SUMMARY
Dopamine action in the nucleus accumbens (NAc) is thought to drive appetitive behavior and Pavlovian reward learning. However, it remains controversial how dopamine achieves these behavioral effects by regulating medium spiny projection neurons (MSNs) of the NAc, especially on a behaviorally relevant timescale. Metabotropic glutamate receptor (mGluR)-induced Ca2+ signaling dependent on the Ca2+- releasing messenger inositol 1,4,5-triphosphate (IP3) plays a critical role in controlling neuronal excitability and synaptic plasticity. Here, we show that transient dopamine application facilitates mGluR/IP3-induced Ca2+ signals within a time window of ~2–10 s in a subpopulation of MSNs in the NAc core. Dopamine facilitation of IP3-induced Ca2+ signaling is mediated by D1 dopamine receptors. In dopamine-insensitive MSNs, activation of A2A adenosine receptors causes enhancement of IP3-evoked Ca2+ signals, which is reversed by D2 dopamine receptor activation. These results show that dopamine differentially regulates Ca2+ signaling on the order of seconds in two distinct MSN subpopulations.
INTRODUCTION
The nucleus accumbens (NAc), which constitutes major part of the ventral striatum, plays a critical role in driving appetitive behavior, Pavlovian reward learning, and the development of addiction (Day and Carelli, 2007; Fields et al., 2007; Ikemoto and Bonci, 2014). It receives massive dopaminergic projections from the ventral tegmental area (VTA), forming the mesolimbic dopaminergic system. Dopamine (DA) neurons in the VTA and the adjacent substantia nigra display transient increases in firing frequency, or bursts, in response to unexpected primary rewards or to reward-predicting environmental cues after repeated cue-reward conditioning (Schultz, 1998), eliciting phasic DA transients lasting several seconds in the NAc and other target areas (Day et al., 2007; Phillips et al., 2003). Ample evidence implicates these DA transients in motivating goal-directed behaviors aimed at rewards or reward-predicting cues and in the learning of cues and behaviors leading to rewards. These behavioral effects are thought to arise from short-term and long-term actions of DA on intrinsic excitability and synaptic transmission in medium spiny neurons (MSNs), projection neurons of the NAc/striatum (Gerfen and Surmeier, 2011; Luscher and Malenka, 2011).
Intracellular Ca2+ signaling, triggered by either action potentials (APs) or synaptic inputs, dynamically controls neuronal activity and plasticity via its actions on plasma membrane ion channels, cytosolic enzymes, and other Ca2+-sensitive proteins (Augustine et al., 2003; Berridge, 1998; Fakler and Adelman, 2008). Although Ca2+ influx through voltage-gated Ca2+ channels or Ca2+-permeable ionotropic neurotransmitter receptors (e.g., NMDA-type glutamate receptors) provides the major source of Ca2+, release of Ca2+ from intracellular stores also make significant contributions. In particular, it has been shown that AP-evoked Ca2+ signals can be amplified by preceding activation of group I metabotropic glutamate receptors (mGluRs) coupled to the generation of inositol-1,4,5-triphosphate (IP3) in DA neurons, hippocampal pyramidal neurons, and cerebellar Purkinje neurons (Cui et al., 2007; Nakamura et al., 2000; Wang et al., 2000). Here, IP3 increases the sensitivity of IP3 receptors (IP3Rs) to Ca2+-dependent activation (Doi et al., 2005; Taylor and Laude, 2002), thereby enhancing Ca2+-induced Ca2+ release (CICR) from intracellular stores triggered by AP-induced Ca2+ influx. Furthermore, strong activation of mGluRs and other IP3-coupled neurotransmitter receptors produces large Ca2+ increases by itself without AP-evoked Ca2+ influx in diverse CNS neurons, frequently causing hyperpolarizations mediated by Ca2+-sensitive K+ conductances (Canepari and Ogden, 2006; El-Hassar et al., 2011; Fiorillo and Williams, 2000; Gulledge and Stuart, 2005; Hagenston et al., 2008; Morikawa et al., 2003; Power and Sah, 2008). These forms of IP3-dependent Ca2+ signaling have been recently reported in MSNs of the dorsal striatum (Clements et al., 2013; Partridge et al., 2014; Plotkin et al., 2013). However, it remains largely unknown how DA regulates IP3-induced Ca2+ signals in MSNs or in any other CNS neurons.
The expression of D1 and D2 DA receptors, two predominant DA receptor subtypes, is mostly segregated into distinct subpopulations of MSNs in the NAc (Bertran-Gonzalez et al., 2008; Bertran-Gonzalez et al., 2010; Frederick et al., 2015; Smith et al., 2013), as has been shown in the dorsal striatum (Gerfen et al., 1990; Gerfen and Surmeier, 2011; Surmeier et al., 1996) [but also see (Perreault et al., 2014) for expression of D1–D2 receptor heteromers in certain MSNs]. In this study, using ex vivo slices from mice, we investigated how DA differentially regulates mGluR/IP3-dependent Ca2+ signaling via D1 and D2 receptors in the NAc core, the subregion predominantly involved in the formation and expression of cue-reward memory (Saddoris et al., 2013; Sesack and Grace, 2010).
RESULTS
Phasic DA Enhances IP3-Induced Ca2+ Signals in a Timing-Dependent Manner in a Subset of MSNs
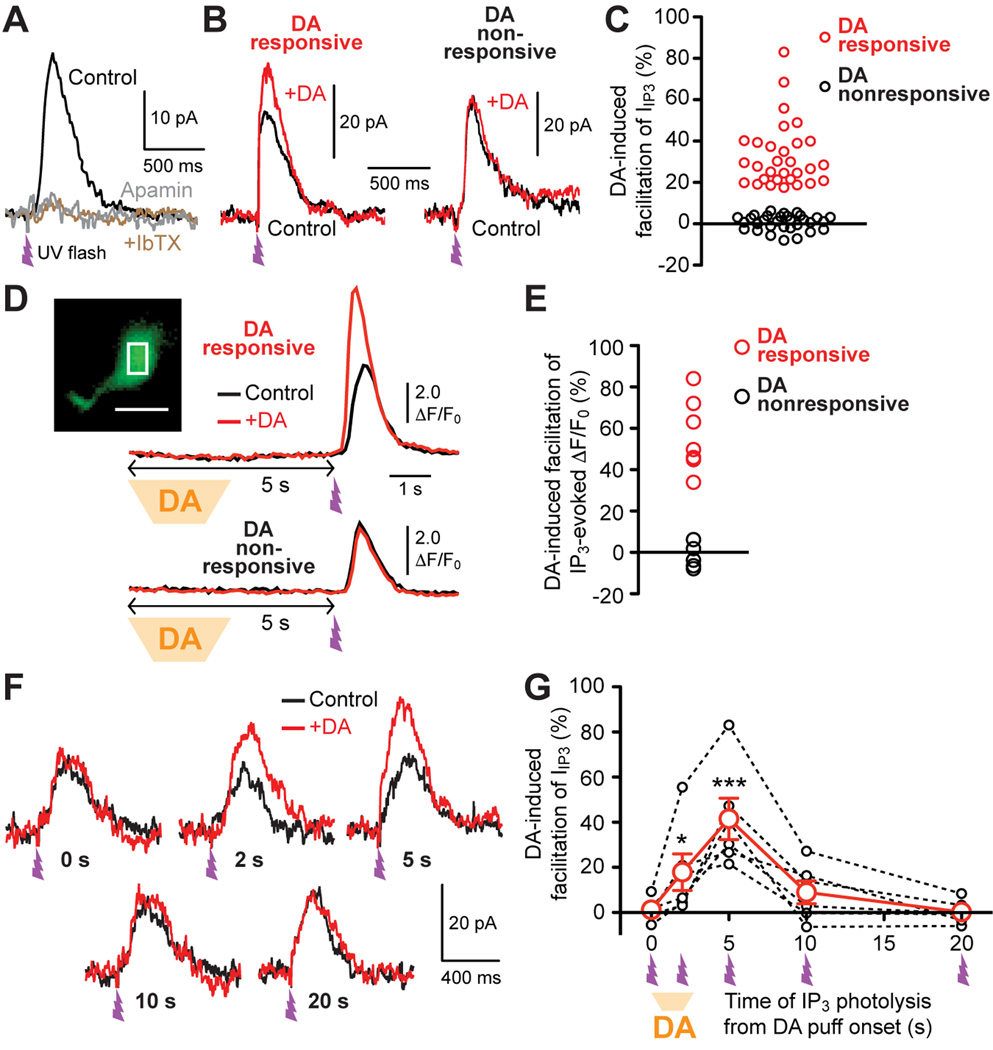
Whole-cell voltage clamp recordings of MSNs were made in the NAc core. MSNs were held at −57 mV, which is close to membrane potentials during the up-state where MSNs frequently fire trains of APs (Stern et al., 1998; Wickens and Wilson, 1998; Wilson and Kawaguchi, 1996). To examine DA action on IP3-dependent Ca2+ signaling, IP3 was directly applied into the cytosol using UV photolysis of caged IP3. Our previous study in striatal MSNs has shown that photolytic IP3 application produces a transient rise in [Ca2+]i, together with a transient outward current via Ca2+-sensitive K+ conductances (termed IIP3), in a concentration-dependent manner when the UV flash intensity is varied (Clements et al., 2013). In the current experiments, UV flash intensity was adjusted to evoke relatively small outward currents (15–60 pA using <EC50 IP3 concentration; see Experimental Procedures for details). IIP3 thus evoked was virtually eliminated by apamin (100 nM, n = 5 cells; Figure 1A), a selective blocker of small-conductance Ca2+-activated K+ (SK) channels, which are solely gated by Ca2+ (Fakler and Adelman, 2008). To mimic phasic DA transients observed in the NAc in behaving animals (Day et al., 2007; Phillips et al., 2003), DA (20 µM) was pressure ejected for 2.5 s from a pipette placed ~100 µm from the recorded MSN. When DA application was made prior to photolytic IP3 application (5 s interval between the onset of 2.5 s DA application and UV flash), DA produced measurable (≥18%) increases in IIP3 in 30 out of 61 recorded MSNs (Figures 1B, 1C, and S1). DA application had no measurable effect on the holding current in these cells (data not shown). We further directly monitored intracellular Ca2+ ([Ca2+]i) at the soma using the low-affinity Ca2+ indicator Fluo-4FF (100 µM) loaded into the cytosol via the whole-cell pipette (>20 min). Phasic DA application increased IP3-evoked Ca2+ transients (with 5 s interval) in 6 out of 11 MSNs tested (Figures 1D and 1E).
