dozen meters or more [23]. This is also consistent with observations that when the two species forage simultaneously in kapok trees (Ceiba pentandra), P. rufus forages in higher branches than E. dupreanum [33]. In regards to δ15N values, the higher δ15N values of E. dupreanum may indicate that it feeds on more commercially grown fruits [21] or in areas that are non-forested and dominated by grasslands and drought-resistant plants [62]. This interpretation accords well with observations that E. dupreanum more readily uses degraded habitats than P. rufus [32].
The diets of both bats shifted similarly–toward higher δ13C and δ15N values–when captured together. Specifically, the diet of P. rufus when the two species co-occurred coincided with the diet of E. dupreanum in the absence of other bats. Further, with P. rufus present, the diet of E. dupreanum shifted to even higher δ13C and δ15N values (Fig 3). Although no clear conclusions can be drawn without baseline data, these results may point to a scenario in which the larger and likely more competitively-dominant P. rufus is able to exclude E. dupreanum from its primary foraging areas in the higher canopy levels of the primary forest, leaving E. dupreanum to forage at a lower canopy height (higher δ13C values) and in more degraded areas including savannahs and agricultural areas (higher δ15N values). In this scenario, when primary forest is unavailable, P. rufus may shift its foraging to more degraded habitats (higher δ15N values) that lack high canopies (higher δ13C values), displacing E. dupreanum to even more degraded habitats (Fig 3). A closer examination of the hunting sites also supports this idea; sites where the two species were caught together had less remnant habitat (9 ± 5% within 15 km and 7 ± 4% within 30 km) than those where they were caught separately (20 ± 11% and 16 ± 11%, respectively).
Our analysis of diet shifts assumed that data on bat captures could be used to infer co-occurrence. However there was a difference between the time frame of the foraging behavior (the shared use of foraging sites was determined for the night captured) and the turnover time for stable isotopes in hair (several months [16]). Thus, while spatial patterns of bat captures are likely to correlate with co-occurrence over time, bias could be introduced if patterns of bat occurrence on the night of capture were not representative of typical patterns of occurrence on a months-long time frame.
We were unable to examine whether R. madagascariensis changed its foraging behavior in the presence of another species. However, it is likely that the diet overlaps with the other species (Fig 2, [14]). This overlap could be maintained through the use of different foraging behavior by R. madagascariensis. Unlike the other two species, R. madagascariensis hovers and removes fruit in flight before bringing it to a nearby feeding roost [13]. This may enable it to access resources that would be inaccessible to the other two species, and thus avoid competition with them even when foraging on the same fruit trees.
Impact of Forest Cover on Bat Diets
The loss of forest cover may impact P. rufus at larger spatial scales (30 km) than E. dupreanum (5–15 km). These data fit well with previous findings that P. rufus is able to forage across a wider range than E. dupreanum [18]. Because P. rufus has access to a wider geographic range [18], it may have more options to choose from in terms of foraging. This increased access may be reflected in the lower δ15N values of P. rufus (compared to E. dupreanum). These δ15N values increase in drought-resistant plants and in agricultural areas [20] where primary forests have been heavily degraded. In such locations, E. dupreanum may have few options but to forage in degraded habitats (because it cannot travel as far as P. rufus [18]), whereas P. rufus, with its greater flight radius, has flexibility to visit farther, more intact habitats. Our dataset was not large enough to analyze the impact of deforestation on R. madagascariensis. However, their short foraging distances relative to the other two Malagasy fruit bat species [37] imply that R. madagascariensis may be responsive to habitat change across smaller spatial scales.
The impact of the interaction between forest cover and annual temperature range on the δ13C values of P. rufus was greater than the impact of forest cover alone. This suggests that P. rufus is vulnerable to the combined impacts of annual temperature range–which may change the availability of fruits and resources–and a decrease in remnant habitat. It may be that P. rufus has the ability to overcome the impacts of annual temperature range or the impacts of forest loss individually, but not the combination of the two.
