Summary
Escalation of anxious behavior while environmentally and socially relevant contextual events amplify the intensity of emotional response produces a testable gradient of anxiety shaped by integrative circuitries. Apprehension of the Stress-Alternatives Model apparatus (SAM) oval open field (OF) is measured by the active latency to escape, and is delayed by unfamiliarity with the passageway. Familiar OF escape is the least anxious behavior along the continuum, which can be reduced by anxiolytics such as icv neuropeptide S (NPS). Social aggression increases anxiousness in the SAM, reducing the number of mice willing to escape by 50%. The apprehension accompanying escape during social aggression is diminished by anxiolytics, such as exercise and corticotropin releasing-factor receptor 1 (CRF1) antagonism, but exacerbated by anxiogenic treatment, like antagonism of α2-adrenoreceptors. What is more, the anxiolytic CRF1 and anxiogenic α2-adrenoreceptor antagonists also modify behavioral phenotypes, with CRF1 antagonism allowing escape by previously submissive animals, and α2-adrenoreceptor antagonism hindering escape in mice that previously engaged in it. Gene expression of NPS and brain-derived neurotrophic factor (BDNF) in the central amygdala (CeA), as well as corticosterone secretion, increased concomitantly with the escalating anxious content of the mouse-specific anxiety continuum. The general trend of CeA NPS and BDNF expression suggested that NPS production was promoted by increasing anxiousness, and that BDNF synthesis was associated with learning about ever-more anxious conditions. The intensity gradient for anxious behavior resulting from varying contextual conditions may yield an improved conceptualization of the complexity of mechanisms producing the natural continuum of human anxious conditions, and potential therapies that arise therefrom.
Keywords: α2 receptors, continuum, CRF1 receptors, exercise, gradient, NPS
1. Introduction
Anxiety covers a spectrum of related disorders affecting as much as 25% of the population, is highly comorbid with depression (Kessler et al., 2010; Reus et al., 2014), and is theoretically constructed along a fear/stress continuum spanning from apprehension to terror (Blanchard and Blanchard, 1989; Estes and Skinner, 1941; Freud, 1953). Recent clinical trials suggest that the predictive power of most single anxiety level/niche tests is low (Haller and Alicki, 2012). Many anxiolytic agents tested in clinical trials have been ineffective (Haller and Alicki, 2012), required a specific narrowing of the treatment group (Holsboer and Ising, 2010), or had adverse effects, sedation, and/or dependence liability (Mohler, 2012). We suggest that for greater translational salience, new models should contiguously examine multiple levels along a gradient of anxiety, and the contextual niches that make up the complex structure of real anxious behavior (Haller and Alicki, 2012). We introduce a testable gradient of anxious behavior and hypothesize that an integrative machinery of stress and decision-making neurocircuitries produce this continuum and putative therapies.
The Stress-Alternatives Model (SAM) assesses anxious and depressive behaviors plus influences on decision-making (Smith et al., 2014), parsing active responses into contextual niches along an anxiety gradient. In the open field test (OF) rodents avoid the open center, which is reversed by anxiolytic drugs (Heredia et al., 2014). Mice in the SAM OF similarly avoid the center, but also actively choose to escape into an unknown chamber (Smith et al., 2014). Basic SAM escape experiments are modified by social aggression to increase the intensity (Koolhaas et al., 1997) of anxious behaviors and stress responsiveness (Smith et al., 2014) due to the uncontrollability and unpredictability of interactions (Koolhaas et al., 1998; Summers et al., 2005). Half the test animals remain submissively, exhibiting fear conditioning and increased corticosterone, and the rest escape. In SAM experiments using trout, rats, or hamsters, increasing social anxiety is accompanied by elevated plasma corticosterone concomitant with altered brain-derived neurotrophic factor (BDNF) and increased neuropeptide S (NPS) in the amygdala (Arendt et al., 2012; Robertson et al., 2015; Smith et al., 2014). As the SAM examines decision-making under socially stressful conditions by giving a choice of behavioral responses (escape or remain submissively), along with OF escape under non-social conditions (Smith et al., 2014), it has the fundamental advantage of examining a range of general and social anxious behaviors, as well as depressive behavior, allowing examination of the hypothetically complex range of circuit chemistry that is thought to produce progressively intense anxious behavior.
Neurocircuitries for stress, fear conditioning, decision-making, anxiety, and depression are highly interwoven, and include regions of the extended amygdalae and hippocampi, linked to periaqueductal gray, and controlled by executive regions of the cortex (Arendt et al., 2012; Herman and Cullinan, 1997; LeDoux et al., 1988; Li et al., 2013; Shin and Liberzon, 2010; Smith et al., 2014). Common to these circuitries, the central amygdala (CeA) is responsible for fearful/anxious signal transfer to other regions which modulate behavioral response, verified optogenetically (Tye et al., 2011). The intra-amygdalar fear/anxiety circuitry includes a glutamatergic pyramidal circuit (lateral = LA, basolateral = BLA) modified by GABAergic modulation and output (BLA, intercalated, CeA), both additionally modified by CRF, orexin (Orx), NPS, norepinephrine (NE), and serotonin (5-HT). There is strong evidence for modification of anxious behavior, and synergistic cross-talk among transmitter and neuromodulatory elements in this circuit (Arendt et al., 2014; Arendt et al., 2013; Cannella et al., 2013; Jungling et al., 2008; Li et al., 2015; Orsini and Maren, 2012; Smith et al., 2014).
Our experiments were designed to investigate an intensity gradient of anxious behavior, and mechanisms that may produce and alleviate varying intensities of anxiety. We postulate that the neural machinery that produces progressively intense anxious behavior integrates numerous neuromodulatory elements of stress and decision-making neurocircuitries. Therefore, both anxiogenic and anxiolytic treatments, some intrinsic to SAM (novel escape route [NER], OF, social interaction and defeat), were included in these studies (Smith et al., 2014). Exogenous drug treatments and physiological enrichment (running wheel), which modify anxious state, were also included. We hypothesized that conditions that promote apprehension (OF, NER, social aggression, social defeat, yohimbine) would delay or inhibit escape from the SAM arena, depending on the intensity of those anxiogenic treatments. In contrast, we hypothesized that anxiolytic treatments (prior escape, running wheel, NPS, antalarmin) would promote escape behavior, and shorten latencies to initial and repeated escapes. As the SAM conditions modify behavior, fear learning, and homeostatic mechanisms of anxiolysis in stress/fear-related neurocircuitry, we hypothesized the anxiogenic treatments would stimulate NPS, BDNF, TrKB and NPS receptor gene expression in the CeA (Smith et al., 2014), and that these changes would be reversed by anxiolytic treatments.
2. Materials and methods
2.1. Animals
Adult (8 week) male C57BL6/N mice weighing ~23-24g were used for treatments and testing. A separate cohort of retired Hsd:ICR (CD1) male breeders weighing ~53g were used to provide aggression. Mice were on a 12:12 reversed light-dark cycle (lights off at 10AM) at 22°C (See the Complete Methods in Supplementary materials).
2.2. Experimental Design
Some anxiogenic elements of the design are intrinsic to the SAM (OF, NER, social interaction); but anxiogenic and anxiolytic treatments were also applied: Antagonists of α2 adrenoreceptor (yohimbine) to enhance, and CRF1 receptors (antalarmin) to reduce, anxious behavior under conditions of high intensity anxiety (social defeat). Yohimbine, an α2 NE receptor antagonist, has been shown to increase anxiety (Charney et al., 1987) and defensive behavior in animals exposed to predator or handling stress (Blanchard et al., 1993). Anxiolytic responses are tested with environmental (running wheel) and drug (antalarmin and NPS) treatments. An endogenous anxiolytic protein, NPS has a broad receptor distribution in regions of the brain associated with anxiety and depression (Guerrini et al., 2010; Han et al., 2014; Jungling et al., 2008; Smith et al., 2014; Xu et al., 2007; Xu et al., 2004). Exercise has been demonstrated to reduce anxiety and depression in humans and animal models, and elicits synthesis and release of endogenous anxiolytics and antidepressant neuromodulators (Heyman et al., 2012; Melancon et al., 2014; Schoenfeld et al., 2013). Exercise, NPS and antalarmin were used as separate anxiolytic treatments to examine low and high intensity anxious behaviors.
