Abstract
IMPORTANCE
Chemotherapy exposure is a known risk factor for cancer-related cognitive impairments. Anthracycline-based regimens are commonly used chemotherapies that have been shown to be associated with cognitive impairment and brain changes in clinical studies.
OBJECTIVE
To directly compare the effects of anthracycline and nonanthracycline regimens on cognitive status and functional brain connectivity.
DESIGN, SETTING, AND PARTICIPANTS
In this observational study, we retrospectively examined cognitive and resting state functional magnetic resonance imaging data acquired from 62 primary breast cancer survivors (mean [SD] age, 54.7 [8.5] years) who were more than 2 years off-therapy, on average. Twenty of these women received anthracycline-based chemotherapy as part of their primary treatment, 19 received nonanthracycline regimens, and 23 did not receive any chemotherapy. Participants were enrolled at a single academic institution (Stanford University) from 2008 to 2014, and the study analyses were performed at this time.
MAIN OUTCOMES AND MEASURES
Cognitive status was measured using standardized neuropsychological tests, and functional brain connectivity was evaluated using resting state functional magnetic resonance imaging with a focus on the brain’s default mode network.
RESULTS
The anthracycline group demonstrated significantly lower verbal memory performance including immediate recall (F = 3.73; P = .03) and delayed recall (F = 11.11; P < .001) as well as lower left precuneus connectivity (F = 7.48; P = .001) compared with the other 2 groups. Patient-reported outcomes related to cognitive dysfunction (F = 7.27; P = .002) and psychological distress (F = 5.64; P = .006) were similarly elevated in both chemotherapy groups compared with the non–chemotherapy-treated controls.
CONCLUSIONS AND RELEVANCE
These results suggest that anthracyclines may have greater negative effects than nonanthracycline regimens on particular cognitive domains and brain network connections. Both anthracycline and nonanthracycline regimens may have nonspecific effects on other cognitive domains as well as certain patient reported outcomes. Further research is needed to identify potential methods for protecting the brain against the effects of various chemotherapeutic agents.
Multiple studies have consistently demonstrated cognitive decline in chemotherapy treated vs non– chemotherapy-treated patients with breast cancer.1–5 These cognitive impairments can be long-lasting and tend to be one of the most common limitations to quality of life and well-being.6,7 There is growing concern that chemotherapy may increase the risk for later neurodegenerative conditions by altering or accelerating brain-aging processes.1,8–10 Accordingly, neuroimaging evidence suggests that breast cancer chemotherapy is associated with diffuse brain injury,11–13 including increased vulnerability of the brain network to neurodegeneration.14
These findings highlight the importance of determining factors that may help predict which patients are at highest risk for adverse cognitive outcome and/or identifying methods for protecting against cognitive effects. Despite chemotherapy treatment being a known risk factor for cognitive decline, very limited information exists regarding the differential cognitive effects of specific chemotherapy agents. Preclinical studies demonstrate direct and indirect neurotoxic effects of conventional breast cancer therapies, including doxorubicin, cisplatin, cyclophosphamide, and fluorouracil,15 but cannot specify whether one of these regimens is more cognitively toxic than others. Studies in humans have been very limited and suggest that regimens containing methotrexate are potentially more neurotoxic.6,16 However, methotrexate is no longer widely used to treat breast cancer.
Combination chemotherapy regimens that include anthracycline (ANTHR)-based agents (eg, doxorubicin) are very commonly and efficaciously used to treat breast cancer.17 ANTHR mechanisms of action include double-strand DNA breaks and free radical damage that affect healthy as well as cancerous cells.18 Clinical studies have demonstrated significant cognitive impairments and brain changes in patients with breast cancer treated exclusively with ANTHR-containing regimens19 as well as in cohorts in which most were treated with ANTHRs.3,12,14 To date, no studies have directly compared ANTHRs with other agents. Therefore, we retrospectively examined the differential effects of ANTHR and non-ANTHR chemotherapies on cognitive status and brain network connectivity in long-term breast cancer survivors.
