Abstract
Timely turnover of RNA is an important element in the control of bacterial gene expression, but relatively few specific targets of RNA turnover regulation are known. Deletion of the Bacillus subtilis pnpA gene, encoding the major 3′ exonuclease turnover enzyme, polynucleotide phosphorylase (PNPase), was shown previously to cause a motility defect correlated with a reduced level of the 32-gene fla/che flagellar biosynthesis operon transcript. fla/che operon transcript abundance has been shown to be inhibited by an excess of the small regulatory protein, SlrA, and here we find that slrA mRNA accumulated in the pnpA-deletion mutant. Mutation of slrA was epistatic to mutation of pnpA for the motility-related phenotype. Further, Rho-dependent termination was required for PNPase turnover of slrA mRNA. When the slrA gene was provided with a Rho-independent transcription terminator, gene regulation was no longer PNPase-dependent. Thus we show that the slrA transcript is a direct target of PNPase and that regulation of RNA turnover is a major determinant of motility gene expression. The interplay of specific transcription termination and mRNA decay mechanisms suggests selection for fine-tuning of gene expression.
INTRODUCTION
Levels of bacterial gene expression depend on the rate of transcription initiation, translation initiation, as well as the rate of messenger RNA decay. In Bacillus subtilis — the best-studied Gram-positive species in terms of mRNA turnover — initiation of mRNA decay is thought to begin most often with endonucleolytic cleavage catalyzed by RNase Y (1–3). Intra-transcript cleavage generates an upstream fragment that is degraded by polynucleotide phosphorylase (PNPase) or another 3′ exonuclease (4), and a downstream fragment that is subject to additional RNase Y-mediated cleavages or processive decay by RNase J1, a 5′ exonuclease (5,6). Decay from a transcript's 5′ end can also occur by the action of RNase J1, provided the 5′-triphosphorylated end has been converted to a monophosphorylated form by an RNA pyrophosphohydrolase (7). Exonucleolytic decay from a transcript's 3′ end is normally hindered by the strong secondary structure that is part of the Rho-independent transcription termination mechanism. Rho-dependent termination, which could generate 3′ ends without this strong structure, is not thought to play a significant role in B. subtilis transcription termination (8). Unlike in Escherichia coli, where about half of the transcription terminators are Rho-dependent and the rho gene is essential (9), the B. subtilis rho gene is not essential (10).
Biochemical evidence suggests that PNPase is the major mRNA turnover enzyme in B. subtilis (11,12). A strain that is deleted for the gene encoding PNPase, the ΔpnpA strain, shows several interesting phenotypes, including growth as non-motile chains of cells in liquid culture, competence deficiency and tetracycline sensitivity (12,13). However, at least in laboratory conditions, the ΔpnpA strain grows only slightly slower than the wild-type, perhaps suggesting that other exonuclease activities compensate in the mRNA turnover process when PNPase is absent. A recent RNA-Seq study analyzed the pattern of decay intermediates in B. subtilis strains that were either wild-type or deleted for the PNPase gene, and found altered levels for many mRNAs in the ΔpnpA strain (14). While many of the changes in gene expression in the ΔpnpA strain are likely due to indirect effects, a direct effect of the lack of PNPase was observed for about 10% of expressed genes, for which there was a significant increase in the level of 5′-proximal reads relative to the level of 3′-proximal reads.
Based on data from the RNA-Seq study, we were able to explain the chain growth phenotype of the ΔpnpA strain by an effect on the fla/che operon, a 27-kb operon containing 32 genes, of which the Sigma D transcription factor gene is the penultimate gene. RNA-Seq analysis revealed a 2-4-fold decrease in fla/che operon read levels overall, with a 3-fold decrease in sigD expression. This, in turn, caused depression of the sigD regulon, including the autolysis genes that are required for separation of daughter cells upon cell division (15,16).
We have now examined the ultimate cause of decreased fla/che operon expression in the ΔpnpA strain. An earlier report demonstrated that fla/che operon RNA levels are controlled by SlrA, a small, 52-amino-acid protein. Insertion in the chromosome of a single extra copy of the slrA gene caused a severe decrease in expression of the fla/che operon (17). The effect was shown to be at a post-transcriptional level, although the mechanism remains undetermined. Here, we show that the level of slrA mRNA increases in the absence of PNPase, resulting in slrA overexpression, as evidenced by chain growth.
MATERIALS AND METHODS
Bacterial strains
The wild-type strain, BG1, is a trpC2 thr-5 derivative of B. subtilis strain 168. BG546, the ΔpnpA strain, was described previously (14). For cell chaining assays, derivatives of the NCIB3610 strain (‘3610') were constructed by SPP1 phage transduction. The ΔslrA and (slrA+) strains were described previously (17). For construction of the Pspac-slrA allele, the slrA transcription unit, from 167 bp upstream of the CDS to 383 bp downstream of the coding sequence (CDS), was amplified with primers containing HindIII and SalI restriction endonuclease sites and cloned downstream of the Pspac promoter in plasmid pDR66 (18), giving pBL24. (The sequences of oligonucleotides used in this study are provided in Supplementary Table S1.) pBL24 DNA was linearized with restriction endonuclease NruI and used to transform the ΔslrA strain. The PslrA-lacZ β-galactosidase reporter gene was constructed as follows: a polymerase chain reaction (PCR) product containing the PslrA promoter was amplified from B. subtilis 3610 chromosomal DNA using the primers containing EcoRI and BamHI sites, and cloned into plasmid pDG268 (19), giving plasmid pDP422. pDP422 DNA was linearized with restriction endonuclease NruI for integration into the amyE locus.
The Δrho strain, a kind gift of J. Helmann, was described previously (20), as was the Δrnr strain (21). For construction of slrATT, complementary 41-mer oligonucleotides representing the ermC transcription terminator sequence were inserted 285 bp downstream of the slrA CDS in pBL24, according to a previously-described mutagenesis procedure (22).
