Abstract
BACKGROUND
Enhanced recovery (ER) protocols are widely used in surgical practice. As protocols are multidisciplinary with multiple components, it is difficult to compare and contrast reports. The present study therefore examined compliance and transferability to clinical practice among ER publications related to colorectal surgery.
METHODS
PubMed, EMBASE and Cochrane databases were searched for current colorectal ER manuscripts. Each publication was assessed for the number of ER elements, whether the element was sufficiently explained so that it could be transferred to clinical practice, and the compliance with the ER element.
RESULTS
Some 50 publications met the reporting criteria for inclusion. There were 22 ERAS elements described altogether. The median number of elements included in each publication was 9 with median number of included patients of 130. The most frequent elements included in ER pathways were early postoperative diet advancement in 49 (98%) and early mobilisation in 47 (94%). Early diet advancement was sufficiently explained in 43 (86%) publications but just 22 (45%) reported compliance. The explanation for early mobilisation was satisfactory in 41 (82%) publications but only 14 (30%) reported compliance. Other ERAS elements had similar rates of explanation and compliance. The most frequently analysed outcome measures were morbidity 49 (98%), length of stay 47 (94%), and mortality in 45 (90%) of publications.
CONCLUSIONS
The current standard of reporting is frequently incomplete. In order to transfer knowledge and facilitate implementation of pathways that demonstrate improvements in perioperative care and recovery, a consistent structured reporting platform is needed.
INTRODUCTION
Although fast track (FT) protocols for surgical care were first described over 20 years ago, it is only in the last 5 years that FT and ER protocols have penetrated large segments of surgical practice1, 2. As ER protocols are multidisciplinary and have multiple components, comparing reports from different centres and across different surgical specialties is not easy.
It seems appropriate that reports should describe the various elements of each ER pathway consistently and with sufficient detail that they can be reproduced elsewhere3. Only through such a rigorous approach will clinicians be able to compare outcomes associated with individual programmes. Without complete descriptions, the ability to translate positive results to other centres will be impossible and the ability to capitalise on the current speed of information transmission lost.
Introduction of a standard reporting template for clinical trials has resulted in a significant improvement in the ability to interpret and translate data from these trials into clinical practice4, 5. Development of a similar structured template for the publication of studies examining outcomes for patients treated on ER protocols is likely to have similar benefits.
The purpose of this project was to examine publications related to ER with colorectal surgery, seeking to critically assess the depth and breadth of description in the protocols used. Colorectal surgery was chosen as it is the area containing the greatest number of publications. Evidence of variability in transparency of describing individual ER elements would strengthen the arguments for a standard reporting dataset for all ER manuscripts. This structured reporting would improve the ability to compare outcomes between experiences, translation of protocols from the author's institution to the reader's institution, and provide a structure for quality assessment of future publications.
METHODS
A systematic review of English language publications was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement6. PubMed, EMBASE and Cochrane Central Register of Controlled Trials databases were searched for the following specific terms: (“Fast Track” OR “Enhanced Recovery” OR “ERAS” OR “Accelerated Recovery” OR “Multimodal Rehabilitation”) AND (“Colon” OR “Rectum” OR “Colorectal”) AND (“Surgery” OR “Operation” OR “Procedure”). The primary search was carried out by a single researcher, with vetting of the studies done in conjunction with a second researcher. Any discrepancies were resolved by an independent third party.
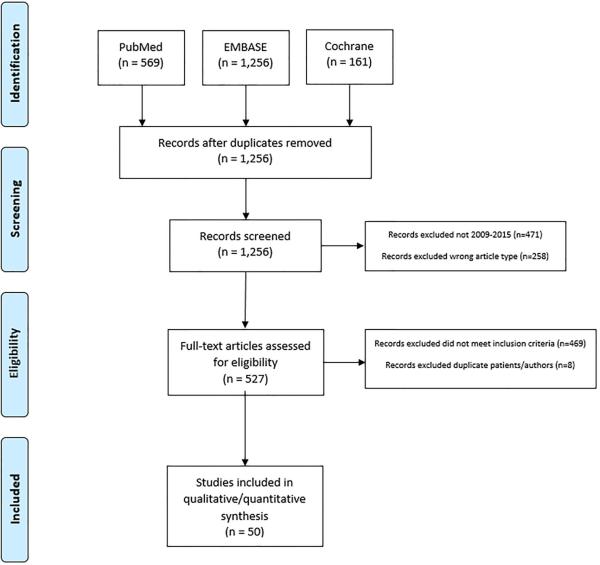
Results from an initial search strategy of the 3 databases were cross-referenced and duplicates removed to create a single list. Secondary filtering was then performed to eliminate older publications (prior to 2009) and non-original manuscripts (meta-analyses and reviews). Remaining records were subject to full text review and included in the final analysis if they met the following criteria: reports comparing outcomes between at least 2 cohorts (one of which must have been an ER pathway cohort and one of which must have been a conventional or traditional pathway cohort); inclusion of at least 50 patients; a confirmed focus on colectomy and/or proctectomy. After eliminating publications with significant overlap in patient population and the same primary or senior authorship, a final analysis list was created and assessed in detail (Figure 1).
Figure 1.
Selection process for identifying publications included in analysis.