Brain regions that show highly correlated functional magnetic resonance imaging (fMRI) signals across time are assumed to be connected in a functional brain network.20 We focused on the brain’s default mode network given previous studies1,21–23 suggesting that this network may be preferentially vulnerable to breast cancer chemotherapy. Default mode network includes precuneus, cingulate, medial frontal, middle temporal, and lateral parietal regions as well as the hippocampus and is believed to support implicit learning, monitoring, and allocation of neural resources to various cognitive processes.22,24,25 The default mode network is an intrinsic functional brain network, meaning that its constituent regions are spontaneously coactive, independent of external stimuli. Such spontaneous, intrinsic connectivity is measured during an extended, task-free “resting” state using fMRI. This resting-state fMRI method is highly sensitive to mechanisms of cognitive dysfunction and decline.26
Methods
Participants
In this observational study, we retrospectively examined cognitive and resting state functional magnetic resonance imaging data acquired from 62 primary breast cancer survivors (mean [SD] age, 54.7 [8.5] years). Of these, 39 women with a history of primary breast cancer (stage I-IIIA, diagnosed from 2003 to 2014) and chemotherapy treatment had completed their primary therapy (surgery, chemotherapy, and radiation) more than 6 months prior to study entry to allow for medical and neurologic stabilization. This chemotherapy group consisted of 20 women who received 4 to 8 cycles of standard-dose ANTHR-based regimens and 19 who received 4 to 8 cycles of standard-dose non-ANTHR regimens. They were compared with 23 non–chemotherapy-treated primary breast cancer survivors. Of the total sample, 41 data sets were included in our previous studies1,27 of intrinsic functional connectivity (all participants for this study were enrolled from 2008 to 2014, and the study and analyses were performed at this time). Participants were recruited using email listserv, Internet, and community flyer postings; local cancer support group advertisements; and physician referrals. All participants were enrolled and evaluated for this study at Stanford University, but, given that they were survivors recruited from the surrounding community, not all of them received their cancer diagnosis and/or treatment at Stanford.
Participants were excluded for evidence of active cancer (n = 0 patients); history of relapse (n = 2); diagnosed psychiatric, neurologic, or comorbid medical conditions that are known to affect cognitive function (n = 5); pregnancy (n = 0); MRI contraindications (n = 7); or major sensory deficits (n = 0). All women underwent surgery with general anesthesia, and some received locoregional breast radiation and/or endocrine therapy (ie, tamoxifen) as part of their treatment regimen (Table 1). ANTHR-based chemotherapy protocols were as follows: doxorubicin, cyclophosphamide, paclitaxel (n = 17 patients); doxorubicin and cyclophosphamide (n = 2); and doxorubicin, cyclophosphamide, and fluorouracil (n = 1). Non-ANTHR regimens were cyclophosphamide, paclitaxel (n = 15) and cyclophosphamide, methotrexate, fluorouracil (n = 4). All participants completed their entire recommended treatment regimen. This study was approved by the Stanford University institutional review board. All participants provided written informed consent and received compensation.
Table 1.
Demographic and Medical Data for 62 Patients
| Variable | Type of Treatment | Omnibus Statistic, F= χ2 | P Value | ||
|---|---|---|---|---|---|
| ANTHR (n = 20) |
Non-ANTHR (n = 19) |
No CT (n = 23) |
|||
| Age, mean (SD) [range], y | 52 (7.6) [36–65] | 53 (8.7) [42–72] | 58 (7.9) [41–71] | 4.39 | .02 |
| Education, mean (SD), y | 17 (2.9) | 17 (2.7) | 17 (2.4) | 0.273 | .76 |
| Chemotherapy cycles, mean (SD), No. | 6 (2.0) | 6 (1.6) | NA | 0.0 | >.99 |
| Breast radiation, % | 50 | 58 | 57 | 0.287 | .87 |
| Endocrine therapy (tamoxifen), % | 60 | 76 | 64 | 1.21 | .55 |
| Disease stage at diagnosis (I, II, III), % | 5, 75, 20 | 8, 76, 16 | 83, 17, 0 | 29.44 | <.001 |
| Time since primary treatment, mean (SD), ya | 2.2 (1.5) | 2.1 (1.6) | 2.8 (1.7) | 1.25 | .30 |
| Postmenopausal, % | 80 | 94 | 86 | 1.71 | .43 |
Abbreviations: ANTHR, anthracycline-based chemotherapy; CT, chemotherapy.
Time since completion of surgery, chemotherapy, and locoregional breast radiation treatments.
Cognitive Status
The Hopkins Verbal Learning Test–Revised (HVLT-R)28 was used to measure verbal memory in all participants. For executive function, participants were administered the Wisconsin Card Sorting Test (WCST)29 or Comprehensive Trail Making Test.30 For verbal fluency, participants were administered Delis-Kaplan Executive Function System Letter Fluency31 or Controlled Oral Word Association.32 We also administered the Behavioral Rating Inventory of Executive Function (BRIEF), a patient-reported measure of executive function,33 and the Clinical Assessment of Depression, a patient-reported measure of depression, anxiety, and fatigue,34 to all participants. Participants received different executive function and verbal fluency tests because the original testing battery was modified to conform to the International Cognition and Cancer Task Force recommendations.35 Testing required approximately 1.5 hours for all participants. There was no difference between the groups in the proportion of participants who received one test battery or the other (P = .30).
Neuroimaging Acquisitions and Preprocessing
Resting-state fMRI and high-resolution anatomic MRI data were obtained using a GE Discovery MR750 3.0 T whole-body scanner (GE Medical Systems) on the same day as the cognitive testing session.