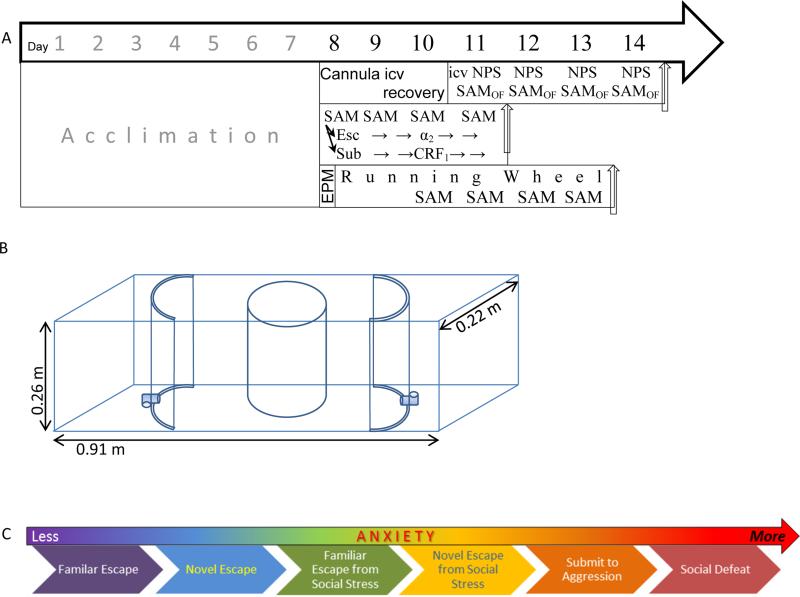
The SAM is designed to produce a variety of anxious conditions that lead to a gradient of generally and socially elicited anxious behavior, tested by the intrinsic properties of the model in combination with anxiogenic and/or anxiolytic treatments. With the exception of the injection procedure, all days (1–4) in the SAM apparatus followed an identical protocol (Figure 1A). The overall process for the experiments took between 11-14 days: Seven days of cage acclimation followed by four days of social interaction in the SAM apparatus were common to all treatment regimens (ip injections, icv injection, and running wheel; Figure 1A). An additional 3 days were added between acclimation and SAM treatment (in the absence of aggression) for implantation of cannulae for icv injection of NPS. Brains and blood were sampled in NPS-treated groups on day 14 after solo SAM treatment (Figure 1A). For animals allowed voluntary exercise, an additional two days were added to the standard 11 day regimen; such that immediately after acclimation, mice were tested for predisposition to anxiety on the elevated plus maze (EPM), then given access to running wheels for 2 days prior, and then also during the four days of SAM social interaction. For anxiogenic (yohimbine) and anxiolytic (antalarmin) pharmacological treatments, SAM social interaction proceeded directly after acclimation. During the first two days of SAM aggressive interaction with a larger CD1, the C57BL6/N mice diverged into cohorts that escaped (N = 25) or remained submissively (N = 23; didn't escape), and constituted experimental groups in addition to undisturbed controls (N = 10). Escaping mice were treated with anxiogenic drug yohimbine, while submissive mice received the anxiolytic drug antalarmin on the third SAM exposure, while control groups were injected with vehicle.
Figure 1.
A) Timelines of the experimental protocols all begin with seven days of acclimation to cages, followed thereafter by anxiogenic or anxiolytic treatments. Interaction with SAM apparatus occurred over four days, and may have taken place in the presence (middle and bottom timelines) or absence (top) of a larger conspecific aggressor. Top) For experiments in which the anxiolytic Neuropeptide S (NPS) is delivered icv, intraventricular cannula placement was executed on day eight followed by recover until icv injection of NPS and interaction with the empty SAM apparatus of each of days eleven through fourteen to test for open field (OF) anxious behavior and escape. Middle) Social anxiety and escape was tested by ip injection of the know anxiogenic α2-adrenoreceptor antagonist yohimbine in escaping animals (determined on days 1 and 2 of SAM interaction; days 8 and 9 overall) or the anxiolytic CRF1 antagonist antalarmin in submitting animals, just prior to day 3 and measuring latency to escape or inhibition of escape on days 3 and 4 of SAM social interactions (days 10 and 11 overall). Bottom) For the voluntary exercise experiments, mice were tested for predisposition for anxious behavior on the EPM on day 8 and then provided a running wheel for 6 days. Social interactions in the SAM begin after 2 days of running, and continue for another 4 days (days 10 – 13 overall). The icv NPS experiment ended on day 14, the yohimbine/antalarmin experiments on day 11, and the running wheel experiments ended on day 13. B) The SAM apparatus includes an open field (OF) arena that can be adjusted for size. The OF includes two escape routes, which lead to safe zones only accessible to small test mice. Test mice are added within the opaque cylindrical divider. Large aggressors are added outside the cylindrical divider. The divider is removed to allow social interaction. C) The continuum or gradient of intensity of anxious behavior as revealed by the Stress-Alternatives Model.
2.2.1. Stress-Alternatives Model (SAM)
In SAM experiments, focal animals are either tested alone or are subject to aggression (see Behavioral procedures below) and based on previous results (Robertson et al., 2015; Smith et al., 2014) and a priori hypotheses, these escapers and non-escapers constituted experimental groups. The SAM (Smith et al., 2014) is a rectangular box (Length:91cm, Width:22cm, Heigth:26cm) that contains two movable semicircular polyvinyl chloride sections (diameter: 22cm, height: 26cm) each with a passage constructed from 19 mm diameter ninety degree polyvinyl chloride tubes that allow for the C57BL6/N to pass, but are too small for CD1 aggressors (Figure 1B). A removable opaque cylinder separates aggressive from test mice prior to behavioral interaction.
2.2.2. NPS icv Injection
Mice were injected intracerebroventricularly (icv, volume = 2μl) under 2% isoflurane anesthesia, following 26 gauge guide cannula (AP −0.1, ML 0.9, DV −2.0) placement and recovery for 3 days, with vehicle (aCSF, N = 13) or NPS (1 nmol; N = 13) 30 min prior to SAM open field exposure (undisturbed controls, N = 6; total N = 32).
2.2.3. CRF1 and α2 Antagonist ip injections
Anxiogenic (via 5mg/kg α2-adrenoreceptor antagonist yohimbine HCl; Y3125, Sigma-Aldrich, N = 16) and anxiolytic effects (via 20mg/kg CRF1 receptor antagonist antalarmin HCl; A8727, Sigma-Aldrich, N = 10) were administered by intraperitoneal injections (ip, 1.5 ml; vehicle N = 14) 30 minutes prior to SAM exposure on day 3 (Cain et al., 2004; Zorrilla et al., 2002).
2.3. Behavior
Behavioral testing in the SAM took place between 10AM and 6PM under red light. Behavioral observations were recorded manually and digitally on video. Experiments included SAM trials either in the presence or absence of a larger CD1 aggressor. For experiments that included social aggression, a CD1 was first placed into the SAM inside the oval area but outside the cylindrical divider. Next, a C57BL6/N test mouse was placed inside an opaque cylindrical divider and allowed 30 seconds to acclimate. For experiments excluding aggression, trials began with the introduction of the test mouse. After acclimation the divider separating the two animals or test mouse from the OF was removed allowing test mice to interact with OF or CD1 mouse for a maximum of 5 min (to minimize injury to the test mouse). A novel CD1 is used for each interaction (used once per day), to limit habituation; mice often display more interest in novel compared to familiar conspecifics (Toth and Neumann, 2013). Behavioral SAM testing took place once per day for four consecutive days for each C57BL6/N test mouse. The duration of the interactions varied, because some animals escaped and some did not, and among those that did there were also differences in individual escape latency. Duration of interaction was defined as the period from removal of the divider (exposure to an aggressor) to the moment that the animal exited the SAM OF, using one of the two available escape holes (also latency to escape), or 5 min of submission. Interactions were scored (two naïve and independent trained scorers) for latency to escape. Once a test animal utilized an escape hole, a cover was placed over the hole for the remainder of the allotted 5 min.