Northern blotting
RNA was isolated from B.subtilis strains grown to mid-logarithmic phase in Luria-Bertani (LB) broth (10 g tryptone, 5 g yeast extract, 5 g NaCl per L), as described (23). RNA was fractionated either on a 1% denaturing agarose MOPS gel and blotted by wicking, or on a 6% denaturing polyacrylamide gel and electroblotted. 5′-end-labeled oligonucleotides were used as probes. The slrA probe was complementary to nts 88-119 of the slrA CDS. For Pspac-slrA transcripts, the probe was complementary to nts 1-27 of the lacO sequence. To control for RNA loading in Northern blot analyses, membranes were stripped and probed for 5S rRNA as described (24) or 16S rRNA using an oligonucleotide complementary to nts 1405–1424 (25). To determine the half-life of full-length RNA, exponential regression analysis (R2 > 0.9) was performed on percentage of RNA remaining versus time. Since decay intermediates are being degraded and simultaneously generated from decay of full-length RNA, the half-life of decay intermediates was corrected based on an approach described previously (26).
3′-RACE
The 3′-RACE protocol was a slight modification of a published method (27). Total RNA from pnpA+ and ΔpnpA strains was isolated as described above. 3′ ends were ligated to pre-adenylated linker (5′-rAppCTGTAGGCACCATCAAT-ddC-3′) by incubation for 2 h at 25°C with truncated T4 RNA ligase 2 (New England BioLabs). The ligated RNA was purified with RNeasy MinElute Cleanup Kit (Qiagen), and the 3′-proximal slrA sequence was amplified by using QIAGEN OneStep RT-PCR Kit with a primer complementary to the 3′ linker and a primer consisting of slrA CDS nts 34-59. PCR products were separated on a 1.5% agarose gel and appropriately-sized bands were excised and cloned into pGEM-T (Promega). For the band from the pnpA+ strain, six clones were sequenced; for the band from the ΔpnpA strain, 12 clones were sequenced.
Phenotype assays
Overnight growth in tetracycline was performed as described (12). Competence was measured by a standard transformation protocol (28), using 1 μg of plasmid pYH250 DNA (24) or 1 μg of chromosomal DNA from a strain that had a chloramphenicol-resistance marker in the amyE locus, and selection for colonies resistant to 4 μg/ml chloramphenicol. Assay of β-galactosidase activity was as described previously (29).
Microscopy
Cells were grown in LB broth to 1 OD600. Isopropyl β-D-thiogalactopyranoside (IPTG, Sigma) was added to the medium at the indicated concentration when appropriate. Phase-contrast microscopy was performed with a Nikon 80i microscope, using a phase-contrast objective Nikon Plan Apo 100×. Images were captured with a Photometrics Coolsnap HQ2 camera and Metamorph image software.
RESULTS
Absence of PNPase affects slrA mRNA levels, causing cell chaining
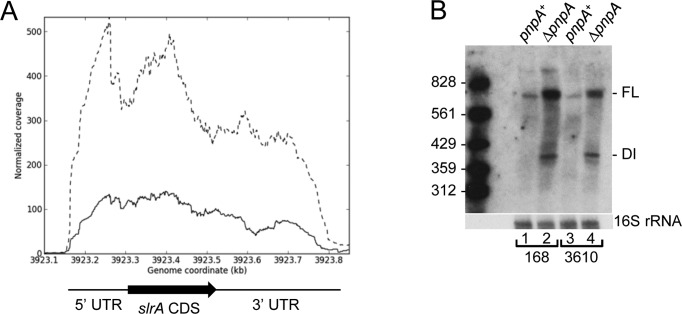
An RNA-Seq analysis of mRNA levels in wild-type and ΔpnpA strains showed decreased levels of fla/che operon mRNA in the strain lacking PNPase (14). As it had been shown earlier that an increased SlrA level correlates with reduced fla/che operon transcript abundance (17), we examined the RNA-Seq data for the slrA transcription unit (Figure 1A). Although the slrA CDS is only 156 nts, the RNA-Seq read data suggested that the transcription unit is about 600 nts, with ∼170-nt 5′ untranslated region (UTR) and ∼330-nt 3′ UTR. The results in Figure 1A showed that slrA read levels were significantly elevated in the ΔpnpA strain, with a 3-4-fold read increase in the part of the transcription unit that includes the CDS, and a smaller increase in reads in the 3′-terminal half. The RNA-seq results were confirmed by Northern blot analysis, using an oligonucleotide probe complementary to a sequence 40 nts from the end of the slrA CDS. We observed ∼3.3-fold increase in the steady-state amount of full-length slrA mRNA in the ΔpnpA strain (average of two independent experiments), as well as an accumulation of decay intermediates, which were long enough to contain the complete slrA CDS (Figure 1B, lanes 1 and 2). Similar results were obtained in the B. subtilis 3610 strain (Figure 1B, lanes 3 and 4), which was used to assay chaining during cell growth (see below). We hypothesized that turnover of slrA mRNA by PNPase affects fla/che operon expression and sigD regulon expression.
Figure 1.
Increased level of slrA mRNA in the absence of PNPase. (A) Read data from RNA-Seq analysis of the slrA gene in pnpA+ (solid line) and ΔpnpA (dashed line) strains. Genome coordinates on the X-axis; normalized reads on the Y-axis. Regions of the slrA transcription unit are indicated below the data. (B) Northern blot analysis of slrA mRNA in Bacillus subtilis 168 and 3610 backgrounds. Ten microgram of total RNA was fractionated on a 6% denaturing polyacrylamide gel. FL, full-length mRNA; DI, decay intermediate. Marker lane contained 5′-end-labeled fragments of a TaqI digest of plasmid pSE420 (51).
