Clinical Question
What are the concerns of patients with glaucoma and how can they be addressed in general practice?
Glaucoma is the leading cause of irreversible blindness worldwide, affecting more than 70 million people, with half unaware they are affected.1–3 As glaucoma cannot be cured, patients require lifelong monitoring and treatment. The aim of this article is to provide an update on glaucoma from the patient’s perspective by addressing some commonly raised questions.
WHAT IS GLAUCOMA?
Glaucoma is an optic neuropathy characterised by loss of retinal ganglion cells and accompanying loss of visual field (Figure 1). Although raised intraocular pressure (IOP) is the major risk factor, some patients develop glaucoma with normal IOP (≤21 mmHg), whereas others have high IOP but do not have glaucoma (ocular hypertension). As glaucoma can be asymptomatic until later stages, for most patients diagnosis follows the unexpected discovery of an abnormality on routine testing at a high-street optometrist. It is therefore important to encourage regular eye examinations.3
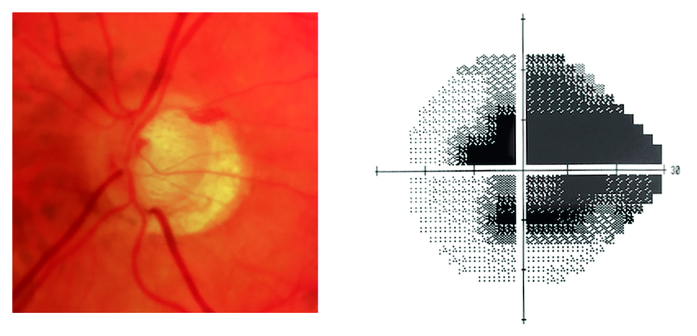
Figure 1.
Optic disc photograph and visual field showing advanced glaucoma in the left eye.
WHAT TYPE OF GLAUCOMA DO I HAVE?
Glaucoma can be divided into open or closed angle, the angle referring to the opening between the iris and cornea, the location of the trabecular meshwork. Both open and angle closure glaucoma can be divided into primary and secondary subtypes. Primary open angle glaucoma (POAG) is the most common type in the UK, affecting 2% of those aged >40 and 10% of those >75 years. Risk factors include age, short sight, family history, African–Caribbean ancestry, and diabetes. Glaucoma can also occur due to medications including systemic or topical steroids.3
Primary angle closure glaucoma (PACG) is less common, affecting 0.17% of those >40 years in the UK. Risk factors include older age, long sight, and East Asian ancestry. Acute angle closure is one of the few ophthalmic emergencies as IOP may become extremely high, risking visual loss unless treated quickly. Although acute angle closure typically produces symptoms including painful red eye, nausea, and blurred vision, often preceded by seeing coloured rings around lights, most patients with PACG do not present acutely and have no symptoms until irreversible visual loss has occurred.
SHOULD I BE REFERRED TO HOSPITAL?
Patients with evidence of optic nerve damage, reproducible visual field defects, or those at risk of angle closure should be referred to secondary eye care services, irrespective of IOP.4 Some patients with ocular hypertension should also be referred due to increased risk of developing glaucoma.4 Around one-third of referrals of patients with suspected glaucoma from optometrists result in a discharge at the first visit.4
WHAT ARE THE TREATMENT OPTIONS?
Treatment of POAG relies on lowering IOP.1 The first-line treatment is usually a prostaglandin analogue eye drop, which lowers IOP by increasing aqueous outflow. Common side effects include red eye, skin pigmentation, and lengthening of the lashes. Other medications include topical beta-blockers, alpha-agonists, or carbonic anhydrase inhibitors. All anti-glaucoma medications, but particularly beta-blockers, have the potential for systemic side effects. Systemic absorption can be reduced by pressing on the side of the nose over the lacrimal sac. If using multiple drops patients should also be advised to wait at least 5 minutes between medications to avoid washing one out with another.
A major problem associated with the use of eye drops is the difficulty of administration. Over half of patients have a poor technique of drop instillation and >10% miss the eye completely.5 Education can improve the ability to instil drops but a surprisingly large number of patients have never been shown how to use them. Compliance aids, which are devices to aid drop delivery, may help some. Other developments include combination and preservative-free medications, which are often better tolerated but can be more challenging to instil as they are often supplied in single-use plastic ampules that are difficult to squeeze.