2.3.1. Elevated Plus Maze
We also measured anxious behavior on the EPM between 10AM and 5PM (during the dark cycle; N = 44), prior to separating the animals into groups, and analyzed by two independent scorers, unaware of treatment.
2.3.2. Running Wheel
Immediately after exposure to the EPM, C57BL6/N mice were singly housed (transparent plastic cages 43×27×15cm) and separated into experimental groups; cage control (no running wheel or SAM exposure, N = 7), running wheel control (running wheel in cage, no SAM exposure; N = 7), SAM (exposure to SAM, but no running wheel N = 15), and Running Wheel SAM (exposure to both running wheel and SAM N = 15). Mice in groups with a running wheel had free access to a 1.08m circumference wheel (Nalgene™ Activity Wheels) for a total of 6 days, with twenty-four hour access except during behavioral testing. Social exposure in the SAM began after two days of wheel running (Running Wheel SAM) or no exercise (SAM).
2.4. Corticosterone Analysis
Fifteen minutes after SAM behavioral testing on day four, plasma corticosterone concentrations were quantified enzyme-linked immunosorbent assay kit (Enzo Life Sciences) as in previous experiments (Arendt et al., 2012; Smith et al., 2014).
2.5. Quantitative rtPCR
Central amygdala was microdissected using a blunt 23 gauge needle, and then analyzed using qrtPCR for Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Mm99999915_g1), BDNF (Mm04230607_s1), TrkB (Mm00435422_m1), NPS (Mm03990645_m1), and NPSR (Mm00558817_m1) using the 2−ΔΔCT method (Livak and Schmittgen, 2001), comparing all samples to the average ΔCT value of the control animals (not exposed to the SAM apparatus) as described previously (Arendt et al., 2012; Smith et al., 2014). Each sample was normalized to the expression of housekeeping gene, GAPDH. Changes in gene expression were quantified by real-time qPCR and analyzed Values for qPCR data were expressed as mean fold change ± standard error of the mean (SEM).
2.6. Statistics
Plasma corticosterone and gene expression results were compared across groups using one-way ANOVA (icv NPS and ip CRF1 and α2 experiments), and two-way ANOVA (for RW experiments). Behavioral comparisons across days (escape latency) were made using one-way repeated measures analyses and between treatments on a specific day by t-test. Significant effects between groups for ANOVA were examined using two post hoc analyses to minimize Type I error (Student–Newman–Keuls) and Type II error (Duncan's) respectively.
3. Results
All C57BL6/N mice exposed to the SAM investigated both the escape holes (within ~5 s) and the novel CD1 following the removal of the cylindrical divider at the start of each interaction.
3.1. Escaping Versus Remaining Submissively
3.1.1. NPS icv injection
Without an aggressive opponent all C57BL6/N test mice escaped when exposed to the SAM open field arena. Escape was not affected by NPS or vehicle icv injections, similar to untreated mice previously (Smith et al., 2014).
3.1.2. Predisposition to anxiety and escape
During SAM trials with a larger, aggressive conspecific, escaping and submissive mice were evenly split similar to previous experiments with this and other species (Carpenter and Summers, 2009; Robertson et al., 2015; Smith et al., 2014), and elevated plus maze anxiety results did not predict outcome of SAM trials (Supplementary Results and Figure S1A, S1B).
3.1.3. Voluntary exercise
Running wheel access (Figure 1A) did not alter phenotypic escape or submissive behavior (Supplementary Results and Figure S1B).
3.1.4. Anxiogenic α2-adrenoreceptor and anxiolytic CRF1 receptor antagonists injection (ip)
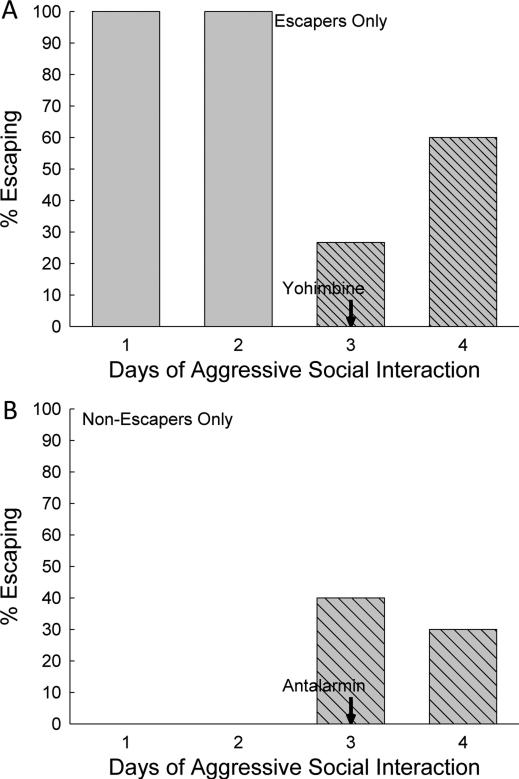
In contrast to the lack of effect of exercise, both anxiogenic and anxiolytic drug treatments did modify individual escape and submission behaviors. In test mice that had previously always escaped, a majority (73.3%) of mice given the anxiogenic α2-adrenoreceptor antagonist yohimbine on day 3 did not escape social aggression in SAM trials on that day (Figure 2A). The inhibition of escape was not universal, as 26.7% of mice did escape (Figures 2A, 3C; triangle, dashed line). Clearance of the drug, 24 hours after the ip yohimbine injection, returned 60% of the mice to their original preference for escaping social aggression in the SAM on day 4.
Figure 2.
Anxiogenic (A) and Anxiolytic (B) drug treatments on day 3 of SAM exposure changed escaping (A) or submissive (B) behavioral phenotypes in a substantial proportion of socially interactive mice. A) The anxiogenic α2-adrenoreceptor antagonist yohimbine inhibited escape behavior. Prior to ip delivery of yohimbine, all of the mice that would receive the anxiogenic drug escaped (light gray wide hatched bars; escaping animals were chosen for this treatment), but on the day of yohimbine administration only approximately one fourth of the animals escaped (dark gray bars with narrow hatching), and over 70% remained submissively. On the following day (4), with no additional α2-adrenoreceptor inhibition, most (60%) returned to escape behavior, with 40% continuing to remain submissively. B) The anxiolytic CRF1 receptor antagonist antalarmin promoted escape behavior in previously non-escaping submissive mice. Prior to ip delivery of antalarmin, none of the mice that would receive the anxiolytic drug escaped (no bars are evident; submissive animals were chosen for this treatment), but on the day of antalarmin administration 40% of the animals escaped (cross hatched bars). On day 4 (no drug treatment) 30% continued to escape.
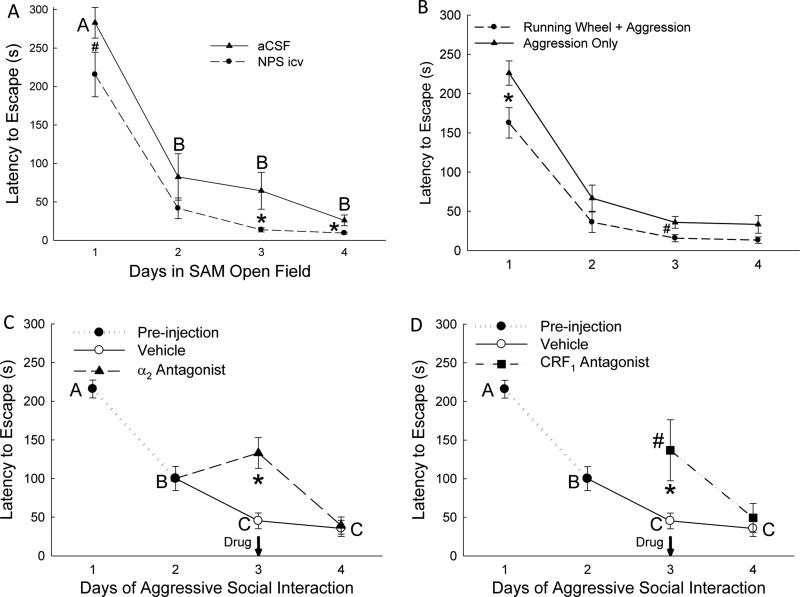
Figure 3.
