Abstract
Maternal consumption of a high fat diet (HFD) during pregnancy is found to stimulate the genesis of hypothalamic orexigenic peptide neurons in the offspring, while HFD intake in adult animals produces a systemic low-grade inflammation which increases neuroimmune factors that may affect neurogenesis and neuronal migration. Building on this evidence and our recent study showing that the inflammatory chemokine, CCL2, stimulates the migration of hypothalamic neurons and expression of orexigenic neuropeptides, we tested here the possibility that prenatal exposure to a HFD affects this chemokine system, both CCL2 and its receptors, CCR2 and CCR4, and alters its actions on hypothalamic neurons, specifically those expressing the neuropeptides, enkephalin (ENK) and galanin (GAL). Using primary dissociated hypothalamic neurons extracted from embryos on embryonic day 19, we found that prenatal HFD exposure compared to chow control actually reduces the expression of CCL2 in these hypothalamic neurons, while increasing CCR2 and CCR4 expression, and also reduces the sensitivity of hypothalamic neurons to CCL2. The HFD abolished the dose-dependent, stimulatory effect of CCL2 on the number of migrated neurons and even shifted its normal stimulatory effect on migrational velocity and distance traveled by control neurons to an inhibition of migration. Further, it abolished the dose-dependent, stimulatory effect of CCL2 on neuronal expression of ENK and GAL. These results demonstrate that prenatal HFD exposure greatly disturbs the functioning of the CCL2 chemokine system in embryonic hypothalamic neurons, reducing its endogenous levels and ability to promote the migration of neurons and their expression of orexigenic peptides.
Keywords: Prenatal high-fat diet, hypothalamus, C-C chemokine ligand 2 (CCL2), enkephalin, galanin, migration
1. Introduction
The ingestion of a diet rich in fat is known to increase caloric intake that promotes obesity (Dourmashkin et al., 2006). This effect can be attributed to changes in the hypothalamus, a region of the brain that is important in controlling consumption and energy homeostasis. Two highly expressed neuropeptides, enkephalin (ENK) and galanin (GAL), are found to be stimulated by the ingestion of a high fat diet (HFD) (Akabayashi et al., 1994, Chang et al., 2007) and are believed to contribute to the overconsumption of this diet, with central injection of these peptides found to preferentially stimulate the consumption dietary fat (Kyrkouli et al., 1990, Naleid et al., 2007). This effect of a HFD in adult animals is similarly evident with prenatal exposure to this diet, which predisposes the offspring to overconsuming dietary fat (Chang et al., 2008) and increases fat mass and body weight (Reynolds et al., 2014). These offspring and embryos exhibited increased expression and number of ENK and GAL neurons in the hypothalamus (Chang et al., 2008). While the molecular mechanisms involved in these changes are not known, recent studies suggest that neuroinflammatory systems may play a role.
The ingestion of a HFD and dietary obesity result in a state of systemic low-grade inflammation (Milanski et al., 2009, Takanabe-Mori et al., 2010). This state is characterized by an increase in the production of immune factors, such as the cytokines, interleukin 1-beta and tumor necrosis factor-alpha, and a superfamily of small chemotactic cytokines, also known as chemokines (Barbarroja et al., 2010, Gregor and Hotamisligil, 2011). Exposure to this diet during the prenatal period additionally increases placental inflammation (Reynolds et al., 2015). Neuroinflammatory mediators, in addition to recruiting immune cells to fight infection, have more recently been shown to affect neuronal function and development. In particular, the chemokine, C-C chemokine ligand 2 (CCL2), also called monocyte chemotactic protein-1 (MCP-1), along with its receptor, C-C chemokine receptor type 2 (CCR2) (Craig and Loberg, 2006), has been singled out as an important regulator of neuropeptide function and development (Banisadr et al., 2005, Edman et al., 2008, Chintawar et al., 2009, Poon et al., 2014). This chemokine, classically known to attract monocytes (Yoshimura et al., 1989, Rollins, 1996) and stimulate migration of these cells during an immune response (Lu et al., 1998), is increased in the brain, uterus and placenta during pregnancy (Meng et al., 1999, He et al., 2007), and its receptors that bind CCL2, both CCR2 and the less studied CCR4, are also expressed in the brain (Banisadr et al., 2002, Banisadr et al., 2005) and found in hippocampal neurons (Meucci et al., 1998). In the hypothalamus, CCL2 is shown to co-localize with the neuropeptides, melanin-concentrating hormone and arginine vasopressin (Banisadr et al., 2005). This chemokine also modulates the activity of neurons in the brain (Gosselin et al., 2005, Guyon et al., 2009a, Guyon et al., 2009b) and has been linked to the migration of neurons into damaged brain areas (Liu et al., 2007, Deng et al., 2008). In addition, our recent in vitro study of hypothalamic neurons cultured from embryonic day 19 (E19) embryos from dams consuming a low-fat chow diet showed that CCR2 co-expresses with ENK and that the colocalization of this chemokine and orexigenic peptide is increased in embryos exposed to a HFD (Poon et al., 2014). Additionally, in normal chow-exposed neurons, CCL2 produced a dose-dependent, stimulatory effect on the migration of neurons and on mRNA expression of ENK and GAL in the hypothalamus (Poon et al., 2014). While reducing or knocking out CCL2 in adult mice is shown to trigger symptoms of increased ingestion and obesity (Inouye et al., 2007), there is other evidence showing that pharmacological blockade of the CCR2 receptor has the opposite effect of improving symptoms of obesity (Rull et al., 2010). These mixed findings leave unclear as to the specific functions of this chemokine system in dietary obesity and its specific relation to the orexigenic peptide neurons in the embryo when exposed to a fat-rich diet.
Thus, the objectives of the present study were to investigate whether prenatal exposure to a HFD affects the CCL2/CCR2 system and its normal functioning in relation to the expression of orexigenic peptides and the migratory behavior of neurons in the hypothalamus. To examine this after removing the endogenous CCL2 stimuli, we extracted and cultured hypothalamic neurons from embryos that were prenatally exposed to a HFD as compared to a low-fat chow diet and determined first whether this HFD exposure affects the expression of CCL2, CCR2, and CCR4 in the hypothalamus and then whether this diet alters the actions of exogenous CCL2 on the migratory behavior of these hypothalamic neurons and on the gene expression of the orexigenic peptides, ENK and GAL.
2. Experimental Procedures
2.1 Animals
Timed-pregnant Sprague-Dawley rats were acquired from Charles River Laboratories (Hartford, CT) on embryonic day 5 (E5). All experimental procedures were performed according to institutionally approved protocols as specified in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and also with approval of the Rockefeller University Animal Care and Use Committee. The dams were individually housed in a fully accredited AAALAC facility (22°C, with a 12:12-h light-dark cycle with lights off at 12 pm). The rats were split into 2 groups of 8 dams each and maintained ad libitum from E7 on either a high-fat diet (HFD; 5.02 kcal/g) with 50% fat or a standard lab chow (3.36 kcal/g) with 13% fat (Purina, St. Louis, MO), as previously described (Dourmashkin et al., 2006, Chang et al., 2008, Poon et al., 2012). In the HFD group, standard lab chow was available for the first 3 days (until E9) before being completely removed, in order for the HFD group to overcome neophobia and adapt to the HFD. Over the course of the experiments, food intake was measured 3 times per week, and body weight was recorded weekly. There was no difference between the HFD and chow dams in their daily caloric intake during pregnancy (70–90 kcals). Dams were sacrificed on embryonic day 19 (E19), as previously described (Poon et al., 2012). The whole hypothalamus was extracted and dissociated for plating into cell culture, as previously described (Poon et al., 2012, Poon et al., 2013), or placed in either RNAlater for mRNA purification. Whole hypothalamus was used since individual regions of the hypothalamus are not fully differentiated at this age.
2.2 Diet
The standard lab chow diet (3.36 kcal/g) consisted of 13% fat and was acquired from Purina (St. Louis, MO), and the HFD used in this report has been described in detail in previous publications (Dourmashkin et al., 2006, Chang et al., 2008, Poon et al., 2012). Briefly, the HFD consisted of 50% fat composed of 75% lard (Armour Star, Peoria, IL) and 25% vegetable oil (Crisco, Orrville, OH), of 25% carbohydrate composed of 30% dextrin (ICN Pharmaceuticals, Costa Mesa, CA), 30% cornstarch (ICN Pharmaceuticals, Costa Mesa, CA), and 40% sucrose (Domino Foods Inc., Yonkers, NY), and of 25% protein from casein (Bio-Serv, Frenchtown, NJ), and it was supplemented with minerals (USP XIV Salt Mixture Briggs; ICN Pharmaceuticals, Costa Mesa, CA) and vitamins (Vitamin Diet Fortification Mixture; ICN Pharmaceuticals, Costa Mesa, CA). This diet is nutritionally complete and is found to have no detrimental effects on the health of the animals.
