Abstract
Objective
To evaluate the effects of a fast-track esophagectomy protocol (FTEP) on esophageal cancer patients' safety, length of hospital stay (LOS) and hospital charges.
Background
FTEP involved transferring patients to the telemetry unit instead of the surgical intensive care unit (SICU) after esophagectomy.
Methods
We retrospectively reviewed 708 consecutive patients who underwent esophagectomy for primary esophageal cancer during the 4 years before (group A; 322 patients) or 4 years after (group B; 386 patients) the institution of an FTEP. Postoperative morbidity and mortality, LOS, and hospital charges were reviewed.
Results
Compared with group A, group B had significantly shorter median LOS (12 days vs 8 days; P < 0.001); lower mean numbers of SICU days (4.5 days vs 1.2 days; P < 0.001) and telemetry days (12.7 days vs 9.7 days; P < 0.001); and lower rates of atrial arrhythmia (27% vs 19%; P = 0.013) and pulmonary complications (27% vs 20%; P = 0.016). Multivariable analysis revealed FTEP to be associated with shorter LOS (P < 0.001) even after adjustment for predictors like tumor histology and location. FTEP was also associated with a lower rate of pulmonary complications (odds ratio = 0.655; 95% confidence interval = 0.456, 0.942; P = 0.022). In addition, the median hospital charges associated with primary admission and readmission within 90 days for group B ($65,649) were lower than that for group A ($79,117; P < 0.001).
Conclusion
These findings suggest that an FTEP reduces patients' LOS, perioperative morbidity and hospital charges.
Introduction
Surgical resection is the mainstay treatment for localized esophageal carcinoma in the absence of medical contraindications.1 However, surgery poses a high risk of complications, which requires that patients be admitted to the intensive care unit (ICU) immediately after surgery. This prolongs their hospital stay and increases costs to both the patients and hospital.
A fast-track surgery protocol first introduced by Kehlet and Wilmore2 represents an advance in postoperative surgical care. In this approach, surgery patients are transferred from the post-anesthesia care unit directly to a monitored (telemetry) unit, eliminating the need for an ICU stay.3 In the telemetry unit, multidisciplinary care is provided by a focused group of surgeons, midlevel providers, and trained surgical nurses. In addition, family members are permitted to be with the patients, and the patients are allowed to ambulate within a few hours after surgery. All of these advantages help reduce the physiological and psychological stress associated with surgery, thereby enhancing patients' postoperative recovery and reducing their length of hospital stay (LOS).2, 4
Fast-track surgery protocols can be cost-effective because they require fewer postoperative ICU admissions, less monitoring, and less nursing care per patient course than traditional surgery protocols do while eliciting the same or better patient outcomes.5, 6 Although numerous studies have investigated such protocols for other procedures, notably colorectal surgery,7 relatively fewer studies have investigated the use of a fast-track surgery protocol for esophagectomy.6, 8-18 Also, only a few fast-track esophagectomy protocol (FTEP) studies conducted in United States have evaluated the impact on hospital charges. An assessment of the safety of the protocol will serve as a performance measure that will help clinicians and policy makers change practice and improve the care of esophageal cancer patients. Therefore, we investigated the effect of a FTEP on patient safety, postoperative recovery, LOS and hospital charges.
Patients and Methods
Study Population
This retrospective study included 708 consecutive patients with histologically confirmed adenocarcinoma or squamous cell carcinoma of the esophagus who underwent esophagectomy in the Department of Thoracic and Cardiovascular Surgery at The University of Texas MD Anderson Cancer Center (MDACC) between March 2004 and March 2012. For patients undergoing esophagectomy from March 17, 2008, a multi-disciplinary decision between hospital administration, nursing support and thoracic surgeons was taken to institute a fast track protocol. Thus, for this study, the patients were divided into 2 groups: Group A consisted of the 322 patients who underwent esophagectomy during 4 years before the institution of the FTEP on March 17, 2008, and group B consisted of the 386 patients who underwent the procedure during 4 years after the institution of the protocol. Group A included patients who were usually transferred to the SICU after esophagectomy, whereas group B primarily included patients who were transferred to a telemetry unit immediately after surgery regardless of whether they were admitted to the ICU during their hospital stay. Only a few patients in group B who required intubation or close monitoring were transferred to the SICU. The study was approved by MDACC Institutional Review Board. Informed consent wasn't obtained from the patients as this was a retrospective data review that involved no diagnostic or therapeutic intervention, as well as no direct patient contact.
Patient Data Retrieval
Patient demographics, tumor characteristics, and short-term postoperative outcomes were obtained from the Esophageal Departmental Database. Patients' postoperative LOS (the total number of days in the hospital after surgery until discharge), immediate postoperative ventilator days (the number of days with mechanical ventilator assistance immediately after surgery), total ventilator days (the total number of days with ventilator assistance between surgery and discharge), total ICU days (the total number of days in the SICU between surgery and discharge), and telemetry days (the number of days in the telemetry unit between surgery and discharge) were also recorded, as were postoperative complications including aspiration, acute respiratory distress syndrome (ARDS), pneumonia, discharge on home oxygen, reintubation, atelectasis, atrial arrhythmia, discharge on a jejunostomy tube (J-tube), barium swallow, ICU readmission, anastomotic leak, hospital readmission within 90 days, reoperation, and 30- and 90-day mortality. Information about the patients' technical hospital charges was collected from MDACC's enterprise information warehouse.
Statistical Analysis
Pearson chi-square and Fisher exact tests were used to analyze differences between groups for significance for categorical variable. A non-parametric Mann Whitney U test was used to analyze differences between groups for significance for continuous variables. Univariable linear regression was used to assess the association between various prognostic predictors and outcomes such as LOS and technical hospital charges. Univariable logistic regression was used to assess the association between various prognostic predictors and pulmonary complications. Factors in univariable analysis with a P-value < 0.25 were entered into a multivariable regression model. The final model for the data set was obtained using Wald's stepwise selection with a P-value of 0.10 as the entry and removal probability. All statistical analyses were performed using the Statistical Package for Social Sciences software version 17 (SPSS, Chicago, IL). P-values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
The characteristics of the 322 patients in group A and the 386 patients in group B are summarized in Table 1. The two groups were similar in terms of gender and age as well as history of chronic obstructive pulmonary disease, coronary artery disease, and diabetes. Although tumor histology, location, grade, and pathological stage did not differ between the 2 groups, the mean tumor size of group A (1.9 cm) was significantly smaller than that of group B (2.5 cm; P = 0.025) (Table 2). There was also difference in the distribution of clinical staging of tumor between the 2 groups. Compared with group B, group A had lower proportions of patients with stage II cancer (39% vs 32%) and stage III cancer (46% vs 42%) and higher proportions of patients with stage I cancer (12% vs 19%) and stage IV cancer (3% vs 6%; P < 0.008) (Table 3). The types of preoperative treatment the 2 groups received also differed, with a greater proportion of patients in group B (85%) than in group A (73%) receiving chemotherapy plus radiation therapy (P < 0.001). The type of surgery also differed between the 2 groups; minimally invasive esophagectomy (MIE) was performed more frequently in group B (22%) than in group A (12%; P < 0.001) (Table 4).