Figure 1. Transient DA application increases IP3-evoked Ca2+ signals in a timingdependent manner in a subpopulation of MSNs.
(A) Example traces of IIP3 showing that apamin (100 nM) virtually abolished IIP3 evoked with submaximal UV flash intensity. UV flash (~1 ms) was applied at the time indicated. Subsequent application of iberiotoxin (IbTX, 100 nM), a selective blocker of large-conductance Ca2+-activated K+ (BK) channels, had no effect in all 5 cells tested for apamin.
(B) Representative IIP3 traces with (+DA; red) or without (control; black) preceding DA puff application (2.5 s) in two MSNs. The onset of DA application was 5 s prior to photolytic IP3 application, as illustrated in (D). Inset (left): Photomicrograph showing the placement of the puff pipette (AC: anterior commissure; scale bar: 200 µm).
(C) Graph plotting the magnitude of IIP3 facilitation caused by preceding DA application in individual MSNs (61 cells). Note that a subset of recorded MSNs (30 cells; red) displayed >18% facilitation, whereas no significant DA effect (<5% facilitation) was observed in the remaining MSNs (31 cells; black). See also Figure S1.
(D) Example traces of IP3-evoked Ca2+ transients with (+DA; red) or without (control; black) preceding DA application in two MSNs. Inset: Confocal fluorescence image illustrating the placement of ROI at the soma for fluorescence monitoring (scale bar: 10 µm).
(E) Graph plotting the magnitude of DA-induced facilitation of IP3-evoked Ca2+ transients in individual MSNs (11 cells). DA application produced >30% facilitation of IP3-evoked Ca2+ transients in a subset (6 cells) of these MSNs.
(F) Example traces illustrating the timing dependence of the effects of DA puff application (2.5 s) on IIP3. All traces are from the same MSN. UV flash was applied at the indicated time after the onset of DA application for each red trace.
(G) Summary graph demonstrating the timing dependence of DA-induced IIP3 facilitation. Data are from 6 cells that were tested for all 5 intervals shown in (A). *p < 0.05, ***p < 0.001 vs. data at 0 s (repeated measures one-way ANOVA followed by Bonferroni post hoc test).
We next varied the interval between phasic DA onset and photolytic IP3 application in DA responsive MSNs (n = 6 cells; Figures 1F and 1G). DA caused IIP3 facilitation with 2–5 s interval, an effect that decayed when the interval was prolonged to 10–20 s. No effect was observed when phasic DA onset was simultaneous with photolytic IP3 application. Therefore, phasic DA facilitates IP3-evoked Ca2+ signals with a time window of ~2–10 s in MSNs of the NAc core.
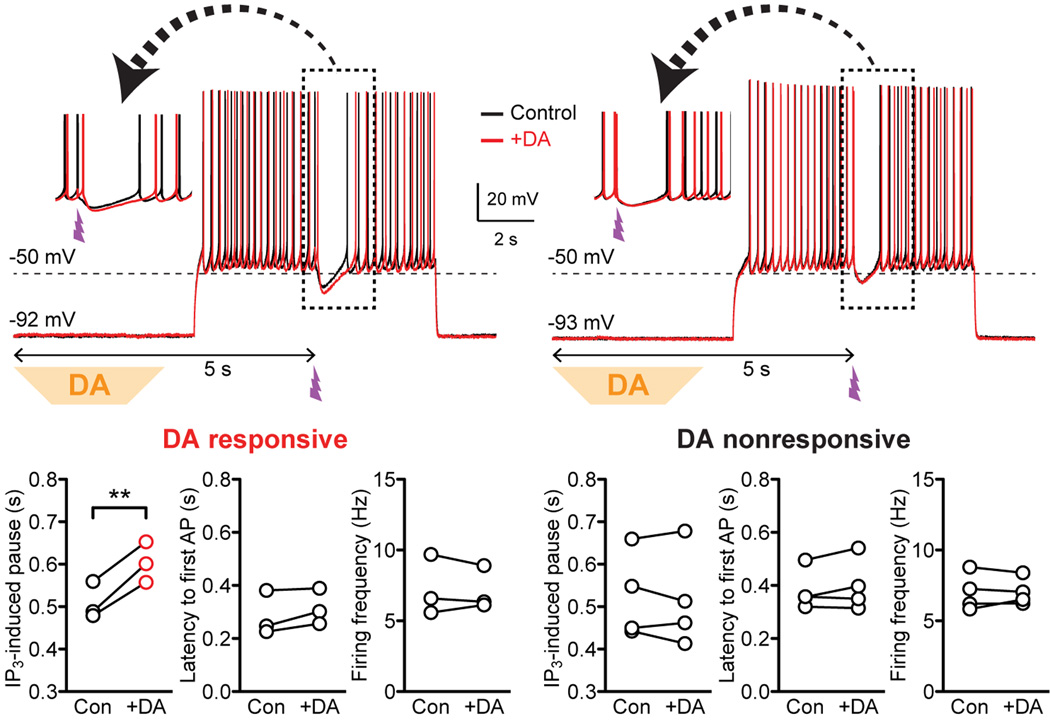
Phasic DA Prolongs IP3-Induced Pauses in MSN Firing
It has been shown in the dorsal striatum that mGluR/IP3-induced Ca2+ signals drive pauses in MSN firing resulting from SK channel-dependent hyperpolarization (Clements et al., 2013). To test the effect of phasic DA on IP3-induced firing pauses, current clamp recordings of MSNs were made in the NAc core. AP firing was evoked by 4 s depolarizing current injections (~200–400 pA; adjusted to produce ~5–10 Hz firing), during which photolytic IP3 application was made 2 s after the onset of current injection (Figure 2; UV flash intensity was adjusted in voltage clamp). Phasic DA application (2.5 s; onset 5 s prior to UV flash, i.e., 3 s prior to the onset of current injection) failed to affect MSN firing preceding IP3 application in all 7 cells tested. However, phasic DA prolonged IP3-evoked pauses in 3 cells that displayed DA-induced IIP3 facilitation in voltage clamp, while DA had no effect in the remaining 4 cells (Figures 2 and S2).
Figure 2. Phasic DA prolongs IP3-induced pauses in MSN firing.