2.3 Cell culture
Hypothalami from E19 embryos were micro-dissected and dissociated, as previously described (Poon et al., 2012, Poon et al., 2013). The cells (1 million / mL) were resuspended in Neurobasal Media containing B27 supplement (Life Technologies, Grand Island, NY) and cultured in either a 6-well plate (BD Biosciences, Sparks, MD), in a cell culture insert (BD Biosciences, Sparks, MD), or on a glass bottom dish (Greiner Bio-One, Monroe, NC). As previously measured (Poon et al., 2014), >95% of the cells were found to be positive for NeuN, a neuronal marker, with the astrocyte marker, GFAP, undetectable. Neurons were then treated with 0, 50, 100, or 200 ng/mL of CCL2, concentrations that are within the EC50 range (Matsushima et al., 1989, Carr et al., 1994, Kao et al., 2012), as previously described (Poon et al., 2014). For each experiment, the hypothalami of embryos were taken from 8 dams consuming either the low-fat chow diet (n=4) or the HFD (n=4), and their neurons were dissociated and placed into culture wells. From each experiment, four cultured wells were used for the no treatment group and for each group treated with CCL2 (50, 100, and 200 ng/mL). While not directly compared here, a study of chow and HFD neurons cultured on the same experimental day has previously revealed a stimulatory effect of HFD on the expression and levels of ENK (Poon et al., 2012).
2.4 Migration Assay
A total of 200,000 neurons were seeded in a cell culture insert (BD Biosciences) and placed into media containing 0, 50, 100, or 200 ng/mL CCL2 for 24 h, as previously described (Poon et al., 2014). The neurons on top of the cell culture insert were carefully removed with a cotton swab, while those attached to the underside were stained with cresyl violet. These neurons were then manually blind-counted under a light microscope (Leitz Dialux 20; Leica Microsystems, Buffalo Grove, IL, USA) using a 10X objective, and the number of migrated neurons was calculated as a percentage relative to the no-treatment control group.
2.5 Real-time Imaging
Cells were plated at a density of 200,000 cells per 0.5 mL, as previously described (Poon et al., 2014). Media containing either 0, 50, 100, or 200 ng/mL of CCL2 was introduced to the plated neurons immediately before imaging. Neurons were continuously imaged every 2.5 minutes for 3 hours using an Olympus LCV110 “VivaView” incubator microscope (Center Valley, PA) with a 20X DIC objective and MetaMorph acquisition software (Molecular Devices, Sunnyvale, CA). Individual neurons were manually tracked to determine the average velocity and distance traversed by treated and non-treated neurons. Approximately 1 microglia was found for every 30 neurons, and these were eliminated from the analysis. Data were analyzed using ImageJ plugins, manual tracking, and chemotaxis and migration tool (Schneider et al., 2012). The velocity and absolute distance travelled by individual cells sampled from each cell culture group were averaged, and the individual movements from four separate cell culture groups were binned into histograms. Although large variations were found, due to the fact that not all neurons respond to CCL2, all data across different cell culture groups yielded similar velocity and absolute distance traveled and thus were analyzed together.
To determine the average movements and the number of independent variables or neuronal populations, the velocity and absolute distance of all neurons was plotted as a frequency count histogram and fitted to a Gaussian distribution. The number of peaks that resulted in an R2 value closest to one was used. The number of fitted distributions to the histogram reflects the number of populations, the median peak of each distribution reflects the mean velocity or distance traveled, with higher velocity or larger distance reflecting faster movements, and the area under the curve reflects the percentage of neurons within that distribution. Between 300 and 400 cells were measured for each group.
2.6 mRNA and qRT-PCR
Primary hypothalamic neuronal cultures were allowed to settle for 5 days before being treated with 0, 50, 100, or 200 ng/mL CCL2 for 3 hours. The mRNA from each micro-dissected sample was purified using a QiagenRNeasy kit (Qiagen, Valencia, CA), cDNA was synthesized using Superscript VILO Master Mix (Life Technologies, Grand Island, NY), and the SYBR Green PCR core reagents kit (Applied Biosystems) was used for qRT-PCR and performed in MicroAmp Optic 96-well Reaction Plates (Applied Biosystems) under the conditions of 2 min at 50°C, 10 min at 95°C, and 40 cycles of 15 s at 95°C and 1 min at 60°C, as previously described (Poon et al., 2014). The levels of target gene expression were quantified relative to the level of cyclophilin-A, using the relative quantification method. Each sample was run in triplicate and included a no-template control and a negative RT control. The primers used were: CCL2 forward: 5′-GTGCTGTCTCAGCCAGATGCAGTT-3′, reverse: 5′-AGTTCTCCAGCCGACTCATTGGG-3′; CCR2 forward: 5′-GGGGGCCACCACACCGTATGAC-3′, reverse: 5′-AGGGAGTAGAGTGGGGGCAGGAT-3′; CCR4 forward: 5′-CACCCAGGATGAAGCCGCGTA-3′, reverse: 5′-CGCCGAACGCCTTGATGCCT-3′; ENK forward: 5′-GGACTGCGCTAAATGCAGCTA-3′, reverse: 5′-GTGTGCATGCCAGGAAGTTG-3′; GAL forward: 5′-TTCCCACCACTGCTCAAGATG-3′, reverse: 5′-TGGCTGACAGGGTTGCAA-3′; cyclophilin-A forward: 5′-GTGTTCTTCGACATCACGGCT-3′, reverse: 5′-CTGTCTTTGGAACTTTGTCTGCA-3′, as previously described (Poon et al., 2014). The specificities of PCR products were confirmed by a single band of corresponding molecular weight revealed by agarose gel electrophoresis. The concentration of all target primers was 100 nM, and the CYC primer was 200 nM. Primers were acquired from Life Technologies (Grand Island, NY).
2.7 Data analysis
For qPCR analysis, the ddCT values were averaged from four neuronal culture wells for each CCL2 treatment and the percent change was calculated relative to the no treatment control. The percent change was then averaged across all four embryonic groups. Statistical analyses with multiple comparisons between CCL2 treatments were completed using a one-way ANOVA, followed by a Bonferroni post-hoc test to determine significant differences between the groups. Statistical analyses comparing multiple mRNA expression from the same chow or HFD samples were completed using a two-way ANOVA, followed by a Bonferroni post-hoc test to determine significant differences for each gene between the two groups. Direct comparison between two groups was made using an unpaired, two-tailed Student’s t-test. The criterion for statistical significance was p < 0.05. Log-dose analysis was performed using the following equation: , and was considered a good fit if it produced significance of p < 0.05. The velocity or absolute distance traveled for each individual neuron from live imaging studies were placed in a histogram and statistically fitted to a Gaussian curve until a max reduced chi-squared value was achieved. This analysis allows theoretical identification of different populations of neurons by using the physical characteristics of migration but does not differentiate specific differences between individual neurons. Only curves producing a significance of p < 0.05 were considered a good fit. To determine the number of independent variables or neuronal populations, the data were plotted as a frequency count and fitted to a Gaussian distribution. The number of peaks that resulted in an R2 value closest to 1 was used. To determine the goodness of fit, X2 values corresponding to p < 0.05 were considered significant. The number of fitted distributions to the histogram reflects the number of populations, the peak of each distribution reflects the average velocity or absolute distance traveled, and the area under the curve reflects the percentage of cells within that distribution. Velocity and absolute distance values were obtained from 500 to 1000 neurons for each condition.