All neuroimaging acquisitions were examined visually, and any suspicious scans were forwarded to a neuroradiologist for clinical review (no scans were found to have neurologic abnormalities). Image preprocessing was performed using Statistical Parametric Mapping 12 (Wellcome Trust Centre) as described in our previous publications1,27,36,37 (see the eMethods in the Supplement for further details).
Default Mode Network Connectivity
To define default mode network, we used the same 19 independent regions of interest (ROIs) that we used in our previous default mode network study involving breast cancer chemotherapy.1 These ROIs were originally defined by Shirer and colleagues.38 Functional connectivity analysis was performed using the CONN Toolbox39 as described in our previous publications.1,27,36 Pearson correlation coefficients were calculated between fMRI time courses for each pair of ROIs and then normalized using Fisher R-Z transformation (see the eMethods in the Supplement for further details).
Statistical Analyses
Group differences in cognitive testing scores were examined using analysis of variance in the R statistical package (R Foundation). Significant (P < .05) omnibus results were further evaluated using pairwise t tests (pooled standard deviation) with Bonferroni correction. Age was included as a covariate in all analyses. “Test” was also included to control for potential confounds related to the different testing batteries. Effect sizes were calculated for pairwise comparisons using Cohen d.
Pairwise between group differences in default mode network connectivity were examined using the general linear model within CONN Toolbox.40 Age was included as a covariate in all analyses, and the α level was set at P < .05 with false discovery rate correction for multiple comparisons. Effect sizes were calculated using Cohen d.
Disease stage was significantly lower in the no-chemotherapy group compared with the chemotherapy groups. We examined the statistical models both with and without including stage as a covariate given that lower disease severity is an expected characteristic of patients who do not receive chemotherapy and may therefore remove the main effect of interest. We also explored the effect of number of chemotherapy cycles and endocrine therapy on cognitive status and default mode network connectivity via 2-tailed, Spearman correlations.
Results
Cognitive Status
As shown in Table 2, and the eFigure in the Supplement, the ANTHR group demonstrated lower verbal memory performance compared with the other 2 groups, especially during a delayed recall trial (P < .001). The 2 chemotherapy groups were relatively similar in terms of patient-reported executive function difficulties and psychological symptoms, but both demonstrated significantly greater complaints in these areas compared with the no-chemotherapy group. Executive function and verbal fluency were not different with respect to chemotherapy type although the no-chemotherapy group showed moderately higher executive function (F = 3.19, P = .08) performance compared with the combined chemotherapy group (ANTHR and non-ANTHR). Cognitive results remained significant with the inclusion of disease stage as a covariate although stage did not contribute to the models (P > .65). Therefore, results are reported from models excluding disease stage. Cognitive status was not associated with number of chemotherapy cycles (P > .50) or endocrine therapy (P > .63).
Table 2.
Neuropsychological Data for 62 Patients
| Measure, Mean (SD), | Patients in Treatment Group | F | P Value | ||
|---|---|---|---|---|---|
| ANTHR (n = 20) |
Non-ANTHR (n = 19) |
No Chemotherapy (n = 23) |
|||
| HVLT-R | |||||
| Total recall | 50 (6.5) | 55 (11.0) | 57 (5.0) | 3.73 | .03a |
| Delayed recall | 48 (5.8) | 56 (7.5) | 56 (4.9) | 11.11 | <.001a |
| Executive function | 49 (9.2) | 49 (11.8) | 53 (8.6) | 1.56 | .22 |
| Verbal fluency | 53 (10.4) | 53 (8.2) | 57 (8.6) | 0.233 | .79 |
| BRIEF | 60 (12.0) | 59 (9.4) | 49 (8.1) | 7.27 | .002b |
| CAD | 52 (11.0) | 52 (10.0) | 42 (10.0) | 5.64 | .006b |
Abbreviations: ANTHR, anthracycline-based chemotherapy; BRIEF, Behavioral Rating Inventory of Executive Function; CAD, Clinical Assessment of Depression; HVLT-R, Hopkins Verbal Learning Test—Revised.
See eFigure in the Supplement for pairwise comparisons.
Higher scores on the BRIEF or CAD indicate elevated symptoms, for all other cognitive measures, higher scores indicate better performance.
Default Mode Network Connectivity
As shown in the Figure, the ANTHR group demonstrated significantly lower left precuneus connectivity compared with the non-ANTHR and no-chemotherapy groups. Specifically, the left precuneus had lower connectivity with the left lateral parietal, left medial frontal, right hippocampal, bilateral middle cingulate, and right superior frontal gyrus. Compared with the no-chemotherapy group, the non-ANTHR group demonstrated lower connectivity between the left precuneus and right middle central gyrus. Because Conn Toolbox can conduct only pairwise comparisons, we confirmed these results post hoc by computing the mean connectivity (based on ROI-to-ROIz score) for the left precuneus with these regions for each participant. We then compared mean left precuneus connectivity between groups using analysis of variance, covaried for age, in R. The omnibus F statistic was significant (F= 7.48, P = .001), and pairwise t tests with Bonferroni correction were consistent with the Conn Toolbox results. Connectivity results remained unchanged (P = .001) with the inclusion of disease stage as a covariate, although stage was not a significant contributor to the model (P = .83). Therefore, results are reported for the model excluding disease stage. Mean left precuneus connectivity was not associated with number of chemotherapy cycles (P = .71) or endocrine therapy (P = .93).