Representative traces (inset: portion in the dashed box shown expanded in timescale) and summary graphs demonstrating the effects of DA puff application on the duration of IP3-evoked pauses in MSN firing and on firing parameters (latency to the first AP after the onset of depolarizing current injection and the average firing frequency before IP3 application). DA responsiveness was first determined in voltage clamp (see Figure S2). **p < 0.01 (paired t test). See also Figure S2.
mGluR Activation Facilitates Ca2+ Signals Evoked by Trains of APs in NAc MSNs
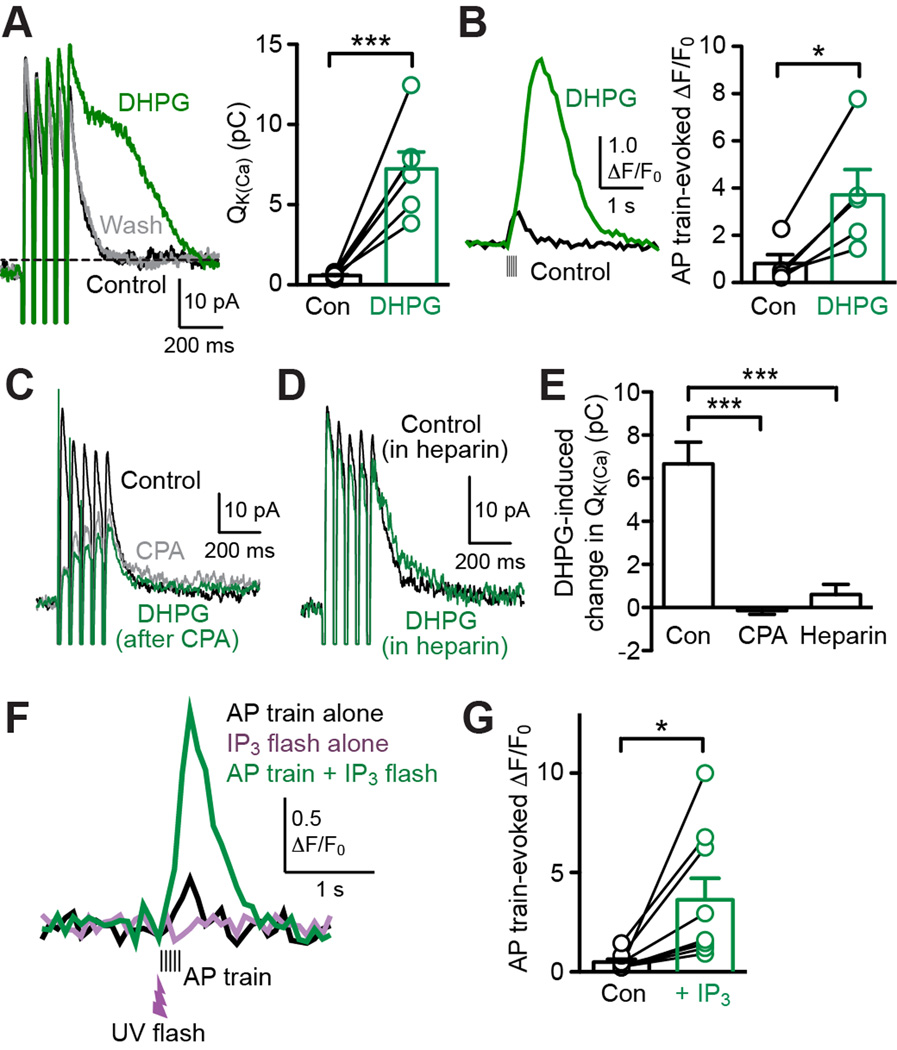
mGluR/IP3-dependent amplification of AP-evoked Ca2+ signals has been implicated in driving synaptic plasticity in CNS neurons (Harnett et al., 2009; Wang et al., 2000). To assess AP-evoked Ca2+ signals in MSNs, 2 ms depolarizing pulses of 30 mV were applied to evoke unclamped APs and the resulting tail outward currents were recorded, similarly to our previous study in the dorsal striatum (Clements et al., 2013). We first confirmed that these tail currents, evoked by a single AP (IAP-single) or a 200 ms AP train (5 APs at 20 Hz; IAP-train), were completely eliminated by TTX (1 µM) in the NAc (n = 4 cells; Figure S3A). Furthermore, IAP-single and IAP-train were suppressed by apamin (100 nM, n = 4 cells; Figure S3B). The apamin-insensitive component of IAP-single and IAP-train mostly decayed within 50 ms and 150 ms, respectively, except for a small component of IAP-train that slowly decayed over ~5–10 s.
To investigate mGluR/IP3-dependent regulation AP-evoked Ca2+ signals, we tested the effect of DHPG, a group I mGluR (mGluR1/5) agonist, on IAP-single and IAP-train. Bath application of DHPG (5 µM; 3–5 min) added a late component to IAP-single in 3 out of 7 cells tested, while IAP-train was consistently augmented and prolonged by DHPG in a reversible manner in all of these cells (Figures 3A and S4). In order to quantify the DHPG effect on IAP-single and IAP-train, we calculated the time integral of these currents after removing the initial 50 ms or 150 ms window, respectively (termed Q-K(Ca); current level at ~600–800 ms after the AP train offset was taken as baseline for IAP-train) (Figure 3A). It should be noted that apamin completely eliminated the DHPG effect on IAP-train (n = 6 cells; Figure S3C), supporting the use of the SK-dependent component of IAP-train as a measure of mGluR-induced Ca2+ signal regulation. In the following experiments, we focused on Ca2+ signals evoked by AP trains (5 APs at 20 Hz) in light of the consistency and robustness of DHPG-induced enhancement.
Figure 3. mGluR activation enhances AP train-evoked Ca2+ signals via IP3-dependent CICR from intracellular Ca2+ stores.
(A) Left: Example traces illustrating the effect of DHPG (5 µM) on IAP-train. Right: Summary graph showing DHPG effect on QK(Ca) for IAP-train (7 cells). ***p < 0.001 (paired t test). See also Figures S3 and S4.
(B) Example traces (left) and summary graph (right) demonstrating DHPG-induced facilitation of AP train-evoked Ca2+ transients monitored with Fluo-4FF (100 µM; 5 cells). *p < 0.05 (paired t test).
(C) Representative traces depicting that DHPG had no effect on IAP-train after depleting intracellular Ca2+ stores with CPA (20 µM).
(D) Example traces illustrating small DHPG effect on IAP-train in a cell loaded with heparin (0.1 mg/ml).
(E) Summary bar graph showing that CPA (n = 5 cells) and heparin (0.1–0.25 mg/ml; n = 5 cells) suppressed DHPG-induced increases in QK(Ca) observed under control condition (n = 7 cells). ***p < 0.001 (one-way ANOVA followed by Bonferroni post hoc test).
(F) Representative traces of Ca2+ transients evoked by an AP train alone (black) or by an AP train paired with preceding photolysis of caged IP3 (green). UV flash (~1 ms) was applied 50 ms prior to the AP train. Note that UV flash intensity was adjusted to evoke no measurable Ca2+ rise by itself (purple trace).
(G) Summary graph showing increases in AP train-evoked Ca2+ signals by preceding subthreshold IP3 application (9 cells). *p < 0.05 (paired t test). See also Figure S5.
When [Ca2+]i was monitored at the soma with Fluo-4FF (100 µM), DHPG (5 µM) produced large increase and prolongation of AP train-evoked Ca2+ signals (n = 5 cells; Figure 3B). Increasing the concentration of Fluo-4FF to 500 µM significantly attenuated the DHPG effect on AP train-evoked Ca2+ transients (n = 4 cells; Figure S5), reflecting an increase in the Ca2+ buffering capacity. Altogether, these results show that mGluR activation amplifies Ca2+ signals triggered by AP trains in MSNs of the NAc core.
To examine whether facilitation of AP train-evoked Ca2+ signals is caused via CICR, we tested cyclopiazonic acid (CPA), which depletes intracellular Ca2+ stores (Seidler et al., 1989). Bath application of CPA (20 µM; >10 min), which inhibited IAP-train by itself, completely eliminated DHPG-induced facilitation (Figures 3C and 3E). Furthermore, intracellular application of heparin (0.1–0.25 mg/ml; >20 min dialysis via the whole-cell pipette), an IP3R antagonist (Ghosh et al., 1988), also largely suppressed the DHPG effect on IAP-train (Figures 3D and 3E). To further demonstrate the role of IP3, we performed UV photolysis of caged IP3 at subthreshold intensity, which produced no measurable rise in [Ca2+]i by itself. However, this subthreshold IP3 caused significant facilitation of AP train-evoked Ca2+ transients when applied 50 ms prior to the AP train (Figures 3F and 3G), in a manner analogous to DHPG perfusion. Therefore, IP3-dependent CICR underlies mGluR facilitation of AP train-evoked Ca2+ signals.
