Abstract
Biochar is increasingly been used as a soil amendment to improve water holding capacity, reduce nutrient leaching, increase soil pH and also as a means to reduce contamination through sorption of heavy metals or organic pollutants. The sorption behavior of three phenylurea herbicides (monuron, diuron, linuron) on five biochars (Enhanced Biochar, Hog Waste, Turkey Litter, Walnut Shell and Wood Feedstock) and an agricultural soil (Yolo silt loam) was investigated using a batch equilibration method. Sorption isotherms of herbicides to biochars were well described by the Freundlich model (R2 = 0.93 -- 0.97). The adsorption KF values ranged from 6.94 to 1306.95 mg kg−1 and indicated the sorption of herbicides in the biochars and Yolo soil was in the sequence of linuron > diuron > monuron and walnut shell biochar > wood feedstock biochar > turkey litter biochar > enhanced biochar > hog waste biochar > Yolo soil. These data show that sorption of herbicides to biochar can have both positive (reduced off-site transport) and negative (reduced herbicide efficacy) implications and specific biochar properties, such as H/C ratio and surface area, should be considered together with soil type, agriculture chemical and climate condition in biochar application to agricultural soil to optimize the system for both agricultural and environmental benefits.
Keywords: Biochar, phenylurea herbicides, monuron, diuron, linuron, sorption isotherm
Introduction
Biochar is a biomass-derived char material intended for soil application. Biochar soil application is regarded as a low risk strategy for sequestering carbon [1] and reducing greenhouse gases emission (e.g. N2O, CH4). [2, 3] Biochar addition to soil can have many agricultural benefits, such as improving soil quality, soil structure, and nutrient availability for plants and microbial populations. [4] In addition, biochar can increase soil water holding capacity, especially in sandy soils. [5] The high specific surface area and cation exchange capacity of biochar compared to soil can be effective in reducing leaching of nutrients such as nitrate, [6] ammonium, [7] and phosphorus. [8]
Biochar is a not fully carbonized product produced by pyrolysis of biomass in a low oxygen environment. [9] Pyrolysis processing factors such as temperature, residence time and oxygen content affect biochar characteristics, such as surface area, [10] functional groups [11] and biochar stability. [12] The effects of biochar soil amendment on, for instance, soil water holding capacity, [13] nutrient retention, [5] herbicide sorption, [14] and reducing heavy metal bioavailability [15] vary with biochar characteristics. The variation in effects of different biochar products presents an opportunity to select or even create a biochar that best matches the needs of a particular agricultural and environmental application.
Phenylurea herbicides are widely utilized for herbaceous and perennial weed control of non-crop areas and for pre-emergent treatment of fruit crops. [16] Globally, they are detected in surface water, ground water, soil and sediment in areas wherever there is extensive use. [17] Herbicides leach through soil into groundwater during rainfall and irrigation and may persist in soil and water for a long period of time. [18] Cost-effective and environmentally friendly technologies, such as biochar amendment, are needed to reduce these losses and impacts of residues in soils.
Our objective was to investigate the potential impact of biochar on phenylurea herbicide environmental behavior and fate. We chose 5 biochars, with representative surface characteristics and produced from different common feedstocks, and monuron, diuron and linuron sorption isotherms on biochars were measured. The three phenylurea herbicides were selected based on their wide application in agriculture and also their representative lipophilicity among phenylurea herbicides.
Materials and methods
Chemicals
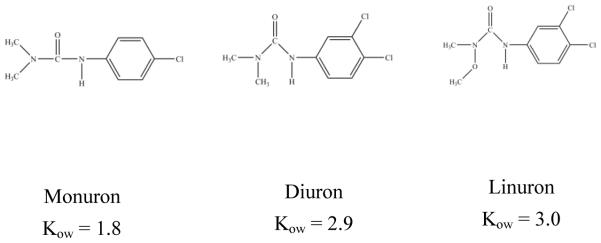
Monuron (3-(4-chlorophenyl)-1-1-dimethylurea) (99.5%), diuron (N-(3,4-dichlorophenyl)-N,N-dimethyl-urea) (99.5%) and linuron (N-(3, 4-dichlorophenyl)-N′-methoxy-N′-methylurea) (99.5%) were obtained from Chem Service (West Chester, PA) (structures in Fig. 1). Stock solutions of these three phenylurea herbicides were prepared in methanol/water solution (50:50, v/v). Water (18.2 MΩ·cm) was supplied by a Barnstead Nanopure water system (Thermo Scientific, OH). All chemicals were used as received.
Figure 1.
Molecular structures of three phenyl urea herbicides and Kow values
Biochars and soil
Five biochars including enhanced biochar (EB), hog waste (HW), turkey litter (TL), walnut shell (WA) and wood feedstock (WF) biochars were obtained from various suppliers. [19] An agricultural soil sample was taken from Plot 6-1 (conventionally managed, irrigated, unfertilized wheat/fallow treatment), from the Russell Ranch Sustainable Agricultural Research Facility on the University of California, Davis campus (32°N, 121°50′W) in Jan. 2012 and air dried. The biochars and soil samples were sieved to pass through a 2 mm mesh, sealed in glass bottles and stored at room temperature until required.