Figure. Default Mode Network Connectivity.
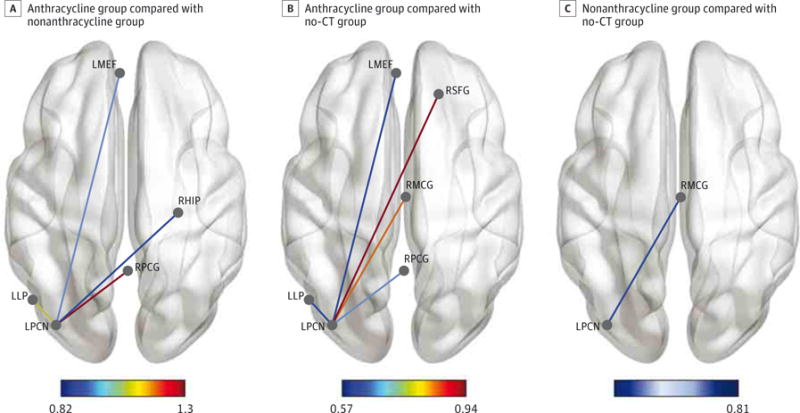
A, The group treated with anthracycline demonstrated significantly lower intrinsic functional connectivity between the left precuneus (LPCN) and several other default mode network regions compared with the nonanthracycline group. B, The anthracycline group also showed lower connectivity in these regions compared with the no-chemotherapy (no-CT) group. C, The nonanthracycline group showed lower connectivity between LPCN and right middle cingulate (RMCG) compared with the no-chemotherapy group. Color bars indicate effect size; LMEF, left medial frontal; LLP, left lateral parietal; RHIP, right hippocampus; RPCG, right posterior cingulate; RSFG, right superior frontal gyrus. Connectivity data are shown overlaid on a smoothed Montreal Neurological Institute space anatomic template using BrainNet Viewer.75
Discussion
Cancer-related cognitive impairment is often referred to as “chemobrain.” While chemotherapy treatment is a known risk factor for cognitive deficits among patients with breast and other cancers, the differential effects of specific chemotherapeutic agents are unclear. We aimed to evaluate the effects of ANTHR-based regimens on cognitive status and default mode network connectivity in breast cancer survivors. Using standardized neuropsychological tests and resting state fMRI, we demonstrated significantly lower verbal memory performance and left precuneus connectivity in participants who received ANTHR regimens compared with those who received non-ANTHR regimens and to participants who did not receive chemotherapy. To our knowledge, this represents the first clinical evidence that ANTHR regimens may be more neurotoxic than non-ANTHR regimens.
The ANTHR group showed relatively lower verbal memory performance on both the Total Recall and Delayed Recall conditions of the HVLT-R. Total Recall is a measure of rote encoding of new information, whereas Delayed Recall assesses retention of learned material. Impairment on these same measures has been consistently observed following breast cancer chemotherapy.14,41,42 Both chemotherapy groups demonstrated lower executive function and increased psychological distress and fatigue compared with the no-chemotherapy group. These results suggest that ANTHRs may be more toxic for brain systems involved in verbal memory, whereas ANTHR and non-ANTHR regimens are associated with similar negative effects on other cognitive-behavioral domains. Replication and refinement of these results may assist clinicians in providing more specific information to patients regarding potential adverse effects of various treatment approaches.
We found that left precuneus connections with the frontal, hippocampal, and lateral parietal regions were the most affected by ANTHR chemotherapy. Disrupted intrinsic connectivity decreases the efficiency of information processing and reduces the brain network’s capacity for dynamic functional response. The precuneus is involved in several cognitive functions, including memory,43 and disrupted connections between the precuneus and frontal-hippocampal regions would particularly affect memory function. Our findings are consistent with those of previous studies that also demonstrated abnormalities in the precuneus, hippocampus, frontal, and parietal regions following exclusively or primarily AN-THR-based treatment regimens.12,19,41,44 These are “hub” regions—areas that participate in a large number of functional interactions.45 This centrality to brain network organization is associated with significantly high metabolic demands.46 Therefore, ANTHR mechanisms may disrupt metabolic resources to a greater extent than other chemotherapies, perhaps via comparatively increased mitochondrial dysfunction.47
Decreased default mode network connectivity is not specific to chemotherapy exposure. Alteration of the default mode network is an important biomarker of several conditions, particularly age-related neurodegeneration.48,49 Therefore, ANTHR mechanisms may exacerbate neurotoxic physiologic cascades that are generally involved in disease and aging. Sanoff and colleagues50 demonstrated significantly elevated molecular markers of aging in patients with breast cancer following ANTHR chemotherapy. However, it is currently unknown if these markers are elevated to a different extent by non-ANTHR regimens.