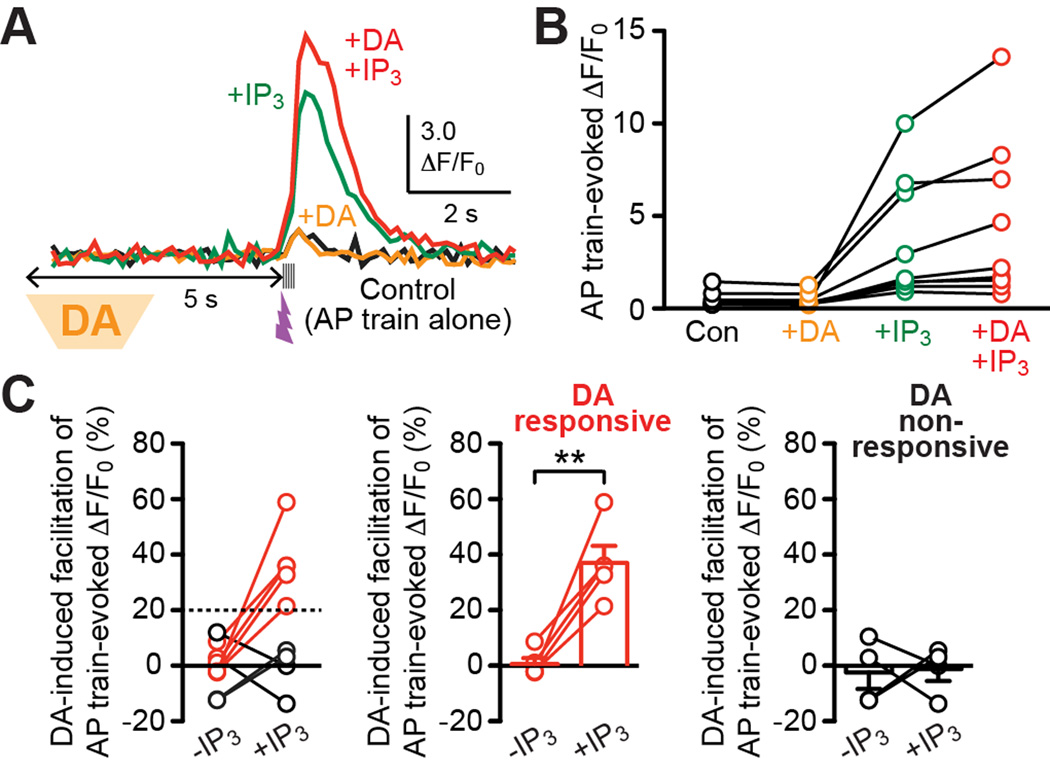
Phasic DA Amplifies IP3 Facilitation of AP Train-Evoked Ca2+ Signals in a Subset of MSNs
We next investigated how phasic DA affects IP3-dependent regulation AP train-evoked Ca2+ signals. Transient DA application (2.5 s, onset 5 s prior to the AP train) failed to exert measurable effect on AP train-evoked Ca2+ transients in 9 cells tested (loaded with 100 µM Fluo-4FF; Figures 4A and 4B). However, DA caused >20% increases in AP train-evoked Ca2+ transients when facilitated by subthreshold IP3 in 5 out of these 9 cells (Figures 4A–4C). Hence, phasic DA can amplify the facilitatory effect of IP3 on AP train-evoked Ca2+ signals in a subset of MSNs in the NAc core.
Figure 4. Phasic DA promotes IP3 facilitation of AP train-evoked Ca2+ signals in a subpopulation of MSNs.
(A) Example traces of Ca2+ transients evoked by an AP train alone (black) or AP trains paired with preceding DA application (orange), with preceding subthreshold IP3 application (green), or with both DA and IP3 applications (red). The onset of DA puff application (2.5 s) was set at 5 s prior to the AP train, while UV flash (~1 ms) was applied 50 ms before the AP train.
(B) Graph plotting AP train-evoked Ca2+ transients in 9 cells tested for the effects of DA/IP3 applications as in (A).
(C) Left: The magnitude of phasic DA-induced facilitation of AP train-evoked Ca2+ transients are plotted under control condition without IP3 application [−IP3; facilitation calculated by comparing black and orange traces/data points in (A) and (B)] and with IP3 application [+IP3; facilitation calculated by comparing green and red traces/data points in (A) and (B)] in all 9 cells shown in (B). Note that DA produced >20% facilitation of AP train-evoked Ca2+ transients with IP3 application in 5 cells (middle), while no measurable DA effect was observed in the remaining 4 cells (right). **p < 0.01 (paired t test).
D1 DA Receptors Mediate Enhancement of IP3-Induced Ca2+ Signals
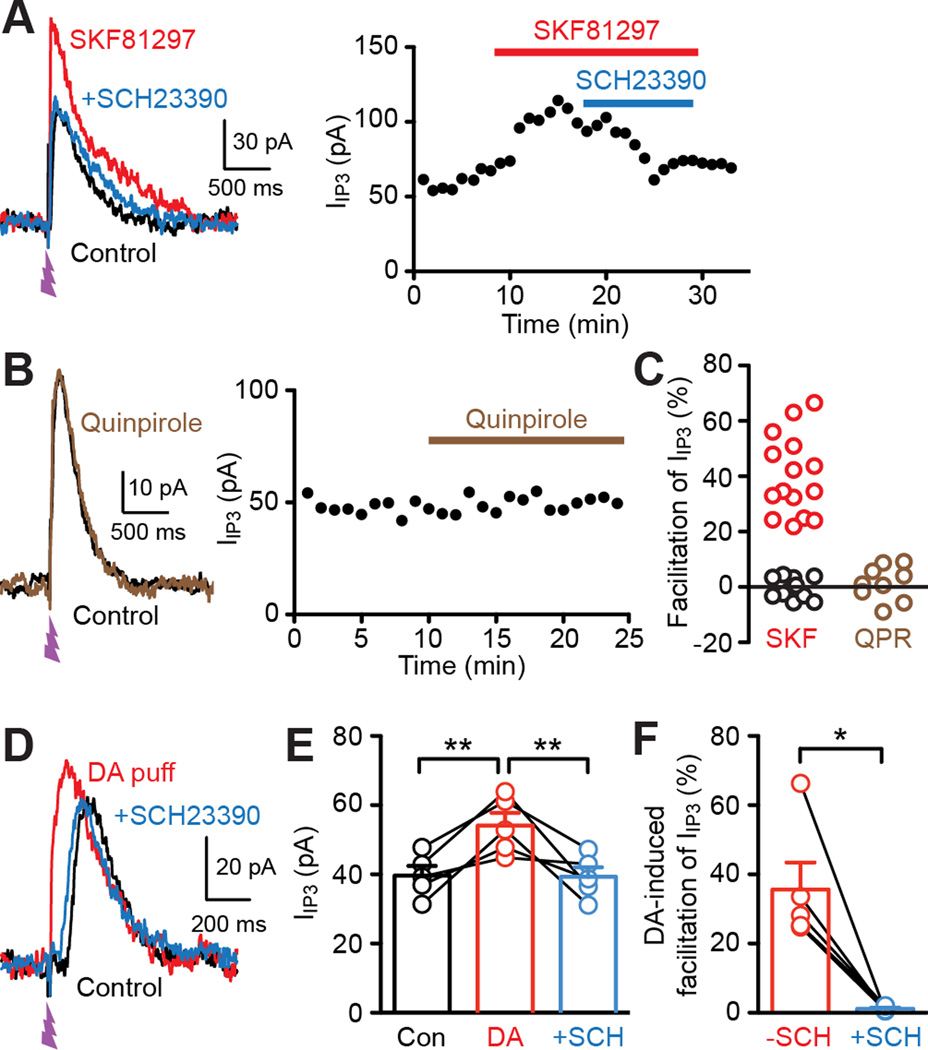
To address the involvement of DA receptor subtypes in Ca2+ signal regulation, we examined the effects of the D1 agonist SKF81297 and the D2 agonist quinpirole on IIP3 (UV flash intensity set as in Figures 1 and 2). Bath application of SKF81297 (1 µM) increased IIP3 by >20% in 15 out of 27 cells tested (Figures 5A and 5C). This SKF8129-induced IIP3 facilitation was reversed by the D1 antagonist SCH23390 (1 µM; n = 7). In contrast, the D2 agonist quinpirole (1 µM) failed to affect IIP3 in all MSNs tested (n = 9; Figures 5B and 5C). Furthermore, phasic DA-induced facilitation of IIP3 was completely blocked by SCH23390 (n = 5; Figures 5D–5F), demonstrating the role of D1 receptors in phasic DA action.
Figure 5. D1 DA receptors mediate facilitation of IP3-induced Ca2+ signals.
(A) Example traces (left) and time graph (right) demonstrating the facilitatory effect of the D1 agonist SKF81297 (1 µM) on IIP3 and its reversal by the D1 antagonist SCH23390 (1 µM).
(B) Example traces (left) and time graph (right) showing that the D2 agonist quinpirole (1 µM) failed to affect IIP3.
(C) Graph plotting the magnitude of IIP3 facilitation produced by SKF81297 (27 cells) or quinpirole (9 cells) in individual MSNs. Data from MSNs showing SKF81297-induced facilitation of IIP3 >20% (15 cells) are marked in red.
(D) Representative traces illustrating the blockade of phasic DA-induced facilitation of IIP3 by SCH23390.
(E) Summary graph plotting IIP3 amplitude in control, with preceding DA application, and with DA application in the presence of SCH23390 (5 cells). **p < 0.01 (repeated measures one-way ANOVA followed by Bonferroni post hoc test).
(F) Summary graph plotting the magnitude of DA-induced facilitation of IIP3 before (−SCH) and after SCH23390 application (+SCH) in the 5 cells shown in (E). *p < 0.05 (paired t test).