Table 1. Patient Characteristics.
| Variable | Group A (N=322) (3/29/2004-3/16/2008) | Group B (N=386) (3/17/2008-3/5/2012) | P-Value |
|---|---|---|---|
| Gender | |||
| Male | 279(87) | 338(88) | 0.716 |
| Female | 43(13) | 48(12) | |
| Age | |||
| Mean | 61 | 61 | 0.385 |
| Median(range) | 62(25-83) | 62(23-84) | |
| COPD | 21(7) | 24(6) | 0.121 |
| CAD | 49(15) | 70(18) | 0.301 |
| Diabetes | 50(16) | 61(16) | 0.920 |
| Any tobacco history | 231(72) | 278(72) | 0.934 |
| Cigarette pack years* | |||
| Median(range) | 30(0.03-137.5) | 29.4(0.2-180) | 0.426 |
| Mean | 34.4 | 32.9 | |
| Smoking cessation time before surgery† | |||
| 0-14 days | 16(5) | 24(6) | 0.258 |
| >14 days;<=1 | 10(3) | 10(3) | |
| >1 mo;<=12 | 33(10) | 60(16) | |
| >12 mo; <=5 y | 21(7) | 23(6) | |
| >5 y | 149(47) | 151(40) | |
| Never smoked | 91(28) | 108(29) | |
| ASA risk score | |||
| 1 | 0(0) | 1(0) | 0.029‡ |
| 2 | 68(21) | 53(14) | |
| 3 | 252(78) | 329(85) | |
| 4 | 2(1) | 3(1) | |
Note: Data are no. of patients (%) unless otherwise indicated.
Data missing for 100 patients in Group A and for 130 patients in Group B.
Data missing for 2 patients in Group A and for 10 patients in Group B.
Statistically significant.
COPD indicates chronic obstructive pulmonary disease; CAD, coronary arterial disease; ASA, American Society of Anesthesiologist.
Table 2. Tumor Characteristics.
| Characteristics | Group A (N=322) (3/29/2004-3/17/2008) | Group B (N=386)(3/17/2008-3/5/2012) | P-Value |
|---|---|---|---|
| Histology | 0.683 | ||
| Adenocarcinoma | 294(91) | 349(90) | |
| Squamous cell carcinoma | 28(9) | 37(10) | |
| Location | 0.387 | ||
| Upper/cervical | 3(1) | 8(2) | |
| Middle | 26(8) | 26(7) | |
| Lower/GEJ | 293(91) | 352(91) | |
| Grade* | 0.061 | ||
| Well differentiated | 7(2) | 2(1) | |
| Moderately differentiated | 136(43) | 129(39) | |
| Poorly differentiated | 170(54) | 201(60) | |
| Undifferentiated | 2(1) | 0(0) | |
| Mean Tumor Size†, cm | 1.9 | 2.5 | 0.025‡ |
| Clinical stage | 0.008‡ | ||
| 0 | 3(1) | 2(1) | |
| I | 62(19) | 46(12) | |
| II | 104(32) | 149(39) | |
| III | 135(42) | 179(46) | |
| IV | 18(6) | 10(3) | |
| Pathological stage | 0.062 | ||
| 0 | 57(18) | 88(23) | |
| 1 | 79(25) | 70(18) | |
| 2 | 122(38) | 132(34) | |
| 3 | 56(17) | 88(23) | |
| 4 | 8(2) | 8(2) |
Note: Data are no. of patients (%) unless otherwise indicated.
Tumor grade data missing for 7 cases in Group A and 54 cases in group B.
Tumor size data missing for 8 cases in Group A and 9 cases in group B.
Statistically significant.
Table 3. Clinical Care Pathway for Traditional and Fast-Track Protocol.
| POD | Traditional Care | Fast-Track Protocol |
|---|---|---|
| 0 |
|
|
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
| 4 |
|
|
| 5 |
|
|
| 6 |
|
|
| 7 |
|
|
| 8 |
|
|
| 9 | ||
| 10 |
|
|
| 11 |
|
|
| 12-14 |
|
POD indicates postoperative day; OR, operation room; ICU, intensive care unit; PACU, post-anesthesia care unit; OOB, out of bed; IV, intravenous; NGT, nasogastric tube; J-tube, jejunostomy tube.
Table 4. Treatment Characteristics.
| Variable | Group A (N=322) (3/29/2004-3/17/2008) | Group B (N=386) (3/17/2008-3/5/2012) | P-Value |
|---|---|---|---|
| Preoperative treatment | <0.001* | ||
| Chemotherapy plus RT | 235(73) | 326(85) | |
| Chemotherapy only | 5(1) | 0(0) | |
| RT only | 1(0) | 1(0) | |
| Other | 2(1) | 0(0) | |
| None | 79(25) | 59(15) | |
| Type of esophagectomy | <0.001* | ||
| Transthoracic (Ivor Lewis) | 213(66) | 249(65) | |
| Transhiatal | 38(12) | 21(5) | |
| Total (3-fold technique) | 28(9) | 21(5) | |
| Minimally invasive | 38(12) | 86(22) | |
| Other | 5(1) | 9(2) |
Note: Data are no. of patients (%) unless otherwise indicated.
Statistically significant.
RT indicates radiotherapy.