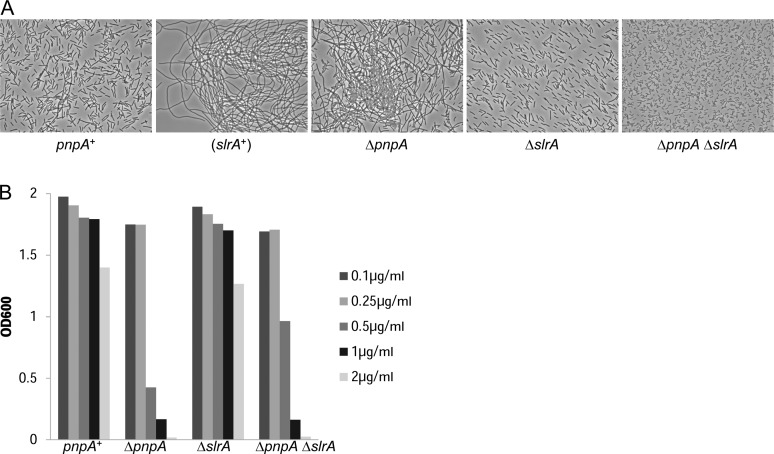
As reported previously, depression of fla/che operon expression in the ΔpnpA mutant, and specifically the 3-fold decrease in sigD expression, was able to explain the chaining phenotype of the ΔpnpA mutant (14). If the effect of the loss of PNPase was due to elevated slrA mRNA, then deletion of the slrA gene should alleviate this effect. We used a previously-constructed ΔslrA strain (17) as well as a newly-constructed double deletion ΔpnpA ΔslrA strain to assay the chaining phenotype. Phase contrast microscopy of logarithmic phase cultures (Figure 2A) showed mostly dividing cells and a few short chains for the pnpA+ strain, while there was extensive chaining in the ‘(slrA+)’ strain, which contains an additional chromosomal copy of the slrA gene (17). Similar extensive chaining was observed in the ΔpnpA strain, but deletion of the pnpA gene did not lead to chaining when the slrA gene was also deleted (Figure 2A; ΔpnpA ΔslrA strain). Thus, mutation of slrA was epistatic to mutation of pnpA for the cell-chaining phenotype.
Figure 2.
slrA mutation is epistatic to pnpA mutation for cell chaining. (A) Phase-contrast microscopy of cell chaining for the indicated strains. pnpA+, wild-type 3610 strain; slrA+, additional copy of slrA gene; ΔpnpA, deletion of gene encoding PNPase; ΔslrA, deletion of gene encoding SlrA; ΔpnpA ΔslrA, deletion of genes encoding PNPase and SlrA. (B) Overnight growth of strains in the presence of increasing concentrations of tetracycline.
Two other phenotypes reported for the ΔpnpA strain are competence deficiency (13) and tetracycline sensitivity (12). We tested whether slrA was epistatic to pnpA for these phenotypes as well. The data for overnight growth in the presence of tetracycline, shown in Figure 2B, indicated no significant difference in tetracycline sensitivity between the ΔpnpA and ΔpnpA ΔslrA strains. Thus, slrA was not epistatic to pnpA for tetracycline sensitivity. On the other hand, the data for competence indicated a partial suppression of the competence deficiency by deletion of slrA (Table 1). For chromosomal and plasmid DNA transformation, the ΔpnpA ΔslrA strain showed 9.8- and 2.7-fold, respectively, higher competence than the ΔpnpA strain (see ‘Discussion’ section).
Table 1. Percent transformants in ΔpnpA strains relative to pnpA+ straina.
| Strains | Chromosomal DNA | Plasmid DNA |
|---|---|---|
| ΔpnpA/pnpA+ | 1.1 (1.1, 1.1) | 9.0 (8.0, 10.0) |
| ΔpnpA ΔslrA/pnpA+ ΔslrA | 10.8 (10.4, 11.1) | 24.4 (27.8, 21.0) |
aValues are average of two independent experiments. Results from the two independent experiments are shown in parentheses.
The effect of PNPase on slrA mRNA is post-transcriptional
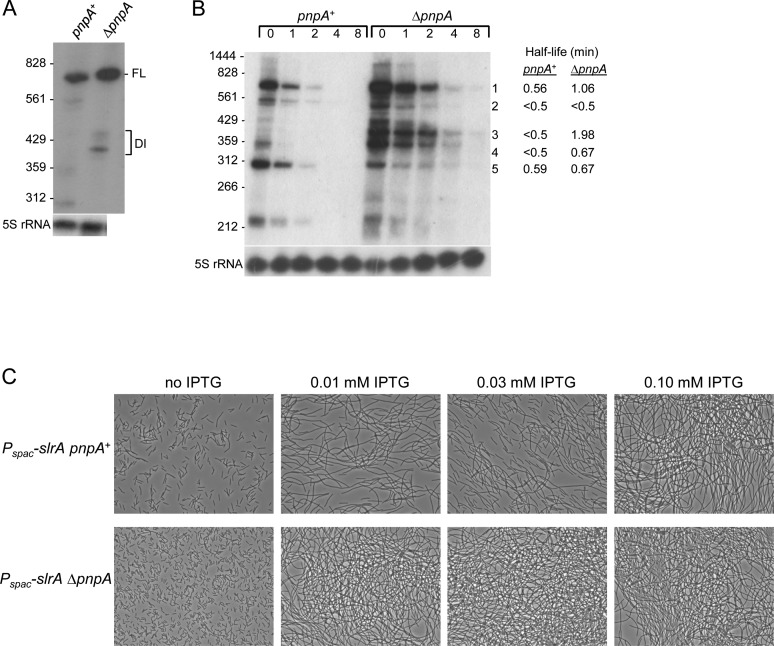
We determined whether the effect of PNPase on slrA mRNA was at the transcriptional or post-transcriptional level. First, Northern blot analysis of lacZ mRNA transcribed from the slrA promoter showed no difference in lacZ mRNA levels in the presence or absence of PNPase (data not shown). Assay of β-galactosidase expression from the PslrA-lacZ construct also showed no significant difference in the presence or absence of PNPase (Table 2). To facilitate analysis of slrA mRNA, which is present at low levels when expressed from the wild-type allele, we constructed a ΔslrA strain that contained a wild-type copy of slrA under control of the IPTG-inducible Pspac promoter integrated at the amyE locus. Phenotypes associated with deletion of pnpA were the same for the Pspac-slrA strain as for strains with native slrA (data not shown). The effect of the pnpA deletion on accumulation of slrA mRNA was recapitulated with the Pspac-slrA construct (Figure 3A), where the amount of full-length slrA mRNA was 2.4-fold higher in the ΔpnpA strain than in the pnpA+ strain, and a similar pattern of decay intermediates was observed. slrA mRNA half-life was measured in the presence of rifampin, and the results showed ∼2-fold increase in the half-life of full-length slrA mRNA in the absence of PNPase (Figure 3B, band 1), as well as increased half-life for two prominent decay intermediates that contain the slrA CDS (Figure 3B, bands 3 and 4).