Doxorubicin has been shown in animal models to increase the release of proinflammatory cytokines causing increased neuroinflammation,51,52 and ANTHRs are more cytokine-inducing in patients with breast cancer than non-ANTHRs.53 Several studies50,54,55 have implicated cytokine-mediated neuroinflammation in cognitive impairment and brain injury among breast cancer survivors treated with ANTHR-containing regimens. However, inflammation and associated cognitive deficits are also present prior to initiation of adjuvant therapies.56 Therefore, further investigation is required regarding the relationship among ANTHR agents, inflammation, and brain injury.
ANTHR agents produce reactive oxygen species that result in oxidative stress,52 a state of reduced capacity for detoxification that has been associated with tissue damage, including neurodegeneration.57 Conroy et al58 demonstrated an association between oxidative stress, direct DNA damage, and brain injury in a cohort of breast cancer survivors, 83% of whom had been treated with doxorubicin. Amyloid β accumulation is toxic effect related to oxidative stress (and inflammation) that represents a critical molecular pathology in neurode-generation.59,60 Default mode network is preferentially vulnerable to amyloid toxicity.61 A potential line of inquiry for future research concerns the differential effects of various chemotherapy agents on amyloid accumulation in the brain. It is also unknown if ANTHRs result in more oxidative stress and/or DNA damage compared with non-ANTHR agents.
ANTHRs are known to cause cardiovascular abnormalities that could have a negative impact on brain function. Increased incidence of cerebral microbleeds has been observed in breast cancer survivors who were treated with non-ANTHR chemotherapies62 but this abnormality has not been examined in association with ANTHR regimens. Participants in the present study were excluded for any known cardiovascular conditions as well as for visible MRI abnormalities. However, microinfarctions are vascular abnormalities believed to play a role in cognitive impairment and decline but are undetectable by conventional neuroimaging.63 Further research is required regarding the potential role of cerebrovascular disease in ANTHR-related cognitive impairment.
ANTHR regimens in this study included non-ANTHR agents (cyclophosphamide, paclitaxel, and fluorouracil) that have also been shown to damage neural progenitor cells, increase neuroinflammation, and induce oxidative stress.53,64 However, the non-ANTHR regimens included these same agents (as well as methotrexate). Although the non-ANTHR group showed higher verbal memory performance and default mode network connectivity than the ANTHR group, comparison with no-chemotherapy controls suggested that non-ANTHR agents are also associated with some neurophysiologic injury, albeit to a lesser degree. Doxorubicin, paclitaxel, cyclophosphamide, and fluorouracil all result in similar suppression of neural proliferation, irrespective of “direct” brain access.65 Agents that are classified as being unable to actively cross the blood-brain barrier, such as doxorubicin, are not completely excluded from the brain but have been shown to be present in low concentrations following intravenous administration.66,67 Dietrich and colleagues68 suggested that even small amounts of chemotherapeutic agents can have clinically significant negative effects on neuroplasticity. However, their study did not include doxorubicin, and therefore additional research is needed to determine if ANTHRs result in greater suppression of neural stem cell division and/or death compared with other agents of similar concentrations.
Although disease stage was significantly lower in the no-chemotherapy group compared with the chemotherapy groups, its inclusion in statistical models did not change our results. It also did not contribute to the models, suggesting that it was not significantly associated with the outcomes of interest. In addition, disease stage would not account for the group differences in performance between the ANTHR and non-ANTHR groups because these did not differ in disease stage. Previous studies3,21,69 have suggested that disease stage is correlated with certain cognitive and neurobiologic measures, whereas other studies19,44 have shown no such association. As noted herein, we believe, based on previous studies, that the default mode network may be less sensitive to disease severity while being preferentially vulnerable to chemotherapy. The present results provide further support for this hypothesis. Continued research regarding these differential brain network vulnerabilities to specific aspects of cancer and its treatments will be crucial for developing syndrome-specific interventions.
We did not observe an association between number of chemotherapy cycles and cognitive performance or default mode network connectivity. Previous studies have suggested greater cognitive and neurobiologic deficits in patients with breast cancer who received high-dose therapy compared with standard-dose chemotherapy.70,71 However, our sample included only women treated with standard-dose regimens, and therefore further research is required to determine dose-response associations of ANTHR regimens. We also did not find an effect on endocrine therapy on cognitive performance or default mode network connectivity, although there may have been too few women who did not receive this therapy to adequately power this analysis.