Mice escape more rapidly (mean latency ± SEM) from the SAM apparatus / SAM social interactions with familiarity, which is enhanced by anxiolytic treatments, and inhibited by anxiogenic treatments. A) Mice injected icv with neuropeptide S (NPS, dashed line with circles) escape significantly faster from the SAM open field (OF) alone (in the absence of social interaction) than aCSF treated controls (solid line/triangles) on the initial trial (#) and during trials on days 3 and 4 (*). B) Mice given access to exercise (running wheel, dashed line/circles) exhibit reduced latency to escape from social aggression on the initial (*) and 3rd trials (#) of SAM social interaction compared to animals that received aggression in the absence of the opportunity for voluntary exercise (solid line/triangles). C) Escaping mice injected ip with the anxiogenic α2-adrenoreceptor antagonist yohimbine on day 3 of SAM social interaction, but continued to escape (See Fig. 3A), did so significantly more slowly (*) on day 3 than mice injected with vehicle. D) Non-escaping submissive mice injected ip with the anxiolytic CRF1 receptor antagonist antalarmin that began escaping (See Fig. 3B) on day 3 did so significantly more rapidly in initial escape (*) than the initial escape (day 1) of escaping animals injected with vehicle, but more slowly than vehicle controls escaped on day 3.
In contrast, for completely submissive C57BL6/N test mice (i.e., 0% escape on days 1 & 2), 40% escaped after treatment with the CRF1 receptor antagonist antalarmin on day 3 d (Figure 2B, 3D; square, dotted line). The effect of anxiolytic CRF1 receptor antagonist was still apparent in 30% for antalarmin treated mice on day 4, one day after the single ip injection on day 3.
3.2. Latency to Escape
3.2.1. NPS icv injection
When no conspecific aggression was present, all mice used the escape tunnels. Mice treated icv with aCSF (vehicle) escaped with a significantly (F3,46 = 37.27, p < 0.001; Figure 3A, solid line) decreased latency after day 1 (~282s) and continued to escape significantly faster (under ~80s) for the duration of the experiment (Figure 3A, solid line). Similarly, NPS injection (icv) also resulted in significantly (F3,46 = 54.89, p < 0.001; Figure 3A, dashed line) decreased escape latency after day 1 (~215s) and continued to escape significantly faster (~42s) for the remainder of the experiment (Figure 3A, dashed line). Animals injected icv with NPS escaped significantly faster than icv aCSF injected mice on day 1 (t18 = 1.98, p < 0.06; Figure 3A#), day 3 (t23 = 2.19, p < 0.038; Figure 3A*) and day 4 (t23 = 2.43, p < 0.023; Figure 3A*).
3.2.2. Voluntary exercise
In the presence of social aggression, mice with voluntary access to a running wheel demonstrated a familiarity-dependent significant (F3, 20 = 24.29, p < 0.001) decrease in escape latency, and escaped significantly faster than mice without running wheels on day 1 (t10 = 2.39, p < 0.03; Figure 3B*) and on day 3 (t10 = 2.08, p < 0.06; Figure 3B#).
3.2.3. Anxiogenic α2-adrenoreceptor and anxiolytic CRF1 receptor antagonists injection (ip)
Escaping mice (day 1 and day 2; see Supplementary Results) were randomly assigned to either vehicle or yohimbine groups on day 3. Treatment with the anxiogenic α2 antagonist reduced the number of escaping mice to only 26.7% (Figure 2A), and among those that did escape, they exhibited escape times on day 3 that were similar to day 2, rather than escaping faster on day 3, as vehicle injected mice did. Additionally, mice treated with anxiogenic α2 antagonist escaped significantly slower on day 3 in comparison with vehicle injected control animals (t16 = 4.13, p < 0.001; Figure 3C*, dashed line). A significant number of α2–inhibited mice returned to escape behavior one day after yohimbine treatment, with escape latencies similar to vehicle-treated mice on day 4, significantly faster than on days 1, 2 and 3 (F3,36 = 9.66, p < 0.001; Figure 3C, dashed line).
For those submissive mice (40%; Figure 2B) that began escaping on day 3 of SAM interactions following antalarmin injection, their first escape (day 3, triangle dash-dotted line) was significantly faster (t20 = 2.62, p < 0.016; Figure 3D#) than the first escape (day 1) of mice pre-injection (solid circle dotted line), but significantly slower (t16 = 3.33, p < 0.004; Figure 3D*, dashed line) than day 3 vehicle-treated escape latencies (open circle solid line; Figure 3D).
3.3. Plasma Corticosterone
3.3.1. NPS icv injection
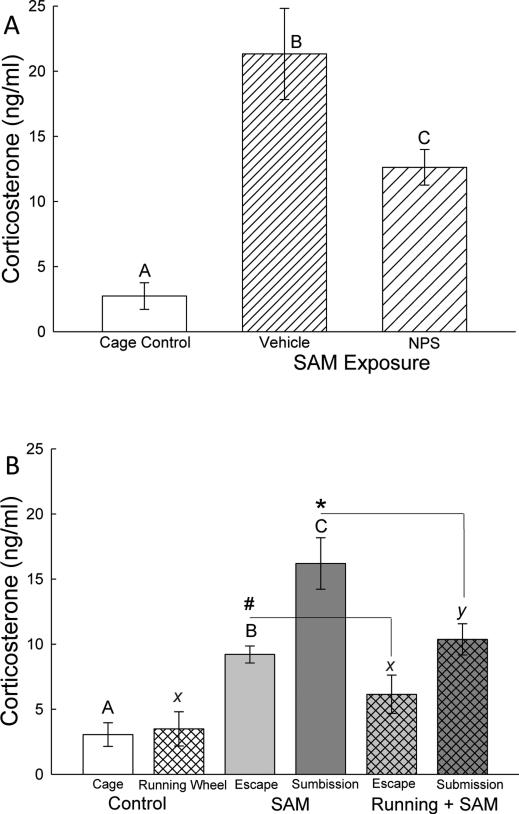
Plasma corticosterone concentrations were significantly elevated for mice exposed to the SAM open field arena and icv injection (F2,25 = 11.6, p < 0.001; Figure 4A) twenty minutes after the final five minute SAM exposure on day 4. Interestingly, NPS injected (icv) mice had significantly (p < 0.017) less plasma corticosterone compared to mice injected with vehicle.
Figure 4.
Plasma corticosterone (mean ± SEM) reflects anxiogenic conditions. A) In the absence of social interaction in the SAM, icv injection (vehicle, hatched bars) significantly elevated corticosterone concentrations, which is reduced somewhat by NPS icv injection (cross hatched bars). B) During social aggressive interactions in the SAM, corticosterone concentrations are elevated; highest in submissive animals (cross hatched bars), and significantly reduced in those that escape (left hatching) compared with controls (open bar). Social stress-induced elevation of corticosterone secretion is ameliorated by access to voluntary exercise on a running wheel for submissive animals (*, gray cross hatched bar) and also for escaping mice (#, gray left hatched bar); while presence of the running wheel in the absence of social interaction did not affect plasma corticosterone concentrations. Bars marked with differing letters above the mean/error bar (e.g. A, B, C; or X and Y) are significantly different, whereas bars marked with the same letter (such as X) are not significantly different. Bars marked # reflect significant differences between escaping animals without access to running wheels and escaping animals with running wheels (Otherwise bars marked A, B, or C, are not compared with bars labeled X, Y, or Z); and * designates significant reduction in corticosterone in submissive animals with running wheels compared to submissive animals without access.
3.3.2. Voluntary exercise
In social SAM interactions without running wheels, both escape (p < 0.014) and submissive (p < 0.001) animals had a rapid and significant plasma corticosterone increase (F2, 19 = 19.81, p < 0.001; Figure 4B) compared to cage controls. Interestingly, in animals with free access to a running wheel, only the submissive animals had significantly (F2, 14 = 6.01, p < 0.016; Figure 4B) elevated plasma corticosterone levels. When comparing the effect of exercise, both escape (t9 = 2.03, p < 0.07; Figure 4B#) and submissive (t11 = 2.15, p < 0.05; Figure 4B*) animals with access to a running wheel had significantly decreased plasma corticosterone when compared to the same group without access.