Articles were entered into an electronic database and their content systematically analysed to determine which elements of ER were stated as being used, whether the stated element was explained, the compliance rate of that element within the study, and the failure rate of the element when applicable. Although all elements were catalogued, particular attention was made to those elements with strong evidence for safety and efficacy7-9. Additionally, the International ERAS Registry variables were reviewed in order to determine which of the elements in this repository were included in the published literature and could, therefore, potentially facilitate audits for future publications.
In order to be given credit for naming an ER element, a publication had to list the element as being involved in their ER pathway in one of the following locations: the manuscript text; a figure, chart, or table within the main publication, appendices or supplements; or an explicit reference to another publication's pathway. If a reference to an outside publication or external website was quoted, this resource was assessed as an extension of the originally reviewed manuscript. The total number of ER elements listed in each publication was determined by the number of discrete elements identified in this way. An element did not have to be implemented in exactly the same manner in different publications to count as the same ER element.
Once an ER element was determined to be named by the publication, it was further reviewed to determine if the element had been explained in detail. Although subjective, the criteria for adequate explanation of an ER element was that the description allowed for sufficient understanding to the level that the reader felt confident that the element could be implemented based on the description. No judgment was made regarding the adequacy or appropriateness of each intervention, only whether it was sufficiently described. For example, a publication that stated ‘preoperative medications were given’ but did not list the medications was not counted as having sufficiently explained this ER element. Publications were also reviewed to determine whether a corresponding element was adequately described as part of a traditional perioperative pathway, to determine what the ER intervention was changing in the authors’ practice.
Level of compliance with the ER interventions was then determined by identifying the number of patients who had received the intervention or evidence that the intervention had been implemented universally across the patient population. For example, a publication which listed the use of oral non-narcotic medications in an effort to limit narcotic administration could satisfy compliance by either listing the percentage of patients that were managed in this manner or by comparing opioid utilisation in the ER group versus the traditional group.
In order to assess ER elements that were subject to failure, transparent reporting of failure rates on an element by element basis was sought covering 5 specific elements. Where nasogastric tube elimination was a component of the ER protocol, was the rate of postoperative nasogastric tube placement disclosed? For those that listed regional/epidural analgesia use, did they indicate the rate of early catheter non-function or haemodynamic lability leading to conversion to intravenous narcotic strategies? Among those that listed early advancement of oral intake, was the rate of postoperative ileus leading to failure of dietary progression stated? Where early and frequent mobilisation were evaluated, was failure to physically progress including adverse events such as falls reported? Finally, where early urinary catheter removal was included, was the rate of urinary retention requiring catheter replacement stated?
Additional information regarding study design, numbers of patients included in each trial, outcomes analysed, whether the number of surgeons or anaesthetists in the practice were listed, implementation process of the ER pathway described, whether discharge criteria were described along with location of the ER pathway were also recorded and analysed.
Quality assessment was performed by using the Downs and Black tool10, as previously recommended for systematic reviews, to address both randomized and non-randomized studies11. Downs and Black scores were grouped into three quality levels: good (greater than 20), fair (15-19) and poor (less than 14).
Statistics
All statistics were descriptive in nature and listed as numbers with percentages in parentheses. Continuous variables are reported as either means or medians with ranges in parentheses. All statistical analysis was performed using SPSS version 22.0.0.0 (IBM Corp., Armonk, NY, USA).
RESULTS
The initial search strategy returned 569 results from PubMed, 1,226 results from Embase, and 161 results from Cochrane Reviews. After cross-referencing and removal of duplicates, 1,256 publications were identified. Secondary filtering to eliminate older and non-original manuscripts left 527 records that were subject to full text review. After eliminating 8 additional publications with overlap in patient population and the same primary or senior author, 50 publications were identified that met the inclusion criteria and these were assessed in detail (Figure 1).
Of these, 20 (40%) were randomized trials comparing ER and non-ER protocols12-31, while the remaining 3032-61 described at least one retrospective cohort or used non-random assignment. Some 22 unique ER elements were identified throughout the publications. The median number of implemented ER elements described in these publications was 9 (range: 3-17 elements). The median number of patients included was 130 (range: 50-1,358 patients). Quality assessment scores ranged from 9 to 24 with a median quality score of 17. There were 6 poor quality studies, 29 fair quality studies, and 15 good quality studies.
Only 19 publications (38%) described the implementation process of that ER pathway and only 14 specifically identified the number of surgeons participating in the study. For this subset of publications the median number of surgeons was 3 (range: 1-8 surgeons). Only a single publication listed the number of anaesthetists involved. Most publications, (90%) detailed their ER protocol within the methods section of the same paper, while the remainder referenced another article as containing the ER protocol in use.