Table 2. lacZ expression driven by slrA promoter.
| Strain | β-galactosidase activity per mg proteina |
|---|---|
| pnpA+ | 241 ± 16 |
| ΔpnpA | 232 ± 10 |
aMean ± standard deviation of three experiments.
Figure 3.
IPTG-induced slrA expression. (A) Northern blot analysis of slrA mRNA transcribed from the Pspac promoter. Ten microgram of total RNA was fractionated on a 6% denaturing polyacrylamide gel. Migration of 5′-end-labeled fragments of TaqI-digested pSE420 indicated at left. (B) Northern blot analysis of slrA mRNA half-life. Above each lane is time (min) after rifampin addition. Measured half-lives (average of two experiments) of specific bands are indicated on the right. Bands 1–4 are large enough to include the full slrA CDS. (C) Phase-contrast microscopy of cell chaining with increasing IPTG concentrations.
slrA expression from the Pspac promoter afforded the opportunity to demonstrate conclusively that the effect of PNPase was not at the level of transcription from the slrA promoter. The chaining phenotype of pnpA+ and ΔpnpA strains was assayed in the presence of increasing concentrations of IPTG. As can be seen in Figure 3C, no chaining was evident in either strain in the absence of IPTG. For the pnpA+ strain, short chains and dividing cells were observed in the presence of 0.01 and 0.03 mM IPTG, while full chaining was observed when 0.10 mM IPTG was present. In the ΔpnpA strain, on the other hand, full chaining was observed even in the presence of 0.01 mM IPTG. These results confirmed that the chain growth that is observed in the absence of PNPase was the result of changes in slrA mRNA levels, independent of transcription from the slrA promoter.
Rho-dependent slrA mRNA 3′ ends
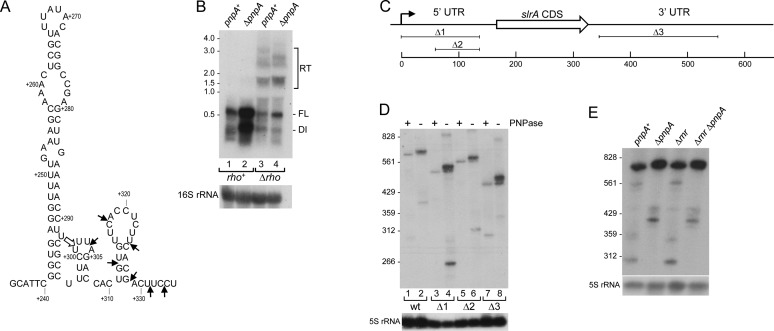
A previous analysis of rpsO mRNA decay in B. subtilis suggested that decay is unlikely to initiate from a 3′ end that is formed by Rho-independent transcription termination (30). Furthermore, in vitro experiments with purified PNPase showed that this enzyme is unable to degrade through the strong secondary structure of a Rho-independent transcription terminator (31). To address how PNPase could be controlling the full-length slrA mRNA half-life, we first used 3′ RACE to map the 3′ ends of slrA mRNA in the pnpA+ and ΔpnpA strains. In the pnpA+ strain, a single 3′ end was mapped at 301 nt downstream of the slrA stop codon (Figure 4A, open arrow). In the ΔpnpA strain, on the other hand, multiple 3′ ends were mapped, ranging from 301 to 333 nts downstream of the slrA stop codon (Figure 4A, short arrows). Immediately upstream of the 301-nt 3′ end is a predicted stem-loop structure that has a ΔG0 value of −18 kcal/mol. The predicted structure is not typical of B. subtilis Rho-independent transcription terminators, almost all of which have completely base-paired, shorter stems (5-12 bp) followed by a 15-nt sequence containing 7–10 U residues (32). We therefore hypothesized that slrA transcription termination might be Rho-dependent. Northern blot analysis of Pspac-slrA RNAs in rho+ and Δrho strains is shown in Figure 4B. The absence of Rho resulted in a decreased intensity of the full-length band in the pnpA+ and ΔpnpA backgrounds, as well as the appearance of higher molecular-weight RNA that is suggestive of transcriptional read-through (Figure 4B, lanes 3 and 4). Together, these results indicated that slrA transcription termination is Rho-dependent and terminates at multiple sites downstream of the stem-loop structure shown in Figure 4A. In the pnpA+ strain, PNPase degrades slrA mRNA from these 3′ ends, although there is a partial block to PNPase processivity 5 nts from the edge of the stem-loop structure (nt 301). In the ΔpnpA strain, slrA mRNAs with various 3′ ends are not efficiently degraded and they accumulate, resulting in an increase in ‘full-length’ slrA mRNA, which is actually a collection of mRNAs of different sizes due to slightly different termination sites. Indeed, higher resolution Northern blot analysis showed several, closely-spaced ‘full-length’ slrA mRNA bands in the ΔpnpA strain that were larger than in the pnpA+ strain (Figure 4D, lanes 1 and 2).
Figure 4.
Rho-dependent termination of slrA transcription. (A) Sequence and predicted secondary structure (52) of 3′-proximal region of slrA 3′ UTR. Open arrow indicates location of major 3′ end at nt 301, mapped by 3′ RACE in the pnpA+ strain. Location of additional 3′ ends mapped in the ΔpnpA strain indicated by short arrows. Numbering is from downstream of the slrA CDS. (B) Northern blot analysis of slrA mRNA in rho+ and Δrho strains. Ten microgram of total RNA was fractionated on a 1.0% MOPS-formaldehye agarose gel. RT, read-through. Migration of unlabeled RNA size markers indicated at left. (C) slrA gene schematic and location of slrA deletion constructs. Scale below is in base-pairs. (D) Northern blot analysis of Pspac-driven slrA mRNA from wild-type slrA gene and from deletion constructs. Ten microgram of total RNA was fractionated on a 6% denaturing polyacrylamide gel. (E) Northern blot analysis of slrA mRNA in the presence and absence of RNase R. Ten microgram of total RNA was fractionated on a 6% denaturing polyacrylamide gel.