3. Results
3.1 Prenatal HFD exposure affects endogenous CCL2 chemokine receptor system in hypothalamic neurons
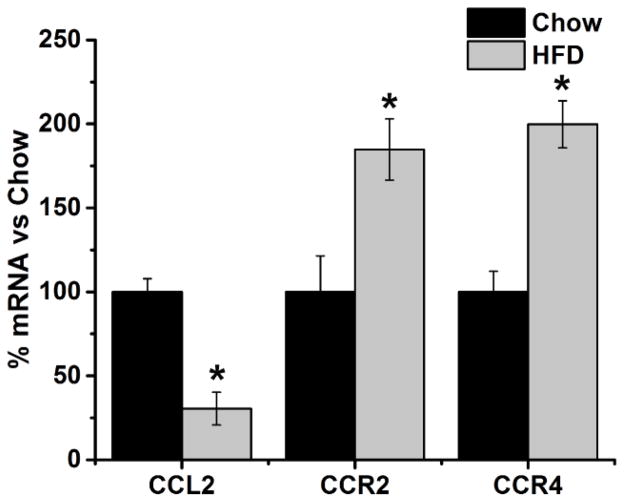
Examination of hypothalamic neurons revealed a significant interaction effect between prenatal HFD exposure and gene expression [F(1, 3) = 507.03, p < 0.001] and a significant main effect of prenatal HFD exposure on the expression of the CCL2 chemokine system and its receptors [F(2, 6) = 12.46, p < 0.05]. The HFD compared to low fat chow diet caused a significant decrease in the expression of CCL2 [−70%, t(6) = 7.18, p < 0.01], which was accompanied by an increase in the expression of CCR2 [+85%, t(6) = 4.64, p < 0.01] and CCR4 [+100%, t(6) = 7.09, p < 0.01] (Fig. 1). These results show that prenatal exposure to a HFD markedly alters the CCL2 chemokine system in hypothalamic neurons during embryonic development, causing a marked reduction in endogenous CCL2.
Figure 1. Effects of prenatal HFD on endogenous CCL2 and its receptors in hypothalamic neurons.
Prenatal exposure to a HFD significantly decreased the expression of CCL2 and increased the expression of CCR2 and CCR4 in cultured hypothalamic neurons. * p < 0.01; n = 4.
3.2 Prenatal HFD exposure reduces the number of migrating hypothalamic neurons toward CCL2
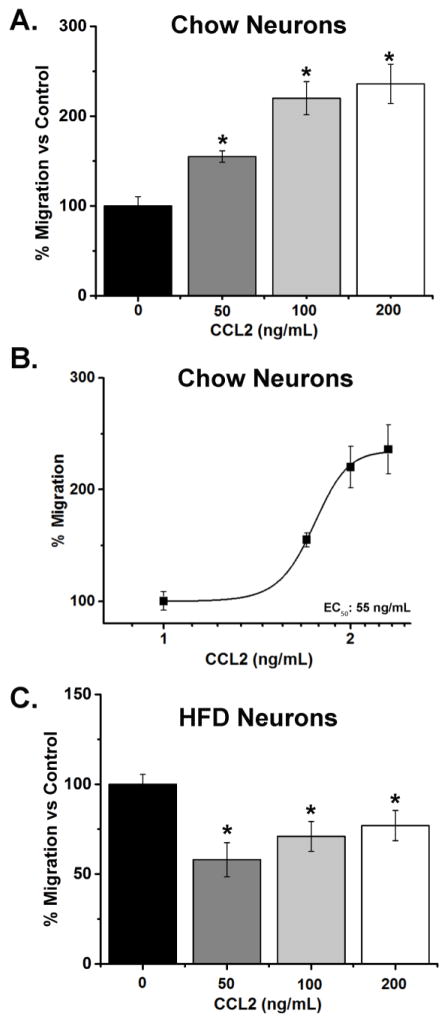
A migration assay using a cell culture insert was used to measure the number of neurons that migrate toward CCL2 in both the chow and HFD-exposed embryos. In chow neurons, a significant effect of CCL2 was observed when comparing the CCL2-treated neurons to the no treatment neurons [F(3,12) = 20.67, p < 0.001; Fig. 2A]. A significant increase in the number of migrated chow neurons was induced by CCL2 at all doses, 50 ng/mL [+55%, t(7) = 9.64, p < 0.01], 100 ng/mL [+120%, t(7) = 6.46, p < 0.01], and 200 ng/mL [+136%, t(7) = 7.07, p < 0.01], with an EC50 value of 55 ng/mL (Fig. 2B), indicating that the hypothalamic neurons are attracted to and migrate toward CCL2 in a dose-dependent manner. The HFD neurons, in contrast, exhibited a very different response to CCL2. While a significant effect was observed between CCL2-treated neurons and no treatment neurons [F(3,16) = 6.77, p < 0.01], this reflected an inhibition of migration at all doses of CCL2 (Fig. 2C). In the HFD-exposed neurons, CCL2 significantly decreased the number of migrated neurons at 50 ng/mL [−42%, t(8) = −5.00, p < 0.01], 100 ng/mL [−29%, t(8) = −3.96, p < 0.01], and 200 ng/mL [−23%, t(8) = −3.10, p < 0.05]. These results show that prenatal HFD exposure negatively impacts the normal stimulatory effect of CCL2 on embryonic hypothalamic neurons, leading this chemokine to cause a reduction in their migration in vitro.
Figure 2. Effects of CCL2 on migration of hypothalamic chow- and HFD-exposed neurons.
A. In chow neurons, treatment with CCL2 at 50, 100 or 200 ng/mL significantly increased the migration of hypothalamic neurons. B. In chow neurons, CCL2 dose-dependently increased the migration of hypothalamic neurons, with an EC50 of 55 ng/mL. C. In HFD neurons, in contrast, CCL2 treatment significantly decreased the migration of hypothalamic neurons. * p < 0.01; n = 4.
3.3 Prenatal HFD exposure reduces migrational behavior of hypothalamic neurons to a CCL2 stimulus
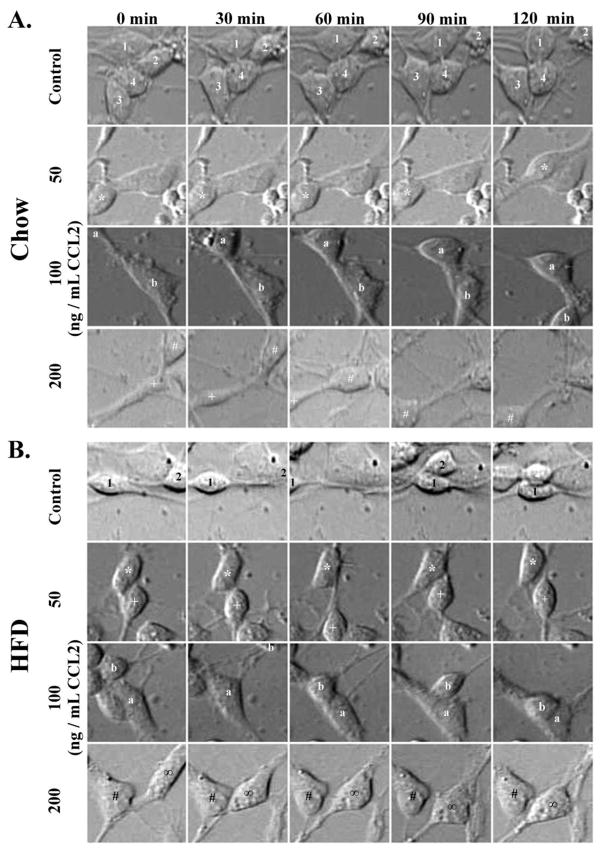
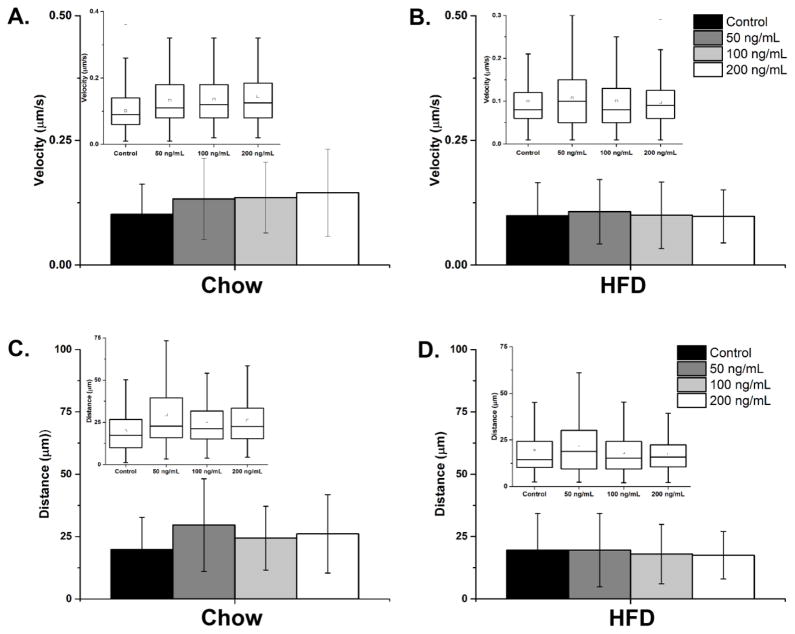
This experiment further examined the effect of CCL2 on the migratory behavior of hypothalamic neurons from the chow- and HFD-exposed embryos. Real-time imaging was used to measure neuronal movements of these neurons, as shown in the animated clip (Supplemental Videos S1–S8), with the cell bodies of neurons tracked to determine their velocity and absolute distance traveled (Fig. 3). In the chow neurons, the results showed a significant effect of CCL2 treatment compared to no treatment on the velocity [F(3,1178) = 19.83, p < 0.001] and absolute distance traveled [F(3,1178) = 13.48, p < 0.001] (Fig. 4A). Post-hoc tests revealed in these chow neurons a significant increase, with effects of similar magnitude at all CCL2 concentrations, in the velocity [50 ng/mL: t(616) = 5.44, p < 0.001; 100 ng/mL: t(625) = 6.44, p < 0.001; 200 ng/mL: t(605) = 7.21, p < 0.001] and the absolute distance traveled [50 ng/mL: t(616) = 5.42, p < 0.001; 100 ng/mL: t(625) = 4.43, p < 0.001; 200 ng/mL: t(605) = 5.39, p < 0.001]. In contrast, in the HFD neurons, analyses of these measures revealed very different results, indicating a strong effect of prenatal HFD exposure on the responsiveness of neurons to CCL2. In the HFD neurons, CCL2 treatment compared to no treatment had no overall effect on velocity [F(3,1301) = 1.26, p = 0.28, not significant] at any of the concentrations examined (Fig. 4B). A similar analysis of absolute distance traveled revealed a significant effect of CCL2 treatment on the HFD neurons [F(3,1302) = 5.42, p < 0.01]. The post-hoc tests showed a significant difference between the doses (p < 0.05), which reflected a decrease in the absolute distance traveled at 100 and 200 ng/mL CCL2 when compared to 50 ng/mL CCL2 (p < 0.01) but no difference when compared to no treatment control (p = 0.27). While this analysis of average velocity and absolute distance travelled across all HFD neurons reveals a significant inhibition only in the latter measure, a further population analysis described in the next section (see 3.4) confirms an inhibitory effect of CCL2 on neuronal migration with both measures. Taken together, these results demonstrate that CCL2, while having a stimulatory effect on the migration of neurons exposed to a low-fat chow diet, has the opposite effect on neurons exposed to a HFD, causing a reduction in their migrational behavior.