Short-Term Postoperative Outcomes
The 2 groups' short-term postoperative outcomes are summarized in Table 5. The median LOS of group A (12 days) was significantly longer than that of group B (8 days; P < 0.001) (see also Figure 1). The mean numbers of total ICU days and telemetry days of group A (4.5 and 12.7, respectively) were also significantly higher than those of group B (1.2 and 9.7, respectively; P < 0.001 for both). In addition, group A had higher mean numbers of postoperative ventilator days (1.3) and total ventilator days (2.5) than did group B (0.3 and 0.8, respectively; P < 0.001 for both). The direct ICU admission rate of group A (71%) was significantly higher than that of group B (7%; P < 0.001). In terms of postoperative complications, the incidences of aspiration, pneumonia, discharge on home oxygen, atelectasis requiring bronchoscopy, anastomotic leak, ICU readmission, and reoperation did not differ significantly between the 2 groups. However, compared with group A, group B had significantly lower incidences of ARDS (6% vs 0.5%; P < 0.001), reintubation (9% vs 4%; P = 0.005), atrial arrhythmia requiring treatment (27% vs 19%; P = 0.015) and overall pulmonary complications (27% vs 20%, with aspiration, ARDS, pneumonia, discharge on home oxygen, and atelectasis grouped together; P = 0.022). The 2 groups' rates of 30-day mortality (3% in group A vs 2% in group B; P = 0.386), 90-day mortality (5% vs 4%; P = 0.377), and 90-day readmission (12% vs 15%; P = 0.134) did not differ significantly. The reasons for readmission within 90 days are summarized in Table 7. Pulmonary complication (38%), gastrointestinal symptoms (16%) like nausea, vomiting and diarrhea, and dysphagia (11%) were the major reasons for readmission. The 2 groups' rates of direct admission to the ICU, total LOS, total ICU days, total ventilator days, and immediate postoperative ventilator days for each year of the study period are summarized in Table 8 and Figure 2. Multivariable analysis revealed that the institution of the FTEP was associated with shorter LOS (β = -6.415; 95% confidence interval (CI) = 8.294, -4.536; P < 0.001) even after adjustment for factors such as tumor location and histology (Table 9). Multivariable analysis also revealed that the institution of the FTEP was associated with fewer pulmonary complications (odds ratio = 0.655; 95% CI = 0.456, 0942; P = 0.022) even after adjustment for other independent predictors such as gender, chronic obstructive pulmonary disease, and type of esophagectomy (Table 10).
Table 5. Short Term Post-Operative Outcomes.
| Outcome | Group A (N=322) (3/29/2004-3/16/2008) | Group B (N=386) (3/17/2008-3/5/2012) | P-Value |
|---|---|---|---|
| Median length of stay | 12(4-153) | 8(0-74) | <0.001* |
| Mean days in SICU | 4.5 | 1.2 | <0.001* |
| Mean total ventilator days | 2.5 | 0.8 | <0.001* |
| Mean postoperative ventilator days | 1.3 | 0.3 | <0.001 * |
| Mean total telemetry days | 12.7 | 9.7 | <0.001* |
| ICU-direct admission | 229(71) | 25(7) | <0.001* |
| Any pulmonary complication† | 88(27) | 76(20) | 0.016* |
| Aspiration | 20(6) | 16(4) | 0.213 |
| ARDS | 18(6) | 2(0.5) | <0.001* |
| Pneumonia | 59(18) | 57(15) | 0.203 |
| Discharge on home oxygen | 26(8) | 26(7) | 0.497 |
| Reintubation | 29(9) | 15(4) | 0.005* |
| Atelectasis requiring bronchoscopy | 18(6) | 14(4) | 0.211 |
| Atrial arrhythmia requiring treatment | 88(27) | 75(19) | 0.013* |
| Readmission to ICU | 34(11) | 33(9) | 0.363 |
| Reoperation | 30(9) | 41(11) | 0.565 |
| Anastomotic leak (All)‡ | 45(14) | 49(13) | 0.581 |
| Grade 1 | 19(6) | 8(2) | |
| Grade 2 | 6(2) | 12(3) | |
| Grade 3 | 13(4) | 16(4) | |
| Grade 4 | 2(1) | 4(1) | |
| 30 days perioperative mortality | 11(3) | 9(2) | 0.386 |
| 90 days perioperative mortality | 16(5) | 14(4) | 0.377 |
| Readmission within 90 days | 38(12) | 59(15) | 0.151 |
| Discharge on J-tube feeding without barium swallow | 99(31) | 251(65) | <0.001* |
Note: Data are no. of patients (%) unless otherwise indicated.
Statistically significant.
Aspiration, ARDS, Pneumonia, Reintubation, Discharge on home oxygen (O2).
Leak grade data missing for 5 cases in Group A and for 9 cases in Group B.
SICU indicates surgical intensive care unit; ICU, intensive care unit; ARDS, acute respiratory distress syndrome; J- tube, jejunostomy tube.
Figure 1.
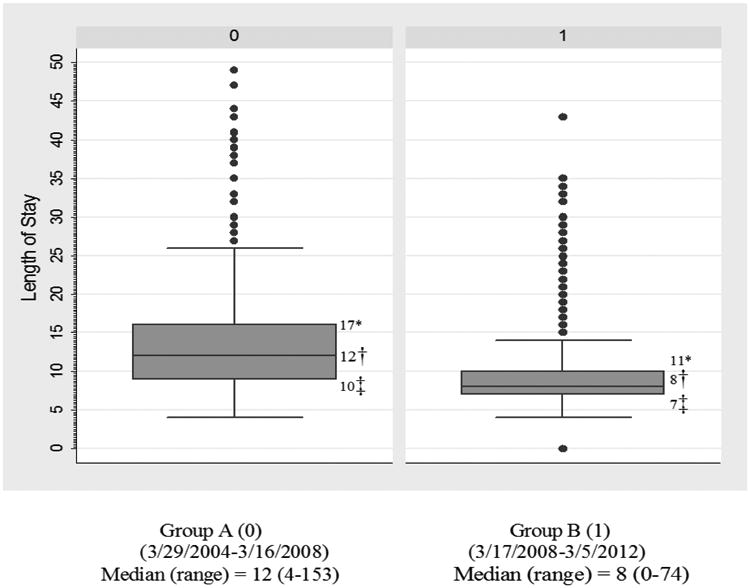
Box and whisker plot for LOS. The box (interquartile range) has 50% of all data while the whiskers extend to the 5th and 95th percentile. Dotes indicate data beyond 5th and 95th percentile. On Y Axis, the unit of measurement for Length of stay is ‘number of days’.
*75th percentile
†50th percentile (median)
‡25th percentile
Table 7. Length of Hospital Stay by Year.
| Group | Year | No. of Patients Admitted Directly to ICU | Mean Total LOS | Mean Total ICU days | Mean Total Ventilator Days | Mean Total Postoperative Ventilator Days |
|---|---|---|---|---|---|---|
| A | 2004-2005 | 88 | 18 | 6 | 3 | 2 |
| 2005-2006 | 79 | 21 | 5 | 3 | 1 | |
| 2006-2007 | 75 | 18 | 5 | 3 | 1 | |
| 2007-2008 | 56 | 14 | 3 | 2 | 1 | |
|
| ||||||
| B | 2008-2009 | 13 | 13 | 1 | 1 | 1 |
| 2009-2010 | 8 | 11 | 1 | 1 | 0 | |
| 2010-2011 | 2 | 10 | 1 | 1 | 0 | |
| 2011-2012 | 3 | 10 | 1 | 0 | 0 | |
ICU indicates intensive care unit.