Biochar and soil characterization
Characteristics of the different biochars are summarized in Table 1. More detailed information on the methods utilized and additional data for characterization is reported elsewhere. [19] The soil was a Yolo silt loam (42.8% sand, 35.20% silt and 22.1% clay) with 1.0% organic C, 20.6 cmol kg−1 cation exchange capacity and a pH of 7.78.
Table 1.
Source material, pyrolysis temperature, moisture content, ash content, BET surface area, pH, cation exchange capacity, elemental composition, atomic ratio of biochars
| Biochar | EB | HW | TL | WS | WF |
|---|---|---|---|---|---|
| Source material | wood and algal digestate |
soft wood | turkey litter | walnut shell | softwood (Mixed Fir)* |
| Pyrolysis temperature (°C) |
600-700 | 600-700 | 700-800 | 900 | 510 |
| BET surface area (m2 g−1) |
2.0 | 25.2 | 21.8 | 227.1 | 165.8 |
| Ash content (%) | 6.4 | 2.4 | 64.0 | 40.4 | 3.0 |
| pH | 6.8 | 7.5 | 10.9 | 9.7 | 7.3 |
| Cation exchange capacity (cmol g−1) |
67.0 | 26.2 | 24.4 | 33.4 | 12.0 |
| C (wt %) | 58.1 | 68.2 | 15.6 | 55.3 | 83.9 |
| H (wt %) | 4.2 | 3.7 | 0.8 | 0.9 | 1.9 |
| H/C | 0.072 | 0.054 | 0.053 | 0.016 | 0.022 |
Predominately Douglas Fir with some White Fir.
Batch sorption experiments
Sorption of monuron, diuron and linuron on all five biochars and soil was determined via batch isotherms. The batch sorption experiments were conducted using 8 mL glass vials with polytetrafluoroethylene (Teflon)-lined screw caps. The initial aqueous phase diuron, linuron and monuron concentrations were 0, 0.5, 1, 5, 25 and 50 mg L−1 with. 0.12 g sorbent in 5.2 mL of background solution (200 mg L−1 CaCl2, 5 mg L−1 NaHCO3 and 200 mg L−1 NaN3 to inhibit biological activity). Prior to addition of herbicides the vials with sorbent and background solution were reacted on an end-over-end shaker (8 rpm) for 48 h to saturate biochar. Following this pre-equilibration step, the pH was adjusted to approximately 7.0 using 0.05 mol L−1 HCl to minimize pH effects on sorption results. Different amount of herbicides and background solution were added into vials to reach the desired concentrations in a total volume of 6.0 mL. Based on our preliminary study, samples were spiked with the pesticides and rotated in the dark on an end-over-end shaker (8 rpm) at 22 ± 1 °C for 48 h.
The supernatants were filtered through 0.45 µm Millipore membrane filter (Millipore Corporation, NH) and the filtrate collected into 2 mL amber LC vials. External standards were similarly filtered to correct for solute loss due to filtration. Control samples with no biochar containing sorbate and with biochar without sorbate were concurrently established with the sorption samples. All experiments were conducted in triplicates.
Analytical methods
Concentrations of monuron, diuron and linuron in in the aqueous phase were analyzed using an Agilent 1200 Series HPLC System with a DAD and an Agilent ZORBAX Eclipse Plus C18 column, 4.6 mm × 250 mm, 5 μm at a flow rate of 0.4 mL min−1 and an injection volume of 5 μL. The ultraviolet DAD was set at 254 nm for diuron, linuron and monuron determination. Isocratic elution was performed with 0.1% formic acid in water and 0.1% formic acid in methanol.
Under these conditions, the elution times were approximately 2.2 min, 3.7 min, and 4.9 min for monuron, diuron, and linuron respectively. Compounds were identified by comparing their retention time values with those of standards. Data was collected and processed using Agilent Chemstation software. The limit of detection for monuron was 1.16 mg L−1, while the limit of detection for diuron was 0.091 mg L−1 for diuron and 0.066 mg L−1 for linuron.
Results and Discussion
Physical and chemical characterization of the adsorbents
The biochars used were pyrolyzed from a variety of feedstocks at temperatures ranging 510 to 900 °C and the measured physical and chemical characteristics of these biochars varied accordingly (Table 1). Pyrolysis temperature is the primary factor for differences in the observed physical and chemical characteristics of biochar. Of these physical characteristics, surface area showed the greatest variability, ranging from 2 to 227.1 m2 g−1. Generally, a high pyrolysis temperature resulted in a higher surface area. The increase in biochar surface area with increasing pyrolysis temperature has been previously reported. [10, 20] However, it should be noted that the surface area of biochars depends not only on pyrolysis temperature, but also on characteristics of source materials, heating rate and reaction time. Increasing pyrolysis temperatures also can result in greater CO2 release, [21] generally resulting in higher ash contents (40.4% for WA and 3% for WF).