3.4. Gene expression for NPS and NPSR
3.4.1. NPS icv injection
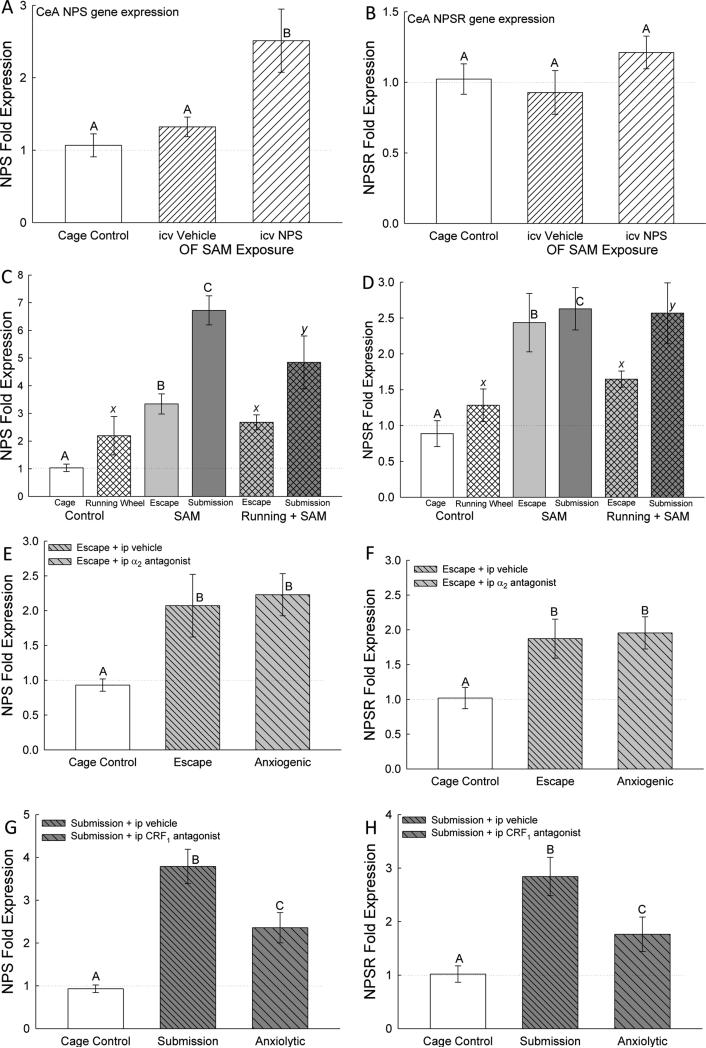
Expression of NPS mRNA in the CeA was significantly (F2,27 = 5.89, p < 0.008) elevated for animals given icv NPS injections (in the absence of social aggression) compared to both controls (Figure 5A).
Figure 5.
Intra-amygdalar (central amygdala, CeA) neuropeptide S (NPS) gene expression (mean ± SEM) is elevated by anxiogenic/stressful conditions, and alleviated by anxiolytic conditions. A) Injection (icv) of NPS (cross hatched bars) stimulated increased NPS mRNA in CeA compared to cage controls (clear bars) and icv aCSF (vehicle) injection (hatched bar), but B) had no effect on NPS receptor (NPSR). C) During social aggressive interactions in the SAM, NPS mRNA is elevated; highest in submissive animals (cross hatched bars; see C, E, G), and significantly reduced in those that escape (left hatching) compared with controls (open bar). Social stress-induced elevation of NPS expression is somewhat ameliorated by access to voluntary exercise on a running wheel for submissive animals; while presence of the running wheel in the absence of social interaction did not affect NPS expression. D) Social aggressive interactions in also stimulated enhanced NPSR mRNA compared with controls (open bar). Presence of the running wheel in the absence of social interaction did not affect NPSR expression. E, F) Social interaction with escape and the anxiogenic drug yohimbine stimulate elevated expression of both NPS and NPSR mRNA. G, H) Socially submissive interaction significantly stimulates elevated NPS and NPSR gene expression (higher for both in submissive mice than escaping mice, see E, F), both of which are ameliorated by the anxiolytic drug antalarmin. Bars marked with differing letters above the mean/error bar (e.g. A, B, C; or X and Y) are significantly different, whereas bars marked with the same letter (such as X) are not significantly different.
3.4.2. Voluntary exercise
Mice in aggressive interactions (but without access to a running wheel) had significantly (F2, 17 = 46.38, p < 0.001; Figure 5C) elevated NPS and NPSR expression for both escaping (p < 0.002) and submissive (p < 0.001) behavior compared to controls. Importantly, mice choosing submissive behavior expressed significantly more (p < 0.001) NPS, but not NPSR, mRNA than mice that escaped. When a running wheel available significantly elevated expression of NPS and NPSR was only found in the CeA submissive animals (NPS: F2, 14 = 4.08, p < 0.045, Figure 5C; NPSR: F2, 18 = 9.43, p < 0.002, Figure 5D).
3.4.3. Anxiogenicα2-adrenoreceptor and anxiolytic CRF1 receptor antagonists injection (ip)
Vehicle treated escaping animals and yohimbine treated animals had significantly elevated (F2,28 = 3.66, p < 0.04; Figure 5E) CeA NPS mRNA expression compared to cage controls, though not significantly different from each other (p > 0.74). Social aggression significantly elevated (F2,22 = 17.75, p < 0.001; Figure 5G) NPS mRNA for both vehicle and antalarmin treated submissive animals compared to cage controls, but antalarmin treated mice had significantly lower (p < 0.005) NPS mRNA expression compared to vehicle treated submissive mice.
Yohimbine treated animals and the vehicle treated escaping animals had significantly elevated (F2,32 = 4.34, p < 0.022; Figure 5F) CeA NPSR mRNA expression compared to cage controls, though they were not significantly different from each other (p > 0.8). Interestingly, vehicle treated submissive mice had significantly elevated (F2,28 = 9.22, p < 0.001; Figure 5H) NPSR mRNA expression compared to antalarmin treated mice and cage controls, but antalarmin treated mice and cage controls were not significantly different (p > 0.094).
3.5. Gene expression for BDNF and TrKB
3.5.1. NPS icv injection
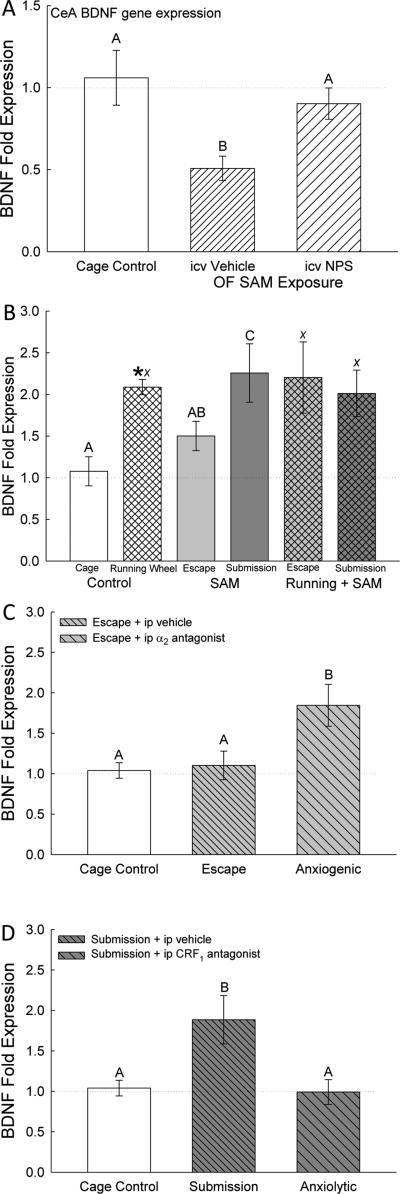
The process of icv injection significantly (F2,31 = 7.06, p < 0.003; Figure 6A) reduced CeA BDNF mRNA in vehicle injected mice exposed to the SAM open field arena only, but not in mice treated with NPS. Gene expression for CeA BDNF was not significantly (p > 0.34) different between NPS injected mice and undisturbed cage controls, suggesting that the stress of icv treatment was ameliorated by NPS.
Figure 6.
