Abstract
Context
X-linked adrenal hypoplasia congenita (AHC) is a rare but important cause of primary adrenal insufficiency and can be associated with significant morbidity and mortality. AHC is caused by mutations within the NROB1 gene that codes for the DAX-1 protein, an orphan nuclear receptor essential for the development of the hypothalamic-pituitary-adrenal axis. Affected individuals typically present in early infancy with adrenal insufficiency and growth is usually normal once medical therapy is instituted. Here we report the first case of growth hormone deficiency in an infant with AHC and a novel NROB1 missense mutation.
Case
A two-week old infant presented with salt-losing adrenal crises and a normal newborn screen. Tests of adrenal function confirmed adrenal hypoplasia congenita and molecular evaluation revealed a novel missense NROB1 mutation. Replacement steroid therapy was promptly initiated, but he subsequently developed growth failure despite optimal nutritional and medical steroid therapy. Further biochemical analyses confirmed isolated idiopathic growth hormone deficiency.
Conclusions
Growth failure in adequately treated infants with adrenal hypoplasia congenita is rare and the role of DAX-1 in the development of pituitary somatotropes is not known. There is variable genotype-phenotype correlation in X-linked adrenal hypoplasia congenita but novel NROB1 missense mutations could offer insight into the function of the various DAX-1 ligand-binding domains.
Keywords: Adrenal Hypoplasia Congenital, Dax-1, NROB1, Missense Mutations, Adrenal Gonadal Disorders, Growth Hormone Deficiency
Introduction
X-linked adrenal hypoplasia congenita (AHC) is a rare cause of primary adrenal insufficiency in infancy and childhood. AHC is caused by a loss of function mutation of the NROB1 (nuclear receptor subfamily 0, group B, member 1) gene that is located on the short arm of chromosome Xp21.3. NROB1 encodes for the nuclear receptor protein, DAX-1 (dosage-sensitive sex-reversal, adrenal hypoplasia congenita, on the X-chromosome, gene 1), which is expressed in the hypothalamus, pituitary gland, urogenital ridge, fetal adrenal gland and the adult adrenal cortex and is hypothesized to be instrumental in the development and function of the hypothalamic-pituitary-adrenal axis[1].
AHC usually occurs as a result of mutations or deletions of NROB1 or as a part of a contiguous deletion of a large segment on the X-chromosome carrying the NROB1, Dystrophyn (DMD) and Glycerol Kinase (GK) genes-thus resulting in a syndrome of adrenal insufficiency hypogonadotropic hypogonadism, Duchenne muscular dystrophy and glycerol kinase deficiency. Genotype-phenotype correlation in isolated AHC is variable and patients with frame shift and missense mutations can present with severe clinical disease [2]. Affected males typically develop salt-wasting crisis within the first few weeks of life or during childhood[3,4]. In contrast, growth failure in infants with isolated AHC who are on replacement therapy is uncommon and poor growth is more likely seen in individuals with glycerol kinase deficiency or contiguous gene deletion syndrome [5]. Isolated growth hormone deficiency is similarly rare with only a few case reports documented in the literature. This case highlights the presentation of AHC in an infant with a novel NROB1 missense mutation and is the first case report of growth hormone deficiency in an infant with AHC.
Case Report
A 12-day-old male infant presented to Texas Children's Hospital with recurrent vomiting, lethargy weight loss and unconjugated hyperbilirubinemia. He was born at term weighing 3.2kg following an uncomplicated pregnancy and delivery. Family history was negative for fetal or infantile demise and he has a healthy 4 year old female sibling. Physical examination was remarkable for 8% weight loss, jaundice and decreased tone. He had hyponatremia and mild hyperkalemia with the following electrolyte concentrations: sodium 120 mmol/L, potassium 6.5 mmol/L, chloride 85 mmol/L, carbon dioxide 23 mmol/L, urea nitrogen 21mg/dl (7.5 mmol/L), and creatinine 0.46 mg/dl (35.08 mol/L). Investigations of adrenal function revealed a low baseline Cortisol level with an elevated adrenocorticotropic hormone level (ACTH), elevated plasma renin activity (Table 1), an undetectable aldosterone level and a flat response to high dose Cosyntropin stimulation (Table 2). Adrenal ultrasonography identified two small glands for age. Primary adrenal insufficiency was diagnosed and he was treated with glucocorticoid, mineralocorticoid and salt supplementation at 2 weeks of life with resolution of symptoms and normalization of plasma renin activity and ACTH level by 2 months of age (Table 1).
Table 1.