Table 8. Reason for readmission within 90 days.
| Complications/Events | N=708 |
|---|---|
| Pulmonary* | 37(38) |
| Nausea/vomiting/diarrhea | 15(16) |
| Esophageal stricture/dysphagia | 11(11) |
| Wound infection | 8(8) |
| Pyloric stenosis | 4(4) |
| Anastomotic leak | 4(4) |
| J-tube malfunction/infection | 4(4) |
| Gastrointestinal bleeding | 3(3) |
| Small bowel obstruction | 3(3) |
| Other† | 8(8) |
|
| |
| Total | 97 |
Note: Data are no. of patients (%) unless otherwise indicated.
pleural effusion, pneumonia, and empyema.
Figure 2. Direct ICU admission and LOS by year.
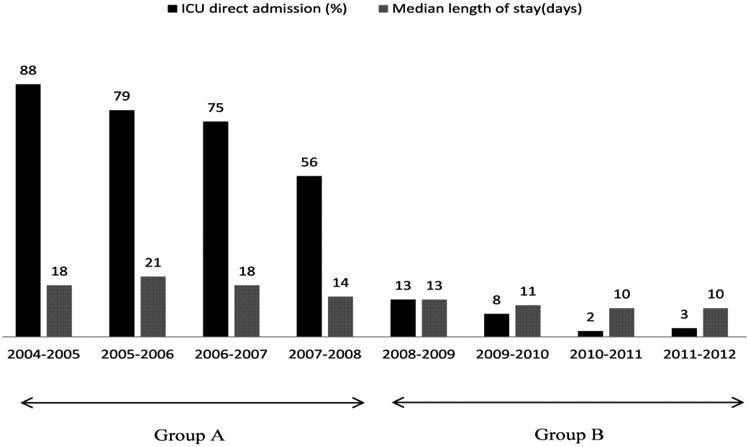
Table 9. Length of Total Hospital Stay (LOS).
| Variables | N | Univariable linear regression | Multivariable linear regression | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 95% CI for β | 95% CI for β | ||||||||
|
|
|
||||||||
| β | Lower | Upper | P-Value | β | Lower | Upper | P-Value | ||
| Group | |||||||||
| A* | 322 | ||||||||
| B | 386 | -6.37 | -8.29 | -4.45 | <0.001† | -6.42 | -8.29 | -4.54 | <0.001 |
| Gender | |||||||||
| Male* | 617 | ||||||||
| Female | 91 | 1.98 | -0.96 | 4.92 | 0.186† | ||||
| Age Preoperative treatment | 708 | 0.11 | 0.01 | 0.20 | 0.028† | ||||
| No* | 140 | ||||||||
| Yes | 568 | -2.028 | -4.50 | -0.44 | 0.107† | ||||
| Diabetes | |||||||||
| No* | 597 | ||||||||
| Yes | 111 | -0.03 | -2.74 | 2.67 | 0.980 | ||||
| COPD | |||||||||
| No* | 663 | ||||||||
| Yes | 45 | 2.70 | -1.33 | 6.73 | 0.189† | ||||
| CAD | |||||||||
| No* | 589 | ||||||||
| Yes | 119 | 1.72 | -0.92 | 4.35 | 0.201† | ||||
| Histology | |||||||||
| Adenocarcinoma* | 643 | ||||||||
| Squamous cell carcinoma | 65 | 8.53 | 5.18 | 11.88 | <0.001† | 5.87 | 1.92 | 9.81 | 0.004 |
| Tumor location | |||||||||
| Upper/cervical/middle* | 63 | ||||||||
| Lower/GEJ | 645 | -8.47 | -11.87 | -5.07 | <0.001† | -5.04 | -9.04 | -1.04 | 0.014 |
| Type of esophagectomy | 0.001† | ||||||||
| Ivor Lewis* | 462 | ||||||||
| Transhiatal | 59 | 1.92 | -1.67 | 5.50 | 0.294 | ||||
| Total | 49 | 7.84 | 3.95 | 11.73 | <0.001 | ||||
| Minimal invasive | 124 | -1.49 | -4.10 | 1.14 | 0.267 | ||||
| N/A | 14 | 0.55 | -6.48 | 7.58 | 0.877 | ||||
| Minimal invasive surgery | |||||||||
| No* | 584 | ||||||||
| Yes | 124 | -2.35 | -4.93 | 0.24 | 0.075† | ||||
| Pathological stage | |||||||||
| 0* | 145 | ||||||||
| I | 149 | -1.50 | -4.56 | 4.56 | 0.337 | ||||
| II | 254 | -0.92 | -3.65 | 1.81 | 0.509 | ||||
| III | 144 | -0.71 | -3.79 | 2.38 | 0.654 | ||||
| IV | 16 | 1.62 | -5.30 | 8.52 | 0.647 | ||||
| Clinical stage | |||||||||
| 0* | 5 | ||||||||
| I | 108 | 4.99 | -7.00 | 16.97 | 0.414 | ||||
| II | 253 | 3.60 | -8.23 | 15.44 | 0.550 | ||||
| III | 314 | 5.22 | -6.60 | 17.03 | 0.386 | ||||
| IV | 28 | 4.94 | -7.78 | 17.67 | 0.446 | ||||
| ASA-risk scale | |||||||||
| 1-2* | 122 | ||||||||
| 3-4 | 586 | 1.293 | -1.31 | 3.90 | 0.330 | ||||
| Salvage operation | |||||||||
| No* | 612 | ||||||||
| Yes | 96 | 2.87 | 0.002 | 5.74 | 0.050† | ||||
Reference group.
Statistically significant for univariable analysis (p<0.25).
βindicates odds ratio; CI, confidence interval; COPD, chronic pulmonary obstructive disease; CA, coronary artery disease; GEJ, gastroesophageal junction; ASA, American Society of Anesthesiologist.