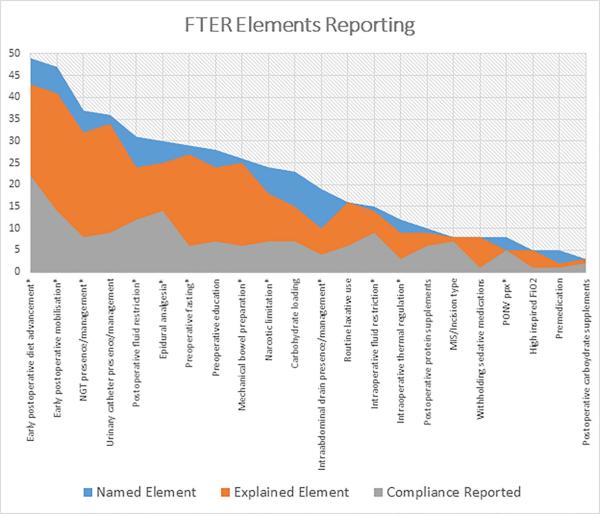
The most frequently utilised ER elements in the reviewed publications included early postoperative diet advancement in 49 (98%) and early mobilisation in 47 (94%). How these elements were implemented varied across publications. The method for diet advancement was explained in 43 publications (86%) and compliance with early initiation of diet advancement was reported in 22 (45%). Similarly, early mobilisation was defined in detail in 41 publications (82%) but compliance reported in only 14 (30%). Descriptions of diet and mobilisation strategies used in the comparative traditional recovery pathways were found in 37 (74%) and 27 publications (54%), respectively. In total, 18 publications disclosed failure rates for post-operative diet advancement and 5 reported whether or not any adverse events related to mobilisation occurred.
After these interventions, the next most commonly described interventions were nasogastric tube management in 37 publications (74%), urinary catheter management in 36 (72%), and epidural anaesthesia utilisation in 31 (62%). These elements were explained in detail in 32 (87%), 34 (94%) and 25 (83%) publications that named them as elements, respectively. The corresponding traditional pathway element or management was explained in 22 (60%), 22 (61%) and 22 (73%) publications. Compliance rates for each of these elements were found in 8 (22%), 9 (25%) and 14 (47%) of the publications, respectively. The rate of postoperative nasogastric tube replacement was reported in only 10 (27%) publications. The rate of urinary retention requiring replacement of a urinary catheter was listed in 8 (22%) and the rate of epidural failure was listed in 9 (30%) reports.
Although fewer publications reported or explained in detail the use of minimally invasive surgery or particular types of incisions, those that did name this element, frequently [7/8 (88%)] reported compliance in the form of surgical approach and the use of specific incisions. Other elements that had high compliance reporting were intraoperative fluid restriction 9/15 (60%), postoperative nausea and vomiting prophylaxis 5/8 (63%), postoperative protein supplements 6/10 (60%), and postoperative carbohydrate supplements 2/3 (67%). The elements that had the lowest compliance reporting were: withholding preoperative sedating medications 1/8 (13%), greater than atmospheric inspired FiO2 treatment 1/5 (20%), premedication administration 1/5 (20%), and reduced preoperative fasting period 6/29 (20%). (Table 1 and Figure 2).
Table 1.
ER and traditional pathway named elements and explanations with level of evidence and inclusion in the ERAS Registry.
| Factor (22 elements) | Evidence-based8, 9 | ERAS registry variable | Named (Total n=50) | ER pathway explained % | Traditional pathway explained % |
|---|---|---|---|---|---|
| Preoperative education | YES* | 28 (56%) | 24 (86%) | 16 (57%) | |
| Bowel preparation | YES | YES* | 26 (52%) | 25 (96%) | 18 (69%) |
| Reduced preoperative fasting | YES | 29 (58%) | 27 (93%) | 20 (67%) | |
| Carbohydrate Loading | YES* | 23 (46%) | 15 (65%) | 19 (83%) | |
| Preoperative medications | YES* | 5 (10%) | 2 (40%) | 4 (80%) | |
| Wi thhold sedating medications preoperatively | YES* | 8 (16%) | 8 (100%) | 6 (75%) | |
| PONV ppx | YES | YES* | 8 (16%) | 5 (63%) | 6 (75%) |
| MIS/Incision | YES | 8 (16%) | 8 (100%) | 6 (75%) | |
| Intraoperative thermal regulation | YES | YES* | 12 (24%) | 9 (69%) | 7 (58%) |
| Narcotic limitation | YES | 24 (48%) | 18 (75%) | 9 (38%) | |
| Increased FiO2 | 5 (10%) | 5 (100%) | 2 (40%) | ||
| NGT management/presence | YES | YES* | 37 (74) | 32 (87%) | 22 (60%) |
| Epidural use | YES | YES* | 30 ( 60%) | 25 (83%) | 22 (73%) |
| Intraop fluid restriction | YES | YES* | 15 (30%) | 14 (93%) | 12 (80%) |
| Postop fluid restriction | YES | YES | 31 (62%) | 24 (77%) | 13 (42%) |
| Routine laxative or prokinetic use | YES* | 16 (32%) | 16 (100%) | 12 (75%) | |
| Postop protein supplements | YES* | 10 (20%) | 9 (90%) | 6 (60%) | |
| Postop carbohydrate supplements | YES* | 3 (6%) | 3 (100%) | 3 (100%) | |
| Early postop diet | YES | YES* | 49 (98%) | 43 (88%) | 37 (76%) |
| Early mobilisation | YES | YES* | 47 (94%) | 41 (87%) | 27 (57%) |
| Urinary catheter management/presence | YES | 36 ( 72%) | 34 (94%) | 22 (61%) | |
| Intraabdominal drain management/presence | YES | YES* | 19 (38%) | 10 (53%) | 8 (42%) |
Named = listed in the manuscript, ER Pathway Explained = enhanced recovery element described with sufficient detail to facilitate transfer to the reader's clinical practice, Traditional pathway explained = traditional pathway element described with sufficient detail to facilitate transfer to the reader's clinical practice. PONV ppx= postop nausea and vomiting prophylaxis, NGT = nasogastric tube.