Conclusions
These results should be considered preliminary given the study limitations of small sample size and retrospective, cross-sectional design. Larger, prospective studies are needed that include pretreatment and posttreatment assessments so that patients’ individual cognitive and neurobiologic trajectories can be evaluated with respect to potential ANTHR-related neurotoxic effects. Continued research regarding the mechanisms by which ANTHRs disrupt neurocircuitry could help identify interventions that will protect against ANTHR-associated neurotoxic effects without reducing the anticancer efficacy of these regimens. For example, certain antioxidants may protect against ANTHR-induced mitochondrial reactive oxygen species,72 whereas others show promise for preventing ANTHR-induced neuroinflammation.73 Preclinical studies are needed to confirm that small amounts of ANTHR agents are able to penetrate the blood-brain barrier and to examine the effects of such concentrations on neural cells compared with other chemotherapies. Animal studies could also examine the effects of potential protective agents on chemotherapy-related cognitive dysfunction as well as disrupted default mode network connectivity given that this network seems to be preserved across species.74
Supplementary Material
At a Glance.
Breast cancer chemotherapy is often associated with persistent cognitive problems that reduce quality of life. It is unclear whether certain regimens are associated with greater cognitive difficulties than others.
Using standardized cognitive tests and functional magnetic resonance imaging, we retrospectively examined cognitive status and functional brain connectivity in 62 long-term breast cancer survivors.
Compared with control groups, patients treated with anthracycline-based chemotherapy demonstrated significantly lower verbal memory function, particularly in retention of information over time (P < .001; effect sizes, 1.2–1.5).
Anthracycline regimens were also associated with significantly lower default mode brain network connectivity (P = .001; effect sizes, 0.6–1.3), suggesting decreased efficiency of information processing.
Patients who receive anthracycline-based therapies may be at increased risk for memory problems and underlying brain injury. These effects seem to persist several years beyond treatment conclusion.
Acknowledgments
Funding/Support: This research was support by grants from the National Institutes of Health (1R01CA172145, 1DP2OD004445, and 1R01NR014195 to Dr Kesler).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Kesler had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Both authors.
Acquisition, analysis, or interpretation of data: Kesler.
Drafting of the manuscript: Both authors.
Critical revision of the manuscript for important intellectual content: Both authors.
Statistical analysis: Kesler.
Obtained funding: Kesler.
Administrative, technical, or material support: Kesler.
Study supervision: Blayney.
Conflict of Interest Disclosures: None reported.
Additional Contributions: Mika Pritchard-Berman, MA, of Stanford University coordinated participant recruitment, MRI scanning and administration of neuropsychological testing. She was a paid staff member of Dr Kesler’s laboratory.
References
- 1.Kesler SR, Wefel JS, Hosseini SM, Cheung M, Watson CL, Hoeft F. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Sci U S A. 2013;110(28):11600–11605. doi: 10.1073/pnas.1214551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013;39(3):297–304. doi: 10.1016/j.ctrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011;68(11):1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quesnel C, Savard J, Ivers H. Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast Cancer Res Treat. 2009;116(1):113–123. doi: 10.1007/s10549-008-0114-2. [DOI] [PubMed] [Google Scholar]
- 5.Stewart A, Collins B, Mackenzie J, Tomiak E, Verma S, Bielajew C. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: a prospective study. Psychooncology. 2008;17(2):122–130. doi: 10.1002/pon.1210. [DOI] [PubMed] [Google Scholar]
- 6.Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30(10):1080–1086. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- 7.Von Ah D, Habermann B, Carpenter JS, Schneider BL. Impact of perceived cognitive impairment in breast cancer survivors. Eur J Oncol Nurs. 2013;17(2):236–241. doi: 10.1016/j.ejon.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Ahles TA. Brain vulnerability to chemotherapy toxicities. Psychooncology. 2012;21(11):1141–1148. doi: 10.1002/pon.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Schagen SB. Late effects of adjuvant chemotherapy for adult onset non-CNS cancer; cognitive impairment, brain structure and risk of dementia. Crit Rev Oncol Hematol. 2013;88(1):87–101. doi: 10.1016/j.critrevonc.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Mandelblatt JS, Hurria A, McDonald BC, et al. Thinking and Living With Cancer Study Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin Oncol. 2013;40(6):709–725. doi: 10.1053/j.seminoncol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccirillo JF, Hardin FM, Nicklaus J, et al. Cognitive impairment after chemotherapy related to atypical network architecture for executive control. Oncology. 2015;88(6):360–368. doi: 10.1159/000370117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lepage C, Smith AM, Moreau J, et al. A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. Springerplus. 2014;3:444. doi: 10.1186/2193-1801-3-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stouten-Kemperman MM, de Ruiter MB, Boogerd W, Veltman DJ, Reneman L, Schagen SB. Very late treatment-related alterations in brain function of breast cancer survivors. J Int Neuropsychol Soc. 2015;21(1):50–61. doi: 10.1017/S1355617714001015. [DOI] [PubMed] [Google Scholar]