Figure 3. Real-time imaging of hypothalamic chow- and HFD-exposed neurons.
A. Representative hypothalamic chow neurons were imaged in real-time, with treatment of CCL2 significantly increasing neuronal movements as compared to no treatment control. B. Representative hypothalamic HFD neurons were imaged in real-time, with no effect of CCL2 on neuronal movement. Numbers, symbols, and letters represent the same neuron across each treatment group.
Figure 4. Average movements of hypothalamic chow- and HFD-exposed neurons.
A. The average velocity traveled by chow neurons with no treatment, or treatment with 50, 100 and 200 ng/mL CCL2. n = 1182. B. The average velocity traveled by HFD neurons with no treatment, or treatment with 50, 100, and 200 ng/mL CCL2. n = 1295. C. The average absolute distance traveled by chow neurons with or without CCL2 treatment. n = 1182. D. The average absolute distance traveled by HFD neurons with or without CCL2 treatment. n = 1295. The inset in each figure shows the average distribution of the velocity or absolute distance for all of the neurons combined.
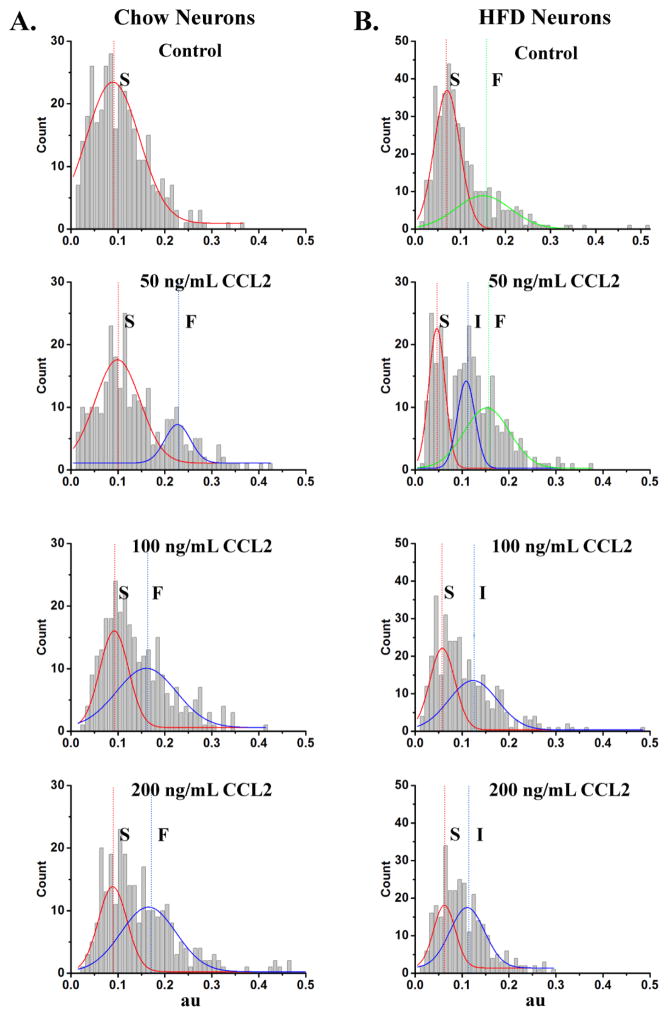
3.4 Prenatal HFD exposure shifts neuronal populations while inhibiting the stimulatory effect of CCL2 on migration
A population analysis was performed here to further characterize the specific neurons that are sensitive to CCL2. The velocity and distance traveled for each individual neuron were plotted as a probability density function (see Methods), to determine whether the chow- and HFD-exposed neurons have specific populations of neurons that are sensitive to CCL2 and also whether they differ in their responsiveness to CCL2. In the chow neurons, this data analysis identified a population of neurons specifically affected by CCL2 (Fig. 5A; Table 1A). Whereas the no-treatment group had only one population of neurons with an average velocity of 0.09 ± 0.01 μm/min assigned as Slow (S), the CCL2-treated groups had two populations of neurons, one that moved with a similar average velocity of 0.09 – 0.10 μm/min assigned as S and the other that moved with a faster average velocity of 0.16 – 0.22 μm/min assigned as Fast (F) (Table 1A). Thus, while the slower moving population of neurons evident in both the treatment and no-treatment groups presumably reflects the basal movements of neurons that were insensitive to CCL2, the second population of faster neurons seen only in the treated groups reflected specific neurons that were affected by CCL2 (Fig. 5A). These same distinct populations of chow neurons were also evident when the measure of absolute distance traveled was similarly analyzed, with the CCL2-treated groups but not the no treatment group having a second population of neurons that traveled a significantly greater absolute distance (Table 1A). These results, consistent with a previous report (Poon et al., 2014), reveal a stimulatory effect of CCL2 on the migration of a specific population of neurons exposed to a low-fat chow diet.
Figure 5. Population analysis of hypothalamic chow- and HFD-exposed neurons.
A. In chow neurons, probability density function analysis of the velocity revealed one population of Slow (S) moving neurons in the no treatment control. This is in contrast to two populations of neurons, S and Fast (F) moving, in the CCL2 groups treated with 50, 100, and 200 ng/mL CCL2, indicating that this chemokine increases velocity and overall movements in chow neurons and that this second F population reflects neurons that are sensitive to CCL2. B. In HFD neurons, probability density function analysis of the velocity revealed two populations of moving neurons, S and F, in the no treatment control. Treatment with 100 or 200 ng/mL CCL2 shifted the populations of neurons to S and Intermediate (I), suggesting that CCL2 reduces velocity and overall movements, consistent with the finding that 50 ng/mL CCL2 treatment group exhibited a trend toward a reduction of movement with an S and I along with the F population. Chow: n = 1182, p < 0.05; HFD: n = 1295, p < 0.05.
Table 1.