Denotes “key” field in registry
Figure 2.
Rate of ER compliance reporting as it relates to ER element naming and explanations. NGT = nasogastric tube, MIS = minimally invasive surgery, PONV ppx = postoperative nausea and vomiting prophylaxis
* indicates elements with strong supporting evidence for safety and efficacy8,9
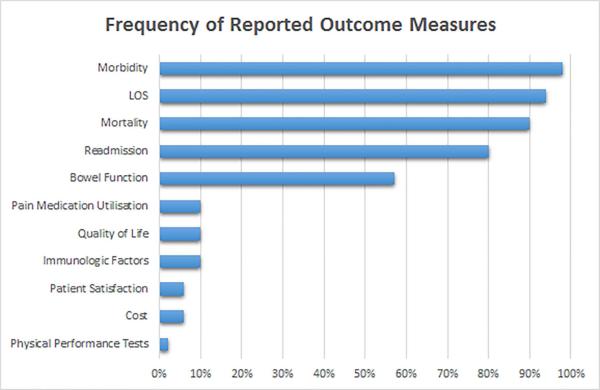
The outcome measures analysed most frequently in the publications were morbidity in 49 (98%) and length of stay (LOS) in 47 (94%). Only 40 (85%) of these publications described utilisation of specific discharge criteria. Mortality was reported in 45 (90%) and readmissions in 41 (82%) publications. These and less frequently reported outcome measures are shown in Figure 3.
Figure 3.
Patient outcome measures assessed as dependent variables in 50 colorectal surgery ER publications. LOS = Length of Stay
DISCUSSION
This project focused on two areas of reporting regarding ER protocols related to colorectal surgery; transparency of reporting individual components of ER protocols and compliance and failure rates with the stated components.
There was significant variation in the completeness of descriptions of ER protocols. The median number of components described was only 9. Despite heterogeneity between studies, most did report a positive effect of ER on short-term outcomes. This may be attributable to the fact that diet advancement and early mobilisation were common to most studies with significant impact on short-term endpoints such as length of stay.
Authors reported inclusion or exclusion of important components such as fluid management, oral non-narcotic analgesia, and routine laxative or prokinetic use, less frequently. When a restrictive fluid management protocol was listed, only rarely did the authors describe their practice, or patient monitoring (e.g. fluid balance or weight monitoring). Importantly, very few manuscripts that recorded neuraxial analgesia reported epidural/regional level, single injection vs. infusion/catheter placement, medications/concentrations used or duration of infusion. The absence of adequate descriptions of ER components significantly impairs the ability to interpret, compare outcomes between studies or translate practice to other centres.
Specific attention to compliance with the most important ER elements also needs to be made clear as not all of the elements (Table 1) are evidence based and known to be important in enhancing recovery8, 9. Compliance and failure rates with stated components of the protocol also varied and few studies actually reported the compliance and/or failure rate of each component of the ER protocol. Without these data it is impossible to determine the efficacy of each step in the pathway and its relative effects on the reported outcome measures. Additionally, detailed description of the corollary traditional pathway was frequently lacking. This is of particular importance when there is no apparent difference between outcome measures for ER and traditional groups.
Recently, there has been a movement towards standard reporting in medical literature. PRISMA, CONSORT and TIDieR recommendations have been proposed for meta-analyses, randomised controlled trials and clinical studies, respectively6, 62, 63. TIDieR guidelines postdate the studies in this review, but additionally do not take into account all of the necessary features in an ER protocol such as component compliance, failure rates, control pathway descriptions, graded complications, and discharge criteria. As such it would not be possible to draw meaningful conclusions about the effectiveness of the applied interventions upon outcome measures using the TIDierR instrument alone.
The present study included a rigorous literature search and strict inclusion criteria that isolated a large number of high-quality studies likely to have high clinical impact. Nevertheless, the non-standardised reporting of these elements meant that some of data collection was subjective, although all findings in the analysis have been transparently reported.
The finding of significant omissions in reporting ER elements in even the highest quality studies including 20 randomised trials, leads to the conclusion that a defined template for reporting these studies would significantly improve the quality of the literature related to ER for colorectal surgery. Based on the deficiencies noted in this analysis a standardised reporting template is proposed (Table 2). While it could prove cumbersome to provide all details within the original publication, the use of appendices or references could be used for complete reporting. Measurement and reporting of compliance for each component has been shown to correlate with patient outcomes64, 65. A clear description of discharge criteria, with differentiation of ‘ready for discharge’ compared to actual length of stay with reasons for non-medical extension of inpatient hospitalization should also be seen as essential66. Adherence with these guidelines should make it easier and more reliable to compare practices and outcomes. It may also reverse the observation that compliance with ER pathway elements drops outside the setting of a clinical trial67.
Table 2.