Intra-amygdalar (central amygdala, CeA) brain-derived neurotrophic factor (BDNF) gene expression (mean ± SEM) is elevated by anxiogenic/stressful conditions, and alleviated by anxiolytic conditions. There were no effects of treatments on BDNF TrKB receptor mRNA. A) Injection (icv) of NPS (cross hatched bars) returned BDNF mRNA in CeA to cage control expression levels (clear bars), compared to reduced expression in icv aCSF (vehicle) injection (hatched bar). B) Running wheels (*) and submission increased BDNF fold expression in the CeA, compared with cage controls (clear bars) and escaping mice without exercise (hatched bars). C) Increased CeA BDNF mRNA in submissive animals (similar to B; cross hatched bars) is ameliorated by anxiolytic CRF1 antagonist antalarmin treatment (gray bars). D) The anxiogenic α2-adrenoreceptor antagonist yohimbine stimulated increased BDNF gene expression in the CeA, compared to cage controls (clear bar) and escaping mice (hatched bar). Bars marked with differing letters above the mean/error bar (e.g. A, B, C; or X and Y) are significantly different, whereas bars marked with the same letter (such as A and AB) are not significantly different.
3.5.2. Voluntary exercise
In running wheel experiments, BDNF mRNA was significantly (F2, 18 = 5.36, p < 0.017; Figure 6B) increased in the no running wheel, SAM only group in submissive (p < 0.015) animals compared to cage controls and escaping animals. Control animals that had access to a running wheel but did not experience social aggression had significantly (t10 = −5.12, p < 0.001; Figure 6B*) elevated BDNF gene expression compared to cage control animals without access to a running wheel. Expression of BDNF was not significantly (F2,16 = 0.098, p > 0.91; Figure 6B) different for animals that experienced social aggression in the SAM (both escaping and submissive mice) and had access to a running wheel compared to cage control animals with a running wheel.
3.5.3. Anxiogenicα2-adrenoreceptor and anxiolytic CRF1 receptor antagonists injection (ip)
Expression of BDNF mRNA in the CeA was significantly increased in escaping mice injected with the anxiogenic α2-adrenoreceptor antagonist yohimbine on day 3 (F2,35 = 4.92, p < 0.014; Figure 6C), and also in submissive test mice (vehicle treated on day 3; F2,31 = 5.08, p < 0.013; Figure 6D) when compared with undisturbed cage controls. These anxiogenic effects showed a stark contrast with BDNF mRNAs in vehicle-treated escaping mice (p > 0.84; Figure 6C), and in submissive mice injected ip with the anxiolytic CRF1 antagonist antalarmin (p > 0.87; Figure 6D), which were not elevated relative to cage controls. Elevated BDNF gene expression for vehicle-injected mice that remained submissively for days 1-4 in the SAM appear to have been ameliorated by the addition of the CRF1 receptor blocker. In contrast, the behavioral anxiolytic action of escaping appears to have been reversed by treatment with the noradrenergic α2 receptor antagonist on day 3.
4. Discussion
The results from these experiments suggest that mice experience a continuum or gradient of apprehensiveness, expressed as distinguishable anxious behaviors, depending on physical and social contexts, and regulated by an integrated circuitry of stress, fear conditioning, decision-making, anxiety and depression. In the Stress-Alternatives Model, all mice react to the SAM open field arena by avoiding the center as they would in any OF test (Smith et al., 2014), but they do not immediately respond by leaving the OF through the escape route suggesting that the novelty of the hole and tunnel are at least mildly anxiogenic. Known anxiolytic processes, including familiarity with the escape route and short-term exercise, as well as neuromodulatory anxiolytics such as icv injection of NPS or antagonism of CRF1 receptors, reduce the delay to use the available escape path, suggesting that latency to escape is a viable tool for assessing actively anxious behavior (Biojone et al., 2011; Sheynin et al., 2014). Stress responsiveness, measured as plasma corticosterone, suggests that social interaction increases the anxiogenic capacity of the OF, and that escape from social aggression occurring in the OF reduces the anxiogenic quality of this condition, which is further reduced by the availability of the opportunity to exercise (when a running wheel is provided, Figure 5B). Based on stress hormone levels, submission to social aggression and defeat are the most anxiogenic conditions encountered in the SAM arena, which is also ameliorated somewhat by the presence and use of the running wheel. Thus, the gradient of anxiety (Figure 1C) extends from familiar escape from an OF to social defeat, with familiar and novel escape from social stress being intermediary anxious behaviors. The trajectory of the anxiety continuum is further corroborated by the effects of known anxiolytic (the CRF1 receptor antagonist, antalarmin) and anxiogenic drugs (the adrenergic α2 receptor antagonist, yohimbine) (Ghitza et al., 2006; Zorrilla et al., 2002). Inhibiting CRF1 receptors not only allows escape behavior from previously defeated animals which only displayed submission, but it also reduces the latency to the first escape on day three when the drug is given (Figure 3D). The anxiogenic effect of yohimbine occurs via increased noradrenergic activity following inhibition of α2 receptor negative feedback, which produces social defeat in previously escaping animals, but also delays the escape of animals given yohimbine which continue to escape (Figure 3C).
Our results suggest that despite the indications that all animals begin SAM testing with equivalent baseline anxious behavior (equal EPM open arm time; Figure S1A), equal preference for edges rather than center of the OF (Smith et al., 2014), and universal escape from the SAM OF in the absence of aggression), during social interactions in the SAM arena divergent behavior emerges (Robertson et al., 2015). Despite the unrelenting, intense aggression from a novel larger conspecific (CD1 mouse), half the test mice do not leave the social arena via the escape hole (Figure S1B). This ratio of submission to escape is consistent over 5 cohorts of C57BL6/N mice in these experiments (Figure S1B), and across species from fish to rodents (Robertson et al., 2015). Even though there appears to be little predisposition for anxious responding (Figure S1A), the test rodents diverge behaviorally very quickly following the initiation of social interaction in the SAM, such that after an initial adoption of submissive behaviors in both groups, one subset of test mice begin exploring the possibility of escape, and eventually make use of the hole to leave the arena. Mice that remain in the arena never reduce the preponderance of submissive behavior throughout the SAM social interactions (Robertson et al., 2015). These phenotypic responses, Escape and Submission, however, are reversible with anxiogenic or anxiolytic drug treatments: α2 adrenergic receptor inhibition limits egress in most escaping mice (Figure 2A), whereas CRF1 receptor inhibition allows some submissive mice (Figure 2B) and trout to escape (Robertson et al., 2015). The results suggest that while anxiety-related behavioral phenotypes may require environmental or social stressors to emerge, and remain relatively consistent, they are not fixed and respond quickly to anxiolytic and anxiogenic conditions and treatments.
This is also true for latency to escape an OF arena, for which familiar escapes were always more rapid (Smith et al., 2014). The latencies for initial-novel and familiar escapes were reduced by anxiolytic treatments, regardless of whether there was an aggressive conspecific present. The endogenous anxiolytic and pro-arousal hypothalamic peptide NPS (Guerrini et al., 2010; Ionescu et al., 2012; Klauke et al., 2014; Xu et al., 2004), when injected icv, reduced OF-only latency to escape (Figure 3A) (Pulga et al., 2012), diminished plasma corticosterone levels (Figure 4A), and stimulated gene expression of NPS in the CeA (Figure 5A). It suggests that while NPS is anxiolytic it also acts through a positive feedback mechanism to enhance NPS synthesis. Interestingly, CeA NPS expression, like corticosterone, increased as the intensity of anxious behavior grew. Expression of NPS in the CeA progressively increased as social interaction became submission and defeat (Figure 5C). In contrast, while the NPS receptor also showed enhanced expression in CeA in any socially interacting individual, submission did not increase NPSR mRNA further (Figure 5D). While the anxiogenic α2 antagonist yohimbine did not raise NPS or NPSR expression beyond that of social interaction (Figure 5E, F), it did delay the escape of mice receiving it via ip injection on day 3 (Figure 3C), and prevented most (67.7%) of the previously escaping mice from escaping on that day (Figure 2A). Like NPS, other anxiolytic treatments reduce the latency to escape, but unlike exogenous icv NPS they also diminish NPS and NPSR expression in CeA. Short-term (6 days) voluntary exercise improved the celerity of escape in both novel and familiar social aggression conditions (Figure 3B), and the anxiolytic CRF1 receptor antagonist antalarmin significantly reduced the latency of escape for first time submissive escapers, compared to the first escape for vehicle treated escaping controls (Figure 3D). In addition, CRF1 antagonism resulted in reduced CeA NPS and NPSR expression (Figures 5G, H). Even short term voluntary exercise reduced social stress-induced plasma corticosterone concentrations, in both escaping and submissive animals (Figure 5B), while also limiting NPS mRNA in submissive mice (Figure 5C) as well as NPSR mRNA in escaping mice (Figure 5D). Social defeat produces the highest levels of corticosterone secretion (Figure 5B), but also the greatest expression of NPS and NPSR in the CeA (Figures 5C, D); these effects are significantly reduced by escape, suggesting that escape is a potent anxiolytic, and its latency to expression is a valid method of measuring the intensity of anxious behavior.