A. In chow neurons, peak values of fits to the probability density histograms for both the velocity and the absolute distance traveled reveal one peak for control neurons and two peaks for those treated with 50, 100, and 200 ng/mL CCL2. B. In HFD neurons, peak values of fits to the probability density histograms for both the velocity and the absolute distance traveled reveal two peaks for no treatment control neurons and two peaks for those treated with 100, and 200 ng/mL CCL2, and three peaks for those treated with 50 ng/mL CCL2.
| Table 1A: Chow Neurons | ||||
|---|---|---|---|---|
| Velocity | Peak 1 | Area 1 (%) | Peak 2 | Area 2 (%) |
|
| ||||
| 0 ng/mL | 0.09 ± 0.01 | 100 | — | — |
| 50 ng/mL | 0.10 ± 0.01 | 87 | 0.22 ± 0.01 | 13 |
| 100 ng/mL | 0.09 ± 0.01 | 42 | 0.16 ± 0.02 | 58 |
| 200 ng/mL | 0.09 ± 0.05 | 40 | 0.17 ± 0.03 | 60 |
|
| ||||
| Distance | Peak 1 | Area 1 (%) | Peak 2 | Area 2 (%) |
|
| ||||
| 0 ng/mL | 14.05 ± 0.78 | 100 | — | — |
| 50 ng/mL | 16.40 ± 0.66 | 61 | 31.16 ± 13.22 | 39 |
| 100 ng/mL | 15.95 ± 0.71 | 34 | 25.62 ± 4.06 | 66 |
| 200 ng/mL | 15.46 ± 1.05 | 44 | 29.51 ± 7.47 | 56 |
| Table 1B: HFD Neurons | ||||||
|---|---|---|---|---|---|---|
| Velocity | Peak 1 | Area 1 (%) | Peak 2 | Area 2 (%) | Peak 3 | Area 3 (%) |
|
| ||||||
| 0 ng/mL | 0.07 ± 0.00 | 66 | — | — | 0.15 ± 0.04 | 34 |
| 50 ng/mL | 0.05 ± 0.00 | 34.5 | 0.11 ± 0.00 | 23.5 | 0.15 ± 0.06 | 42 |
| 100 ng/mL | 0.06 ± 0.01 | 46 | 0.12 ± 0.05 | 54 | — | — |
| 200 ng/mL | 0.06 ± 0.01 | 39 | 0.11 ± 0.05 | 61 | — | — |
|
| ||||||
| Distance | Peak 1 | Area 1 (%) | Peak 2 | Area 2 (%) | Peak 3 | Area 3 (%) |
|
| ||||||
| 0 ng/mL | 11.83 ± 0.22 | 70 | — | — | 33.20 ± 2.60 | 30 |
| 50 ng/mL | 7.78 ± 0.35 | 39 | 18.63 ± 0.57 | 28 | 32.38 ± 1.73 | 33 |
| 100 ng/mL | 9.53 ± 1.10 | 48 | 21.43 ± 11.77 | 52 | — | — |
| 200 ng/mL | 10.54 ± 2.65 | 47 | 19.73 ± 8.69 | 53 | — | — |
In the neurons exposed to a HFD, very different results were obtained when these measures of velocity and distance traveled were plotted as a probability density function (Fig. 5B; Table 1B). For velocity, this analysis of the no treatment group revealed two populations of HFD neurons, one with an average velocity of 0.07 ± 0.00 μm/min assigned as S and a second population with a faster velocity of 0.15 ± 0.04 μm/min assigned as F (Fig. 5B). Comparison of the neurons under conditions of diet manipulation alone reveal that this second population of neurons exists only in the HFD neurons and was not evident in the chow neurons (Fig. 5A). Further analysis of the HFD neurons under conditions of CCL2 treatment revealed, in stark contrast to chow neurons, an inhibitory effect of CCL2 on neuronal movement (Fig. 5B). This was most evident in the HFD neurons treated with 100 or 200 ng/mL of CCL2 and had only S and Intermediate (I) populations, with average velocities respectively of 0.06 μm/min and 0.11 – 0.12 μm/min, but no population of F neurons. This trend toward a reduction in velocity was also evident in the HFD neurons treated with 50 ng/mL of CCL2, which had an additional intermediate (I) population with an average velocity of 0.11 ± 0.00 μm/min along with the S (0.05 ± 0.00 μm/min) and F (0.15 ± 0.06 μm/min) populations seen in the untreated HFD neurons (Fig. 5B). A similar analysis performed on the measure of absolute distance traveled yielded similar results, showing that CCL2 treatment of HFD neurons reduces migrational behavior (Table 1B). These findings demonstrate once again that, while CCL2 treatment of chow neurons normally stimulates neuronal migration activity, prenatal exposure to a HFD, in addition to inducing a second population of fast neurons, actually disrupts the actions of CCL2 and shifts its effect on hypothalamic neurons toward an inhibition of neuronal movement.
3.5 Prenatal HFD exposure reduces changes in neuropeptide mRNA expression induced by CCL2
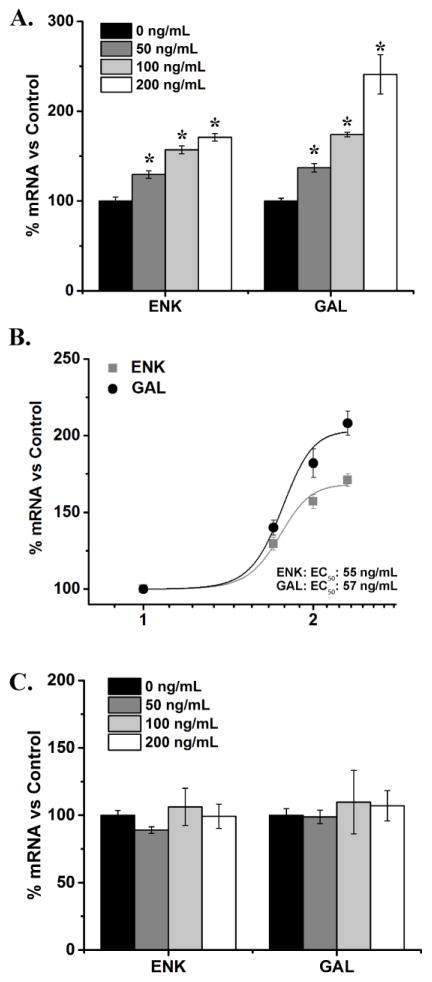
This experiment tested whether prenatal HFD exposure also alters the effects of CCL2 on specific hypothalamic neurons that express the orexigenic neuropeptides, ENK and GAL. In chow neurons, there was a significant effect of CCL2 at the three concentrations on the expression of both ENK [F(3, 10) = 68.24, p < 0.001] and GAL [F(3, 10) = 49.11, p < 0.001] (Fig. 6A). Post-hoc analysis revealed a significant increase in levels of ENK mRNA [50 ng/mL: t(6) = 7.04, p < 0.001; 100 ng/mL: t(5) = 13.00, p < 0.001; 200 ng/mL: t(5) = 17.82, p < 0.001], as well as in levels of GAL mRNA [50 ng/mL: t(6) = 8.11, p < 0.001; 100 ng/mL: t(6) = 8.87, p < 0.001; 200 ng/mL: t(4) = 11.91, p < 0.001]. This increase in peptide expression was found to be dose dependent, with EC50 values of 55 ng/mL for ENK and 57 ng/mL for GAL (Fig. 6B). Again, very different results were obtained in the HFD-exposed neurons. Compared to no treatment control, there was no significant effect of CCL2 treatment on these neurons, on the expression of either ENK [F(3, 12) = 0.74, p = 0.55] or GAL [F(3, 12) = 0.16, p = 0.92], at any of the doses tested (Fig. 6C). These results, consistent with the other experiments, show prenatal HFD exposure to reduce the sensitivity of hypothalamic neurons to exogenous CCL2.
Figure 6. CCL2 and changes in mRNA levels of orexigenic peptides in hypothalamic neurons.
A. In chow neurons, treatment with 50, 100 or 200 ng/mL CCL2 significantly increased the expression of both enkephalin (ENK) and galanin (GAL). B. In chow neurons, CCL2 dose-dependently increased ENK and GAL mRNA, with EC50 of 55 ng/mL and 57 ng/mL, respectively. C. In HFD neurons, in contrast, treatment with CCL2 had no effect on the expression of either ENK or GAL. * p < 0.01; n = 4.