Recommended reporting elements for studies comparing ER to traditional pathways
| 1. Tabular reporting of all elements included in the examined ER pathway and corresponding elements of the traditional pathway. |
| 2. Explain all ER elements clearly with particular attention to reporting specific algorithms and pathways used in clinical management where applicable (e.g. IV fluid rates and criteria for goal directed fluid therapy; epidural/regional level, single injection vs. infusion/catheter placement, medications/concentrations used, duration of infusion; analgesia escalation strategies; drain placement algorithms). |
| 3. Report compliance for all elements named as part of the ER protocol. |
| 4. When failure of an ER element is possible, it should be reported and explained, including adverse events that may be related to an ER element. |
| 5. When length of stay is used as an outcome measure, discharge criteria or the lack thereof should be reported. If a substitute for length of stay such as “readiness for discharge” is used, there should be a report of actual length of stay and reasons for non-medical extension of hospitalisation listed. |
| 6. When morbidity is used as an outcome measure, efforts should be made to grade complications and stratify them according to severity using a standard system69, 70 |
ER = Enhanced Recovery
Another important finding in this analysis was the character of the dependent variables assessed in these studies. These were usually crude inpatient indicators of recovery such as morbidity, return of bowel function and length of stay. Patient reported outcomes and information related to costs of care are lacking. Biologic and molecular correlates that might inform future modifications in ER pathways were rarely included. This deficiency has been highlighted before and it does not appear that reporting of these elements has improved68.
ER protocols involve multiple components that will naturally have variable success rates. In order to transfer knowledge and facilitate implementation of pathways that demonstrate improvements in perioperative care and recovery, a structured reporting platform needs to be agreed and implemented.
Acknowledgments
Source of Funding: This research was supported in part by National Institutes of Health Grant, CA016672.
References
- 1.Kehlet H, Wilmore DW. Fast-track surgery. Br J Surg. 2005;92(1):3–4. doi: 10.1002/bjs.4841. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H. Fast-track colonic surgery: status and perspectives. Recent Results Cancer Res. 2005;165:8–13. doi: 10.1007/3-540-27449-9_2. [DOI] [PubMed] [Google Scholar]
- 3.Basse L, Thorbol JE, Lossl K, Kehlet H. Colonic surgery with accelerated rehabilitation or conventional care. Dis Colon Rectum. 2004;47(3):271–277. doi: 10.1007/s10350-003-0055-0. discussion 277-278. [DOI] [PubMed] [Google Scholar]
- 4.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63(8):e1–37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Brolmann FE, Eskes AM, Sumpio BE, Mayer DO, Moore Z, Agren MS, et al. Fundamentals of randomized clinical trials in wound care: reporting standards. Wound Repair Regen. 2013;21(5):641–647. doi: 10.1111/wrr.12087. [DOI] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Slim K, Kehlet H. Commentary: Fast track surgery: the need for improved study design. Colorectal Dis. 2012;14(8):1013–1014. doi: 10.1111/j.1463-1318.2012.03114.x. [DOI] [PubMed] [Google Scholar]
- 8.Kehlet H. Fast-track colorectal surgery. Lancet. 2008;371(9615):791–793. doi: 10.1016/S0140-6736(08)60357-8. [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. World J Surg. 2013;37(2):259–284. doi: 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 10.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samoocha D, Bruinvels DJ, Elbers NA, Anema JR, van der Beek AJ. Effectiveness of web-based interventions on patient empowerment: a systematic review and meta-analysis. J Med Internet Res. 2010;12(2):e23. doi: 10.2196/jmir.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frontera D, Arena L, Corsale I, Francioli N, Mammoliti F, Buccianelli E. Fast track in colo-rectal surgery. Preliminary experience in a rural hospital. G Chir. 2014;35(11-12):293–301. [PMC free article] [PubMed] [Google Scholar]
- 13.Feng F, Li XH, Shi H, Wu GS, Zhang HW, Liu XN, et al. Fast-track surgery combined with laparoscopy could improve postoperative recovery of low-risk rectal cancer patients: a randomized controlled clinical trial. J Dig Dis. 2014;15(6):306–313. doi: 10.1111/1751-2980.12142. [DOI] [PubMed] [Google Scholar]