There are several considerations and factors that were not accounted for in our statistical analysis, but which could have affected our interpretation of the data. First, stable isotope values can be affected by changes in the dominant forest type. However, given that all hunting sites in our study had the same dominant forest type (Table 3), we hypothesize that the dietary changes observed are due primarily to a loss in forest cover, not a change in forest type or geographic variation. Second, in the spatial analysis of forest cover, we used hunting sites as centroids, which could introduce bias if hunting sites were actually near the edge of a bat’s nightly foraging range and if forest cover differed substantially outside the foraging range. However, the possibility for such bias seems limited since hunting sites for bats in Madagascar are often at or near roost sites [30,32]. Finally, although each of the bat species appear tolerant of degraded areas [31], P. rufus dietary breadth may decrease substantially in degraded areas. As such additional research is needed to understand to what extent bats are negatively affected by the restriction of diets associated with loss of forest and if so, how much forest loss the bats can withstand.
Conservation Implications
Our data suggest that the three species had broadly overlapping diets but that there were differences in the diets of P. rufus and E. dupreanum. These diets shifted when the species co-occurred, suggesting resource partitioning across habitats and vertical strata within the canopy to avoid competition, with P. rufus perhaps foraging more in native forests and at a higher forest strata compared to E. dupreanum. Diets changed as the percent of forest cover decreased, though at a larger spatial scale in P. rufus than in E. dupreanum. These results suggest that the species exhibit differing responses to habitat change, highlight the threats fruit bats face from habitat change, and clarify the spatial scales at which conservation efforts could be implemented. Specifically, our data provide evidence that the scale at which conservation should be implemented may differ for the three species. For example, based on the finding of the significant effect of forest cover on a 30 km scale, a conservation strategy aimed at protecting P. rufus might include a focus on protecting sufficient patches of primary forest within ~30 km of its roots to maintain sufficient foraging resources. Conversely, programs aimed at protecting R. madagascariensis and E. dupreanum, could focus on maintaining foraging resources at smaller (5–15 km) scales. It is important to note that such an emphasis on maintaining foraging habitat is important but not sufficient for conserving fruit bats in Madagascar; for example, hunting at roost locations is a primary threat facing these species [29,31]. Promisingly, our sampling of bat hair via the wild meat trade confirms the presence of bats near known roost sites [32] and suggests the presence of previously unknown roosts (e.g., near Andriba and Antsiafabositra).
Supporting Information
Includes species identification, city of purchase, location hunted (including GPS coordinates), % of forest cover (within 5km, 15km, and 30km radius), city population, annual precipitation, annual temperature, temperature seasonality, and precipitation seasonality.
(XLSX)
Calculated using a Bayesian approach (105 posterior draws).
(JPEG)
(DOCX)
Acknowledgments