We asked whether the unusually long 5′ and 3′ UTRs of slrA mRNA were relevant to PNPase-mediated decay. Deletion constructs were made in the slrA 5′ UTR (Δ1 and Δ2) and 3′ UTR (Δ3), as shown in Figure 4C. Northern blot analysis of a higher resolution gel (Figure 4D) showed for all constructs the presence of a single full-length band in the pnpA+ strain, which was the predicted length based on the size of the deletion, and additional bands of slightly larger size in the ΔpnpA strain. Furthermore, a higher level of slrA mRNA in the ΔpnpA background was observed for all deletion constructs. The data suggested that the 5′ and 3′ UTR sequences do not play a role in slrA mRNA decay.
We next tested whether the other known processive 3′ exonuclease of B. subtilis, RNase R, was involved in slrA mRNA turnover. Northern blot analysis of Pspac-slrA mRNA was performed in Δrnr and Δrnr ΔpnpA strains. As shown in Figure 4E, there was little difference in the steady-state patterns of slrA mRNA, with or without RNase R; full-length slrA mRNA accumulated in the absence of PNPase, whether or not RNase R was present. We conclude that primarily PNPase is responsible for efficient turnover from the 3′ ends of slrA mRNA generated by Rho-dependent transcription termination.
Rho-independent termination of slrA transcription phenocopies the ΔpnpA strain
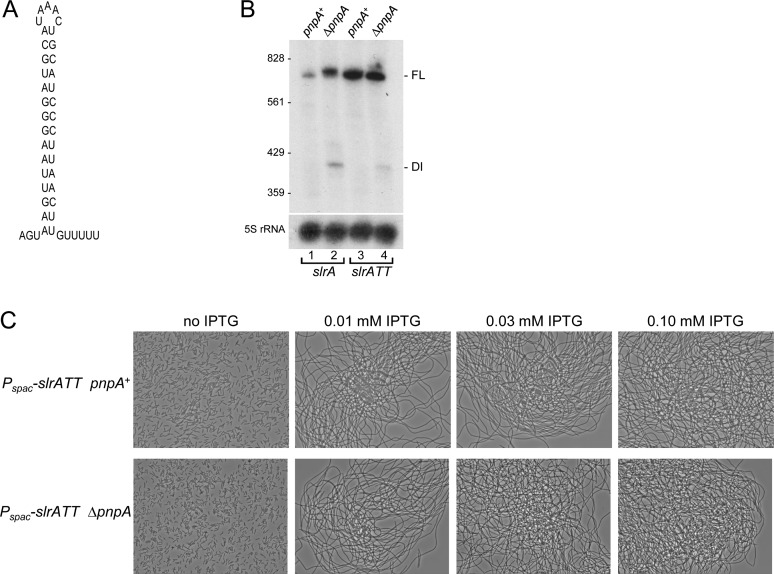
To test the hypothesis that Rho-dependent termination of slrA transcription allows access to PNPase-mediated turnover from the 3′ end, a derivative of the Pspac-slrA construct was created that had a Rho-independent transcription terminator sequence inserted near the end of the 3′ UTR. The terminator was that of the ermC gene, which is predicted to form a strong secondary structure (ΔG0 = −24.1 kcal/mol) followed by five U residues (Figure 5A). The slrA gene with the ermC transcription terminator sequence is referred to as slrATT. The expectation was that the presence of 3′-terminal structure would block PNPase decay, and even in the pnpA+ strain there would be an accumulation of slrA mRNA and inhibition of fla/che operon expression. Northern blot analysis showed that slrATT mRNA was detected as a single band in both pnpA+ and ΔpnpA strains (Figure 5B). Importantly, slrATT mRNA was present at a higher level than slrA mRNA in the pnpA+ strain (∼8-fold), and, unlike slrA mRNA that was terminated in a Rho-dependent manner, there was no difference in steady-state mRNA level between the pnpA+ and ΔpnpA strains. These results suggested that slrATT mRNA was not a substrate for PNPase-mediated decay, which likely resulted in a higher level of SlrA protein. To show the effect of protecting slrA mRNA from decay, the chain growth phenotype of the Pspac-slrATT strain was tested. We showed above that when the Pspac-slrA strain was grown in the presence of 0.01 mM IPTG, there was limited chaining when PNPase was present and a high degree of chaining when PNPase was absent (Figure 3C). In contrast, the Pspac-slrATT strain showed massive chaining even when only 0.01 mM IPTG was added and PNPase was present (Figure 5C). Thus, Rho-mediated termination of slrA transcription, in concert with PNPase-mediated mRNA decay, is required to set a suitable level of slrA expression that allows motile B. subtilis growth.
Figure 5.
slrA with Rho-independent terminator. (A) Predicted secondary structure of the ermC transcription terminator. (B) Northern blot analysis of slrA mRNA carrying the ermC transcription terminator (slrATT). Ten microgram of total RNA was fractionated on a 6% denaturing polyacrylamide gel. (C) Phase-contrast microscopy of slrATT strains with increasing IPTG concentrations.
DISCUSSION
The results presented here indicate that timely turnover of slrA mRNA is required for precise regulation of the fla/che operon (32 genes) and, indirectly, the rest of the sigD regulon (50 genes). Thus, the expression level of over 80 genes is determined by the ability of PNPase to degrade slrA mRNA and confer on it a relatively short half-life. PNPase-mediated decay of bacterial regulatory RNAs, such as small RNAs (sRNAs) and leader RNAs, has been documented previously: the decay (or stability) of a number of E. coli and Salmonella typhimurium sRNAs are regulated by PNPase (see (33) and references therein); control of E. coli C biofilm formation by PNPase is hypothesized to occur via degradation of small regulatory RNAs (34); autoregulation of the E. coli pnp gene relies on efficient degradation of the pnp leader region RNA by PNPase (35); and we have shown that regulation of B. subtilis trp operon gene expression requires efficient degradation of trp leader RNA by PNPase (36). However, we are not aware of another report in which PNPase-mediated decay of a full-length mRNA is crucial for the control of many genes.