- 14.Kesler SR, Watson CL, Blayney DW. Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiol Aging. 2015;36(8):2429–2442. doi: 10.1016/j.neurobiolaging.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seigers R, Schagen SB, Van Tellingen O, Dietrich J. Chemotherapy-related cognitive dysfunction: current animal studies and future directions. Brain Imaging Behav. 2013;7(4):453–459. doi: 10.1007/s11682-013-9250-3. [DOI] [PubMed] [Google Scholar]
- 16.Kesler SR, Bennett FC, Mahaffey ML, Spiegel D. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin Cancer Res. 2009;15(21):6665–6673. doi: 10.1158/1078-0432.CCR-09-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner N, Biganzoli L, Di Leo A. Continued value of adjuvant anthracyclines as treatment for early breast cancer. Lancet Oncol. 2015;16(7):e362–e369. doi: 10.1016/S1470-2045(15)00079-0. [DOI] [PubMed] [Google Scholar]
- 18.Hortobágyi GN. Anthracyclines in the treatment of cancer: n overview. Drugs. 1997;54(suppl 4):1–7. doi: 10.2165/00003495-199700544-00003. [DOI] [PubMed] [Google Scholar]
- 19.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30(20):2500–2508. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horwitz B. The elusive concept of brain connectivity. Neuroimage. 2003;19(2, pt 1):466–470. doi: 10.1016/s1053-8119(03)00112-5. [DOI] [PubMed] [Google Scholar]
- 21.Hosseini SM, Kesler SR. Multivariate pattern analysis of FMRI in breast cancer survivors and healthy women. J Int Neuropsychol Soc. 2014;20(4):391–401. doi: 10.1017/S1355617713001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kesler SR. Default mode network as a potential biomarker of chemotherapy-related brain injury. Neurobiol Aging. 2014;35(suppl 2):S11–S19. doi: 10.1016/j.neurobiolaging.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumas JA, Makarewicz J, Schaubhut GJ, et al. Chemotherapy altered brain functional connectivity in women with breast cancer: a pilot study. Brain Imaging Behav. 2013;7(4):524–532. doi: 10.1007/s11682-013-9244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raichle ME. The restless brain. Brain Connect. 2011;1(1):3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruno J, Hosseini SM, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48(3):329–338. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test–Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12(1):43–55. [Google Scholar]
- 29.Heaton RK. Wisconsin Card Sorting Test Computer Version 4—Research Edition (WCST:CV4) Odessa, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 30.Moses JA., Jr Test review-Comprehensive Trail Making Test (CTMT) Arch Clin Neuropsychol. 2004;19(5):703–708. doi: 10.1016/j.acn.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Homack S, Lee D, Riccio CA. Test review: Delis-Kaplan executive function system. J Clin Exp Neuropsychol. 2005;27(5):599–609. doi: 10.1080/13803390490918444. [DOI] [PubMed] [Google Scholar]
- 32.Barry D, Bates ME, Labouvie E. FAS and CFL forms of verbal fluency differ in difficulty: a meta-analytic study. Appl Neuropsychol. 2008;15(2):97–106. doi: 10.1080/09084280802083863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roth RM, Isquith PK, Gioia G. Behavioral Rating Inventory of Executive Function—Adult Version. Lutz, FL: Psychological Assessment Resources; 2005. [Google Scholar]
- 34.Aghakhani A, Chan EK. Test Reviews: Bracken, B. A., & Howell, K. (2004). Clinical Assessment of Depression. Odessa, FL: Psychological Assessment Resources. J Psychoed Assess. 2007;25(4):416–422. doi: 10.1177/0734282907300383. [DOI] [Google Scholar]
- 35.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 36.Kesler SR, Gugel M, Pritchard-Berman M, et al. Altered resting state functional connectivity in young survivors of acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61(7):1295–1299. doi: 10.1002/pbc.25022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosseini SM, Kesler SR. Comparing connectivity pattern and small-world organization between structural correlation and resting-state networks in healthy adults. Neuroimage. 2013;78:402–414. doi: 10.1016/j.neuroimage.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22(1):158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59(2):1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 41.Kesler S, Janelsins M, Koovakkattu D, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(suppl):S109–S116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116(14):3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 43.Zhang S, Li CS. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012;59(4):3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123(3):819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. Neuroimage. 2010;49(4):3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Lord LD, Expert P, Huckins JF, Turkheimer FE. Cerebral energy metabolism and the brain’s functional network architecture: an integrative review. J Cereb Blood Flow Metab. 2013;33(9):1347–1354. doi: 10.1038/jcbfm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mordente A, Meucci E, Silvestrini A, Martorana GE, Giardina B. Anthracyclines and mitochondria. Adv Exp Med Biol. 2012;942:385–419. doi: 10.1007/978-94-007-2869-1_18. [DOI] [PubMed] [Google Scholar]