Table 10. Pulmonary Complication.
| Variables | N | Univariable logistic regression | Multivariable logistic regression | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 95% C.I for OR | 95% C.I for OR | ||||||||
|
|
|
||||||||
| OR | Lower | Upper | P-Value | OR | Lower | Upper | P-Value | ||
| Group | |||||||||
| A* | 322 | 1.00 | |||||||
| B | 386 | 0.65 | 0.46 | 0.93 | 0.017† | 0.66 | 0.46 | 0.94 | 0.022 |
| Gender | |||||||||
| Male* | 617 | 1.00 | |||||||
| Female | 91 | 1.48 | 0.91 | 2.41 | 0.117† | ||||
| Age | 708 | 1.03 | 1.01 | 1.05 | 0.001† | 1.03 | 1.01 | 1.05 | 0.001 |
| Preoperative treatment | |||||||||
| No* | 140 | 1.00 | |||||||
| Yes | 568 | 0.64 | 0.42 | 0.97 | 0.033† | ||||
| Diabetes | |||||||||
| No* | 597 | 1.00 | |||||||
| Yes | 111 | 1.28 | 0.81 | 2.03 | 0.294 | ||||
| COPD | |||||||||
| No* | 663 | 1.00 | |||||||
| Yes | 45 | 1.92 | 1.02 | 3.63 | 0.045† | ||||
| CAD | |||||||||
| No* | 589 | ||||||||
| Yes | 119 | 1.15 | 0.73 | 1.81 | 0.562 | ||||
| Histology | |||||||||
| Adenocarcinoma* | 643 | 1.00 | 1.00 | ||||||
| Squamous cell carcinoma | 65 | 1.42 | 0.81 | 2.50 | 0.226† | 1.77 | 0.92 | 3.38 | 0.087 |
| Tumor location | |||||||||
| Upper/cervical/middle* | 63 | 1.00 | |||||||
| Lower/GEJ | 645 | 0.61 | 0.35 | 1.08 | 0.093† | ||||
| Type of esophagectomy | 0.045† | 0.052 | |||||||
| Ivor Lewis* | 462 | 1.00 | 1.00 | ||||||
| Transhiatal | 59 | 1.61 | 0.88 | 2.95 | 0.126 | 1.36 | 0.73 | 2.54 | 0.329 |
| Total | 49 | 2.30 | 1.24 | 1.30 | 0.009 | 2.27 | 1.20 | 4.29 | 0.011 |
| Minimal invasive | 124 | 1.32 | 0.83 | 2.10 | 0.24 | 1.44 | 0.89 | 2.31 | 0.137 |
| N/A | 14 | 2.20 | 0.72 | 6.73 | 0.17 | 2.48 | 0.80 | 7.74 | 0.117 |
| Minimal invasive surgery | |||||||||
| No* | 584 | 1.00 | |||||||
| Yes | 124 | 1.13 | 0.72 | 1.77 | 0.594 | ||||
| Pathological stage | |||||||||
| 0* | 145 | 1.00 | |||||||
| I | 149 | 0.77 | 0.45 | 1.32 | 0.34 | ||||
| II | 254 | 0.90 | 0.56 | 1.45 | 0.67 | ||||
| III | 144 | 0.77 | 0.44 | 1.33 | 0.346 | ||||
| IV | 16 | 1.75 | 0.60 | 5.15 | 0.31 | ||||
| Clinical stage | |||||||||
| 0* | 5 | 1.00 | |||||||
| I | 108 | 0.55 | 0.09 | 3.47 | 0.52 | ||||
| II | 253 | 0.37 | 0.06 | 2.27 | 0.28 | ||||
| III | 314 | 0.48 | 0.08 | 2.92 | 0.42 | ||||
| IV | 28 | 0.50 | 0.07 | 3.63 | 0.49 | ||||
| ASA-risk scale | |||||||||
| 1-2* | 122 | 1.00 | |||||||
| 3-4 | 586 | 1.21 | 0.75 | 1.95 | 0.442 | ||||
| Salvage operation | |||||||||
| No* | 612 | ||||||||
| Yes | 96 | 1.62 | 1.01 | 2.60 | 0.045† | ||||
Reference group.
Statistically significant for univariable analysis (p<0.25).
OR indicates odds ratio; CI, confidence interval; COPD, chronic pulmonary obstructive disease; CA, coronary artery disease; GEJ, gastroesophageal junction; ASA, American Society of Anesthesiologist.
Technical Hospital Charges
The median technical hospital charge associated with primary admission after surgery for group B ($63,406) was significantly lower than that for group A ($76,685; P < 0.001) (Table 6). Multivariable analysis revealed that the institution of the FTEP decreased hospital charges (β = -41714.3; 95% CI = -63706.3, -19722.3; P < 0.001) even after adjustment for predictors such as tumor histology and location (Table 11). Also, there was no significant difference in median hospital charges associated with readmission within 90 days between the two groups ($13,336 in group A vs $22,373 in group B, P = 0.275). However, when we compared combined technical hospital charges associated with both primary admission after surgery and readmissions within 90 days of discharge, group B ($65,649) still had significantly lower charges than those for group A ($79,117; P < 0.001)(Table 6).
Table 6. Technical Hospital Charges.
| Variable | Group A (N=322) (3/29/2004-3/17/2008) | Group B (N=386) (3/17/2008-3/5/2012) | P-Value |
|---|---|---|---|
| Charges (U.S. dollars) | <0.001 | ||
| Median | 76,685 | 68,406 | |
| Range | 40,740- 1,695,956 | 26,528 – 962,474 | |
| Mean | 134,983 | 93,858 | |
| 90 days readmission charges(U.S dollars) | 0.275 | ||
| Median | 13,336 | 22,373 | |
| Range | 584-293,959 | 115-319,734 | |
| Mean | 32,580 | 42,913 | |
| Combined Charges† (U.S. dollars) | <0.001* | ||
| Median | 79,117 | 65,649 | |
| Range | 40,740- 1,695,956 | 26,528 – 962,474 | |
| Mean | 138,828 | 100,417 |
Statistically significant.
Combined charges associated with primary admission after surgery and readmission within 90 days of discharge.
Table 11. Technical Hospital Charges.