- 14.Jia Y, Jin G, Guo S, Gu B, Jin Z, Gao X, et al. Fast-track surgery decreases the incidence of postoperative delirium and other complications in elderly patients with colorectal carcinoma. Langenbecks Arch Surg. 2014;399(1):77–84. doi: 10.1007/s00423-013-1151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li K, Li JP, Peng NH, Jiang LL, Hu YJ, Huang MJ. Fast-track improves post-operative nutrition and outcomes of colorectal surgery: a single-center prospective trial in China. Asia Pac J Clin Nutr. 2014;23(1):41–47. doi: 10.6133/apjcn.2014.23.1.09. [DOI] [PubMed] [Google Scholar]
- 16.Mari GM, Costanzi A, Maggioni D, Origi M, Ferrari GC, De Martini P, et al. Fast-track versus standard care in laparoscopic high anterior resection: a prospective randomized-controlled trial. Surg Laparosc Endosc Percutan Tech. 2014;24(2):118–121. doi: 10.1097/SLE.0b013e3182a50e3a. [DOI] [PubMed] [Google Scholar]
- 17.Compagna R, Aprea G, De Rosa D, Gentile M, Cestaro G, Vigliotti G, et al. Fast track for elderly patients: is it feasible for colorectal surgery? Int J Surg. 2014;12(Suppl 2):S20–22. doi: 10.1016/j.ijsu.2014.08.389. [DOI] [PubMed] [Google Scholar]
- 18.Lee SM, Kang SB, Jang JH, Park JS, Hong S, Lee TG, et al. Early rehabilitation versus conventional care after laparoscopic rectal surgery: a prospective, randomized, controlled trial. Surg Endosc. 2013;27(10):3902–3909. doi: 10.1007/s00464-013-3006-4. [DOI] [PubMed] [Google Scholar]
- 19.Wang G, Jiang Z, Zhao K, Li G, Liu F, Pan H, et al. Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J Gastrointest Surg. 2012;16(7):1379–1388. doi: 10.1007/s11605-012-1880-z. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Suo J, Jiang J, Wang C, Zhao YQ, Cao X. Effectiveness of fast-track rehabilitation vs conventional care in laparoscopic colorectal resection for elderly patients: a randomized trial. Colorectal Dis. 2012;14(8):1009–1013. doi: 10.1111/j.1463-1318.2011.02855.x. [DOI] [PubMed] [Google Scholar]
- 21.Ren L, Zhu D, Wei Y, Pan X, Liang L, Xu J, et al. Enhanced Recovery After Surgery (ERAS) program attenuates stress and accelerates recovery in patients after radical resection for colorectal cancer: a prospective randomized controlled trial. World J Surg. 2012;36(2):407–414. doi: 10.1007/s00268-011-1348-4. [DOI] [PubMed] [Google Scholar]
- 22.Veenhof AA, Vlug MS, van der Pas MH, Sietses C, van der Peet DL, de Lange-de Klerk ES, et al. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg. 2012;255(2):216–221. doi: 10.1097/SLA.0b013e31824336e2. [DOI] [PubMed] [Google Scholar]
- 23.Yang D, He W, Zhang S, Chen H, Zhang C, He Y. Fast-track surgery improves postoperative clinical recovery and immunity after elective surgery for colorectal carcinoma: randomized controlled clinical trial. World J Surg. 2012;36(8):1874–1880. doi: 10.1007/s00268-012-1606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubner M, Schafer M, Demartines N, Muller S, Maurer K, Baulig W, et al. Impact of restrictive intravenous fluid replacement and combined epidural analgesia on perioperative volume balance and renal function within a Fast Track program. J Surg Res. 2012;173(1):68–74. doi: 10.1016/j.jss.2010.08.051. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Botello S, Canovas de Lucas R, Tornero C, Escamilla B, Espi-Macias A, Esclapez-Valero P, et al. [Implementation of a perioperative multimodal rehabilitation protocol in elective colorectal surgery. A prospective randomised controlled study]. Cir Esp. 2011;89(3):159–166. doi: 10.1016/j.ciresp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg. 2011;254(6):868–875. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- 27.van Bree SH, Vlug MS, Bemelman WA, Hollmann MW, Ubbink DT, Zwinderman AH, et al. Faster recovery of gastrointestinal transit after laparoscopy and fast-track care in patients undergoing colonic surgery. Gastroenterology. 2011;141(3):872–880. e871–874. doi: 10.1053/j.gastro.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 28.Lee TG, Kang SB, Kim DW, Hong S, Heo SC, Park KJ. Comparison of early mobilization and diet rehabilitation program with conventional care after laparoscopic colon surgery: a prospective randomized controlled trial. Dis Colon Rectum. 2011;54(1):21–28. doi: 10.1007/DCR.0b013e3181fcdb3e. [DOI] [PubMed] [Google Scholar]
- 29.Serclova Z, Dytrych P, Marvan J, Nova K, Hankeova Z, Ryska O, et al. Fast-track in open intestinal surgery: prospective randomized study (Clinical Trials Gov Identifier no. NCT00123456). Clin Nutr. 2009;28(6):618–624. doi: 10.1016/j.clnu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Ionescu D, Iancu C, Ion D, Al-Hajjar N, Margarit S, Mocan L, et al. Implementing fast-track protocol for colorectal surgery: a prospective randomized clinical trial. World J Surg. 2009;33(11):2433–2438. doi: 10.1007/s00268-009-0197-x. [DOI] [PubMed] [Google Scholar]