Thanks to Haley Randell for project management; Melissa Schaefer for assistance in the field; and Totozafy Eric Janvier, Sahondra Hanitriniaina, Tertius Rodriguez Belalahy, and Claudesse Barat for serving as a translators and research assistants. Thanks to Samuel Georgian and Alanna Durkin for assistance with statistical analyses. Thanks to two anonymous reviewers for edits that substantially increased the quality of the manuscript. The authors are indebted to the host communities for their hospitality and assistance with the research, and to the Madagascar Institute for the Conservation of Tropical Environments for assistance with permitting matters.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship (https://www.nsfgrfp.org/) under Grant No. (DGE-1144462) to KER, a National Science Foundation grant (DEB-1257916) to BJS (http://www.nsf.gov/div/index.jsp?div=DEB), an Explorers Club Grant to KER (https://explorers.org/expeditions/funding/expedition_grants), and a Temple University Faculty Senate grant to BJS (https://www.temple.edu/research/facinitiatives/facini_int_funding.html). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lamb D, Erskine PD, Parrotta JA. Restoration of degraded tropical forest landscapes. Science 2005;310: 1628–1632. [DOI] [PubMed] [Google Scholar]
- 2.Wright SJ. Tropical Forests in a Changing Environment. Trends Ecol Evol 2005;20: 553–560. [DOI] [PubMed] [Google Scholar]
- 3.Cosson JF, Pons JM, Masson D. Effects of forest fragmentation on frugivorous and nectarivorous bats in French Guinea. J Trop Ecol 1999; 15:515–534. [Google Scholar]
- 4.Kunz TH, Braun de Torrez E, Bauer D, Lobova T, Fleming TH. Ecosystem services provided by bats. Ann N Y Acad Sci 2011;1223: 1–38. 10.1111/j.1749-6632.2011.06004.x [DOI] [PubMed] [Google Scholar]
- 5.Mildenstein TL, Stier SC, Nuevo-Diego CE, Mills LS. Habitat selection of endangered and endemic large flying-foxes in Subic Bay, Philippines. Biol Conserv 2005;126: 93–102. [Google Scholar]
- 6.Luskin MS. Flying foxes prefer to forage in farmland in a tropical dry forest landscape mosaic in Fiji. Biotropica 2010;42: 246–250. [Google Scholar]
- 7.Sewall BJ, Granek EF, Trewhella WJ. The endemic Comores Islands fruit bat Rousettus obliviosus: ecology, conservation, and Red List status. Oryx 2003;37: 344–352. [Google Scholar]
- 8.Gorchov DL, Cornejo F, Ascorra C, Jaramillo M. The role of seed dispersal in the natural regeneration of rain forest after strip-cutting in the Peruvian Amazon. Vegetatio 1993;107/108: 339–349. [Google Scholar]
- 9.Medellin RA, Gaona O. Seed dispersal by bats and birds in forest and disturbed habitats of Chiapas, Mexico. Biotropica 1999;31: 478–485. [Google Scholar]
- 10.Bravo A, Harms KE, Emmons LH. Preference for Collpa water by frugivorous bats (Artibeus): an experimental approach. Biotropica 2010;42: 276–280. [Google Scholar]
- 11.Soto-Centeno JA, Kurta A. Diet of two nectarivorous bats, Erophylla sezekorni and Monophyllus redmani (Phyllostomidae), on Puerto Rico. J Mammal 2006;87: 19–26. [Google Scholar]
- 12.Bollen A, Van Elsacker L. Feeding ecology of Pteropus rufus (Pteropodidae) in the littoral forest of Sainte Luce, SE Madagascar. Acta Chiropt 2002;4: 33–47. [Google Scholar]
- 13.Sewall BJ, Freestone AL, Hawes JE, Andriamanarina E. Size-Energy Relationships in Ecological Communities. PLOS ONE 2013;8: e68657 10.1371/journal.pone.0068657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dammhahn M, Goodman SM. Trophic niche differentiation and microhabitat utilization revealed by stable isotope analyses in a dry-forest bat assemblage at Ankarana, northern Madagascar. J Trop Ecol 2014;30: 97–109. [Google Scholar]
- 15.Marshall AG. Old World phytophagous bats (Megachiroptera) and their food plants: a survey. Zool J Linn Soc 1985;83: 351–369. [Google Scholar]
- 16.Crawford K, McDonald RA, Bearhop S. Applications of stable isotope techniques to the ecology of mammals. Mamm Rev 2008;38: 87–107. [Google Scholar]
- 17.Fraser EE, Longstaffe EJ, Fenton MB. Moulting matters: the importance for understanding moutling cycles in bats when using fur for endogenous marker analysis. Can J Zool 2013;91: 533–544. [Google Scholar]
- 18.Garbutt N. Mammals of Madagascar: A complete guide USA: Yale University Press; 2007. [Google Scholar]
- 19.Boecklen WJ, Yarnes CT, Cook BA, James AC. On the use of stable isotopes in trophic ecology. Annu Rev Ecol Evol Syst 2011;42: 411–440. [Google Scholar]
- 20.Voigt CC, Matt F. Nitrogen stress caused unpredictable enrichments of 15N in two nectar-feeding bat species. J Exp Biol 2004;207: 1741–1748. [DOI] [PubMed] [Google Scholar]