| Variables | N | Univariable Linear regression | Multivariable linear regression | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 95% C.I for β | 95% C.I for β | ||||||||
|
|
|
||||||||
| β | Lower | Upper | P-Value | β | Lower | Upper | P-Value | ||
| Group | |||||||||
| A* | 322 | ||||||||
| B | 386 | -41125 | -63623 | -18627 | 0.000† | -41714 | -63706 | -19722 | 0.000 |
| Gender | |||||||||
| Male* | 617 | ||||||||
| Female | 91 | 35479 | 1803 | 69154 | 0.039† | ||||
| Age | 708 | 953 | -150 | 2057 | 0.090† | ||||
| Preoperative treatment | |||||||||
| No* | 140 | ||||||||
| Yes | 568 | -17911 | -46263 | 10441 | 0.215† | ||||
| Diabetes | |||||||||
| No* | 597 | ||||||||
| Yes | 111 | -485 | -31577 | 30607 | 0.976 | ||||
| COPD | |||||||||
| No* | 663 | ||||||||
| Yes | 45 | 39700 | -7549 | 84849 | 0.101† | ||||
| CAD | |||||||||
| No* | 589 | ||||||||
| Yes | 119 | 3022 | -27209 | 33253 | 0.844 | ||||
| Histology | |||||||||
| Adenocarcinoma* | 643 | ||||||||
| Squamous cell carcinoma | 65 | 105620 | 67255 | 143984 | 0.000† | 77707 | 31525 | 123889 | 0.001 |
| Tumor location | |||||||||
| Upper/cervical/middle* | 63 | ||||||||
| Lower/GEJ | 645 | -96793 | -1.5849 | -57738 | 0.000† | -51569 | -98401 | -4737 | 0.031 |
| Type of esophagectomy | 0.001† | ||||||||
| Ivor Lewis* | 462 | ||||||||
| Transhiatal | 59 | 185575 | -22562 | 59714 | 0.376 | ||||
| Total | 49 | 95169 | 50463 | 139875 | 0.000 | ||||
| Minimal invasive | 124 | -1567 | -31661 | 28528 | 0.919 | ||||
| N/A | 14 | 26220 | -54501 | 106941 | 0.524 | ||||
| Minimal invasive surgery | |||||||||
| No* | 584 | ||||||||
| Yes | 124 | -12056 | -41786 | 17672 | 0.426 | ||||
| Pathological stage | 0.460 | ||||||||
| 0* | 145 | ||||||||
| I | 149 | -21222 | -56297 | 13852 | 0.235 | ||||
| II | 254 | -13090 | -44385 | 18205 | 0.412 | ||||
| III | 144 | 1774 | -33599 | 37147 | 0.922 | ||||
| IV | 16 | 34742 | -4464 | 113947 | 0.389 | ||||
| Clinical stage | |||||||||
| 0* | 5 | ||||||||
| I | 108 | 34574 | -103001 | 172150 | 0.622 | ||||
| II | 253 | 34545 | -101275 | 170365 | 0.618 | ||||
| III | 314 | 54507 | -81057 | 190071 | 0.430 | ||||
| IV | 28 | 44978 | -101036 | 190991 | 0.546 | ||||
| ASA-risk scale | |||||||||
| 1-2* | 122 | ||||||||
| 3-4 | 586 | 16962 | -12946 | 46870 | 0.266 | ||||
| Salvage operation | |||||||||
| No* | 612 | ||||||||
| Yes | 96 | 22361 | -10617.6 | 55340 | 0.184* | ||||
Reference group.
Statistically significant for univariable analysis (p<0.25).
βindicates the regression coefficient; CI, confidence interval; COPD, chronic pulmonary obstructive disease; CAD, coronary artery disease; GEJ, gastroesophageal junction; ASA, American Society of Anesthesiologist.
Discussion
In our study, we demonstrated that a fast-track setup significantly reduced LOS and technical hospital charges as well as the incidence of pulmonary complications without affecting rates of hospital readmission and 30- and 90-day mortality. Taken together, our findings show that the institution of the FTEP was safe and reduced hospital charges.
In the face of escalating healthcare costs, reducing patients' length of postoperative stay and number of postoperative complications is key to using medical resources optimally. Despite major advancements in the perioperative management of esophageal cancer patients, esophagectomy remains significantly associated with high incidences of mortality and morbidity. 19 Over the last decade, fast-track protocols have been used successfully for several surgical specialties4, 7, 20-22 as well as for esophagectomy. In 1998, Brodner et al. conducted a retrospective cohort study that showed that a multimodal approach can be used to enhance recovery after esophagectomy.23 In 2003, Chandrashekhar et al., who suggested that patients could be safely transferred to and managed on a high-dependency unit following immediate extubation after 2-stage esophagectomy, were the first to mention an FTEP.24 In 2004, Cerfolio et al. were the first to publish a study of a fast-track protocol for esophagectomy.10 Later retrospective cohort studies showed that FTEP was associated with reduced LOS.6, 11, 14, 17 A recent single-institution, randomized clinical trial also showed that an enhanced recovery protocol for esophagectomy resulted in a small but significant reduction in LOS.12
Detailed descriptions of the clinical care pathways of the traditional esophagectomy protocol and the FTEP used at our institution are given in Table 3. As part of the FTEP, patients were immediately extubated after surgery, transferred to the post-anesthesia care unit only for a few hours, and then transferred to the telemetry unit. However, 7% of patients on FTEP still went to ICU because these were high risk salvage esophageal resection patients who had other co-morbidities that required ventilator support immediately after surgery. In the telemetry unit, the patients' vital signs, chest tube output, and urine output were monitored hourly, and the patients were allowed to ambulate within 4 hours of their arrival to the floor. Early ambulation reduces postoperative stress and fatigue and facilitates recovery.25, 26 The reduced LOS could be attributed to close monitoring and keeping patients ambulatory in the telemetry unit.
Perioperative fluid therapy plays an important role in the care of patients after esophagectomy. Patients on a FTEP are typically recommended to receive a balanced rather than restrictive regimen of preoperative fluid therapy as well as epidural analgesia for postoperative pain management.13 In our study, there was not much difference in perioperative fluid therapy and postoperative pain management between patients on the traditional esophagectomy protocol and those on the FTEP. For perioperative fluid therapy, patients on the traditional esophagectomy protocol as well as those on the FTEP received 5% dextrose in half the amount of normal saline (0.45% w/v of sodium chloride) at a rate of 125 ml/hour on the day of surgery and postoperative day (POD) 1 and POD 2. After POD 2, the volume of fluid administered was reduced gradually, from 125 ml/hour on POD 3 to 75 ml/hour on POD 4, 50 ml/hour on POD 5, 21 ml/hour on POD 6, and finally to saline lock on POD 7. For their postoperative pain, patients received epidural analgesia with 5 mcg/ml hydromorphone administered with 0.075% bupivacaine at a rate of 10 ml/hour continuously, with patient boluses of 3 ml administered every 10 minutes and clinician boluses of 5 ml administered every 3 hours as needed. This pain management regimen was continued for a maximum of 7 days or stopped early if the patient's chest tubes were removed before that time. The proportion of patients who were discharged on J-tube feeding without barium swallow in the FTEP group (65%) was significantly larger than that in the traditional protocol group (31%; P < 0.001). For patients on the FTEP, a barium swallow was performed between 10-15 days after discharge on an outpatient basis and the J-tube was removed 4-6 weeks after discharge, whenever the patient could take in most of the calories by mouth. Although J-tube feeding has been found to be a safe and effective method of providing postoperative nutritional support,27, 28 it has not been part of the FTEPs other studies have investigated.6, 8, 9, 11-18 Thus, additional studies to investigate the use of J-tube feeding in this setting are warranted. Also, no additional outpatient care was required for fast track patients over the standard pathway. We had a standard follow-up for all esophagectomy patients. First follow-up was done at 6 weeks after discharge, next 3 follow-ups were done every 6 months and later a yearly follow-up was done.