Pyrolysis temperature also has a distinct impact on chemical properties of the biochars, with aromaticity increasing with pyrolysis temperature as indicated by the decreasing H/C ratios. The atomic ratio of H/C is an index for aromaticity and polarity [22] and this result is in agreement with other studies also showing the ratio of aromatic carbon increases with an increase in pyrolysis temperature. [23]
Sorption isotherms
The sorption isotherm data for the phenylurea herbicides to the various biochars were analyzed using both the Langmuir and Freundlich isotherm models. The linearized Langmuir isotherm is described in Equation 1:
| (1) |
where qe (in mg g−1) is the amount of solute adsorbed per unit mass of adsorbent, ce (in mg L−1) the equilibrium concentration of solute, qm (in mg g−1) the amount of solute adsorbed per unit mass of adsorbent required for monolayer coverage of the surface, b (in L mg−1) a constant related to the heat of adsorption. The Freundlich isotherm equation in linearized form is Equation 2:
| (2) |
where qe and ce have the same definitions as in Langmuir equation above. The Freundlich constant, KF, in units of (mg kg−1) and 1/n represents the degree of non-linerarity of the isotherm. When intraparticle retardation increases as the concentration inside the particle declines, n in the Freundlich model is unequal to unity-the sorption isotherm is nonlinear. [24]
The values of Kd (the partition coefficient) were calculated from the fit of the experimental sorption isotherms at selected Ce (5 and 50 mg L−1) using the formula given in Equation 3:
| (3) |
The Kd values indicated the order of herbicide sorption capacity of sorbents. The WS biochar showed the greatest sorption to monuron (Table 2).
Table 2.
Partition coefficient (Kd) for sorption isotherms of herbicides on soil and biochars.
| Adsorbent | monuron | diuron | linuron | |
|---|---|---|---|---|
| Yolo soil |
Kd5 Kd50 |
0.0075±0.0029 0.0025±0.00074 |
0.016±0.0024 0.011±0.0037 |
0.069±0.0056 0.02±0.0031 |
| EB |
Kd5 Kd50 |
0.2±0.043 0.052±0.0037 |
0.27±0.094 0.092±0.012 |
0.24±.044 0.078±.016 |
| HW |
Kd5 Kd50 |
0.041±0.0083 0.026±0.0010 |
0.16±0.034 0.034±0.0013 |
0.21±0.0051 0.043±0.0056 |
| TL |
Kd5 Kd50 |
0.68±0.093 0.15±0.042 |
0.61±0.0068 0.23±0.054 |
-*
0.28±0.058 |
| WF |
Kd5 Kd50 |
- 0.15±0.045 |
- 0.11±0.0071 |
- 0.18±0.070 |
| WS |
Kd5
Kd50 |
- 2.5±0.85 |
- - |
- - |
The equilibrium concentration was below detection limit due to the high sorption capacity of biochar.
Most of the data describing the sorption isotherms fit well to the Freundlich equation (Tables 3, 4); however, for some combinations of biochar and herbicides, the fit to the Freundlich equations was relatively poor and the Langmuir model provided a better fit.
Table 3.
Freundlich parameters (KF and n) and Langmuir parameters (qm and b) for sorption isotherms of herbicides on Yolo soil.
| Adsorbent | Herbicide | Freundlich Model | Langmuir Model | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| KF (mg kg−1) | n | R2 | qm (mg kg−1) | b | R2 | ||
| monuron | 6.94±0.16 | 1.3±0.08 | 0.95 | 11.79±1.90 | 0.08±0.03 | 0.73 | |
| Yolo Soil | diuron | 22.22±0.45 | 1.31±0.06 | 0.97 | 32.39±8.23 | 0.11±0.08 | 0.51 |
| linuron | 63.67±2.20 | 1.41±0.08 | 0.96 | 86.93±6.99 | 0.09±0.02 | 0.93 | |
Table 4.
Freundlich parameters (KF and n) and Langmuir parameters (qm and b) for sorption isotherms of herbicides on biochars.