Markers of adrenal function at baseline and after 2 months of treatment.
| Test | Baseline | Post-treatment | Normal Range |
|---|---|---|---|
| ACTH (pg/ml) | 1761 | 47.4 | 6-48 |
| Plasma renin activity (ng/ml/hr) | 53.4 | 27.7 | 2-35 |
| 11-desoxycortisol (ng/dl) | 1092 | <10 | 10-156 |
Table 2.
Investigation of adrenal function at baseline and after Co-syntropin stimulation.
| Test | Baseline | Stimulated | Normal Range |
|---|---|---|---|
| Cortisol | 2900 | 2900 | >18,000 |
| Deoxy-corticosterone | 34 | 36 | 7-49 |
| 11 -desoxycortisol | 1092 | 1126 | <10-156 |
| 17OH pregnenolone | 39 | 42 | 36-763 |
| Progesterone | <10 | <10 | <10-15 |
| 17OH progesterone | 101 | 133 | <91 |
| Androstenedione | 56 | 60 | <10-37 |
| DHEA | 29 | <20 | <20 |
| Testosterone | 124 | 189 | 75-400 |
Values expressed in ng/dl
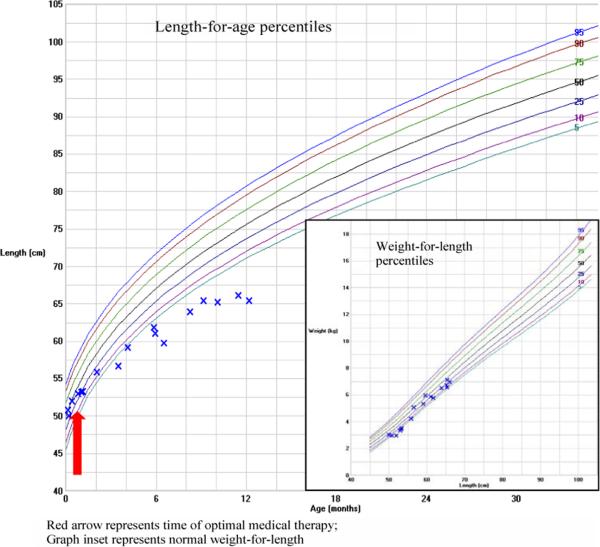
Growth failure was noted at 4 months of age with poor linear growth despite normal weight for length and optimization of nutrition and medical therapy-hydrocortisone 7mg/m2/day and fludrocortisone O.lmg daily (Figure 1). Biochemical evaluation of growth factors at 9 months age showed low insulin-like growth factor 1 (IGF-1) and insulin-like growth factor binding protein 3 (IGFBP-3) concentrations for age, as well as a low peak stimulated growth hormone concentration after glucagon stimulation (Table 3). Skeletal survey was unremarkable without evidence of metaphyseal dysplasia; magnetic resonance imaging of the brain revealed normal appearing pituitary gland and sella turcica. Growth hormone deficiency was diagnosed and growth hormone therapy initiated at a dose of 0.2 mg/kg/week.
Figure 1.
Growth chart illustrating decreased height velocity at 4 months of age
Table 3.
Growth Evaluation at 9 months of age.
| Test | Value | Reference Range |
|---|---|---|
| IGF-1 | 36 | 55-327 ng/dl |
| IGFBP-3 | 1.7 | 0.7-3.6 mg/L |
| Stimulated GH* | ≥ 10 ng/ml | |
| • Baseline | 1.3 | |
| • 45 minutes | 1.1 | |
| • 90 minutes | 5.8 | |
| • 120 minutes | 4.0 | |
| • 150 minutes | 3.3 | |
| • 180 minutes | 4.3 |
Growth hormone concentrations after 0.03mg/kg glucagon stimulation test
Data expressed in conventional units
IGF-1 – insulin-like growth factor 1
IGFBP-3 – insulin like growth factor binding protein 3
GH – growth hormone
Molecular diagnostic evaluation included a chromosomal microarray, which showed a copy number loss of chromosome band 10q21.1, but no deletion of the Xp22-p21 region that is seen with contiguous gene deletion syndrome. Subsequent DNA sequencing analysis of NROBldetected a sequence variant nucleotide change of c.1094T>C with an amino acid change of p.Leu365Pro. The patient's mother was heterozygous for this mutation.