We show here that slrA mRNA decay by PNPase depends on Rho-dependent transcription termination (Figures 4 and 5). A recent transcriptome analysis suggested that deletion of the rho gene causes extended transcription of many mRNAs (37), and, indeed, the slrA gene is one of these. However, only a few transcripts acted on by Rho have been examined in any detail: the rho gene itself (8), the trp operon (38), and, very recently, the rplJL operon (39). In these cases, Rho acts either in a leader region or in the first gene of an operon to terminate transcription before synthesis of protein coding sequences. For slrA, Rho apparently binds downstream of a coding region to cause transcription termination that leaves 3′ ends susceptible to PNPase decay. PNPase is unable to degrade strong stem-loop structures in vitro (31), and it is not known whether in vivo association with the RNA helicase CshA (40) allows it to degrade Rho-independent transcription terminator sequences. It is also not known whether an iterative polyadenylation process, which in E. coli is catalyzed by poly(A) polymerase and confers susceptibility of 3′-terminal RNA fragments to decay (41), exists in B. subtilis. Experiments to test this have not yet been possible, since the gene encoding a B. subtilis poly(A) polymerase remains elusive (42). Recent evidence in our laboratory suggests that 3′-terminal mRNA fragments are degraded for the most part by RNase J1 (our unpublished data). It is therefore assumed that initiation of decay for mRNAs with a Rho-independent terminator occurs either by endonucleolytic cleavage catalyzed by RNase Y, or by 5′-to-3′ exonuclease activity of RNase J1 acting on a 5′-monophosphate end (6). It is expected that only for mRNAs whose transcription termination is Rho-dependent could efficient decay occur by a 3′ exonuclease activity starting from a native 3′ end. Although B. subtilis contains at least four 3′ exoribonucleases (4), in the case of slrA mRNA it appears that primarily PNPase engages in this degradative function (Figure 4E).
An interesting aspect of slrA mRNA is its unusually long 3′ UTR. A survey of the location of Rho-independent transcription terminators, relative to an upstream stop codon, determined that 93% occur within 100 bp of the stop codon, and many of the remaining terminators may be functioning in transcription termination of convergently transcribed genes (32). For slrA, termination occurs ∼330 nts downstream of the stop codon, and the next predicted gene is in the same orientation and starts 100 bp away. A 208-nt deletion starting 18 nts downstream of the slrA CDS (Δ3, Figure 4C) gave the same pattern of multiple ‘full-length’ slrA mRNA bands as the wild-type gene and as two deletions in the 5′ UTR (Figure 4D). The implication is that a Rho binding site exists in the remaining 75 nts of the Δ3 construct. For E. coli, the primary characteristics of Rho binding sites are ribosome-free, unstructured and C-rich (43). B. subtilis Rho has similar biochemical characteristics to E. coli Rho (8), suggesting that it will have similar requirements for binding. However, we were unable to recognize specific attributes of the 3′-proximal 75-nt sequence that would explain Rho binding. This sequence has an equal distribution of the four deoxyribonucleotides, and 56 nts of the sequence can be predicted to form a stable secondary structure (Figure 4A). The presence of a ‘C > G bubble,’ defined as a relatively C-rich and G-poor region over a length of 78 nts that precedes a Rho-dependent transcription termination site in many cases (44), was not observed in the corresponding region of the slrA gene (see Supplementary Figure S1). Clearly, there is much to be learned about the mechanism of B. subtilis Rho activity. Our discovery here of Rho-dependent transcription termination for a full-length mRNA, as well as the recent finding that the rho gene affects expression of genes involved in antibiotic resistance (20), should prompt additional study of the requirements for Rho activity in B. subtilis.
We showed that slrA was epistatic to pnpA for the chaining phenotype (Figure 2A). This, as well as chaining in the presence of increasing IPTG concentration for Pspac-promoted slrA (Figure 3C), are strong evidence for the hypothesis that the cause of the chaining in the absence of PNPase is due to a post-transcriptional effect on slrA expression. Two other phenotypes caused by the loss of PNPase did not appear to involve slrA mRNA. Tetracycline-sensitivity of the ΔpnpA strain, the basis of which is unknown, was not affected by the deletion of slrA (Figure 2B). Interestingly, competence deficiency of the ΔpnpA strain was partially suppressed by the slrA knockout (Table 1). Characterization of com gene expression in the ΔpnpA strain by Dubnau et al. suggested that loss of PNPase affected regulated expression of several competence genes, including comG, comK and srfAA (comS) (13). A simple explanation for the observed partial suppression in the ΔslrA strain is that the protein complex required for DNA uptake is located at the cell poles (45,46). Cells growing in long chains would have fewer poles available for presentation of transforming DNA to the DNA-binding apparatus. Alleviation of the chain growth by deletion of slrA may thus explain partial restoration of the competence defect caused by loss of PNPase.
The finding that efficient decay of an mRNA figures prominently in the regulation of a large number of genes supports the concept that models of gene expression networks must take into account not only transcriptional and translational control, but also control at the level of mRNA decay (47). In the case of slrA, the involvement of PNPase as a controlling factor relies on the generation of PNPase-susceptible 3′ ends by the action of Rho. slrA is not only required for fla/che operon regulation, but is part of a gene network that controls biofilm formation (48–50). Fine-tuning of slrA expression is likely necessary for its functions, and there has been selection for this control to involve an unusual form of mRNA decay. We expect that more examples of the interplay of Rho-dependent transcription termination and mRNA turnover will be discovered.
Supplementary Material
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Institutes of Health [GM-100137 to D.H.B., GM-093030 to D.B.K.]. Funding for open access charge: National Institutes of Health [GM-100137 D.H.B.].
Conflict of interest statement. None declared.