| biochar | herbicide | Freundlich model | Langmuir model | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| KF (mg kg−1) | n | R2 | qm (mg kg−1) | b (mg L−1) | R2 | ||
| monuron | 60.89±7.43 | 0.93±0.16 | 0.7 | −0.98±1.53 | −0.13±0.05 | −0.04 | |
| EB | diuron | 156.61±9.02 | 1.13±0.07 | 0.95 | 153.41±46.07 | 0.17±0.10 | 0.42 |
| linuron | 235.81±11.17 | 1.56±0.08 | 0.98 | 198.73±17.10 | 0.13±0.03 | 0.94 | |
| monuron | 44.09±1.56 | 1.15±0.07 | 0.95 | 80.91±27.49 | 0.08±0.05 | 0.35 | |
| HW | diuron | 94.46±6.12 | 1.31±0.12 | 0.91 | 132.01±14.33 | 0.09±0.02 | 0.88 |
| linuron | 208.16±5.48 | 1.89±0.06 | 0.99 | 135.25±10.09 | 0.16±0.04 | 0.96 | |
| monuron | 261.36±37.16 | 1.08±0.12 | 0.85 | 61.44±41.89 | −2.69±16.88 | 0.08 | |
| TL | diuron | 550.1±112.3 | 1.48±0.19 | 0.88 | 256.16±23.7 | 0.36±0.09 | 0.94 |
| linuron | 647.53±203.52 | 1.75±0.26 | 0.9 | 303.99±56.18 | 0.25±0.11 | 0.85 | |
| monuron | 1236.97±293.84 | 6.52±1.19 | 0.85 | 177.92±11.02 | 9.34±50.64 | 0.98 | |
| WF | diuron | 1216.58±92.59 | 7.8±0.47 | 0.98 | 177.15±1.70 | 2.36±0.59 | 1 |
| linuron | 1306.95±261.59 | 6.8±1.4 | 0.82 | 178.66±10.79 | −27.88±404.95 | 0.98 | |
* The aqueous equilibrium concentrations of WS biochar sorption experiments were below detection limit for most samples due to its high sorption capacity and the data are therefore not presented here.
Herbicide sorption to Yolo soil
All data for the soil sorption isotherms fit well to a Freundlich model (Table 3), as indicated by the high regression coefficients (R2 = 0.95 – 0.97) and nonlinear adsorption isotherms (1/n ≠ 1) observed.
The KF values indicate that sorption of herbicides in Yolo soil follows the sequence of linuron > diuron > monuron, which is the same order that was observed for the octanol-water partition coefficient (Kow) (Fig. 1, Kow values were from literature [25]) of the three herbicides. Kow value is an index of the adsorptive hydrophobicity. Among the three herbicides, the Kow of linuron was the highest. Herbicides with high Kow values would be expected to be adsorbed on the hydrophobic solid phase due to its greater hydrophobicity and van der Waals interaction. [26] The functional groups on herbicides, for instance –CH3 and –Cl which are hydrogen bonding donors, also increases adsorption affinity. [27] The Freundlich model parameters (1/n < 1) suggested that sorption of the herbicides to Yolo soil is to both the clay fraction and soil organic matter. [28-30]
Herbicide sorption to biochar
The data describing herbicide sorption to the five biochars fit well to a Freundlich model (Table 4). The one exception was WF biochar, which fit better to the Langmuir model. The WS biochar has the largest capacity of the biochars for herbicide adsorption and the equilibrium concentrations for all sorption experiments were below detection limit. According to the Freundlich model, nonlinear sorption isotherms (1/n ≠ 1) were observed in all cases.
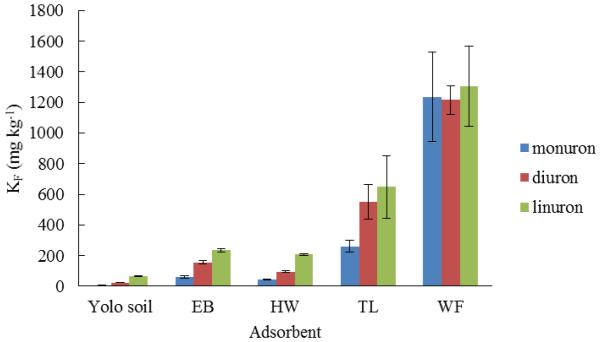
The KF values (as shown in Fig. 2) suggest that sorption of herbicides in biochars decreased in intensity according to the following sequence: linuron > diuron > monuron and the sorption of monuron, diuron and linuron in biochars decreased in intensity according to the following sequence: WS > WF > TL > EB > HW. The KF values are all greater than those observed for Yolo soil. These data demonstrate that the herbicides had a higher affinity for all biochars than for Yolo silt loam soil. Adding biochar into soil has potential to significantly increase phenylurea herbicide sorption, which could impact the transmission, fate and effectiveness of herbicides.
Figure 2.