- 31.Muller S, Zalunardo MP, Hubner M, Clavien PA, Demartines N, Zurich Fast Track Study G. A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology. 2009;136(3):842–847. doi: 10.1053/j.gastro.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 32.Ehrlich A, Kellokumpu S, Wagner B, Kautiainen H, Kellokumpu I. Comparison of laparoscopic and open colonic resection within fast-track and traditional perioperative care pathways: Clinical outcomes and in-hospital costs. Scand J Surg. 2014 doi: 10.1177/1457496914557016. [DOI] [PubMed] [Google Scholar]
- 33.Lohsiriwat V. Enhanced recovery after surgery vs conventional care in emergency colorectal surgery. World J Gastroenterol. 2014;20(38):13950–13955. doi: 10.3748/wjg.v20.i38.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller TE, Thacker JK, White WD, Mantyh C, Migaly J, Jin J, et al. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg. 2014;118(5):1052–1061. doi: 10.1213/ANE.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 35.Khreiss W, Huebner M, Cima RR, Dozois ER, Chua HK, Pemberton JH, et al. Improving conventional recovery with enhanced recovery in minimally invasive surgery for rectal cancer. Dis Colon Rectum. 2014;57(5):557–563. doi: 10.1097/DCR.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 36.Arroyo A, Ramirez JM, Callejo D, Vinas X, Maeso S, Cabezali R, et al. Influence of size and complexity of the hospitals in an enhanced recovery programme for colorectal resection. Int J Colorectal Dis. 2012;27(12):1637–1644. doi: 10.1007/s00384-012-1497-4. [DOI] [PubMed] [Google Scholar]
- 37.Fierens J, Wolthuis AM, Penninckx F, D'Hoore A. Enhanced recovery after surgery (ERAS) protocol: prospective study of outcome in colorectal surgery. Acta Chir Belg. 2012;112(5):355–358. doi: 10.1080/00015458.2012.11680851. [DOI] [PubMed] [Google Scholar]
- 38.Keane C, Savage S, McFarlane K, Seigne R, Robertson G, Eglinton T. Enhanced recovery after surgery versus conventional care in colonic and rectal surgery. ANZ J Surg. 2012;82(10):697–703. doi: 10.1111/j.1445-2197.2012.06139.x. [DOI] [PubMed] [Google Scholar]
- 39.Gouvas N, Gogos-Pappas G, Tsimogiannis K, Tsimoyiannis E, Dervenis C, Xynos E. Implementation of fast-track protocols in open and laparoscopic sphincter-preserving rectal cancer surgery: a multicenter, comparative, prospective, non-randomized study. Dig Surg. 2012;29(4):301–309. doi: 10.1159/000342554. [DOI] [PubMed] [Google Scholar]
- 40.Huibers CJ, de Roos MA, Ong KH. The effect of the introduction of the ERAS protocol in laparoscopic total mesorectal excision for rectal cancer. Int J Colorectal Dis. 2012;27(6):751–757. doi: 10.1007/s00384-011-1385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haverkamp MP, de Roos MA, Ong KH. The ERAS protocol reduces the length of stay after laparoscopic colectomies. Surg Endosc. 2012;26(2):361–367. doi: 10.1007/s00464-011-1877-9. [DOI] [PubMed] [Google Scholar]
- 42.McNicol FJ, Kennedy RH, Phillips RK, Clark SK. Laparoscopic total colectomy and ileorectal anastomosis (IRA), supported by an enhanced recovery programme in cases of familial adenomatous polyposis. Colorectal Dis. 2012;14(4):458–462. doi: 10.1111/j.1463-1318.2011.02683.x. [DOI] [PubMed] [Google Scholar]
- 43.Walter CJ, Watson JT, Pullan RD, Kenefick NJ, Mitchell SJ, Defriend DJ. Enhanced recovery in major colorectal surgery: safety and efficacy in an unselected surgical population at a UK district general hospital. Surgeon. 2011;9(5):259–264. doi: 10.1016/j.surge.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Archibald LH, Ott MJ, Gale CM, Zhang J, Peters MS, Stroud GK. Enhanced recovery after colon surgery in a community hospital system. Dis Colon Rectum. 2011;54(7):840–845. doi: 10.1007/DCR.0b013e31821645bd. [DOI] [PubMed] [Google Scholar]
- 45.Poon JT, Fan JK, Lo OS, Law WL. Enhanced recovery program in laparoscopic colectomy for cancer. Int J Colorectal Dis. 2011;26(1):71–77. doi: 10.1007/s00384-010-1059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christensen HK, Thaysen HV, Rodt SA, Carlsson P, Laurberg S. Short hospital stay and low complication rate are possible with a fully implemented fast-track model after elective colonic surgery. Eur Surg Res. 2011;46(3):156–161. doi: 10.1159/000324406. [DOI] [PubMed] [Google Scholar]
- 47.Schwarzbach M, Hasenberg T, Linke M, Kienle P, Post S, Ronellenfitsch U. Perioperative quality of care is modulated by process management with clinical pathways for fast-track surgery of the colon. Int J Colorectal Dis. 2011;26(12):1567–1575. doi: 10.1007/s00384-011-1260-2. [DOI] [PubMed] [Google Scholar]
- 48.Teeuwen PH, Bleichrodt RP, de Jong PJ, van Goor H, Bremers AJ. Enhanced recovery after surgery versus conventional perioperative care in rectal surgery. Dis Colon Rectum. 2011;54(7):833–839. doi: 10.1007/DCR.0b013e318216067d. [DOI] [PubMed] [Google Scholar]