Discussion
We report a novel missense mutation of NROB1 in a patient whose clinical phenotype is consistent with AHC. This infant was subsequently noted to have poor linear growth and this is the first reported case of growth hormone deficiency in an infant with NROB1 mutation. X-linked adrenal hypoplasia congenita has a broad clinical spectrum with over 60% of boys presenting with life-threatening adrenal crisis in the first month of life [3,4]. As in our patient, diagnosis can be delayed for 1-2 weeks after birth because of the lack of physical features, the normal newborn screen and variable clinical presentation as some boys have sufficient adrenal reserve early in life [6]. A high degree of clinical suspicion is necessary to ensure prompt diagnosis of AHC, especially since hyperkalemia is only present in up to 50% of cases of salt-losing adrenal crises at initial presentation [7]. In our patient, the absence of marked hyperkalemia and the rapid normalization of his mild elevation in plasma potassium concentration with rehydration highlights the need for high degree of clinical suspicion when interpreting laboratory values in patients with clinical evidence of adrenal insufficiency. Also of note is that our patient's clinical course was not complicated by other hallmark features of primary adrenal insufficiency such as hypoglycemia, acidosis or hyperpigmentation.
We propose that this novel mutation is the likely cause of AHC disease as it is expected to result in a change in protein structure and function in a child with the characteristic phenotype. The DNA change in this patient results in a leucine residue substitution with a proline in one of the helices of the ligand-binding domain. The proline cyclical ring structure tends to be inflexible, thus disrupting secondary structures—particularly alpha helices—and it is also highly hydrophilic, unlike the hydrophobic leucine. This residue is highly conserved among several mammalian species and is within the second of three known mutation clusters in the critical ligand-binding domain thought to be essential to the DAX-1 function of transcriptional repression [8]. Polyphen-2 analysis, a software program designed to predict functional effects of human nsSNPs, yielded two scores of confidence. The HumDiv analysis showed that the mutation is probably damaging with a score 0.979 (sensitivity: 0.74; specificity 0.96) while the more stringent HumVar predicted the mutation to be possibly damaging with a score of 0.791(sensitivity: 0.74; specificity 0.87). A query of the Human Genome mutation database revealed this DNA change has not been previously reported. In addition, less than 20% of the mutations within the coding region for DAX-1 are missense mutations yet these rearrangements help to define important domains and determine DAX-1 protein function [8].
Interestingly, the decline in growth velocity observed in our patient after resolution of his acute clinical illness, and while he was receiving adequate nutrition and optimal medical therapy with glucocorticoid and mineralocorticoid replacementhas not been previously reported. Short final height has infrequently been reported in children with AHC although the exact cause of short stature has not been fully elucidated [9-11]. The cause of the short stature in these cases was unclear but was presumed to be linked to underlying pituitary dysfunction. Specifically in one case, the clinical picture was compounded by the presence of traumatic brain injury in infancy and an abnormally small pituitary gland [9].
Growth hormone deficiency could be an important contributing factor to short stature in patients with AHC and was recently reported in two children with NROB1 mutations [10, 11]. Both children were normal weight at birth but subsequently presented in adolescence (age 13 and 18years) with declining growth velocity, delayed bone age and suboptimal response to growth hormone evaluation. In addition, after initiating recombinant growth hormone therapy, the children were diagnosed with hypothyroidism and started on L-thyroxine therapy.
Growth hormone deficiency is not a characteristic feature of patients with AHC, but our case and others highlight that it should be an important consideration in any child with poor growth. The mechanism of growth hormone deficiency in AHC is unknown, but could be associated with an underlying defect in pituitary development. Under-development of pituitary gonadotropes resulting in hypogonadotropic hypogonadism in patients with AHC is well established and maybe secondary to decreased DAX-1 expression within the pituitary gonadotropes. Decreased DAX-1 expression within the ventromedial hypothalamic nucleus and pituitary gonadotropes is hypothesized to result in the well-known association of hypogonadotropic hypogonadism with AHC [12]. However, it is unknown whether pituitary somatotropes specifically respond to DAX-1 and if this transcriptional repressor is instrumental in their development. Since DAX-1 is expressed in the hypothalamus and pituitary early in development, it could influence other pituitary cell differentiation and maturation [13]. Further histochemical and molecular analysis would be needed to elucidate the role of the DAX-1 protein in the development of the growth hormone axis.
Conclusion
This is the first case of documented growth hormone deficiency in an infant with AHC who had a novel missense NROB1 mutation and was on optimal nutrition and steroid replacement therapy. This novel missense mutation, located within the NROB1 carboxy-terminal, is likely disease-causing and should be considered when defining mutations associated with AHC. Growth should be closely monitored in infants with AHC and growth hormonal evaluation considered if growth failure arises despite optimization of therapy.
Acknowledgements
We thank the subject's parents for giving permission to discuss this case. STC is currently supported by the intramural department of the National Institutes of Health.