Plot showing Freundlich constant (KF) values of biochars and soil
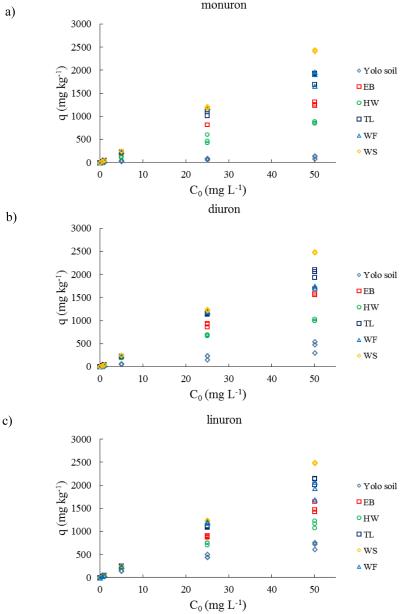
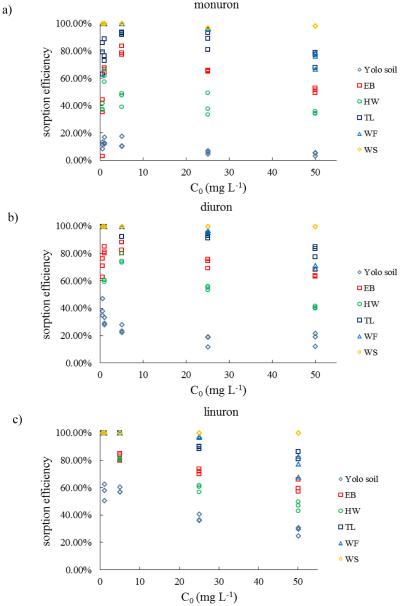
WS biochar shows excellent sorptive properties among biochars. The relationship between initial concentration of herbicides in aqueous solution and the amount of herbicide adsorbed per unit mass of adsorbent also reflects the sorption capacity of adsorbents (Fig. 3). The results indicate that the sorption capacity of WS biochar to all three kinds of herbicides were the highest among biochars. The parameter qe in Freundlich model indicated the highest sorption capacity of WS biochar among all the biochars for all three herbicides. Sorption efficiency of WS biochar remained 100% for all initial concentrations (0.5 to 50 mg L−1) of diuron and linuron and greater than 95% for monuron (0.5 to 50 mg L−1) (Fig. 4).
Figure 3.
Relationship between initial concentration and amount of sorbed herbicides: (a) monuron, (b) diuron, (c) linuron
Figure 4.
Sorption efficiency of adsorbents toward herbicides: (a) monuron, (b) diuron, (c) linuron
The biochars used in this study contained both carbonized and non-carbonized domains, which potentially can express varied reactivities with sorbates and thus represent present different sorption mechanisms. [31] Analysis of the sorption data suggests that monuron, diuron and linuron are likely binding to the biochars via multiple sorption mechanisms.
The nonlinearity of absorption isotherms varied between biochars. The nonlinearity of sorption isotherms for monuron, diuron and linuron observed on the biochars is a characteristic of sorption processes arising from site-specific interactions (for 1/n < 1) occurring on the carbonized phase of the biochar. [32] The carbonized fraction of biochars is sometimes referred to as a “glassy” domain, whereas the non-carbonized soil organic matter is a rubbery domain. [24, 33, 34] Generally, the sorption of organic compounds such as herbicides on carbonized phase of biochar can be characterized by nonlinear adsorption [20]; however, sorption on the noncarbonized phase is better described by a partitioning mechanism that follows a linear isotherm. [35] A lower nonlinearity was observed in the low temperature biochar (e.g. EB biochar) sorption results and higher nonlinearity was observed in the high temperature biochar (e.g. WF biochar). These results indicate that a “glassy” domain sorption mechanism is involved in sorption of phenyluea herbicides to biochar produced under high temperatures. The mechanism of low temperature produced biochar sorption is similar to that involved in sorption to soil organic matter. The incomplete carbonization of low temperature biochar results in biochar with larger amounts of non-carbonized (rubbery domain) carbon than high temperature biochar. The microbial availability of carbon associated with the rubbery domain of low temperature biochar is relatively higher than that associated with the carbonized phase of higher temperature biochar. [36] Hence, the sorption capacity of phenylurea herbicides to high rubbery domain biochar may be reduced over time due to degradation of the rubbery domain as biochar ages after field application.
Abundance of rubbery and glassy domains can also be inferred from the biochar H/C ratios. Biochars with high H/C ratios, such as EB, contain larger amounts of the original organic residues. A decrease in H/C ratio indicates more complete carbonization and higher saturation in the biochar. The 1/n value for diuron and linuron sorption data increased with the atomic H/C ratio of biochars, which indicates that the higher the aromaticity of sorbent, the higher the nonlinearity of the sorption isotherms. It is noted that this positive correlation was observed in the higher Kow herbicides (e.g. linuron and diuron), but not in the lower Kow herbicide (e.g. monuron). This indicates that glassy domain of biochars plays an important role in high lipophilic herbicide sorption.
Positive and negative effects of biochar application in soil related to herbicides
The high sorption capacity of biochars for the phenylurea herbicides reported in this study is consistent with previously published data. [25, 37] Biochar amendment to agricultural soil significantly enhanced sorption of linuron and diuron [38, 39] and reduced leaching of 12 kinds of phenylurea herbicides from soil to groundwater. [25] The large capacity for biochars to adsorb herbicides also substantially reduced leaching of linuron, alachlor, and metalaxyl in a sandy soil. [40]
Sorption capacity of herbicides to biochar amended soil can be lower than theoretical sorption capacity based on biochar and soil sorption capacity measured by batch sorption experiments. Organo-mineral interactions between soil and biochar can compete binding sites on biochar surface with herbicides, which can diminish biochar herbicide sorption capacity. [41,42] During the ageing of biochar, the organo-mineral interactions can also convert binding sites on biochar surface, which can also influence herbicide sorption capacity of biochar amended soil, both positive and negative impacts reported previously. [42,43]
On the other hand, biochar amendment can reduce the effectiveness of pesticides in soil [44] and has been shown to reduce the bioavailability of herbicides to weeds in soils. [45] This could require increased inputs of herbicides and increased costs of agricultural management. However, the increased adsorption capacity, if managed correctly, could possibly provide a mechanism that would permit a slow release source of herbicide from biochar and thus lengthen the period of effectiveness of the herbicide application.