D2 DA Receptors Mediate Reversal of A2A Adenosine Receptor-Induced Ca2+ Signal Facilitation in D1-Insensitive MSNs
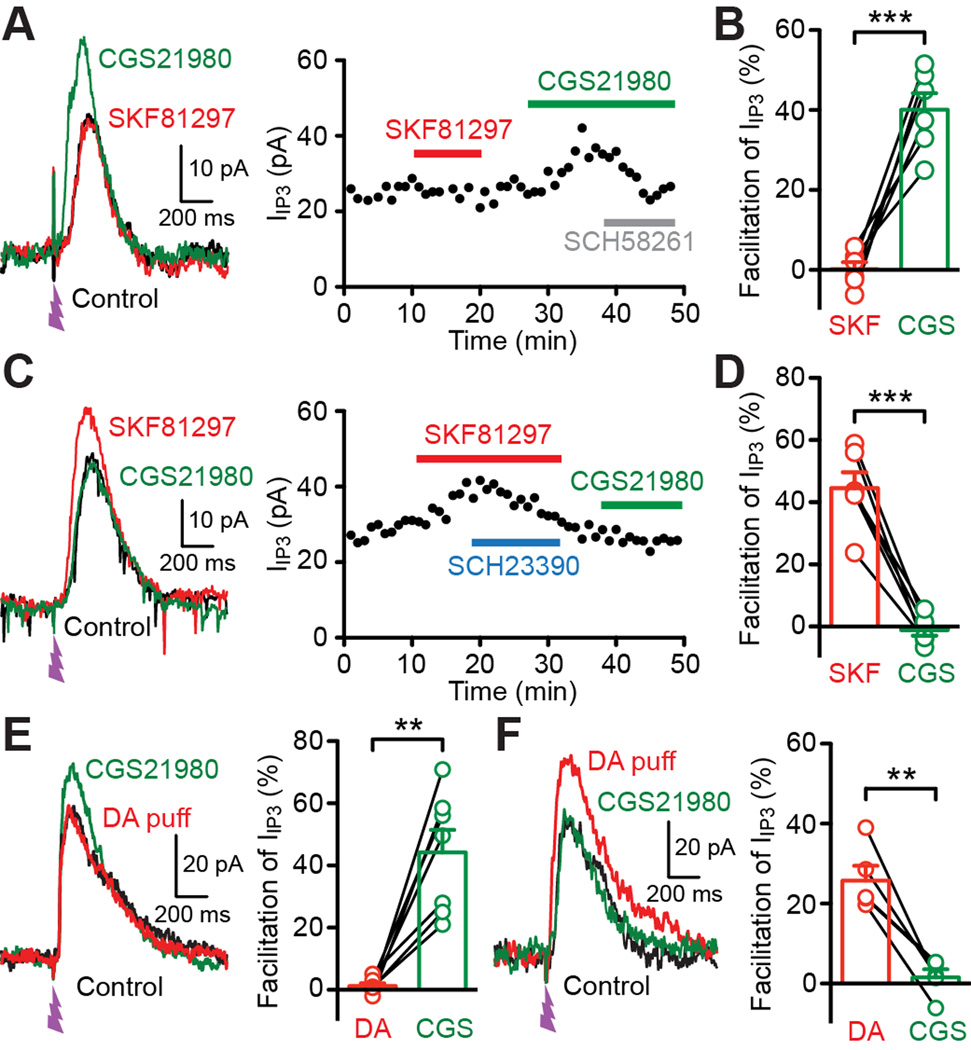
D1 and D2 receptors are coupled to Gs- and Gi-subtype G proteins, which stimulate and inhibit the adenylyl cyclase-cAMP-PKA (protein kinase A) pathway, respectively. Adensoine A2A receptors, which are coupled to Gs like D1 receptors, are selectively expressed in D2 receptor-positive MSNs, where they frequently exert effects opposing those mediated by Gi-coupled D2 receptors (Higley and Sabatini, 2010; Schiffmann et al., 2007; Shen et al., 2008). Thus we examined the effect of the A2A receptor agonist CGS21680 (1 µM) on IIP3. In 12 cells tested for both SKF81297 and CGS21680 (SKF81297 tested first in 8 cells, CGS21680 tested first in 4 cells), we found that CGS21680 augmented IIP3 in 6 cells that displayed no response to SKF81297 (Figures 6A and 6B), while CGS21680 had no effect in 6 cells in which SKF81297 facilitated IIP3 (Figures 6C and 6D). CGS21680 increased IIP3 in 5 out of 11 cells where CGS21680 was tested without prior SKF81297 application. CGS21680-induced IIP3 facilitation was reversed by the A2A receptor antagonist SCH58261 (1 µM, n = 4; Figure 6A). Furthermore, in 12 MSNs tested for phasic DA effect on IIP3, CGS21680 increased IIP3 in 7 cells in which phasic DA application had no effect (Figure 6E), whereas CGS21680 was ineffective in 5 cells that responded to phasic DA (Figure 6F). These results demonstrate that D1 DA receptors and A2A adenosine receptors positively regulate IP3-dependent Ca2+ signaling in distinct, non-overlapping subpopulations of MSNs.
Figure 6. Adenosine A2A receptor activation drives facilitation of IP3-induced Ca2+ signals in MSNs insensitive to D1 DA receptor activation.
(A) Representative traces (left) and time graph (right) showing that the A2A agonist CGS21680 (1 µM) augmented IIP3 in an MSN that exhibited no response to the D1 antagonist SCH23390 (1 µM). Note in the time graph that the A2A antagonist SCH58261 (1 µM) reversed the effect of CGS21680.
(B) Graph plotting the magnitude of IIP3 facilitation in MSNs where IIP3 was insensitive to SCH23390 but sensitive to CGS21680 (6 cells).
(C) Example traces (left) and time graph (right) illustrating that CGS21680 failed to affect IIP3 in an MSN in which SCH23390 facilitated IIP3.
(D) Graph plotting the magnitude of IIP3 facilitation in MSNs where IIP3 was sensitive to SCH23390 but insensitive to CGS21680 (6 cells).
(E) Example traces (left) and summary graph (right) showing that CGS21680 facilitated IIP3 in MSNs in which DA puff application (2.5 s, onset 5 s before UV flash) failed to affect IIP3 (7 cells).
(F) Example traces (left) and summary graph (right) showing that CGS21680 failed to affect IIP3 in MSNs where IIP3 was enhanced by preceding DA application (5 cells).
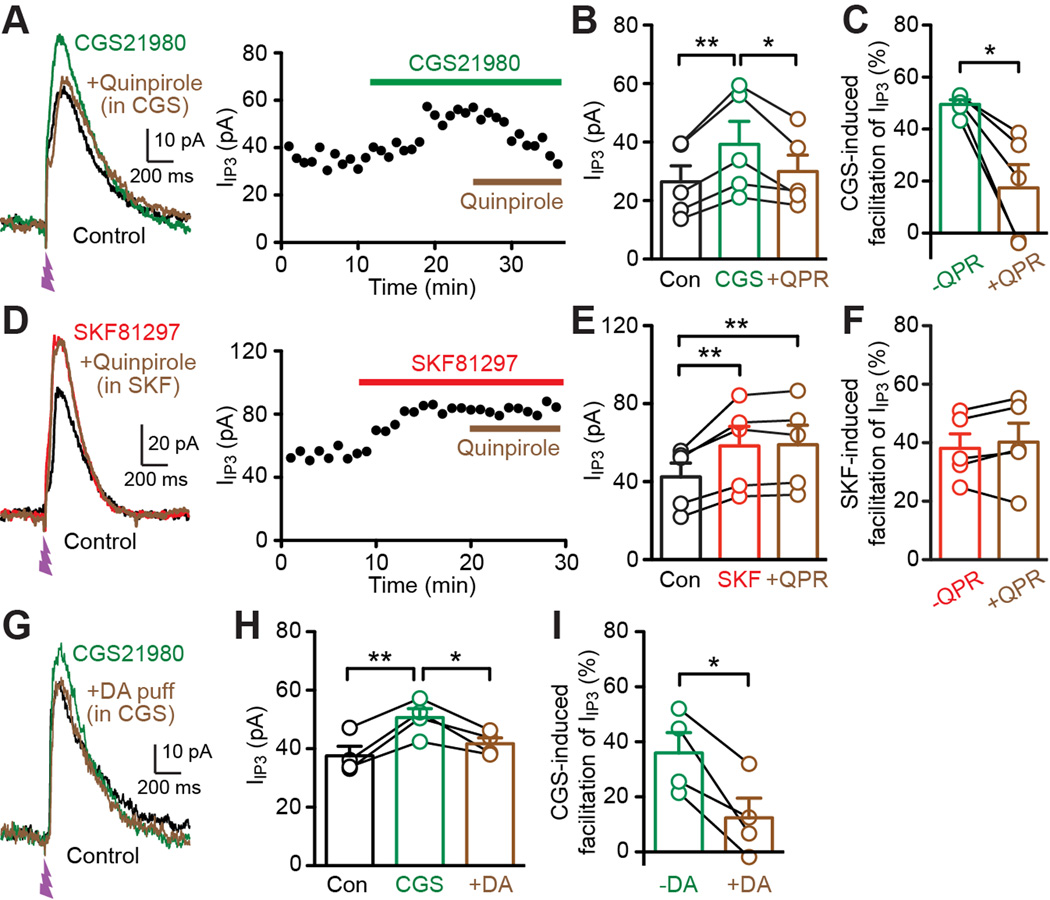
Finally, the D2 agonist quinpirole, which had no effect on IIP3 by itself (Figures 5B and 5C), was capable of reversing CGS21680-induced IIP3 facilitation, at least partially, in all 5 cells tested (Figures 7A–7C), while quinpirole failed to reverse the SKF81297 effect (5 cells; Figures 7D–7F). Similarly, phasic DA application caused reversal of CGS21680-induced IIP3 facilitation (Figures 7G–7I; tested in 4 cells out of the 7 cells shown in Figure 7F where phasic DA failed to affect IIP3 by itself). These results are consistent with the selective expression of D2 receptors in A2A receptor-positive MSNs, where activation of D2 receptors counteracts A2A receptor-mediated enhancement of IP3-dependent Ca2+ signaling.