Selective laser trabeculoplasty (SLT) is an alternative first-line treatment that involves directing laser to the trabecular meshwork. Surgical treatments include trabeculectomy, which involves creating a drainage pathway through the sclera to a reservoir area under the conjunctiva. There are also a growing number of new operations collectively known as minimally invasive glaucoma surgery (MIGS). These procedures seem to carry lower risk but also lower efficacy than conventional glaucoma surgery.1
Although PACG carries a higher risk of blindness, it can be prevented. Angle closure occurs when there is contact between the trabecular meshwork and iris, which can be detected prior to the onset of glaucoma. Angle closure is often due to age-related enlargement of the natural lens causing obstruction to aqueous outflow through the pupil, leading to build-up of aqueous behind the iris, bowing forward of the iris, and obstruction to the trabecular meshwork. Laser can be used to create a hole in the iris (iridotomy) and bypass the obstruction.
WILL I GO BLIND?
The major risk factor for blindness is late presentation, although other risk factors include angle closure and family history of glaucoma blindness. Age at diagnosis is also important and a younger person with slowly progressive disease may be at higher risk of blindness than an older person progressing more rapidly. If diagnosed early though, there are effective treatments, and most patients will not become blind. For those with impaired vision, referral to a low vision clinic may be beneficial, and they may be eligible for sight impaired (partially sighted) or severely sight impaired (blind) registration. Patients require lifelong ophthalmology follow-up and will typically be monitored by regular visual field and optic disc assessments.
WHY HAVE I DEVELOPED GLAUCOMA WITH A NORMAL PRESSURE?
Why some patients develop glaucoma with a ‘normal’ pressure, whereas others seem resistant to high pressure, is not understood, but some patients may have more fragile optic nerves rendering them sensitive to damage at otherwise physiological pressures. IOP can also vary through the day and other factors may contribute including systemic hypotension, which may be associated with reduced ocular blood flow.
CAN I CONTINUE DRIVING?
Drivers diagnosed with glaucoma are at increased risk of collisions6 and if diagnosed in both eyes have an obligation to inform the DVLA, who will notify the patient of the need to have a binocular Esterman visual field test.7 This will be conducted at a high-street optometrist. Some patients with visual field loss will be advised by their ophthalmologist not to drive, but many can continue driving until the DVLA advises otherwise.
HOW CAN MY GP HELP?
GPs can play an important role in glaucoma detection by encouraging patients to have regular eye examinations. In England and Wales individuals aged ≥40 years with a first-degree family history of glaucoma are entitled to free eye examinations. GPs may also be confronted by patients experiencing side effects from their eye drops, ranging from red eye due to drop allergy — which may be misdiagnosed as infective conjunctivitis — to systemic side effects such as bradycardia. Liaise with your local ophthalmology department if you suspect drop side effects.
Like many chronic diseases, glaucoma is associated with reduced quality of life, but this is compounded by reduction in vision that affects the ability to perform daily tasks like driving and increases risk of falls. It is therefore important to be aware of services available to individuals with visual impairment. Low vision services are a valuable resource, able to offer advice and equipment, which may help prolong safe mobility and maintain independent living. Some patients may also benefit from contact with patient support groups such as the International Glaucoma Association.8 Knowledge of these factors will help support those who may seek advice and reassurance about this common blinding condition.
Funding
Andrew Tatham is funded by an NHS Scotland Career Research Fellowship.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence . Glaucoma: diagnosis and management. London: NICE; 2009. p. CG85. https://www.nice.org.uk/guidance/cg85 (accessed 4 Apr 2016). [Google Scholar]
- 3.Quigley HA. Glaucoma. Lancet. 2011;377(9774):1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 4.Scottish Intercollegiate Guidelines Network . Glaucoma referral and safe discharge. Edinburgh: SIGN; 2015. SIGN publication No. 144 http://sign.ac.uk/guidelines/fulltext/144/index.html (accessed 4 Apr 2016). [Google Scholar]
- 5.Tatham AJ, Sarodia U, Gatrad F, Awan A. Eye drop instillation technique in patients with glaucoma. Eye. 2013;27(11):1293–1298. doi: 10.1038/eye.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gracitelli CP, Tatham AJ, Boer ER, et al. Predicting risk of motor vehicle collisions in patients with glaucoma: a longitudinal study. PLoS One. 2015;10(10):e0138288. doi: 10.1371/journal.pone.0138288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driver and Vehicle Licensing Agency . Assessing fitness to drive — a guide for medical professionals. Swansea: DVLA; 2016. https://www.gov.uk/government/publications/assessing-fitness-to-drive-a-guide-for-medical-professionals (accessed 4 Apr 2016). [Google Scholar]
- 8.International Glaucoma Association http://www.glaucoma-association.com (accessed 4 Apr 2016).