Based on both lab and field scale experiments, the transport of herbicides in soil depends not only on soil properties but also climatic conditions, especially hydrological processes, such as rainfall events and soil moisture condition. [46-48] These two factors can also impact the long term effects of biochar soil amendment and interact in the ageing of biochar. Sorption capacity of aged biochar has been observed in some cases to decrease with time [43] and, in other cases, remain similar to the behavior of freshly added biochar. [49] Based on the results above, herbicide application rates may need to be adjusted depending on how a particular biochar ages and particular environmental conditions; this topic deserves more research.
Conclusions
Adsorption isotherms data of monuron, diuron, linuron on five biochars and Yolo soil were well described by the Freundlich model. The adsorption KF values decreased in magnitude across the different herbicides: linuron > diuron > monuron- and for the different biochars and soil: WS > WF > TL > EB > HW > Yolo soil. These data confirm that broad generalizations for all agrochemical interactions in soil with all biochars cannot be made. This study demonstrates that specific biochar properties, for instance H/C ratio and surface area, should be utilized along with soil type, target chemical and climatic conditions to optimize a management system for pesticide application to soil in order to achieve both agricultural and environmental benefits.
Acknowledgment
This publication was made possible by grant number 5 P42 ES004699 from the National Institute of Environmental Health Sciences (NIEHS), NIH and the contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH. Additional funding was provided by the United States Department of Agriculture (USDA) National Institute of Food and Agriculture (NIFA) through Hatch Formula Funding (provided to their respective institutions) and multistate regional project W-2082.
References
- [1].Lehmann J. A handful of carbon. Nature. 2007447(7147):143–144.Q. doi: 10.1038/447143a. [DOI] [PubMed] [Google Scholar]
- [2].Bruun EW, Ambus P, Egsgaard H, Hauggaard-Nielsen H. Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol. Biochem. 2012;46:73–79. [Google Scholar]
- [3].Liu Y, Yang M, Wu Y, Wang H, Chen Y, Wu W. Reducing CH4 and CO2 emissions from waterlogged paddy soil with biochar. J. Soils Sediment. 2011;11(6):930–939. [Google Scholar]
- [4].Sohi SP, Krull E, Lopez-Capel E, Bol R. Sparks DL, editor. A review of biochar and its use and function in soil. Adv Agron. 2010:47–82. [Google Scholar]
- [5].Uzoma KC, Inoue M, Andry H, Zahoor A, Nishihara E. Influence of biochar application on sandy soil hydraulic properties and nutrient retention. J. Food Agric. Environ. 2011;9(3-4):1137–1143. [Google Scholar]
- [6].Ventura M, Sorrenti G, Panzacchi P, George E, Tonon G. Biochar reduces short-term nitrate leaching from a horizon in an apple orchard. J. Environ. Qual. 2013;42(1):76–82. doi: 10.2134/jeq2012.0250. [DOI] [PubMed] [Google Scholar]
- [7].Zheng J, Stewart CE, Cotrufo MF. Biochar and nitrogen fertilizer alters soil nitrogen dynamics and greenhouse gas fluxes from two temperate soils. J. Environ. Qual. 2012;41(5):1361–1370. doi: 10.2134/jeq2012.0019. [DOI] [PubMed] [Google Scholar]
- [8].Parvage MM, Ulen B, Eriksson J, Strock J, Kirchmann H. Phosphorus availability in soils amended with wheat residue char. Biol. Fert. Soils. 2013;49(2):245–250. [Google Scholar]
- [9].Lehmann J. Bio-energy in the black. Front. Ecol. Environ. 2007;5(7):381–387. [Google Scholar]
- [10].Ahmad M, Lee SS, Dou XM, Mohan D, Sung JK, Yang JE, Ok YS. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresource Technol. 2012;118:536–544. doi: 10.1016/j.biortech.2012.05.042. [DOI] [PubMed] [Google Scholar]
- [11].Uchimiya M, Wartelle LH, Klasson KT, Fortier CA, Lima IM. Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J. Agr. Food Chem. 2011;59(6):2501–2510. doi: 10.1021/jf104206c. [DOI] [PubMed] [Google Scholar]