Figure 7. D2 DA receptor activation counteracts adenosine A2A receptor-mediated facilitation of IP3-induced Ca2+ signals.
(A) Representative traces (left) and time graph (right) illustrating the reversal of CGS21680 (1 µM)-induced facilitation of IIP3 by the D2 agonist quinpirole (1 µM).
(B) Graph plotting IIP3 amplitude in control, in CGS21680, and in quinpirole in the continued presence of CGS21680 (5 cells). *p < 0.05, **p < 0.01 (repeated measures one-way ANOVA followed by Bonferroni post hoc test).
(C) Graph plotting the magnitude of CGS21680-induced facilitation of IIP3 before (−QPR) and after quinpirole application (+QPR) in the 5 cells shown in (B). *p < 0.05 (paired t test).
(D) Representative traces (left) and time graph (right) depicting the ineffectiveness of quinpirole in reversing SKF81297-induced facilitation of IIP3.
(E) Graph plotting IIP3 amplitude in control, in SKF81297, and in quinpirole in the continued presence of SKF81297 (5 cells). **p < 0.01 (repeated measures one-way ANOVA followed by Bonferroni post hoc test).
(F) Graph plotting the magnitude of SKF81297-induced facilitation of IIP3 before (−QPR) and after quinpirole application (+QPR) in the 5 cells shown in (E).
(G) Example traces illustrating that DA puff application (2.5 s, onset 5 s prior to UV flash) inhibited IIP3 enhanced by CGS21680 perfusion.
(H) Graph plotting IIP3 amplitude in control, in CGS21680, and with preceding DA application in the continued presence of CGS21680 (4 cells). *p < 0.05, **p < 0.01 (repeated measures one-way ANOVA followed by Bonferroni post hoc test).
(I) Graph plotting the magnitude of CGS21680-induced facilitation of IIP3 without (−DA) and with DA puff application (+DA) in the 4 cells shown in (H). *p < 0.05 (paired t test).
DISCUSSION
Previous studies in the dorsal striatum have shown that D1 DA receptors and A2A adenosine receptors mediate facilitation of NMDA receptor-induced Ca2+ signals in distinct subpopulations of MSNs in the dorsal striatum, with A2A receptor-mediated facilitation being reversed by D2 receptor activation (Higley and Sabatini, 2010; Plotkin et al., 2011). The present study demonstrates that mGluR/IP3-induced Ca2+ signaling undergoes similar regulation by these receptors (D1 vs. A2A/D2) in two distinct MSN subpopulations in the NAc. Thus DA controls two types of Ca2+ signals that play critical roles in regulating MSN excitability and plasticity. Intriguingly, DA puff application (2.5 s), mimicking phasic DA transients observed in behaving animals (Day et al., 2007; Phillips et al., 2003), triggered D1 receptor-mediated facilitation of IP3-evoked Ca2+ signals when its onset preceded IP3 application by ~2–10 s. This type of timing dependence of DA action in the order of seconds might be related to the timing dependence of reward action displayed during reward-based appetitive behavior and conditioning (Day and Carelli, 2007; Fields et al., 2007; Schultz, 1998).
Differential Regulation of IP3-Evoked Ca2+ Signals in Two Subpopulations of MSNs
Phasic DA application enhanced IP3-evoked Ca2+ signals in ~50% of MSNs (30 out of 61 cells) in the NAc core. This DA effect is mediated by D1 receptors, as it was blocked by a D1 antagonist and reproduced by a D1 agonist. We further found that, in the remaining subpopulation of MSNs displaying no D1 response, A2A adenosine receptors drive facilitation of IP3-evoked Ca2+ signals, which can be reversed by D2 receptor activation, attained with either phasic DA or a D2 agonist. In contrast to many recent brain slice recording studies using transgenic mice in which MSNs expressing D1 or D2 receptors are selectively labeled with markers (e.g., green fluorescent proteins) (Higley and Sabatini, 2010; Lim et al., 2012; Pascoli et al., 2014; Plotkin et al., 2011), recordings were done in NAc slices from wild-type mice in the present study. Thus the experimenter was "blind" in terms of the MSN subtypes (D1 receptor-expressing vs. D2 receptor-expressing) during recording and still was able to detect two distinct, nonoverlapping subsets of MSNs: one showing D1-mediated response and the other displaying counteracting effects of A2A/D2 receptors. Coexpression of D1 and D2 receptors has been shown in a small fraction of MSNs (2–6 %) in the NAc core in transgenic mice (Bertran-Gonzalez et al., 2008; Kupchik et al., 2015). We failed to observe MSNs displaying both D1 and D2 responses, i.e., D1-mediated Ca2+ signal facilitation reversed by D2 activation, or MSNs responsive to both D1 and A2A activation, with latter being reversed by D2 activation. It should also be noted that phasic DA application caused no Ca2+ rises by itself in the present study, in contrast to the evidence for the existence of D1–D2 heteromers coupled to IP3-induced Ca2+ signaling in certain MSNs in rats (Hasbi et al., 2010). Thus, DA regulation of IP3-induced Ca2+ signaling may display regional difference (NAc core vs. NAc shell vs. dorsal striatum) and/or species difference based on the degree of D1–D2 coexpression/heterodimerization (Bertran-Gonzalez et al., 2010; Perreault et al., 2014).
Mechanistically, PKA phosphorylation of IP3Rs is known to increase their IP3 sensitivity (Tang et al., 2003; Wagner et al., 2008). Alternatively, recent studies indicate that cAMP can directly increase IP3R sensitivity independent of PKA phosphorylation (Taylor et al., 2014; Tovey et al., 2010). These mechanisms most likely underlie the differential regulation via D1 receptors (Gs-coupled) vs. A2A receptors (Gs-coupled)/D2 receptors (Gi-coupled) in two MSN subpopulations. The ineffectiveness of D2 activation in regulating basal IP3-evoked Ca2+ signals (i.e., without A2A-induced facilitation) suggests low basal cAMP levels/PKA activity in MSNs, which may be reflecting low adenosine tone in ex vivo slices [(Plotkin et al., 2011); but also see (Higley and Sabatini, 2010)].
Regulation of AP-Evoked Ca2+ Signals: Interaction between D1 DA receptors and mGluRs Coupled to IP3 Generation
Our data demonstrate that mGluR activation facilitates AP train-evoked Ca2+ signals via IP3-dependent CICR in MSNs of the NAc core, as has been reported in DA neurons, hippocampal pyramidal neurons, and cerebellar Purkinje neurons (Cui et al., 2007; Nakamura et al., 2000; Wang et al., 2000), as well as in MSNs of the dorsal striatum (Plotkin et al., 2013). mGluR action on single AP-evoked Ca2+ signals was inconsistent among MSNs, in contrast to the robust mGluR-induced facilitation of single AP-evoked Ca2+ signals observed in DA neurons of the substantia nigra (Cui et al., 2007). Thus, in MSNs, large Ca2+ influx associated with multiple APs is necessary to reliably trigger Ca2+-dependent activation of IP3Rs driving regenerative CICR, even when their Ca2+ sensitivity is increased by IP3 generated via mGluR activation.
As expected from the D1-dependent regulation of IP3 signaling, phasic DA is capable of promoting IP3 facilitation of AP train-evoked Ca2+ signals in a subset of MSNs, while having no effect on basal AP train-evoked Ca2+ signals. Ryanodine receptors (RyRs), another type of Ca2+-activated receptors on intracellular Ca2+ stores, have been shown to selectively mediate the CICR-dependent component of basal AP train-evoked Ca2+ transients in MSNs of the dorsal striatum (Clements et al., 2013). Our data suggest that RyR-mediated CICR cannot be directly regulated by D1 activation; however RyRs may well act to boost IP3R-dependent CICR (Plotkin et al., 2013), particularly when the latter is facilitated by D1 activation.