4. Discussion
In our prior studies, we uncovered a stimulatory effect in vivo of prenatal exposure to a HFD on the genesis and expression of hypothalamic orexigenic peptide neurons (Chang et al., 2008). We have also observed in vitro a similar role for CCL2 in stimulating the migration of hypothalamic neurons and the expression of these peptides, suggesting its involvement in the normal development of these neurons (Poon et al., 2014). Building on these findings, we investigated here whether prenatal HFD exposure affects the endogenous CCL2 chemokine system and its normal functioning within the hypothalamus. While replicating the stimulatory effects of CCL2 on neuronal migration and peptide expression in chow neurons, the experiments conducted in this study consistently showed that prenatal exposure to a HFD actually disrupts the normal functioning of the hypothalamic CCL2 chemokine system. Prenatal HFD exposure compared to chow significantly decreased hypothalamic CCL2 expression, an effect accompanied by an increase in its receptors. The HFD exposure also markedly reduced the sensitivity of hypothalamic neurons to the effect of CCL2 on neuronal migration and migratory behavior and on their expression of the orexigenic neuropeptides. These findings demonstrate for the first time that prenatal HFD exposure greatly disrupts both the development and functioning of the endogenous CCL2 chemokine system in embryonic hypothalamic neurons.
4.1 Prenatal HFD exposure affects endogenous hypothalamic CCL2 chemokine receptor system
A few studies, including our previous report, have shown that CCL2 and one of its receptors, CCR2, are highly expressed in neurons of the hypothalamus (Banisadr et al., 2002, Banisadr et al., 2005, Poon et al., 2014). With HFD ingestion known to induce a state of systemic low grade inflammation, the present report examined, in embryonic hypothalamic neurons, whether prenatal HFD intake affects the inflammatory mediator, CCL2, and its receptors. Whereas previous studies have found maternal obesity or prenatal HFD exposure to increase both protein and mRNA levels of CCL2 in the gut or adipose tissue (Yan et al., 2011, Segovia et al., 2014), our results here show that prenatal HFD exposure actually decreases the expression of CCL2 in embryonic hypothalamic neurons, indicating that neuronal systems in the brain respond very differently from those in peripheral organs. In contrast, our measurements of the CCR2 receptor expression revealed a stimulatory effect of prenatal HFD exposure in hypothalamic neurons, similar to that observed in adipose tissue (Murabayashi et al., 2013). With the only neuronal study of CCR4 showing this receptor to exist in hippocampal neurons (Meucci et al., 1998), the present findings are the first to reveal its existence in hypothalamic neurons and a stimulatory effect of a prenatal HFD on its expression. These HFD-induced changes are very similar to those found with prenatal ethanol exposure, which also increases both mRNA and protein levels of CCR2 (Chang et al., 2015). This HFD-induced increase in both CCR2 and CCR4 receptors in embryonic hypothalamic neurons may have some relation to the significant suppression of endogenous CCL2 expression, as discussed below.
4.2 Prenatal HFD reduces migratory behavior of neurons toward CCL2
The chemokine, CCL2, is a known chemotactic agent that is highly prevalent in the embryonic brain at different stages of development (Meng et al., 1999, Rezaie et al., 2002). It induces the migration of immune cells during an immune response (Rollins et al., 1991, Carr et al., 1994, Ge and Pachter, 2004) and stimulates the migration of neurons during cellular damage both in vitro and in the brain (Deng et al., 2008, Magge et al., 2009). The results of the present study, similar to our previous report (Poon et al., 2014), demonstrate in chow-exposed embryos that CCL2 dose-dependently stimulates the migration of hypothalamic neurons towards this chemokine and also increases the velocity and absolute distance traveled by the population of neurons that is sensitive to this chemokine. These findings further reinforce the important role of CCL2 in stimulating the migration of neurons as they develop normally under conditions involving a low-fat diet. Under conditions of a HFD, however, a very different neuronal response is evident. In embryonic neurons exposed to a HFD, CCL2 actually reduces the number of migrating neurons and the overall movements of these neurons. There is evidence that prenatal HFD exposure decreases in embryos the migration and maturation of neuronal precursors from the third ventricle (Stachowiak et al., 2013), the site where hypothalamic neurons are born (Markakis, 2002, Bouret, 2010). With the importance of chemokine systems in the normal development and migration of neurons, this decreased migratory behavior may possibly be attributed to the HFD-induced disturbances in the CCL2 chemokine system demonstrated here.
4.3 Prenatal HFD alters the effect of CCL2 on orexigenic neuropeptide expression
Evidence to date shows that CCL2 co-expresses and stimulates orexigenic neuropeptides in hypothalamic neurons (Banisadr et al., 2005, Poon et al., 2014) and affects neuronal function–(Guyon et al., 2009b, Zhou et al., 2011). Consistent with this are our results here in low-fat chow-exposed embryos, showing that CCL2 compared to no treatment dose-dependently increases the expression of ENK and GAL in normally developing hypothalamic neurons. Of particular interest are our additional findings that embryonic neurons exposed to a HFD are clearly different and apparently insensitive to CCL2, showing no effect of this chemokine at all doses on the expression of these neuropeptides. One explanation for this decreased sensitivity to CCL2 may be a feedback mechanism caused by an extended inflammatory state induced by the HFD. The ingestion of a HFD increases circulating levels of CCL2 (Chen et al., 2005, Segovia et al., 2014), with some studies in adipose tissue showing prolonged exposure to CCL2 to negatively affect proteins that are involved in ingestive behavior, such as downregulating PPARγ (Gerhardt et al., 2001) or desensitizing mu-opioid receptors (Zhang et al., 2004). Also, the prenatal HFD exposure, by already increasing the expression of ENK and GAL as shown previously (Poon et al., 2012), may cause a ceiling effect that prevents further stimulation by CCL2. While the exact cause for this phenomenon is still unknown, the present findings further substantiate the idea that the prenatal environment caused by HFD exposure is effective in blunting the sensitivity of hypothalamic neurons to exogenous CCL2.
4.4 Prenatal HFD alters CCL2 chemokine system while affecting hypothalamic neuronal response to CCL2
Our results here demonstrate that hypothalamic embryonic neurons developing under normal, low-fat chow conditions are highly sensitive to the stimulatory effects of CCL2 on neuronal migration and peptide expression, consistent with our prior study (Poon et al., 2014). Additionally, hypothalamic neurons exposed to a HFD, in addition to increasing protein levels of CCR2 and colabeling with ENK neurons (Poon et al., 2014), also stimulates neurogenesis and peptide expression (Chang et al., 2008, Poon et al., 2012). These HFD neurons, however, respond very differently to CCL2, which actually inhibits neuronal migration and is unable to stimulate expression of the peptides. There may be several possible mechanisms, one of which may involve the HFD-induced decrease in CCL2 and increase of both CCR2 and CCR4 expression in hypothalamic neurons. Several studies suggest that CCR2 and possibly CCR4 are scavengers of CCL2, with binding to CCR2 to produce rapid desensitization (Aragay et al., 1998, Ajram et al., 2014) or internalization of CCL2 (Zhang and Rollins, 1995, Tylaska et al., 2002, Mahad et al., 2006, Volpe et al., 2012), thus regulating its levels and reducing the strength of the CCL2 signal. This internalization of CCL2 by its receptors may reflect a feedback mechanism against the disruptive effects of a HFD on embryonic hypothalamic neurons, designed to reduce CCL2 signaling and expression and thus attenuate its actions on neuronal function. Additionally, other studies have shown long-term inflammation to decrease CCL2 levels in the brain and spinal cord (Franciotta et al., 2001, Sorensen et al., 2001) and to be inhibited by other neurochemicals or hormones, such as glucocorticoids (Melgarejo et al., 2009). Future studies will be needed to shed light on the exact mechanism underlying this phenomenon.
4.5 Prenatal HFD effects on CCL2 chemokine system contribute to the increase in HFD intake
Systemic changes in the CCL2 chemokine system have been suggested to affect ingestive behavior, as shown by studies in adipose tissue of mice consuming a HFD. In the obese state or during HFD intake, CCL2 is found to be increased in adipose tissue (Chen et al., 2005, Kanda et al., 2006, Inouye et al., 2007), and CCL2 treatment of cultured adipocytes decreases the fat content of these cells (Gerhardt et al., 2001). Also, reducing or knocking out CCL2 in adult mice has the opposite effect of triggering symptoms of obesity and increasing ingestive behavior (Inouye et al., 2007), indicating that a suppression or absence of CCL2 contributes to an increase in HFD intake. With regards to the receptor, there is evidence that blocking or knocking down CCR2 reduces HFD intake and attenuates the development of obesity (Weisberg et al., 2006, Rull et al., 2010), indicating that an upregulation of this receptor as shown here may have the same effect as reducing CCL2, causing an overall increase in HFD intake. Whereas more studies directly relating the CCR2 and CCR4 receptors to intake and metabolism are needed, the available evidence, along with our findings here that prenatal HFD exposure decreases CCL2 signaling while stimulating its receptors, suggests that the effects of this HFD during embryonic development may contribute, over the long term, to an increase in ingestive behavior and an obesogenic phenotype in the offspring.