- [12].Bruun EW, Hauggaard-Nielsen H, Ibrahim N, Egsgaard H, Ambus P, Jensen PA, Dam-Johansen K. Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass Bioenerg. 2011;35(3):1182–1189. [Google Scholar]
- [13].Kinney TJ, Masiello CA, Dugan B, Hockaday WC, Dean MR, Zygourakis K, Barnes RT. Hydrologic properties of biochars produced at different temperatures. Biomass Bioenerg. 2012;41:34–43. [Google Scholar]
- [14].Lu J, Li J, Li Y, Chen B, Bao Z. Use of rice straw biochar simultaneously as the sustained release carrier of herbicides and soil amendment for their reduced leaching. J. Agr. Food Chem. 2012;60(26):6463–6470. doi: 10.1021/jf3009734. [DOI] [PubMed] [Google Scholar]
- [15].Park JH, Choppala GK, Bolan NS, Chung JW, Chuasavathi T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil. 2011;348(1-2):439–451. [Google Scholar]
- [16].Gupta PK. Chapter 51 - Toxicity of herbicides. In: Ramesh CG Dvm, Mvsc, Phd, Dabt and Facta2, Ramesh C, Gupta DMPDF, editors. Veterinary Toxicology. Oxford; Academic Press: 2007. pp. 567–586. [Google Scholar]
- [17].Kotrikla A, Gatidou G, Lekkas TD. Monitoring of triazine and phenylurea herbicides in the surface waters of Greece. J. Environ. Sci. Heal. B. 2006;41(2):135–144. doi: 10.1080/03601230500364336. [DOI] [PubMed] [Google Scholar]
- [18].Weldon LW, Timmons FL. Penetration and persistence of diuron in soil. Weeds. 1961;9(2):195–203. [Google Scholar]
- [19].Mukome FND, Zhang X, Silva LCR, Six J, Parikh SJ. Use of chemical and physical characteristics to investigate trends in biochar feedstocks. J. Agr. Food Chem. 2013 doi: 10.1021/jf3049142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang G, Zhang Q, Sun K, Liu X, Zheng W, Zhao Y. Sorption of simazine to corn straw biochars prepared at different pyrolytic temperatures. Environ. Pollut. 2011;159(10):2594–2601. doi: 10.1016/j.envpol.2011.06.012. [DOI] [PubMed] [Google Scholar]
- [21].Mohan D, Pittman CU., Jr Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007;142(1-2):1–53. doi: 10.1016/j.jhazmat.2007.01.006. [DOI] [PubMed] [Google Scholar]
- [22].Wu M, Pan B, Zhang D, Xiao D, Li H, Wang C, Ning P. The sorption of organic contaminants on biochars derived from sediments with high organic carbon content. Chemosphere. 2013;90(2):782–788. doi: 10.1016/j.chemosphere.2012.09.075. [DOI] [PubMed] [Google Scholar]
- [23].Sun K, Zhang Z, Gao B, Wang Z, Xu D, Jin J, Liu X. Adsorption of diuron, fluridone and norflurazon on single-walled and multi-walled carbon nanotubes. Sci. Total Environ. 2012;439:1–7. doi: 10.1016/j.scitotenv.2012.08.022. [DOI] [PubMed] [Google Scholar]
- [24].Pignatello JJ, Xing BS. Mechanisms of slow sorption of organic chemicals to natural particles. Environ. Sci. Technol. 1996;30(1):1–11. [Google Scholar]
- [25].Navarro S, Hernandez-Bastida J, Cazana G, Perez-Lucas G, Fenoll J. Assessment of the leaching potential of 12 substituted phenylurea herbicides in two agricultural soils under laboratory conditions. J. Agr. Food Chem. 2012;60(21):5279–5286. doi: 10.1021/jf301094c. [DOI] [PubMed] [Google Scholar]
- [26].Lagaly G. Pesticide-clay interactions and formulations. Appl. Clay Sci. 2001;18(5-6):205–209. [Google Scholar]
- [27].Senesi N. Binding mechanisms of pesticides to soil humic substances. Sci. Total Environ. 1992;123:63–76. doi: 10.1016/0048-9697(92)90133-d. [DOI] [PubMed] [Google Scholar]
- [28].Chaplain V, Brault A, Tessier D, Defossez P. Soil hydrophobicity: a contribution of diuron sorption experiments. Eur. J. Soil Sci. 2008;59(6):1202–1208. [Google Scholar]
- [29].Undabeytia T, Recio E, Maqueda C, Sánchez-Verdejo T, Balek V. Slow diuron release formulations based on clay–phosphatidylcholine complexes. Appl. Clay Sci. 2012;55:53–61. [Google Scholar]
- [30].Xing B, Pignatello JJ, Gigliotti B. Competitive sorption between atrazine and other organic compounds in soils and model sorbents. Environ. Sci. Technol. 1996;30(8):2432–2440. [Google Scholar]
- [31].Lehmann J, Joseph S. Biochar for environmental management: science and technology. Earthscan. 2009 [Google Scholar]