Patients' baseline characteristics such as age and comorbidities including diabetes, chronic obstructive pulmonary disease, and coronary artery disease are significant predictors of morbidity and mortality after esophagectomy.29, 30 In our study, groups A and B had similar proportions of patients with these predictors. In terms of perioperative treatment, the proportion of patients who received neoadjuvant chemoradiotherapy in group B (85%) was higher than that in group A (73%). One meta-analysis showed that, compared with surgery alone, neoadjuvant chemoradiotherapy plus surgery is associated with a higher risk of mortality.31, 32 With regards to tumor characteristics, the patients in group B had larger, more clinically advanced tumors than the patients in group A did. Although these characteristics put patients in group B at a higher risk of poor outcomes, these patients had better outcomes than the patients in group A did even after adjustment for these predictors in the multivariable analysis.
One recent trend in localized esophageal carcinoma surgery is MIE, which utilizes a combined thoracoscopic and laparoscopic approach or a hybrid approach.33 Systematic reviews comparing MIE with conventional methods of open surgery (i.e., transthoracic and transhiatal esophagectomy) found MIE to be safe and associated with better postoperative outcomes.34-39 In the present study, MIE was not part of the FTEP. Given that MIE is a recent development, the proportion of patients who underwent MIE in group B was unsurprisingly higher than that in group A. Multivariable analysis revealed MIE to be associated with pulmonary complications but not LOS.
The anastomotic leak rate (14% in group A vs 13% in group B, P=0.581) seems high because leaks were calculated including clinically non-significant leaks which required no intervention (Table 5). Anastomotic leak was classified as: grade 1, small contained leak in barium or CT requiring no intervention or basic treatment, such as giving antibiotic, or observation; grade 2, small contained leak requiring minimum intervention, such as stent or drainage placement; grade 3, leak requiring a repeat operation; and grade 4, conduit loss requiring conduit resection (Table 5). For patients who had an anastomotic leak, the mean LOS increased by 2 days but there was no difference in 30 days perioperative mortality [2.1% in leak group vs 2.9% in no leak group, P =1.000].
Our study, which included patients treated on a traditional esophagectomy protocol during a 4-year period immediately before the institution of a FTEP as well as patients treated on a FTEP during its first 4 years of implementation, enabled us to thoroughly assess postoperative outcomes in a large group of patients over a long period. Owing to the introduction of a FTEP in March 2008, we reduced the proportion of patients who were immediately transferred to the SICU after esophagectomy from 71% in March 2004 to just 7% in March 2012 (Table 5.) Ours is one of the first studies conducted in the United States to assess the impact of a FTEP on hospital charges. In our study, there was about 17% reduction in the median technical charges and 31% reduction in the mean technical charges after institution of a FTEP. The reduction in hospital charges can be contributed to decreased SICU days, LOS and post-operative complication rate in group B patients. This suggested that a FTEP reduced hospital charges without compromising the safety of the patients.
Our study had a few potential limitations. Although we assessed important outcomes such as patient safety and LOS, we did not assess patients' satisfaction with the new protocol because this information had not been collected for all esophagectomy patients at their time of discharge and follow-up visits. Patients' satisfaction is an important indicator of quality of care to help evaluate efficacy of a new protocol. For FTEP, patient satisfaction may be driven by various factors including pain management, ability to swallow, early ambulation, fewer postoperative complications, and enhanced recovery. However, we had collected data on difficulty in swallowing noted during the patient's post-operative follow-up visits and found it to be similar across both Group A(11%) and Group B(9%, P=0.151). In the future, survey studies should be conducted to assess patients' satisfaction and approval of the fast-track protocol. Another potential limitation of our study is that in the cost-effectiveness analysis, we performed only univariable and multivariable analyses to compare the overall technical charges in group A with those in group B. A detailed analysis of the cost-effectiveness of a FTEP using a more systematic approach is beyond the scope of this paper and will be published separately. More prospective randomized studies are needed to support the use of a fast-track protocol for esophageal cancer patients and provide evidence of the protocol's cost-effectiveness.
Conclusion
The results of this study confirm existing data regarding the safety of FTEPs. We found that various components of a FTEP, including the avoidance of direct ICU admission after surgery, initiation of mobilization shortly after transfer to a telemetry unit, early initiation of pulmonary toileting, and minimal use of nasogastric tubes and drains, helped reduce patients' postoperative morbidity and LOS. We also found FTEP to be as safe as the traditional esophagectomy protocol in terms of postoperative mortality. Thus, a FTEP has positive clinical and financial implications for esophageal cancer patients, and its use should be extended to other esophageal surgical centers.
Acknowledgments
We thank the nursing staff of the telemetry unit and Wanda Reese for help in preparation of the manuscript.
Sources of Support: This work was supported in part by the Homer Flower Gene Therapy Fund, the Charles Rogers Gene Therapy Fund, the Margaret W Elkins Endowed Research Fund and the Phalan Thoracic Gene Therapy Fund.