- 21.Herrera LG, Korine C, Fleming TH, Arad Z. Dietary implications of intrapopulation variation in nitrogen isotope composition of an old world fruit bat. J Mammal 2008;89: 1184–1990. [Google Scholar]
- 22.Voigt CC, Voigt-Heucke SL, Kretzschmar AS. Isotopic evidence for seed transfer from successional areas into forests by short-tailed fruit bats (Carollia spp.; Phyllostomidae). J Trop Ecol 2012;28: 181–186. [Google Scholar]
- 23.Rex K, Michener R, Kunz TH, Voigt CC. Vertical stratification of neotropical leaf-nosed bats (Chiroptera: Phyllostomidae) revealed by stable carbon isotopes. J Trop Ecol 2011;27: 211–222. [Google Scholar]
- 24.Scanlon A, Sophie TP, da S. Stenberg L. Insectivory in Fijian flying foxes (Pteropodidae). Aust J Zool 2013;61: 342–349. [Google Scholar]
- 25.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature 2000;403: 853–858. [DOI] [PubMed] [Google Scholar]
- 26.Irwin MT, Wright PC, Birkinshaw C, Fisher BL, Gardner CJ, Glos J, et al. Patterns of species change in anthropogenically disturbed forests of Madagascar. Biol Conserv 2010;143: 2351–2362. [Google Scholar]
- 27.Baum DA. The comparative pollination and floral biology of baobabs (Adansonia- Bombacaceae). Ann Mo Bot Gard 1995;82: 322–348. [Google Scholar]
- 28.The IUCN Red List of Threatened Species. Version 2013.2. 2013. Available: http://www.iucnredlist.org
- 29.Jenkins RKB, Racey PA, Andriafidison D, Razafindrakoto N, Raxafimahatratra E, Rabearivelo A, et al. Not rare, but threatened: the endemic Madagascar flying fox Pteropus rufus in a fragmented landscape. Oryx 2007;41: 263–271. [Google Scholar]
- 30.Cardiff DG, Ratrimomanarivo FH, Rembert G, Goodman SM. Hunting, disturbance and roost persistence of bats in caves at Ankarana, northern Madagascar. Afr J Ecol 2009;47: 640–649. [Google Scholar]
- 31.Long E, Racey PA. An exotic plantation crop as a keystone resource for an endemic megachiropteran, Pteropus rufus, in Madagascar. J Trop Ecol 2007;23: 397–407. [Google Scholar]
- 32.MacKinnon JL, Hawkins CE, Racey PA. Pteropodidae In: Goodman SM, Benstead JP, editors. The natural history of Madagascar. Chicago: University of Chicago Press; 2003. pp. 1299–1302. [Google Scholar]
- 33.Andriafidison D, Andrianaivoarivelo RA, Ramilijaona OR, Razanahoera MR, MacKinnon J, Jenkins RKB, et al. Nectarivory by endemic Malagasy fruit bats during the dry season. Biotropica 2006;38: 85–90. [Google Scholar]
- 34.Ossa G, Kramer-Schadt S, Peel AJ, Scharf AK, Voigt CC. The Movement Ecology of the Straw-Colored Fruit Bat, Eidolon helvum, in Sub-Saharan Africa Assessed by Stable Isotope Ratios. PLOS ONE 2012;7: e45729 10.1371/journal.pone.0045729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tidemann CR, Nelson JE. Long-distance movements of the grey-headed flying-fox (Pteropus polioce phalus). J Zool 2004;263: 141–146. [Google Scholar]
- 36.Oleksy R, Racey PA, Jones G. High-resolution GPS tracking reveals habitat selection and the potential for long-distance seed dispersal by Madagascan flying foxed Pteropus rufus. Global Ecology and Conservation 2015;3: 678–692. [Google Scholar]
- 37.Goodman SM, Andriafidison D, Andrianaivoarivelo R, Cardiff SG, Ifiticene E, Jenkins RKB, et al. The distribution and conservation of bats in the dry regions of Madagascar. Anim Conserv 2005;8: 153–165. [Google Scholar]
- 38.Andrianaivoarivelo RA, Ramilijaona OR, Racey PA, Razafindrakoto N, Jenkins RKB. Feeding ecology, habitat use and reproduction of Rousettus madagascariensis Grandidier, 1928 (Chiroptera: Pteropodidae) in eastern Madagascar. Mammalia 2011;75: 69–78. [Google Scholar]
- 39.Picot M, Jenkins RKB, Ramilijaona O, Racey PA, Carrière SM. The feeding ecology of Eidolon dupreanum (Pteropodidae) in eastern Madagascar. Afr J Ecol 2007;45: 645–650. [Google Scholar]
- 40.Reuter KE, Gilles H, Wills AR, Sewall BJ. Live capture and ownership of lemurs in Madagascar: extent and conservation implications. Oryx 2015 [Google Scholar]
- 41.Reuter KE. Natural Resource Use in Madagascar. Doctoral Dissertation. Temple University. 2015. Retrieved from ProQuest Dissertation and Theses.