- 48.Ouchi Y, Kikuchi M. A review of the default mode network in aging and dementia based on molecular imaging. Rev Neurosci. 2012;23(3):263–268. doi: 10.1515/revneuro-2012-0029. [DOI] [PubMed] [Google Scholar]
- 49.Damoiseaux JS. Resting-state fMRI as a biomarker for Alzheimer’s disease? Alzheimers Res Ther. 2012;4(2):8. doi: 10.1186/alzrt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106(4):dju057. doi: 10.1093/jnci/dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tangpong J, Cole MP, Sultana R, et al. Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: insight into the mechanism of chemobrain. J Neurochem. 2007;100(1):191–201. doi: 10.1111/j.1471-4159.2006.04179.x. [DOI] [PubMed] [Google Scholar]
- 52.Joshi G, Aluise CD, Cole MP, et al. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: implications for oxidative stress-mediated chemobrain. Neuroscience. 2010;166(3):796–807. doi: 10.1016/j.neuroscience.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janelsins MC, Mustian KM, Palesh OG, et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Support Care Cancer. 2012;20(4):831–839. doi: 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pomykala KL, Ganz PA, Bower JE, et al. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013;7(4):511–523. doi: 10.1007/s11682-013-9243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30(suppl):S99–S108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel SK, Wong AL, Wong FL, et al. Inflammatory biomarkers, comorbidity, and neurocognition in women with newly diagnosed breast cancer. J Natl Cancer Inst. 2015;107(8):1–7. doi: 10.1093/jnci/djv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niedzielska E, Smaga I, Gawlik M, et al. Oxidative stress in neurodegenerative diseases [published online July 22, 2015] Mol Neurobiol. doi: 10.1007/s12035-015-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conroy SK, McDonald BC, Smith DJ, et al. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat. 2013;137(2):493–502. doi: 10.1007/s10549-012-2385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herrup K. Reimagining Alzheimer’s disease: an age-based hypothesis. J Neurosci. 2010;30(50):16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hernández-Zimbrón LF, Rivas-Arancibia S. Oxidative stress caused by ozone exposure induces β-amyloid 1-42 overproduction and mitochondrial accumulation by activating the amyloidogenic pathway. Neuroscience. 2015;304:340–348. doi: 10.1016/j.neuroscience.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 61.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koppelmans V, Vernooij MW, Boogerd W, et al. Prevalence of cerebral small-vessel disease in long-term breast cancer survivors exposed to both adjuvant radiotherapy and chemotherapy. J Clin Oncol. 2015;33(6):588–593. doi: 10.1200/JCO.2014.56.8345. [DOI] [PubMed] [Google Scholar]
- 63.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11(3):272–282. doi: 10.1016/S1474-4422(11)70307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dietrich J. Chemotherapy associated central nervous system damage. Adv Exp Med Biol. 2010;678:77–85. doi: 10.1007/978-1-4419-6306-2_11. [DOI] [PubMed] [Google Scholar]
- 65.Janelsins MC, Roscoe JA, Berg MJ, et al. IGF-1 partially restores chemotherapy-induced reductions in neural cell proliferation in adult C57BL/6 mice. Cancer Invest. 2010;28(5):544–553. doi: 10.3109/07357900903405942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rousselle C, Clair P, Lefauconnier J-M, Kaczorek M, Scherrmann J-M, Temsamani J. New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy. Mol Pharmacol. 2000;57(4):679–686. doi: 10.1124/mol.57.4.679. [DOI] [PubMed] [Google Scholar]
- 67.Rousselle C, Smirnova M, Clair P, et al. Enhanced delivery of doxorubicin into the brain via a peptide-vector-mediated strategy: saturation kinetics and specificity. J Pharmacol Exp Ther. 2001;296(1):124–131. [PubMed] [Google Scholar]
- 68.Dietrich J, Han R, Yang Y, Mayer-Pröschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5(7):22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110(1):143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stouten-Kemperman MM, de Ruiter MB, Koppelmans V, Boogerd W, Reneman L, Schagen SB. Neurotoxicity in breast cancer survivors ≥10 years post-treatment is dependent on treatment type. Brain Imaging Behav. 2015;9(2):275–284. doi: 10.1007/s11682-014-9305-0. [DOI] [PubMed] [Google Scholar]
- 71.Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FS. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J Natl Cancer Inst. 2006;98(23):1742–1745. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- 72.Chaturvedi RK, Beal MF. Mitochondrial approaches for neuroprotection. Ann N Y Acad Sci. 2008;1147:395–412. doi: 10.1196/annals.1427.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aluise CD, Miriyala S, Noel T, et al. 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-α release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic Biol Med. 2011;50(11):1630–1638. doi: 10.1016/j.freeradbiomed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 74.Stafford JM, Jarrett BR, Miranda-Dominguez O, et al. Large-scale topology and the default mode network in the mouse connectome. Proc Natl Acad Sci U S A. 2014;111(52):18745–18750. doi: 10.1073/pnas.1404346111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