Previous studies have shown that D2 activation, either by focal DA puff application or bath perfusion of the D2 agonist quinpirole, suppresses AP-evoked Ca2+ transients in dendritic spines of D2 receptor-expressing MSNs in the dorsal striatum (Day et al., 2008; Higley and Sabatini, 2010), while another study has reported that optogenetic stimulation of dopaminergic fibers has no effect on AP-evoked Ca2+ transients in dendritic spines of D1 receptor-expressing MSNs in the NAc core (Yagishita et al., 2014). Although out of the scope of the current study, it would be interesting to fully determine DA actions on AP-evoked Ca2+ transients, both basal and IP3-facilitated, in different subcellular locations of MSNs, as has been demonstrated for DA-induced activation of PKA (soma/proximal dendrites vs. distal dendrites) in the Yagishita et al. study.
Timing Dependence of DA Effect on IP3 Signaling: Potential Relevance to Phasic DA Actions in Behaving Animals
In behaving animals, presentation of primary rewards or reward-predicting sensory stimuli evokes transient DA rises in the NAc core lasting ~1–10 s (Day and Carelli, 2007; Day et al., 2007; Phillips et al., 2003). Although the critical roles of these phasic DA signals in driving reward-based learning and motivated behavior is widely agreed upon, the precise mechanisms underlying DA actions in the NAc/striatum remain to be a matter of debate (Fields et al., 2007; Surmeier et al., 2014; Tritsch and Sabatini, 2012). How exactly do phasic DA signals affect MSNs? The answer to this question has been elusive, as DA exerts no clear effects on MSNs in the order of seconds. For example, a recent study reported a form of phasic DA-timing-dependent synaptic plasticity, where phasic DA paired with synaptic activity within a time window of ~1 s, while having no immediate effects on MSN activity or synaptic transmission by itself, leads to persistent synaptic potentiation after the pairing (Wieland et al., 2015). In line with the lack of acute DA effects on MSNs, another recent study has exogenously expressed G protein-coupled inwardly rectifying K+ channels (GIRKs) as a "sensor" to detect phasic DA-evoked activation of D2 receptors with high temporal resolution (i.e., in the order of seconds) in MSNs (Marcott et al., 2014). Our data demonstrate that activation of D1 receptors with local DA application (2.5 s) leads to transient facilitation of IP3-evoked Ca2+ signaling within 2 s from the onset of DA application, peaking at 5 s, and lasting for ~10 s to 20 s at most. Intriguingly, phasic DA activation of D1 receptors, attained with photolytic DA application (0.1–1 s), has been shown to produce PKA activation within seconds, peaking at ~20–30 s, and lasting for ~1–3 min in striatal MSNs (Castro et al., 2013). Similarly, phasic DA release via optogenetic stimulation of DA fibers (0.3 s), when paired with AP train-evoked Ca2+rises necessary to activate Ca2+-sensitive adenylyl cyclase, causes D1-dependent PKA activation lasting ~1 min in dendritic spines of MSNs in the NAc core (Yagishita et al., 2014). Based on the difference in the onset/offset kinetics of DA action, direct cAMP regulation of IP3Rs (Taylor et al., 2014; Tovey et al., 2010), not PKA phosphorylation of IP3Rs (Tang et al., 2003; Wagner et al., 2008), may underlie the relatively rapid and short-lasting DA effect on IP3-induced Ca2+ signaling demonstrated in the present study.
Firing pauses in a subpopulation of NAc neurons have been linked to reward-directed behavior (Krause et al., 2010; Taha and Fields, 2006). Furthermore, mGluR/IP3-dependent amplification of AP-evoked Ca2+ signals is known to mediate the induction of glutamatergic synaptic plasticity in DA neurons and cerebellar Purkinje neurons (Harnett et al., 2009; Wang et al., 2000). We propose that DA-timing-dependent regulation of IP3 signaling (i.e., IP3-evoked pauses in firing and IP3-induced facilitation of AP train Ca2+ transients) might play important roles during reward-based learning and behavior.
EXPERIMENTAL PROCEDURES
Animals
Male C57BL/6J mice (4–5 weeks old) were obtained from Jackson Laboratory and were housed under a 12 h light/dark cycle. Food and water were available ad libitum. All animal procedures were approved by the University of Texas Institutional Animal Care and Use Committee.
Electrophysiology
Mice were sacrificed by cervical dislocation under isoflurane anesthesia and the brain was quickly dissected out. Coronal or sagittal slices (200–250 µm) containing the NAc were cut in an ice-cold solution containing (in mM): 205 sucrose, 2.5 KCl, 1.25 NaH2PO4, 7.5 MgCl2, 0.5 CaCl2, 10 glucose, and 25 NaHCO3, saturated with 95% O2 and 5% CO2 (~300 mOsm/kg) and incubated > 1 h at 35°C in physiological saline containing (in mM): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 11 glucose, and 25 NaHCO3, saturated with 95% O2 and 5% CO2 (pH 7.4, ~295 mOsm/ kg). MK-801 (10 µM) was added to the incubation chamber to prevent NMDA receptor-mediated toxicity. Recordings were made at 34–35°C in the same saline (without MK-801) perfused at ~2.5 ml/min. The majority of experiments were performed in the presence of DNQX (10 µM) and picrotoxin (100 µM) to block AMPA/GABAA-mediated synaptic inputs.
Cells were visualized using an upright microscope (Olympus) with infrared/oblique illumination optics. Recordings were performed in the NAc core, which was located using anterior commissure as the landmark. Whole-cell recordings were made with borosilicate glass pipettes (2.0–2.6 MΩ) filled with internal solution containing (in mM): 115 K-gluconate, 20 KCl, 1.5 MgCl2, 10 HEPES, 0.025 EGTA, 2 Mg-ATP, 0.2 Na2-GTP, and 10 Na2-phosphocreatine (pH 7.2, ~285 mOsm/kg), unless indicated otherwise. Series resistance was continuously monitored but left uncompensated during voltage-clamp recording experiments. Recordings were discarded if the series resistance increased beyond 18 MΩ. Bridge balance was adjusted periodically (~every 2–3 min) during current-clamp recording experiments. The membrane potential was corrected for a liquid junction potential of −7 mV. Axopatch 1D or Multiclamp 700B amplifier (Molecular Devices) and AxoGraph X software (AxoGraph Scientific) were used to record and collect data (filtered at 1–10 kHz and digitized at 2–20 kHz for voltage-clamp recordings, filtered at 10 kHz and digitized at 20 kHz for current-clamp recordings). MSNs, which comprise the vast majority (>90–95% in rodents) of neurons in the NAc/striatum, were identified by their electrophysiological properties, including hyperpolarized resting membrane potentials (~−80 to –95 mV), low input resistance at resting potential (~40–100 MΩ), prominent inward rectification, and slow depolarizing ramp preceding AP firing during threshold depolarizations, as in our previous studies (Beatty et al., 2012; Clements et al., 2013).
Ca2+ Imaging
Fluorescence imaging of [Ca2+]i was done using low-affinity Ca2+ indicator Fluo-4FF (Kd = 9.7 µM). Fluo-4FF (100 or 500 µM; no EGTA in these experiments) was loaded into the cell via the whole-cell pipette (>20 min dialysis after break-in). Images were taken at ~7–15 Hz using the Olympus Disk Spinning Unit Imaging System. After raw fluorescent signals from selected regions of interest (ROIs) were background subtracted at each time point, Ca2+ transients were expressed as ΔF/F0 = (F(t) − F0)/F0, where F0 was determined as an average of F(t) over a 1 s baseline period. Brief artifacts accompanying UV flashes are omitted from the fluorescence traces in flash photolysis experiments.
Flash Photolysis
Cells were dialyzed with caged IP3 (50 µM) through the whole-cell pipette. A brief UV flash (~1 ms) was applied with a xenon arc lamp driven by a photolysis system (Cairn Research) to rapidly photolyze caged IP3 (~3 ms) (Walker et al., 1989). The UV flash was focused through a 60× objective onto a 350 µm area surrounding the recorded cell. The Cairn system has the capacity to vary the intensity of UV flash, which was measured at the tip of the objective (expressed in µJ). The degree of photolysis of caged compounds is known to be proportional to the UV flash intensity (McCray et al., 1980), thus the concentration of IP3 released into the cytosol can be expressed in µM·µJ. In the experiments testing suprathreshold IP3 responses, UV flash intensity was adjusted to evoke IIP3 with amplitude ~50% of that evoked by the largest flash intensity in our setup (~200 µJ, i.e., ~10,000 µM·µJ IP3; IIP3 shows no sign of saturation). This produced IIP3 in the range of 15–60 pA (with UV intensity in the range of ~25–50 µJ, i.e., ~1250–2500 µM·µJ IP3) in the majority of recorded MSNs. Cells with IIP3 outside of this range were excluded from the analysis. It should be noted that IP3 concentration up to 80,000 µM·µJ failed to evoke saturating IP3 responses in MSNs of the dorsal striatum in our previous study (Clements et al., 2013).