- 42.Jenkins RKB, Racey PA. Bats as bushmeat in Madagascar. Madag Conserv Dev 2008;3: 22–30. [Google Scholar]
- 43.Owens JR. Ecology and behavior of the Bioko Island Drill (Mandrillus leucophaeous poensis). Ph.D. Dissertation, Drexel University. 2013. Available: http://search.proquest.com.libproxy.temple.edu/docview/1496774454?accountid=14270
- 44.Ilo Program. Recensement des Communes 2001. 2003. Available: http://www.ilo.cornell.edu/ilo/data.html
- 45.Crowley BE, Thoren S, Rasoazanabary E, Vogel ER, Barrett MA, Zohdy S, et al. Explaining geographical variation in the isotope composition of mouse lemurs (Microcebus). J Biogeogr 2011;38: 2106–2121. [Google Scholar]
- 46.Yohannes E, Hansson B, Lee RW, Waldenstrom J, Westerdahl H, Akesson M, et al. Isotope signatures in winter moulted feathers predict malaria prevalence in a breeding avian host. Oecologia 2008;158: 299–306. 10.1007/s00442-008-1138-3 [DOI] [PubMed] [Google Scholar]
- 47.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol 2001;26: 32–46. [Google Scholar]
- 48.Cabanellas-Reborado M, Deudero S, Blanco A. Stable-isotope signatures (δ13C and δ15N) of different tissues of Pinna Nobilis Linneaus, 1758 (Bivalvia): isotopic variations among tissues and between seasons. J. Mollus. Stud. 2009;75: 343–349. [Google Scholar]
- 49.Deudero S, Cabanellas M, Blanco A, Tejada S. Stable isotope fractionation in the digestive gland, muscle, and gills tissues of the marine mussel Mytilus galloprovincialis. J Exp Mar Bio Ecol 2009;368: 181–188. [Google Scholar]
- 50.Dethier MN, Sosik E, Galloway AWE, Duggins DO, Simenstad CA. Addressing assumptions: variation in stable isotopes and fatty acids of marine macrophytes can confound conclusions of food web studies. Mar Ecol Prog Ser. 2013;468: 1–14. [Google Scholar]
- 51.Jackson AL, Inger R, Parnell AC, Bearhop S. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J Anim Ecol 2011;80: 595–602. 10.1111/j.1365-2656.2011.01806.x [DOI] [PubMed] [Google Scholar]
- 52.Fisher R. Statistical methods for research workers Oxford, UK: Oxford University Press; 1925. [Google Scholar]
- 53.U.S. Department of the Interior, U.S. Geological Survey. The USGS Earth Explorer. 2014. Available: http://earthexplorer.usgs.gov/
- 54.ESRI. ArcGIS Desktop: Release 10. Redlands, USA: Environmental Systems Research Institute; 2011. [Google Scholar]
- 55.Asner GP, Knapp DE, Balaji A, Paez-Acosta. Automated mapping of tropical deforestation and forest degradation: CLASlist. J Appl Remote Sens 2009;3: 33524–33543. [Google Scholar]
- 56.Allnutt TF, Asner GP, Golden CD, Powell GVN. Mapping recent deforestation and forest disturbance in northeastern Madagascar. Trop Conserv Sci 2013;6: 1–15. [Google Scholar]
- 57.Moat J, Smith PP. Atlas of the vegetation of Madagascar / Atlas de la vegetation de Madagascar London, UK: Royal Botanic Gardens; 2007. [Google Scholar]
- 58.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 2005;25: 1965–1978. [Google Scholar]
- 59.Hobson KA. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 1999;120: 314–326. [DOI] [PubMed] [Google Scholar]
- 60.Hurvich CM, Tsai C-L. Regression and time series model selection in small samples. Biometrika 1989;76: 297–307. [Google Scholar]
- 61.Burnham KP, Anderson DR. Model selection and multimodel inference: A practical information-theoretic approach USA: Springer Science & Business Media; 2002. [Google Scholar]
- 62.Winter K. δ13C values of some succulent plants from Madagascar. Oecologia 1979;40: 103–112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Includes species identification, city of purchase, location hunted (including GPS coordinates), % of forest cover (within 5km, 15km, and 30km radius), city population, annual precipitation, annual temperature, temperature seasonality, and precipitation seasonality.
(XLSX)
Calculated using a Bayesian approach (105 posterior draws).
(JPEG)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.