4.6 Conclusions
In addition to increasing inflammation, maternal consumption of a HFD during pregnancy greatly alters the neurochemistry of the hypothalamus in the offspring that in turn may further increase the intake of a HFD. The present in vitro study, while possibly not reflecting the in vivo situation, examined one aspect of inflammation that may be affected by this diet exposure. The results demonstrate that prenatal HFD exposure, in addition to changing the endogenous CCL2 chemokine system, alters the responsiveness of hypothalamic neurons to CCL2, decreasing their overall migratory behavior and attenuating its effects on expression of hypothalamic peptides. The systemic consequence of this change in chemokine levels and function may contribute to increased HFD intake as adults, with a disturbance in normal functioning of the CCL2 chemokine receptor system in embryonic hypothalamic neurons increasing the propensity of the offspring to overconsume a fat-rich diet. Future studies on other neuropeptides as well as on the behavior of postnatal offspring exposed to this chemokine will shed further light on the physiological effects of CCL2 on development.
Supplementary Material
Highlights.
Hypothalamic neurons from normally developing embryos exposed to chow dose-dependently respond to CCL2
Prenatal high fat diet exposure decreases expression of CCL2 and increases expression of CCR2, CCR4 in hypothalamic neurons
Prenatal high fat diet exposure decreases migration in response to CCL2 in embryonic hypothalamic neurons
Prenatal high fat diet exposure reduces sensitivity of embryonic hypothalamic neurons to CCL2 on neuropeptide expression
Acknowledgments
The authors would like to thank The Rockefeller University’s Bio-Imaging Resource Center for the use of their equipment.
Grants
This work was supported by grants from the National Institutes of Health, F32DK100058 (KP) and 1R21 AA020593 (SFL). This work was additionally supported by grant # UL1 TR000043 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health Clinical and Translational Science Award (CTSA) program (The Rockefeller University TTCL Resource Center).
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kinning Poon, Email: kpoon@rockefeller.edu.
Dzhamilya Abramova, Email: Jamiex250@gmail.com.
Hui Tin Ho, Email: hth2101@gmail.com.
Sarah Leibowitz, Email: leibow@rockefeller.edu.
References
- Ajram L, Begg M, Slack R, Cryan J, Hall D, Hodgson S, Ford A, Barnes A, Swieboda D, Mousnier A, Solari R. Internalization of the chemokine receptor CCR4 can be evoked by orthosteric and allosteric receptor antagonists. Eur J Pharmacol. 2014;729:75–85. doi: 10.1016/j.ejphar.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akabayashi A, Koenig JI, Watanabe Y, Alexander JT, Leibowitz SF. Galanin-containing neurons in the paraventricular nucleus: a neurochemical marker for fat ingestion and body weight gain. Proc Natl Acad Sci U S A. 1994;91:10375–10379. doi: 10.1073/pnas.91.22.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragay AM, Mellado M, Frade JM, Martin AM, Jimenez-Sainz MC, Martinez AC, Mayor F., Jr Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:2985–2990. doi: 10.1073/pnas.95.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Mechighel P, Rostene W, Kitabgi P, Melik Parsadaniantz S. Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. The Journal of comparative neurology. 2005;492:178–192. doi: 10.1002/cne.20729. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Queraud-Lesaux F, Boutterin MC, Pelaprat D, Zalc B, Rostene W, Haour F, Parsadaniantz SM. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. Journal of neurochemistry. 2002;81:257–269. doi: 10.1046/j.1471-4159.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Barbarroja N, Lopez-Pedrera R, Mayas MD, Garcia-Fuentes E, Garrido-Sanchez L, Macias-Gonzalez M, El Bekay R, Vidal-Puig A, Tinahones FJ. The obese healthy paradox: is inflammation the answer? Biochem J. 2010;430:141–149. doi: 10.1042/BJ20100285. [DOI] [PubMed] [Google Scholar]
- Bouret SG. Development of hypothalamic neural networks controlling appetite. Forum Nutr. 2010;63:84–93. doi: 10.1159/000264396. [DOI] [PubMed] [Google Scholar]
- Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Gaysinskaya V, Marwil Z, Leibowitz SF. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: role of circulating triglycerides. American journal of physiology Endocrinology and metabolism. 2007;292:E561–570. doi: 10.1152/ajpendo.00087.2006. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Leibowitz SF. Prenatal exposure to ethanol stimulates hypothalamic CCR2 chemokine receptor system: Possible relation to increased density of orexigenic peptide neurons and ethanol drinking in adolescent offspring. Neuroscience. 2015;310:163–175. doi: 10.1016/j.neuroscience.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Mumick S, Zhang C, Lamb J, Dai H, Weingarth D, Mudgett J, Chen H, MacNeil DJ, Reitman ML, Qian S. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes Res. 2005;13:1311–1320. doi: 10.1038/oby.2005.159. [DOI] [PubMed] [Google Scholar]
- Chintawar S, Cayrol R, Antel J, Pandolfo M, Prat A. Blood-brain barrier promotes differentiation of human fetal neural precursor cells. Stem cells. 2009;27:838–846. doi: 10.1002/stem.25. [DOI] [PubMed] [Google Scholar]
- Craig MJ, Loberg RD. CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Cancer metastasis reviews. 2006;25:611–619. doi: 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- Deng Y, Lu J, Sivakumar V, Ling EA, Kaur C. Amoeboid microglia in the periventricular white matter induce oligodendrocyte damage through expression of proinflammatory cytokines via MAP kinase signaling pathway in hypoxic neonatal rats. Brain pathology. 2008;18:387–400. doi: 10.1111/j.1750-3639.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourmashkin JT, Chang GQ, Hill JO, Gayles EC, Fried SK, Leibowitz SF. Model for predicting and phenotyping at normal weight the long-term propensity for obesity in Sprague-Dawley rats. Physiol Behav. 2006;87:666–678. doi: 10.1016/j.physbeh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Edman LC, Mira H, Arenas E. The beta-chemokines CCL2 and CCL7 are two novel differentiation factors for midbrain dopaminergic precursors and neurons. Experimental cell research. 2008;314:2123–2130. doi: 10.1016/j.yexcr.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Franciotta D, Martino G, Zardini E, Furlan R, Bergamaschi R, Andreoni L, Cosi V. Serum and CSF levels of MCP-1 and IP-10 in multiple sclerosis patients with acute and stable disease and undergoing immunomodulatory therapies. Journal of neuroimmunology. 2001;115:192–198. doi: 10.1016/s0165-5728(01)00261-2. [DOI] [PubMed] [Google Scholar]
- Ge S, Pachter JS. Caveolin-1 knockdown by small interfering RNA suppresses responses to the chemokine monocyte chemoattractant protein-1 by human astrocytes. The Journal of biological chemistry. 2004;279:6688–6695. doi: 10.1074/jbc.M311769200. [DOI] [PubMed] [Google Scholar]
- Gerhardt CC, Romero IA, Cancello R, Camoin L, Strosberg AD. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol. 2001;175:81–92. doi: 10.1016/s0303-7207(01)00394-x. [DOI] [PubMed] [Google Scholar]
- Gosselin RD, Varela C, Banisadr G, Mechighel P, Rostene W, Kitabgi P, Melik-Parsadaniantz S. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. Journal of neurochemistry. 2005;95:1023–1034. doi: 10.1111/j.1471-4159.2005.03431.x. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Guyon A, Conductier G, Rovere C, Enfissi A, Nahon JL. Melanin-concentrating hormone producing neurons: Activities and modulations. Peptides. 2009a;30:2031–2039. doi: 10.1016/j.peptides.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Guyon A, Skrzydelski D, De Giry I, Rovere C, Conductier G, Trocello JM, Dauge V, Kitabgi P, Rostene W, Nahon JL, Melik Parsadaniantz S. Long term exposure to the chemokine CCL2 activates the nigrostriatal dopamine system: a novel mechanism for the control of dopamine release. Neuroscience. 2009b;162:1072–1080. doi: 10.1016/j.neuroscience.2009.05.048. [DOI] [PubMed] [Google Scholar]
- He YY, Du MR, Guo PF, He XJ, Zhou WH, Zhu XY, Li DJ. Regulation of C-C motif chemokine ligand 2 and its receptor in human decidual stromal cells by pregnancy-associated hormones in early gestation. Human reproduction. 2007;22:2733–2742. doi: 10.1093/humrep/dem208. [DOI] [PubMed] [Google Scholar]