REFERENCES
- 1.Shahbabian K., Jamalli A., Zig L., Putzer H. RNase Y, a novel endoribonuclease, initiates riboswitch turnover in Bacillus subtilis. EMBO J. 2009;28:3523–3533. doi: 10.1038/emboj.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehnik-Habrink M., Schaffer M., Mader U., Diethmaier C., Herzberg C., Stulke J. RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y. Mol. Microbiol. 2011;81:1459–1473. doi: 10.1111/j.1365-2958.2011.07777.x. [DOI] [PubMed] [Google Scholar]
- 3.Durand S., Gilet L., Bessieres P., Nicolas P., Condon C. Three essential ribonucleases-RNase Y, J1, and III-control the abundance of a majority of Bacillus subtilis mRNAs. PLoS Genet. 2012;8:e1002520. doi: 10.1371/journal.pgen.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oussenko I.A., Abe T., Ujiie H., Muto A., Bechhofer D.H. Participation of 3′-to-5′ exoribonucleases in the turnover of Bacillus subtilis mRNA. J. Bacteriol. 2005;187:2758–2767. doi: 10.1128/JB.187.8.2758-2767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daou-Chabo R., Mathy N., Benard L., Condon C. Ribosomes initiating translation of the hbs mRNA protect it from 5′-to-3′ exoribonucleolytic degradation by RNase J1. Mol. Microbiol. 2009;71:1538–1550. doi: 10.1111/j.1365-2958.2009.06620.x. [DOI] [PubMed] [Google Scholar]
- 6.Lehnik-Habrink M., Lewis R.J., Mader U., Stulke J. RNA degradation in Bacillus subtilis: an interplay of essential endo- and exoribonucleases. Mol. Microbiol. 2012;84:1005–1017. doi: 10.1111/j.1365-2958.2012.08072.x. [DOI] [PubMed] [Google Scholar]
- 7.Richards J., Liu Q., Pellegrini O., Celesnik H., Yao S., Bechhofer D.H., Condon C., Belasco J.G. An RNA pyrophosphohydrolase triggers 5′-exonucleolytic degradation of mRNA in Bacillus subtilis. Mol. Cell. 2011;43:940–949. doi: 10.1016/j.molcel.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingham C.J., Dennis J., Furneaux P.A. Autogenous regulation of transcription termination factor Rho and the requirement for Nus factors in Bacillus subtilis. Mol. Microbiol. 1999;31:651–663. doi: 10.1046/j.1365-2958.1999.01205.x. [DOI] [PubMed] [Google Scholar]
- 9.Ciampi M.S. Rho-dependent terminators and transcription termination. Microbiology. 2006;152:2515–2528. doi: 10.1099/mic.0.28982-0. [DOI] [PubMed] [Google Scholar]
- 10.Quirk P.G., Dunkley E.A., Jr, Lee P., Krulwich T.A. Identification of a putative Bacillus subtilis rho gene. J. Bacteriol. 1993;175:647–654. doi: 10.1128/jb.175.3.647-654.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deutscher M.P., Reuven N.B. Enzymatic basis for hydrolytic versus phosphorolytic mRNA degradation in Escherichia coli and Bacillus subtilis. Proc. Natl. Acad. Sci. U.S.A. 1991;88:3277–3280. doi: 10.1073/pnas.88.8.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W., Bechhofer D.H. Properties of a Bacillus subtilis polynucleotide phosphorylase deletion strain. J. Bacteriol. 1996;178:2375–2382. doi: 10.1128/jb.178.8.2375-2382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luttinger A., Hahn J., Dubnau D. Polynucleotide phosphorylase is necessary for competence development in Bacillus subtilis. Mol. Microbiol. 1996;19:343–356. doi: 10.1046/j.1365-2958.1996.380907.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu B., Deikus G., Bree A., Durand S., Kearns D.B., Bechhofer D.H. Global analysis of mRNA decay intermediates in Bacillus subtilis wild-type and polynucleotide phosphorylase-deletion strains. Mol. Microbiol. 2014;94:41–55. doi: 10.1111/mmi.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marquez L.M., Helmann J.D., Ferrari E., Parker H.M., Ordal G.W., Chamberlin M.J. Studies of sigma D-dependent functions in Bacillus subtilis. J. Bacteriol. 1990;172:3435–3443. doi: 10.1128/jb.172.6.3435-3443.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R., Guttenplan S.B., Blair K.M., Kearns D.B. Role of the sigmaD-dependent autolysins in Bacillus subtilis population heterogeneity. J. Bacteriol. 2009;191:5775–5784. doi: 10.1128/JB.00521-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cozy L.M., Phillips A.M., Calvo R.A., Bate A.R., Hsueh Y.H., Bonneau R., Eichenberger P., Kearns D.B. SlrA/SinR/SlrR inhibits motility gene expression upstream of a hypersensitive and hysteretic switch at the level of sigma(D) in Bacillus subtilis. Mol. Microbiol. 2012;83:1210–1228. doi: 10.1111/j.1365-2958.2012.08003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ireton K., Rudner D.Z., Siranosian K.J., Grossman A.D. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev. 1993;7:283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- 19.Antoniewski C., Savelli B., Stragier P. The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. J. Bacteriol. 1990;172:86–93. doi: 10.1128/jb.172.1.86-93.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y.H., Helmann J.D. Mutations in the primary sigma factor sigmaA and termination factor rho that reduce susceptibility to cell wall antibiotics. J. Bacteriol. 2014;196:3700–3711. doi: 10.1128/JB.02022-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oussenko I.A., Bechhofer D.H. The yvaJ gene of Bacillus subtilis encodes a 3′-to-5′ exoribonuclease and is not essential in a strain lacking polynucleotide phosphorylase. J. Bacteriol. 2000;182:2639–2642. doi: 10.1128/jb.182.9.2639-2642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W., Malcolm B.A. Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange Site-Directed Mutagenesis. Biotechniques. 1999;26:680–682. doi: 10.2144/99264st03. [DOI] [PubMed] [Google Scholar]
- 23.Bechhofer D.H., Oussenko I.A., Deikus G., Yao S., Mathy N., Condon C. Analysis of mRNA decay in Bacillus subtilis. Methods Enzymol. 2008;447:259–276. doi: 10.1016/S0076-6879(08)02214-3. [DOI] [PubMed] [Google Scholar]
- 24.Sharp J.S., Bechhofer D.H. Effect of translational signals on mRNA decay in Bacillus subtilis. J. Bacteriol. 2003;185:5372–5379. doi: 10.1128/JB.185.18.5372-5379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sulthana S., Deutscher M.P. Multiple exoribonucleases catalyze maturation of the 3′ terminus of 16S ribosomal RNA (rRNA) J. Biol. Chem. 2013;288:12574–12579. doi: 10.1074/jbc.C113.459172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebbole D.J., Zalkin H. Detection of pur operon-attenuated mRNA and accumulated degradation intermediates in Bacillus subtilis. J. Biol. Chem. 1988;263:10894–10902. [PubMed] [Google Scholar]