References
- 1.Wu PC, Posner MC. The role of surgery in the management of oesophageal cancer. Lancet Oncol. 2003;4:481–8. doi: 10.1016/s1470-2045(03)01167-7. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630–41. doi: 10.1016/s0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 3.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189–98. doi: 10.1097/SLA.0b013e31817f2c1a. [DOI] [PubMed] [Google Scholar]
- 4.Watkins AC, White PF. Fast-tracking after ambulatory surgery. J Perianesth Nurs. 2001;16:379–87. doi: 10.1053/jpan.2001.28887. [DOI] [PubMed] [Google Scholar]
- 5.Jakobsen D, Sonne E, Kehlet H. Nursing workload and fast track colonic surgery. J Adv Perioperat Care. 2006;2:177. [Google Scholar]
- 6.Lee L, Li C, Ferri LE, et al. Mo1720 Economic Impact of an Enhanced Recovery Pathway for Esophagectomy. Gastroenterol. 2013;144:S–1099. doi: 10.1002/bjs.9224. [DOI] [PubMed] [Google Scholar]
- 7.Kehlet H. Fast-track colonic surgery: status and perspectives. Recent Results Cancer Res. 2005;165:8–13. doi: 10.1007/3-540-27449-9_2. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Ferri LE, Mulder DS, et al. An enhanced recovery pathway decreases duration of stay after esophagectomy. Surg. 2012 doi: 10.1016/j.surg.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Preston S, Markar S, Baker C, et al. Impact of a multidisciplinary standardized clinical pathway on perioperative outcomes in patients with oesophageal cancer. Br J Surg. 2013;100:105–112. doi: 10.1002/bjs.8974. [DOI] [PubMed] [Google Scholar]
- 10.Cerfolio RJ, Bryant AS, Bass CS, et al. Fast tracking after ivor lewis esophagogastrectomy*. CHEST. 2004;126:1187–1194. doi: 10.1378/chest.126.4.1187. [DOI] [PubMed] [Google Scholar]
- 11.Blom RL, van Heijl M, Bemelman WA, et al. Initial Experiences of an Enhanced Recovery Protocol in Esophageal Surgery. World J Surg. 2013;37:2372–2378. doi: 10.1007/s00268-013-2135-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhao G, Cao S, Cui J. Fast-track surgery improves postoperative clinical recovery and reduces postoperative insulin resistance after esophagectomy for esophageal cancer. Support Care Cancer. 2013:1–8. doi: 10.1007/s00520-013-1979-0. [DOI] [PubMed] [Google Scholar]
- 13.Findlay JM, Gillies RS, Millo J, et al. Enhanced Recovery for Esophagectomy: A Systematic Review and Evidence-Based Guidelines. Ann Surg. 2013 doi: 10.1097/SLA.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 14.Munitiz V, Martinez-de-Haro L, Ortiz A, et al. Effectiveness of a written clinical pathway for enhanced recovery after transthoracic (Ivor Lewis) oesophagectomy. Br J Surg. 2010;97:714–718. doi: 10.1002/bjs.6942. [DOI] [PubMed] [Google Scholar]
- 15.Jiang K, Cheng L, Wang JJ, et al. Fast track clinical pathway implications in esophagogastrectomy. World J Gastroenterol. 2009;15:496. doi: 10.3748/wjg.15.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Low DE, Kunz S, Schembre D, et al. Esophagectomy—it's not just about mortality anymore: standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. J Gastrointest Surg. 2007;11:1395–1402. doi: 10.1007/s11605-007-0265-1. [DOI] [PubMed] [Google Scholar]
- 17.Cao S, Zhao G, Cui J, et al. Fast-track rehabilitation program and conventional care after esophagectomy: a retrospective controlled cohort study. Support Care Cancer. 2013;21:707–14. doi: 10.1007/s00520-012-1570-0. [DOI] [PubMed] [Google Scholar]
- 18.Tang J, Humes D, Gemmil E, et al. Reduction in length of stay for patients undergoing oesophageal and gastric resections with implementation of enhanced recovery packages. Ann R Coll Surg Engl. 2013;95:323–328. doi: 10.1308/003588413X13629960046039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson MK, Martin TR, Reeder LB, et al. Mortality after esophagectomy: risk factor analysis. World J Surg. 1997;21:599–603. doi: 10.1007/s002689900279. discussion 603-4. [DOI] [PubMed] [Google Scholar]
- 20.Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg. 2001;122:318–24. doi: 10.1067/mtc.2001.114352. [DOI] [PubMed] [Google Scholar]
- 21.Collier PE. Fast tracking carotid endarterectomy: practical considerations. Semin Vasc Surg. 1998;11:41–5. [PubMed] [Google Scholar]
- 22.Wang G, Jiang ZW, Xu J, et al. Fast-track rehabilitation program vs conventional care after colorectal resection: a randomized clinical trial. World J Gastroenterol. 2011;17:671–6. doi: 10.3748/wjg.v17.i5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodner G, Pogatzki E, Van Aken H, et al. A multimodal approach to control postoperative pathophysiology and rehabilitation in patients undergoing abdominothoracic esophagectomy. Anesth & Analg. 1998;86:228–234. doi: 10.1097/00000539-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Chandrashekar MV, Irving M, Wayman J, et al. Immediate extubation and epidural analgesia allow safe management in a high-dependency unit after two-stage oesophagectomy. Results of eight years of experience in a specialized upper gastrointestinal unit in a district general hospital. Br J Anaesth. 2003;90:474–9. doi: 10.1093/bja/aeg091. [DOI] [PubMed] [Google Scholar]
- 25.Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- 26.Jakobsen DH, Sonne E, Basse L, et al. Convalescence after colonic resection with fast-track versus conventional care. Scand J Surg. 2004;93:24–28. doi: 10.1177/145749690409300105. [DOI] [PubMed] [Google Scholar]
- 27.Gerndt SJ, Orringer MB. Tube jejunostomy as an adjunct to esophagectomy. Surg. 1994;115:164. [PubMed] [Google Scholar]
- 28.Gupta V. Benefits versus risks: a prospective audit. World J Surg. 2009;33:1432–1438. doi: 10.1007/s00268-009-0019-1. [DOI] [PubMed] [Google Scholar]
- 29.Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75:217–22. doi: 10.1016/s0003-4975(02)04368-0. discussion 222. [DOI] [PubMed] [Google Scholar]
- 30.Wright CD, Kucharczuk JC, O'Brien SM, et al. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg. 2009;137:587–95. doi: 10.1016/j.jtcvs.2008.11.042. discussion 596. [DOI] [PubMed] [Google Scholar]
- 31.Fiorica F, Di Bona D, Schepis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925–30. doi: 10.1136/gut.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaklamanos IG, Walker GR, Ferry K, et al. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta-analysis of randomized clinical trials. Ann Surg Oncol. 2003;10:754–61. doi: 10.1245/aso.2003.03.078. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen NT, Follette DM, Wolfe BM, et al. Comparison of minimally invasive esophagectomy with transthoracic and transhiatal esophagectomy. Arch Surg. 2000;135:920. doi: 10.1001/archsurg.135.8.920. [DOI] [PubMed] [Google Scholar]
- 34.Verhage RJ, Hazebroek E, Boone J, et al. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir. 2009;64:135. [PubMed] [Google Scholar]
- 35.Gemmill E, McCulloch P. Systematic review of minimally invasive resection for gastro-oesophageal cancer. Br J Surg. 2007;94:1461–1467. doi: 10.1002/bjs.6015. [DOI] [PubMed] [Google Scholar]
- 36.Biere S, Cuesta M, Van Der Peet D. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir. 2009;64:121–133. [PubMed] [Google Scholar]
- 37.Sgourakis G, Gockel I, Radtke A, et al. Minimally invasive versus open esophagectomy: meta-analysis of outcomes. Dig Dis Sci. 2010;55:3031–3040. doi: 10.1007/s10620-010-1153-1. [DOI] [PubMed] [Google Scholar]
- 38.Decker G, Coosemans W, De Leyn P, et al. Minimally invasive esophagectomy for cancer. Eur J Cardiothorac Surg. 2009;35:13–21. doi: 10.1016/j.ejcts.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 39.Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis Surg Endosc. 2010;24:1621–1629. doi: 10.1007/s00464-009-0822-7. [DOI] [PubMed] [Google Scholar]