- Inouye KE, Shi H, Howard JK, Daly CH, Lord GM, Rollins BJ, Flier JS. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes. 2007;56:2242–2250. doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of clinical investigation. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao DJ, Li AH, Chen JC, Luo RS, Chen YL, Lu JC, Wang HL. CC chemokine ligand 2 upregulates the current density and expression of TRPV1 channels and Nav1.8 sodium channels in dorsal root ganglion neurons. Journal of neuroinflammation. 2012;9:189. doi: 10.1186/1742-2094-9-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrkouli SE, Stanley BG, Seirafi RD, Leibowitz SF. Stimulation of feeding by galanin: anatomical localization and behavioral specificity of this peptide’s effects in the brain. Peptides. 1990;11:995–1001. doi: 10.1016/0196-9781(90)90023-x. [DOI] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang RL, Gregg SR, Wang L, Yier T, Chopp M. Chemokine ligand 2 (CCL2) induces migration and differentiation of subventricular zone cells after stroke. Journal of neuroscience research. 2007;85:2120–2125. doi: 10.1002/jnr.21359. [DOI] [PubMed] [Google Scholar]
- Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magge SN, Malik SZ, Royo NC, Chen HI, Yu L, Snyder EY, O’Rourke DM, Watson DJ. Role of monocyte chemoattractant protein-1 (MCP-1/CCL2) in migration of neural progenitor cells toward glial tumors. Journal of neuroscience research. 2009;87:1547–1555. doi: 10.1002/jnr.21983. [DOI] [PubMed] [Google Scholar]
- Mahad D, Callahan MK, Williams KA, Ubogu EE, Kivisakk P, Tucky B, Kidd G, Kingsbury GA, Chang A, Fox RJ, Mack M, Sniderman MB, Ravid R, Staugaitis SM, Stins MF, Ransohoff RM. Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain. 2006;129:212–223. doi: 10.1093/brain/awh655. [DOI] [PubMed] [Google Scholar]
- Markakis EA. Development of the neuroendocrine hypothalamus. Frontiers in neuroendocrinology. 2002;23:257–291. doi: 10.1016/s0091-3022(02)00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. The Journal of experimental medicine. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melgarejo E, Medina MA, Sanchez-Jimenez F, Urdiales JL. Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Cell Biol. 2009;41:998–1001. doi: 10.1016/j.biocel.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Meng SZ, Oka A, Takashima S. Developmental expression of monocyte chemoattractant protein-1 in the human cerebellum and brainstem. Brain & development. 1999;21:30–35. doi: 10.1016/s0387-7604(98)00065-5. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murabayashi N, Sugiyama T, Zhang L, Kamimoto Y, Umekawa T, Ma N, Sagawa N. Maternal high-fat diets cause insulin resistance through inflammatory changes in fetal adipose tissue. Eur J Obstet Gynecol Reprod Biol. 2013;169:39–44. doi: 10.1016/j.ejogrb.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. Am J Physiol Regul Integr Comp Physiol. 2007;293:R99–105. doi: 10.1152/ajpregu.00675.2006. [DOI] [PubMed] [Google Scholar]
- Poon K, Barson JR, Fagan SE, Leibowitz SF. Developmental changes in embryonic hypothalamic neurons during prenatal fat exposure. Am J Physiol Endocrinol Metab. 2012;303:E432–441. doi: 10.1152/ajpendo.00238.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K, Ho HT, Barson JR, Leibowitz SF. Stimulatory role of the chemokine CCL2 in the migration and peptide expression of embryonic hypothalamic neurons. Journal of neurochemistry. 2014;131:509–520. doi: 10.1111/jnc.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K, Mandava S, Chen K, Barson JR, Buschlen S, Leibowitz SF. Prenatal exposure to dietary fat induces changes in the transcriptional factors, TEF and YAP, which may stimulate differentiation of peptide neurons in rat hypothalamus. PloS one. 2013;8:e77668. doi: 10.1371/journal.pone.0077668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CM, Vickers MH, Harrison CJ, Segovia SA, Gray C. High fat and/or high salt intake during pregnancy alters maternal meta-inflammation and offspring growth and metabolic profiles. Physiol Rep. 2014;2 doi: 10.14814/phy2.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CM, Vickers MH, Harrison CJ, Segovia SA, Gray C. Maternal high fat and/or salt consumption induces sex-specific inflammatory and nutrient transport in the rat placenta. Physiol Rep. 2015;3 doi: 10.14814/phy2.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie P, Trillo-Pazos G, Everall IP, Male DK. Expression of beta-chemokines and chemokine receptors in human fetal astrocyte and microglial co-cultures: potential role of chemokines in the developing CNS. Glia. 2002;37:64–75. doi: 10.1002/glia.1128. [DOI] [PubMed] [Google Scholar]
- Rollins BJ. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today. 1996;2:198–204. doi: 10.1016/1357-4310(96)88772-7. [DOI] [PubMed] [Google Scholar]
- Rollins BJ, Walz A, Baggiolini M. Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood. 1991;78:1112–1116. [PubMed] [Google Scholar]
- Rull A, Camps J, Alonso-Villaverde C, Joven J. Insulin resistance, inflammation, and obesity: role of monocyte chemoattractant protein-1 (or CCL2) in the regulation of metabolism. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/326580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia SA, Vickers MH, Gray C, Reynolds CM. Maternal obesity, inflammation, and developmental programming. Biomed Res Int. 2014;2014:418975. doi: 10.1155/2014/418975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen TL, Sellebjerg F, Jensen CV, Strieter RM, Ransohoff RM. Chemokines CXCL10 and CCL2: differential involvement in intrathecal inflammation in multiple sclerosis. Eur J Neurol. 2001;8:665–672. doi: 10.1046/j.1468-1331.2001.00327.x. [DOI] [PubMed] [Google Scholar]
- Stachowiak EK, Srinivasan M, Stachowiak MK, Patel MS. Maternal obesity induced by a high fat diet causes altered cellular development in fetal brains suggestive of a predisposition of offspring to neurological disorders in later life. Metab Brain Dis. 2013;28:721–725. doi: 10.1007/s11011-013-9437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanabe-Mori R, Ono K, Sowa N, Wada H, Takaya T, Horie T, Satoh-Asahara N, Shimatsu A, Fujita M, Sawamura T, Hasegawa K. Lectin-like oxidized low-density lipoprotein receptor-1 is required for the adipose tissue expression of proinflammatory cytokines in high-fat diet-induced obese mice. Biochem Biophys Res Commun. 2010;398:576–580. doi: 10.1016/j.bbrc.2010.06.123. [DOI] [PubMed] [Google Scholar]
- Tylaska LA, Boring L, Weng W, Aiello R, Charo IF, Rollins BJ, Gladue RP. Ccr2 regulates the level of MCP-1/CCL2 in vitro and at inflammatory sites and controls T cell activation in response to alloantigen. Cytokine. 2002;18:184–190. doi: 10.1006/cyto.2002.1031. [DOI] [PubMed] [Google Scholar]
- Volpe S, Cameroni E, Moepps B, Thelen S, Apuzzo T, Thelen M. CCR2 acts as scavenger for CCL2 during monocyte chemotaxis. PloS one. 2012;7:e37208. doi: 10.1371/journal.pone.0037208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. The Journal of clinical investigation. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Huang Y, Wang H, Du M, Hess BW, Ford SP, Nathanielsz PW, Zhu MJ. Maternal obesity induces sustained inflammation in both fetal and offspring large intestine of sheep. Inflamm Bowel Dis. 2011;17:1513–1522. doi: 10.1002/ibd.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989;169:1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Rogers TJ, Caterina M, Oppenheim JJ. Proinflammatory chemokines, such as C-C chemokine ligand 3, desensitize mu-opioid receptors on dorsal root ganglia neurons. Journal of immunology. 2004;173:594–599. doi: 10.4049/jimmunol.173.1.594. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rollins BJ. A dominant negative inhibitor indicates that monocyte chemoattractant protein 1 functions as a dimer. Mol Cell Biol. 1995;15:4851–4855. doi: 10.1128/mcb.15.9.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Tang H, Liu J, Dong J, Xiong H. Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. Journal of neurochemistry. 2011;116:406–414. doi: 10.1111/j.1471-4159.2010.07121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.