- 27.Zhang J., Olsen G.J. Messenger RNA processing in Methanocaldococcus (Methanococcus) jannaschii. RNA. 2009;15:1909–1916. doi: 10.1261/rna.1715209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J. Mol. Biol. 1971;56:209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- 29.Dubnau D. Induction of ermC requires translation of the leader peptide. EMBO J. 1985;4:533–537. doi: 10.1002/j.1460-2075.1985.tb03661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao S., Bechhofer D.H. Initiation of decay of Bacillus subtilis rpsO mRNA by endoribonuclease RNase Y. J. Bacteriol. 2010;192:3279–3286. doi: 10.1128/JB.00230-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deikus G., Bechhofer D.H. Initiation of decay of Bacillus subtilis trp leader RNA. J. Biol. Chem. 2007;282:20238–20244. doi: 10.1074/jbc.M702747200. [DOI] [PubMed] [Google Scholar]
- 32.de Hoon M.J., Makita Y., Nakai K., Miyano S. Prediction of transcriptional terminators in Bacillus subtilis and related species. PLoS Comput. Biol. 2005;1:e25. doi: 10.1371/journal.pcbi.0010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saramago M., Barria C., Dos Santos R.F., Silva I.J., Pobre V., Domingues S., Andrade J.M., Viegas S.C., Arraiano C.M. The role of RNases in the regulation of small RNAs. Curr. Opin. Microbiol. 2014;18:105–115. doi: 10.1016/j.mib.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Carzaniga T., Antoniani D., Deho G., Briani F., Landini P. The RNA processing enzyme polynucleotide phosphorylase negatively controls biofilm formation by repressing poly-N-acetylglucosamine (PNAG) production in Escherichia coli C. BMC Microbiol. 2012;12:270–281. doi: 10.1186/1471-2180-12-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jarrige A.C., Mathy N., Portier C. PNPase autocontrols its expression by degrading a double-stranded structure in the pnp mRNA leader. EMBO J. 2001;20:6845–6855. doi: 10.1093/emboj/20.23.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deikus G., Babitzke P., Bechhofer D.H. Recycling of a regulatory protein by degradation of the RNA to which it binds. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2747–2751. doi: 10.1073/pnas.0307343101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicolas P., Mader U., Dervyn E., Rochat T., Leduc A., Pigeonneau N., Bidnenko E., Marchadier E., Hoebeke M., Aymerich S., et al. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science. 2012;335:1103–1106. doi: 10.1126/science.1206848. [DOI] [PubMed] [Google Scholar]
- 38.Yakhnin H., Babiarz J.E., Yakhnin A.V., Babitzke P. Expression of the Bacillus subtilis trpEDCFBA operon is influenced by translational coupling and Rho termination factor. J. Bacteriol. 2001;183:5918–5926. doi: 10.1128/JB.183.20.5918-5926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yakhnin H., Yakhnin A.V., Babitzke P. Ribosomal protein L10(L12)4 autoregulates expression of the Bacillus subtilis rplJL operon by a transcription attenuation mechanism. Nucleic Acids Res. 2015;43:7032–7043. doi: 10.1093/nar/gkv628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehnik-Habrink M., Pfortner H., Rempeters L., Pietack N., Herzberg C., Stulke J. The RNA degradosome in Bacillus subtilis: identification of CshA as the major RNA helicase in the multiprotein complex. Mol. Microbiol. 2010;77:958–971. doi: 10.1111/j.1365-2958.2010.07264.x. [DOI] [PubMed] [Google Scholar]
- 41.Regnier P., Hajnsdorf E. Poly(A)-assisted RNA decay and modulators of RNA stability. Prog. Mol. Biol. Transl. Sci. 2009;85:137–185. doi: 10.1016/S0079-6603(08)00804-0. [DOI] [PubMed] [Google Scholar]
- 42.Campos-Guillen J., Bralley P., Jones G.H., Bechhofer D.H., Olmedo-Alvarez G. Addition of poly(A) and heteropolymeric 3′ ends in Bacillus subtilis wild-type and polynucleotide phosphorylase-deficient strains. J. Bacteriol. 2005;187:4698–4706. doi: 10.1128/JB.187.14.4698-4706.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richardson J.P. Loading Rho to terminate transcription. Cell. 2003;114:157–159. doi: 10.1016/s0092-8674(03)00554-3. [DOI] [PubMed] [Google Scholar]
- 44.Alifano P., Rivellini F., Limauro D., Bruni C.B., Carlomagno M.S. A consensus motif common to all Rho-dependent prokaryotic transcription terminators. Cell. 1991;64:553–563. doi: 10.1016/0092-8674(91)90239-u. [DOI] [PubMed] [Google Scholar]
- 45.Hahn J., Maier B., Haijema B.J., Sheetz M., Dubnau D. Transformation proteins and DNA uptake localize to the cell poles in Bacillus subtilis. Cell. 2005;122:59–71. doi: 10.1016/j.cell.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kidane D., Graumann P.L. Intracellular protein and DNA dynamics in competent Bacillus subtilis cells. Cell. 2005;122:73–84. doi: 10.1016/j.cell.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 47.Silva I.J., Saramago M., Dressaire C., Domingues S., Viegas S.C., Arraiano C.M. Importance and key events of prokaryotic RNA decay: the ultimate fate of an RNA molecule. Wiley Interdiscip. Rev. RNA. 2011;2:818–836. doi: 10.1002/wrna.94. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi K. SlrR/SlrA controls the initiation of biofilm formation in Bacillus subtilis. Mol. Microbiol. 2008;69:1399–1410. doi: 10.1111/j.1365-2958.2008.06369.x. [DOI] [PubMed] [Google Scholar]
- 49.Chai Y., Kolter R., Losick R. Paralogous antirepressors acting on the master regulator for biofilm formation in Bacillus subtilis. Mol. Microbiol. 2009;74:876–887. doi: 10.1111/j.1365-2958.2009.06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newman J.A., Lewis R.J. Exploring the role of SlrR and SlrA in the SinR epigenetic switch. Commun. Integr. Biol. 2013;6:e25658. doi: 10.4161/cib.25658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brosius J. Compilation of superlinker vectors. Meth. Enzymol. 1992;216:469–483. doi: 10.1016/0076-6879(92)16043-j. [DOI] [PubMed] [Google Scholar]
- 52.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.