- [32].Zheng W, Guo M, Chow T, Bennett DN, Rajagopalan N. Sorption properties of greenwaste biochar for two triazine pesticides. J. Hazard. Mater. 2010;181(1-3):121–126. doi: 10.1016/j.jhazmat.2010.04.103. [DOI] [PubMed] [Google Scholar]
- [33].Huang W, Weber WJ. A distributed reactivity model for sorption by soils and sediments. 10. Relationships between desorption, hysteresis, and the chemical characteristics of organic domains. Environ. Sci. Technol. 1997;31(9):2562–2569. [Google Scholar]
- [34].Chiou CT, Porter PE, Schmedding DW. Partition equilibriums of nonionic organic compounds between soil organic matter and water. Environ. Sci. Technol. 1983;17(4):227–231. doi: 10.1021/es00110a009. [DOI] [PubMed] [Google Scholar]
- [35].Chen B, Zhou D, Zhu L. Transitional Adsorption and Partition of Nonpolar and Polar Aromatic Contaminants by Biochars of Pine Needles with Different Pyrolytic Temperatures. Environ. Sci. Technol. 2008;42(14):5137–5143. doi: 10.1021/es8002684. [DOI] [PubMed] [Google Scholar]
- [36].Zimmerman AR. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar) Environ. Sci. Technol. 2010;44(4):1295–1301. doi: 10.1021/es903140c. [DOI] [PubMed] [Google Scholar]
- [37].Liu Y, Xu Z, Wu X, Gui W, Zhu G. Adsorption and desorption behavior of herbicide diuron on various Chinese cultivated soils. J. Hazard. Mater. 2010;178(8):462–468. doi: 10.1016/j.jhazmat.2010.01.105. [DOI] [PubMed] [Google Scholar]
- [38].Yang YN, Sheng GY. Enhanced pesticide sorption by soils containing particulate matter from crop residue burns. Environ. Sci. Technol. 2003;37(16):3635–3639. doi: 10.1021/es034006a. [DOI] [PubMed] [Google Scholar]
- [39].Yu X-Y, Ying G-G, Kookana RS. Sorption and desorption behaviors of diuron in soils amended with charcoal. J. Agr. Food Chem. 2006;54(22):8545–8550. doi: 10.1021/jf061354y. [DOI] [PubMed] [Google Scholar]
- [40].Rodriguez-Cruz MS, Ordax JM, Arienzo M, Sanchez-Martin MJ. Enhanced retention of linuron, alachlor and metalaxyl in sandy soil columns intercalated with wood barriers. Chemosphere. 2011;82(10):1415–1421. doi: 10.1016/j.chemosphere.2010.11.059. [DOI] [PubMed] [Google Scholar]
- [41].Joseph SD, Camps-Arbestain M, Lin Y, Munroe P, Chia CH, Hook J, van Zwieten L, Kimber S, Cowie A, Singh BP, Lehmann J, Foidl N, Smernik RJ, Amonette JE. An investigation into the reactions of biochar in soil. Soil Res. 2010;48(7):501–515. [Google Scholar]
- [42].Lin Y, Munroe P, Joseph S, Kimber S, Van Zwieten L. Nanoscale organo-mineral reactions of biochars in ferrosol: an investigation using microscopy. Plant Soil. 2012;357(1-2):369–380. [Google Scholar]
- [43].Martin SM, Kookana RS, Van Zwieten L, Krull E. Marked changes in herbicide sorption–desorption upon ageing of biochars in soil. J. Hazard. Mater. 2012;(231–232):70–78. doi: 10.1016/j.jhazmat.2012.06.040. [DOI] [PubMed] [Google Scholar]
- [44].Nag SK, Kookana R, Smith L, Krull E, Macdonald LM, Gill G. Poor efficacy of herbicides in biochar-amended soils as affected by their chemistry and mode of action. Chemosphere. 2011;84(11):1572–1577. doi: 10.1016/j.chemosphere.2011.05.052. [DOI] [PubMed] [Google Scholar]
- [45].Sopena F, Semple K, Sohi S, Bending G. Assessing the chemical and biological accessibility of the herbicide isoproturon in soil amended with biochar. Chemosphere. 2012;88(1):77–83. doi: 10.1016/j.chemosphere.2012.02.066. [DOI] [PubMed] [Google Scholar]
- [46].Alister C, Kogan M. Rainfall effect on dissipation and movement of diuron and simazine in a vineyard soil. Planta Daninha. 2010;28:1059–1071. [Google Scholar]
- [47].Gouy V, Dur JC, Calvet R, Belamie R, Chaplain V. Influence of adsorption-desorption phenomena on pesticide run-off from soil using simulated rainfall. Pestic. Sci. 1999;55(2):175–182. [Google Scholar]
- [48].Gaillardon P. Influence of soil moisture on long-term sorption of diuron and isoproturon by soil. Pestic. Sci. 1996;47(4):347–354. [Google Scholar]
- [49].Jones DL, Edwards-Jones G, Murphy DV. Biochar mediated alterations in herbicide breakdown and leaching in soil. Soil Biol. Biochem. 2011;43(4):804–813. [Google Scholar]