Anxiety appears to be an important factor influencing BDNF expression the amygdala, as maternal anxiety impacts DNA methylation of Met/Met genotype of the BDNF Val66Met gene variant in infants and effects amygdalar volume (Chen et al., 2015). Alcohol treatment during early postnatal rat development enhances BDNF and GDNF expression in the central extended amygdala (Balaszczuk et al., 2013), and ETOH withdrawal in adult rats leads to decreased BDNF gene expression and dendritic spine density in the CeA along with increased anxious behavior (You et al., 2014). Social defeat stress simulates BDNF immunoreactivity in the medial amygdala of rats (Nikulina et al., 2012), so it is possible that the social stress in our paradigm stimulates increased BDNF expression generally in the amygdala. Extinction of conditioned taste aversion leads to increased BDNF gene expression in BLA but not CeA (Xin et al., 2014). In a previous experiment, we demonstrated that fear conditioning using the SAM apparatus resulted in decreased BDNF gene expression in both BLA and CeA (Smith et al., 2014), but in the experiments reported herein, in the absence of a conditioned stimulus (tone) or testing for the conditioned response, four days of social aggression stimulated CeA BDNF gene expression (Figure 6B). The results suggest that BDNF expression generally, and in the CeA specifically, can be modified by both stressful conditions and anxious behavior, but also by classical conditioning, especially in fear or aversion related circumstances. Our results suggest that subordination stress increases BDNF mRNA in CeA, which can also be increased by the anxiogenic drug yohimbine (Figure 6C), and reversed by anxiolytic behavior like escape or conditioning and anxiolytic drugs like the CRF1 antagonist antalarmin (Figure 6D). However, running also stimulates BDNF gene expression (Figure 6B), and anxiolytic NPS reverses depressed CeA BDNF expression caused by the stress of icv injection (Figure 6A). For the most part BDNF gene expression is raised by high level anxiogenic treatments and conditions, and reversed by anxiolytic mechanisms, which suggests that expression of CeA BDNF may enhance fear learning or expression of anxious behaviors.
5. Conclusions
Our results suggest that a recognizable gradient of intensity underlies the generation and expression of anxious behavior. The intensity gradient for anxious behavior was modifiable by a variety of neuromodulatory systems, which include CRF, NPS, NE, as well as Orx and 5-HT, known to modify anxious behavior and synergistically interact within the appropriate circuits (Arendt et al., 2014; Arendt et al., 2013; Cannella et al., 2013; Jungling et al., 2008; Li et al., 2015; Orsini and Maren, 2012; Smith et al., 2014). The results of experiments utilizing the SAM apparatus suggest that in addition to Pavlovian fear conditioning and decision-making (Smith et al., 2014), this model is an effective way to examine the escalation of anxious behavior as environmentally and socially relevant contextual events amplify the intensity of emotional response along a gradient of anxiety (Figure 1C). Apprehension of the OF is measured by latency to escape, which is effectively delayed by unfamiliarity with the route for egress. Familiar OF escape is the least anxious behavior along the continuum, but the apprehension of even this low level mouse-appropriate anxiety can be reduced by anxiolytics such as icv NPS. Social aggression adds anxious content to the SAM experience, with a reduction by approximately 50% of the mice willing to engage in escape from the OF. The apprehension associated with latency to escape during social aggression can also be modified by anxiolytics, such as exercise and CRF1 antagonism, but can also be exacerbated by anxiogenic antagonism of α2-adrenoreceptors. What is more, the anxiolytic CRF1 and anxiogenic α2-adrenoreceptor antagonists are capable of modifying behavioral phenotypes based on the stress-coping strategies of social submission and escape; with CRF1 antagonism allowing a substantial proportion of submissive animals to escape and α2-adrenoreceptor antagonism blocking escape in a substantial percentage of previously escaping mice. Endogenous corticosterone secretion and gene expression of NPS and BDNF in the CeA increased following the growing anxious content of each level of the mouse-specific anxiety continuum. Although BDNF mRNA was amplified by exercise, the general trend of CeA NPS and BDNF expression suggested that NPS production was promoted by increasing anxiousness, and that BDNF synthesis was associated with learning about ever-more anxious conditions. The resulting continuum of anxiety derived from varying contextual conditions may yield a more complete understanding of the complexity of the mechanisms that produce a natural gradient of human anxious conditions, and the putative therapies that may be effective.
Supplementary Material
Highlights.
Stress-Alternatives Model examines behavioral escalation along gradient of anxiety
Escape anxiety during aggression is diminished by exercise and CRF1 antagonism
SAM escape/submission phenotypes are altered by anxiolytic or anxiogenic treatments
Anxious Intensity is revealed in corticosterone, central amygdalar NPS & BDNF mRNA
SAM general and social anxieties reflect a natural gradient of human conditions
Acknowledgments
We would like to thank Tangi R. Summers for contributions to writing and editing the manuscript. Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R15MH104485, and by NIH grant and P20 RR15567, a CBBRe Research Enhancement Pilot grant, and by an anonymous donor to the Summers’ lab via the USD Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States Government.
Role of the funding sources
This research was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R15MH104485, and by NIH grant and P20 RR15567, a CBBRe Research Enhancement Pilot grant, and by an anonymous donor to the Summers’ lab via the USD Foundation. These funding sources had no further role in the study design, collection, analysis and interpretation of data, writing of the report, or in the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
There are no conflicts of interest with any author in the conduct or reporting of the research contained in the manuscript.