Abbreviations
- ACTH
Adrenocorticotropic hormone level
- AHC
X-linked adrenal hypoplasia congenital
- DAX-1
Dosage-sensitive sex-reversal, adrenal hypoplasia congenita, onthe X-chromosome, gene 1
- IGF-1
Insulin-like growth factor 1
- IGFBP-3
Insulin-like growth factor binding protein 3
References
- 1.Achermann JC, Meeks JJ, Jameson JL. Phenotypic spectrum of mutations in DAX-1 and SF-1. Molecular and cellular endocrinology. 2001;185(1-2):17–25. doi: 10.1016/s0303-7207(01)00619-0. [DOI] [PubMed] [Google Scholar]
- 2.Lin L, Gu WX, Ozisik G, To WS, Owen CJ, et al. Analysis of DAX1 (NROB1) and steroidogenic factor-1 (NR5A1) in children and adults with primary adrenal failure: Ten years’ experience. J Clin Endocr Metab. 2006;91(8):3048–3054. doi: 10.1210/jc.2006-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reutens AT, Achermann JC, Ito M, Ito M, Gu WX, Habiby RL, et al. Clinical and functional effects of mutations in the DAX-1 gene in patients with adrenal hypoplasia congenita. J Clin Endocr Metab. 1999;84(2):504–511. doi: 10.1210/jcem.84.2.5468. [DOI] [PubMed] [Google Scholar]
- 4.Peter M, Viemann M, Partsch CJ, Sippell WG. Congenital adrenal hypoplasia: Clinical spectrum, experience with hormonal diagnosis, and report on new point mutations of the DAX-1 gene. J Clin Endocr Metab. 1998;83(8):2666–2674. doi: 10.1210/jcem.83.8.5027. [DOI] [PubMed] [Google Scholar]
- 5.Savage MO, Lebrethon MC, Blair JC, Ho JTF, Johnston LB, et al. Growth abnormalities associated with adrenal disorders and their management. Horm Res. 2001;56(Suppl 1):19–23. doi: 10.1159/000048129. [DOI] [PubMed] [Google Scholar]
- 6.Achermann JC, Silverman BL, Habiby RL, Jameson JL. Presymptomatic diagnosis of X-linked adrenal hypoplasia congenita by analysis of DAX1. Journal of Pediatrics. 2000;137(6):878–881. doi: 10.1067/mpd.2000.108567. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh S, White PC. Presentation of Primary Adrenal Insufficiency in Childhood. J Clin Endocr Metab. 2011;96(6):E925–E928. doi: 10.1210/jc.2011-0015. [DOI] [PubMed] [Google Scholar]
- 8.Achermann JC, Ito M, Silverman BL, Habiby RL, Pang S, et al. Missense mutations cluster within the carboxyl-terminal region of DAX-1 and impair transcriptional repression. J Clin Endocr Metab. 2001;86(7):3171–3175. doi: 10.1210/jcem.86.7.7660. [DOI] [PubMed] [Google Scholar]
- 9.Engiz O, Ozon A, Riepe F, Alikasifoglu A, Gone N, et al. Growth hormone deficiency due to traumatic brain injury in a patient with X-linked congenital adrenal hypoplasia. Turkish J Pediatr. 2010;52(3):312–316. [PubMed] [Google Scholar]
- 10.Pérez Rodríguez O, Ruibal Francisco JL, Loidi Fernández de Trocóniz L, Parajes Castro S, Martín Rojas-Marcos P. [Gene as a cause of adrenal hypoplasia, hypogonadism and short height novel mutation of DAX-1 gene (pGly168fsX17)]. Anales de pediatría. 2006;64(6):591–594. doi: 10.1157/13089927. [DOI] [PubMed] [Google Scholar]
- 11.Calliari LEP, Rocha M, Monte O, Longui C. Mild adrenal insufficiency due to a NROB1 (DAX1) gene mutation in a boy presenting an association of hypogonadotropic hypogonadism, reduced final height and attention deficit disorder. Arquivos brasileiros de endocrinologia emetabologia. 2013;57(7):562–565. doi: 10.1590/s0004-27302013000700011. [DOI] [PubMed] [Google Scholar]
- 12.Jadhav U, Harris RM, Jameson JL. Hypogonadotropic hypogonadism in subjects with DAX1 mutations. Molecular and cellular endocrinology. 2011;346(1-2):65–73. doi: 10.1016/j.mce.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer AK, McCabe ERB. Molecular mechanisms of DAX1 action. Mol Genet Metab. 2004;83(1-2):60–73. doi: 10.1016/j.ymgme.2004.07.018. [DOI] [PubMed] [Google Scholar]