- 49.Kahokehr A, Sammour T, Zargar-Shoshtari K, Srinivasa S, Hill AG. Recovery after open and laparoscopic right hemicolectomy: a comparison. J Surg Res. 2010;162(1):11–16. doi: 10.1016/j.jss.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 50.Baird G, Maxson P, Wrobleski D, Luna BS. Fast-track colorectal surgery program reduces hospital length of stay. Clin Nurse Spec. 2010;24(4):202–208. doi: 10.1097/NUR.0b013e3181e3604c. [DOI] [PubMed] [Google Scholar]
- 51.Larson DW, Batdorf NJ, Touzios JG, Cima RR, Chua HK, Pemberton JH, et al. A fast-track recovery protocol improves outcomes in elective laparoscopic colectomy for diverticulitis. J Am Coll Surg. 2010;211(4):485–489. doi: 10.1016/j.jamcollsurg.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Lloyd GM, Kirby R, Hemingway DM, Keane FB, Miller AS, Neary P. The RAPID protocol enhances patient recovery after both laparoscopic and open colorectal resections. Surg Endosc. 2010;24(6):1434–1439. doi: 10.1007/s00464-009-0795-6. [DOI] [PubMed] [Google Scholar]
- 53.Al Chalabi H, Kavanagh DO, Hassan L, Donnell KO, Nugent E, Andrews E, et al. The benefit of an enhanced recovery programme following elective laparoscopic sigmoid colectomy. Int J Colorectal Dis. 2010;25(6):761–766. doi: 10.1007/s00384-010-0902-0. [DOI] [PubMed] [Google Scholar]
- 54.Tsikitis VL, Holubar SD, Dozois EJ, Cima RR, Pemberton JH, Larson DW. Advantages of fast-track recovery after laparoscopic right hemicolectomy for colon cancer. Surg Endosc. 2010;24(8):1911–1916. doi: 10.1007/s00464-009-0871-y. [DOI] [PubMed] [Google Scholar]
- 55.Aboulian A, Hassan Z, Lin MY, Kaji AH, Kumar RR. Successful enhanced recovery program after colorectal surgery in a county institution. Am Surg. 2010;76(10):1158–1162. [PubMed] [Google Scholar]
- 56.Branagan G, Richardson L, Shetty A, Chave HS. An enhanced recovery programme reduces length of stay after rectal surgery. Int J Colorectal Dis. 2010;25(11):1359–1362. doi: 10.1007/s00384-010-1032-4. [DOI] [PubMed] [Google Scholar]
- 57.de Aguilar-Nascimento JE, Bicudo-Salomao A, Caporossi C, Silva Rde M, Cardoso EA, Santos TP, et al. Multimodal approach in colorrectal surgery without mechanical bowel cleansing. Rev Col Bras Cir. 2009;36(3):204–209. [PubMed] [Google Scholar]
- 58.Nygren J, Soop M, Thorell A, Hausel J, Ljungqvist O, Grp E. An Enhanced-Recovery Protocol Improves Outcome After Colorectal Resection Already During the First Year: A Single-Center Experience in 168 Consecutive Patients. Diseases of the Colon & Rectum. 2009;52(5):978–985. doi: 10.1007/DCR.0b013e31819f1416. [DOI] [PubMed] [Google Scholar]
- 59.Mohn AC, Bernardshaw SV, Ristesund SM, Hovde Hansen PE, Rokke O. Enhanced recovery after colorectal surgery. Results from a prospective observational two-centre study. Scand J Surg. 2009;98(3):155–159. doi: 10.1177/145749690909800305. [DOI] [PubMed] [Google Scholar]
- 60.Feo CV, Lanzara S, Sortini D, Ragazzi R, De Pinto M, Pansini GC, et al. Fast track postoperative management after elective colorectal surgery: a controlled trail. Am Surg. 2009;75(12):1247–1251. [PubMed] [Google Scholar]
- 61.Wang G, Jiang ZW, Xu J, Gong JF, Bao Y, Xie LF, et al. Fast-track rehabilitation program vs conventional care after colorectal resection: a randomized clinical trial. World J Gastroenterol. 2011;17(5):671–676. doi: 10.3748/wjg.v17.i5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63(8):834–840. doi: 10.1016/j.jclinepi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 64.Feroci F, Lenzi E, Baraghini M, Garzi A, Vannucchi A, Cantafio S, et al. Fast-track colorectal surgery: protocol adherence influences postoperative outcomes. Int J Colorectal Dis. 2013;28(1):103–109. doi: 10.1007/s00384-012-1569-5. [DOI] [PubMed] [Google Scholar]
- 65.Alcantara-Moral M, Serra-Aracil X, Gil-Egea MJ, Frasson M, Flor-Lorente B, Garcia-Granero E, et al. Observational cross-sectional study of compliance with the fast track protocol in elective surgery for colon cancer in Spain. Int J Colorectal Dis. 2014;29(4):477–483. doi: 10.1007/s00384-013-1825-3. [DOI] [PubMed] [Google Scholar]
- 66.Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82(6):679–684. doi: 10.3109/17453674.2011.636682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed J, Khan S, Gatt M, Kallam R, MacFie J. Compliance with enhanced recovery programmes in elective colorectal surgery. Br J Surg. 2010;97(5):754–758. doi: 10.1002/bjs.6961. [DOI] [PubMed] [Google Scholar]
- 68.Neville A, Lee L, Antonescu I, Mayo NE, Vassiliou MC, Fried GM, et al. Systematic review of outcomes used to evaluate enhanced recovery after surgery. Br J Surg. 2014;101(3):159–170. doi: 10.1002/bjs.9324. [DOI] [PubMed] [Google Scholar]
- 69.Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250(2):177–186. doi: 10.1097/SLA.0b013e3181afde41. [DOI] [PubMed] [Google Scholar]
- 70.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]