References
- Arendt DH, Hassell J, Li H, Achua JK, Guarnieri DJ, Dileone RJ, Ronan PJ, Summers CH. Anxiolytic function of the orexin 2/hypocretin A receptor in the basolateral amygdala. Psychoneuroendocrinology. 2014;40:17–26. doi: 10.1016/j.psyneuen.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt DH, Ronan PJ, Oliver KD, Callahan LB, Summers TR, Summers CH. Depressive behavior and activation of the orexin/hypocretin system. Behavioral neuroscience. 2013;127:86–94. doi: 10.1037/a0031442. [DOI] [PubMed] [Google Scholar]
- Arendt DH, Smith JP, Bastida CC, Prasad MS, Oliver KD, Eyster KM, Summers TR, Delville Y, Summers CH. Contrasting hippocampal and amygdalar expression of genes related to neural plasticity during escape from social aggression. Physiology & behavior. 2012;107:670–679. doi: 10.1016/j.physbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaszczuk V, Bender C, Pereno G, Beltramino CA. Binge alcohol-induced alterations in BDNF and GDNF expression in central extended amygdala and pyriform cortex on infant rats. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2013;31:287–296. doi: 10.1016/j.ijdevneu.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Biojone C, Casarotto PC, Resstel LB, Zangrossi H, Jr., Guimaraes FS, Moreira FA. Anti-aversive effects of the atypical antipsychotic, aripiprazole, in animal models of anxiety. J Psychopharmacol. 2011;25:801–807. doi: 10.1177/0269881110376690. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. Journal of comparative psychology. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Taukulis HK, Rodgers RJ, Magee LK, Blanchard DC. Yohimbine potentiates active defensive responses to threatening stimuli in Swiss-Webster mice. Pharmacology, biochemistry, and behavior. 1993;44:673–681. doi: 10.1016/0091-3057(93)90185-v. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learning & memory. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N, Kallupi M, Ruggeri B, Ciccocioppo R, Ubaldi M. The role of the neuropeptide S system in addiction: focus on its interaction with the CRF and hypocretin/orexin neurotransmission. Progress in neurobiology. 2013;100:48–59. doi: 10.1016/j.pneurobio.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Carpenter RE, Summers CH. Learning strategies during fear conditioning. Neurobiology of learning and memory. 2009;91:415–423. doi: 10.1016/j.nlm.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Woods SW, Goodman WK, Heninger GR. Neurobiological mechanisms of panic anxiety: biochemical and behavioral correlates of yohimbine-induced panic attacks. The American journal of psychiatry. 1987;144:1030–1036. doi: 10.1176/ajp.144.8.1030. [DOI] [PubMed] [Google Scholar]
- Chen L, Pan H, Tuan TA, Teh AL, MacIsaac JL, Mah SM, McEwen LM, Li Y, Chen H, Broekman BF, Buschdorf JP, Chong YS, Kwek K, Saw SM, Gluckman PD, Fortier MV, Rifkin-Graboi A, Kobor MS, Qiu A, Meaney MJ, Holbrook JD, Gusto Study G. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism influences the association of the methylome with maternal anxiety and neonatal brain volumes. Development and psychopathology. 2015;27:137–150. doi: 10.1017/S0954579414001357. [DOI] [PubMed] [Google Scholar]
- Estes WK, Skinner BF. Some quantitative properties of anxiety. Journal of Experimental Psychology. 1941;29:390–400. [Google Scholar]
- Freud S. Inhibitions, symptoms and anxiety, The Standard Edition of the Complete Psychological works of Sigmund Freud. Hogarth Press; London: 1953. [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini R, Salvadori S, Rizzi A, Regoli D, Calo G. Neurobiology, Pharmacology, and Medicinal Chemistry of Neuropeptide S and Its Receptor. Med Res Rev. 2010;30:751–777. doi: 10.1002/med.20180. [DOI] [PubMed] [Google Scholar]
- Haller J, Alicki M. Current animal models of anxiety, anxiety disorders, and anxiolytic drugs. Current opinion in psychiatry. 2012;25:59–64. doi: 10.1097/YCO.0b013e32834de34f. [DOI] [PubMed] [Google Scholar]
- Han RW, Xu HJ, Zhang RS, Wang P, Chang M, Peng YL, Deng KY, Wang R. Neuropeptide S interacts with the basolateral amygdala noradrenergic system in facilitating object recognition memory consolidation. Neurobiology of learning and memory. 2014;107:32–36. doi: 10.1016/j.nlm.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Heredia L, Torrente M, Colomina MT, Domingo JL. Assessing anxiety in C57BL/6J mice: A pharmacological characterization of the open-field and light/dark tests. Journal of pharmacological and toxicological methods. 2014;69:108–114. doi: 10.1016/j.vascn.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in neurosciences. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Heyman E, Gamelin FX, Goekint M, Piscitelli F, Roelands B, Leclair E, Di Marzo V, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans-Possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–851. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Stress hormone regulation: biological role and translation into therapy. Annual review of psychology. 2010;61:81–109. C101–111. doi: 10.1146/annurev.psych.093008.100321. [DOI] [PubMed] [Google Scholar]
- Ionescu IA, Dine J, Yen YC, Buell DR, Herrmann L, Holsboer F, Eder M, Landgraf R, Schmidt U. Intranasally administered neuropeptide S (NPS) exerts anxiolytic effects following internalization into NPS receptor-expressing neurons. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1323–1337. doi: 10.1038/npp.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Ruscio AM, Shear K, Wittchen HU. Epidemiology of anxiety disorders. Current topics in behavioral neurosciences. 2010;2:21–35. [PubMed] [Google Scholar]
- Klauke B, Deckert J, Zwanzger P, Baumann C, Arolt V, Pauli P, Reif A, Domschke K. Neuropeptide S receptor gene (NPSR) and life events: G x E effects on anxiety sensitivity and its subdimensions. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2014;15:17–25. doi: 10.3109/15622975.2011.646302. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta physiologica Scandinavica. Supplementum. 1997;640:69–72. [PubMed] [Google Scholar]
- Koolhaas JM, Everts H, de Ruiter AJ, de Boer SF, Bohus B. Coping with stress in rats and mice: differential peptidergic modulation of the amygdala-lateral septum complex. Progress in brain research. 1998;119:437–448. doi: 10.1016/s0079-6123(08)61586-1. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nature neuroscience. 2013;16:332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MS, Peng YL, Jiang JH, Xue HX, Wang P, Zhang PJ, Han RW, Chang M, Wang R. Neuropeptide S Increases locomotion activity through corticotropin-releasing factor receptor 1 in substantia nigra of mice. Peptides. 2015;71:196–201. doi: 10.1016/j.peptides.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Melancon MO, Lorrain D, Dionne IJ. Changes in markers of brain serotonin activity in response to chronic exercise in senior men. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2014;39:1250–1256. doi: 10.1139/apnm-2014-0092. [DOI] [PubMed] [Google Scholar]
- Mohler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Lacagnina MJ, Fanous S, Wang J, Hammer RP., Jr. Intermittent social defeat stress enhances mesocorticolimbic DeltaFosB/BDNF co-expression and persistently activates corticotegmental neurons: implication for vulnerability to psychostimulants. Neuroscience. 2012;212:38–48. doi: 10.1016/j.neuroscience.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neuroscience and biobehavioral reviews. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulga A, Ruzza C, Rizzi A, Guerrini R, Calo G. Anxiolytic- and panicolytic-like effects of Neuropeptide S in the mouse elevated T-maze. The European journal of neuroscience. 2012;36:3531–3537. doi: 10.1111/j.1460-9568.2012.08265.x. [DOI] [PubMed] [Google Scholar]
- Reus GZ, Dos Santos MA, Abelaira HM, Quevedo J. Animal models of social anxiety disorder and their validity criteria. Life sciences. 2014;114:1–3. doi: 10.1016/j.lfs.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Robertson JM, Prince MA, Achua JK, Carpenter RE, Arendt DH, Smith JP, Summers TL, Summers TR, Blanchard DC, Summers CH. Nuance and behavioral cogency: How the Visible Burrow System inspired the Stress-Alternatives Model and conceptualization of the continuum of anxiety. Physiology & behavior. 2015;146:86–97. doi: 10.1016/j.physbeh.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Rada P, Pieruzzini PR, Hsueh B, Gould E. Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:7770–7777. doi: 10.1523/JNEUROSCI.5352-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheynin J, Beck KD, Servatius RJ, Myers CE. Acquisition and extinction of human avoidance behavior: attenuating effect of safety signals and associations with anxiety vulnerabilities. Front Behav Neurosci. 2014;8:323. doi: 10.3389/fnbeh.2014.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, Achua JK, Summers TR, Ronan PJ, Summers CH. Neuropeptide S and BDNF gene expression in the amygdala are influenced by social decision-making under stress. Front Behav Neurosci. 2014;8:121, 1–13. doi: 10.3389/fnbeh.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CH, Forster GL, Korzan WJ, Watt MJ, Larson ET, Overli O, Hoglund E, Ronan PJ, Summers TR, Renner KJ, Greenberg N. Dynamics and mechanics of social rank reversal. Journal of comparative physiology. A, Neuroethology, sensory, neural, and behavioral physiology. 2005;191:241–252. doi: 10.1007/s00359-004-0554-z. [DOI] [PubMed] [Google Scholar]
- Toth I, Neumann ID. Animal models of social avoidance and social fear. Cell and tissue research. 2013;354:107–118. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J, Ma L, Zhang TY, Yu H, Wang Y, Kong L, Chen ZY. Involvement of BDNF signaling transmission from basolateral amygdala to infralimbic prefrontal cortex in conditioned taste aversion extinction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:7302–7313. doi: 10.1523/JNEUROSCI.5030-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. The Journal of comparative neurology. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- You C, Zhang H, Sakharkar AJ, Teppen T, Pandey SC. Reversal of deficits in dendritic spines, BDNF and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014;17:313–322. doi: 10.1017/S1461145713001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain research. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.