DA Pressure Ejection
Patch pipettes (~2–3 µm tip diameter), filled with DA (20 µM) and ascorbate (1.3 mM) dissolved in physiological saline, were placed ~100 µm from recorded cells, and pressure of 15 p.s.i. was applied using a TooheySpritzer Pressure System IIe. The speed and spatial range of puff application can be seen in Movie S1, where both the puff pipette and the whole-cell recording pipette are loaded with Alexa Fluor 594 (50 µM). DA solutions were made fresh every 1 hr or after each recording.
Drugs
Apamin, iberiotoxin, cyclopiazonic acid, DHPG, DNQX, picrotoxin, quinpirole, SKF81297, SCH23390, CGS21680, and SCH58261 were obtained from Tocris Biosciences. TTX was obtained from Alomone Labs. Fluo-4FF and Alexa Fluor 594 were purchased from Life Technologies. Caged IP3 was a generous gift from Dr. Kamran Khodakhah at Albert Einstein College of Medicine. All other chemicals were from Sigma-RBI.
Data Analysis
Data are expressed as mean ± SEM. Statistical significance was determined by Student's t test or ANOVA followed by Bonferroni post hoc test. The difference was considered significant at p < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Kamran Khodakhah for the generous gift of caged IP3 made in his lab. We also thank Dr. Michael Clements for acquiring some preliminary data and Jeremiah Lee for providing NAc slices for certain experiments. This work was funded by NIH grants DA015687 and AA015521.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
I.S. and B.B. conducted the experiments and analyzed the data. I.S. and H.M. designed the experiments and wrote the paper.
REFERENCES
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- Beatty JA, Sullivan MA, Morikawa H, Wilson CJ. Complex autonomous firing patterns of striatal low-threshold spike interneurons. J Neurophysiol. 2012;108:771–781. doi: 10.1152/jn.00283.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. What is the Degree of Segregation between Striatonigral and Striatopallidal Projections? Frontiers in neuroanatomy. 2010;4 doi: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepari M, Ogden D. Kinetic, pharmacological and activity-dependent separation of two Ca2+ signalling pathways mediated by type 1 metabotropic glutamate receptors in rat Purkinje neurones. J Physiol. 2006;573:65–82. doi: 10.1113/jphysiol.2005.103770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro LR, Brito M, Guiot E, Polito M, Korn CW, Herve D, Girault JA, Paupardin-Tritsch D, Vincent P. Striatal neurones have a specific ability to respond to phasic dopamine release. J Physiol. 2013;591:3197–3214. doi: 10.1113/jphysiol.2013.252197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements MA, Swapna I, Morikawa H. Inositol 1,4,5-triphosphate drives glutamatergic and cholinergic inhibition selectively in spiny projection neurons in the striatum. J Neurosci. 2013;33:2697–2708. doi: 10.1523/JNEUROSCI.4759-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Bernier BE, Harnett MT, Morikawa H. Differential regulation of action potential- and metabotropic glutamate receptor-induced Ca2+ signals by inositol 1,4,5-trisphosphate in dopaminergic neurons. J Neurosci. 2007;27:4776–4785. doi: 10.1523/JNEUROSCI.0139-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Carelli RM. The nucleus accumbens and Pavlovian reward learning. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2007;13:148–159. doi: 10.1177/1073858406295854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28:11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Kuroda S, Michikawa T, Kawato M. Inositol 1,4,5-trisphosphatedependent Ca2+ threshold dynamics detect spike timing in cerebellar Purkinje cells. J Neurosci. 2005;25:950–961. doi: 10.1523/JNEUROSCI.2727-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hassar L, Hagenston AM, D'Angelo LB, Yeckel MF. Metabotropic glutamate receptors regulate hippocampal CA1 pyramidal neuron excitability via Ca(2)(+) wave-dependent activation of SK and TRPC channels. J Physiol. 2011;589:3211–3229. doi: 10.1113/jphysiol.2011.209783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron. 2008;59:873–881. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Cholinergic inhibition of ventral midbrain dopamine neurons. J Neurosci. 2000;20:7855–7860. doi: 10.1523/JNEUROSCI.20-20-07855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick AL, Yano H, Trifilieff P, Vishwasrao HD, Biezonski D, Meszaros J, Urizar E, Sibley DR, Kellendonk C, Sonntag KC, et al. Evidence against dopamine D1/D2 receptor heteromers. Mol Psychiatry. 2015;20:1373–1385. doi: 10.1038/mp.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annual review of neuroscience. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh TK, Eis PS, Mullaney JM, Ebert CL, Gill DL. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988;263:11075–11079. [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Cholinergic inhibition of neocortical pyramidal neurons. J Neurosci. 2005;25:10308–10320. doi: 10.1523/JNEUROSCI.2697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenston AM, Fitzpatrick JS, Yeckel MF. MGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cereb Cortex. 2008;18:407–423. doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett MT, Bernier BE, Ahn KC, Morikawa H. Burst-timing-dependent plasticity of NMDA receptor-mediated transmission in midbrain dopamine neurons. Neuron. 2009;62:826–838. doi: 10.1016/j.neuron.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, O'Dowd BF, George SR. Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms. Curr Opin Pharmacol. 2010;10:93–99. doi: 10.1016/j.coph.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13:958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Bonci A. Neurocircuitry of drug reward. Neuropharmacology. 2014;76(Pt B):329–341. doi: 10.1016/j.neuropharm.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci. 2010;30:4746–4756. doi: 10.1523/JNEUROSCI.0197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nature neuroscience. 2015;18:1230–1232. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcott PF, Mamaligas AA, Ford CP. Phasic dopamine release drives rapid activation of striatal D2-receptors. Neuron. 2014;84:164–176. doi: 10.1016/j.neuron.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray JA, Herbette L, Kihara T, Trentham DR. A new approach to time-resolved studies of ATP-requiring biological systems; laser flash photolysis of caged ATP. Proc Natl Acad Sci U S A. 1980;77:7237–7241. doi: 10.1073/pnas.77.12.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Khodakhah K, Williams JT. Two intracellular pathways mediate metabotropic glutamate receptor-induced Ca2+ mobilization in dopamine neurons. J Neurosci. 2003;23:149–157. doi: 10.1523/JNEUROSCI.23-01-00149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Nakamura K, Lasser-Ross N, Barbara JG, Sandler VM, Ross WN. Inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release evoked by metabotropic agonists and backpropagating action potentials in hippocampal CA1 pyramidal neurons. J Neurosci. 2000;20:8365–8376. doi: 10.1523/JNEUROSCI.20-22-08365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Lewin AE, Yasko JR, Vicini S. Contrasting actions of group I metabotropic glutamate receptors in distinct mouse striatal neurones. J Physiol. 2014;592:2721–2733. doi: 10.1113/jphysiol.2014.272773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O'Connor EC, Luscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, O'Dowd BF, George SR. Heteromeric dopamine receptor signaling complexes: emerging neurobiology and disease relevance. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:156–168. doi: 10.1038/npp.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Plotkin JL, Day M, Surmeier DJ. Synaptically driven state transitions in distal dendrites of striatal spiny neurons. Nat Neurosci. 2011;14:881–888. doi: 10.1038/nn.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin JL, Shen W, Rafalovich I, Sebel LE, Day M, Chan CS, Surmeier DJ. Regulation of dendritic calcium release in striatal spiny projection neurons. J Neurophysiol. 2013;110:2325–2336. doi: 10.1152/jn.00422.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Sah P. Competition between calcium-activated K+ channels determines cholinergic action on firing properties of basolateral amygdala projection neurons. J Neurosci. 2008;28:3209–3220. doi: 10.1523/JNEUROSCI.4310-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Cacciapaglia F, Carelli RM. Rapid dopamine dynamics in the accumbens core and shell: learning and action. Front Biosci (Elite Ed) 2013;5:273–288. doi: 10.2741/e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Curr Opin Neurobiol. 2013;23:546–552. doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern EA, Jaeger D, Wilson CJ. Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo. Nature. 1998;394:475–478. doi: 10.1038/28848. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Graves SM, Shen W. Dopaminergic modulation of striatal networks in health and Parkinson's disease. Current opinion in neurobiology. 2014;29:109–117. doi: 10.1016/j.conb.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J Neurosci. 2006;26:217–222. doi: 10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TS, Tu H, Wang Z, Bezprozvanny I. Modulation of type 1 inositol (1,4,5)-trisphosphate receptor function by protein kinase a and protein phosphatase 1alpha. J Neurosci. 2003;23:403–415. doi: 10.1523/JNEUROSCI.23-02-00403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Laude AJ. IP3 receptors and their regulation by calmodulin and cytosolic Ca2+ Cell Calcium. 2002;32:321–334. doi: 10.1016/s0143416002001859. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Tovey SC, Rossi AM, Lopez Sanjurjo CI, Prole DL, Rahman T. Structural organization of signalling to and from IP3 receptors. Biochem Soc Trans. 2014;42:63–70. doi: 10.1042/BST20130205. [DOI] [PubMed] [Google Scholar]
- Tovey SC, Dedos SG, Rahman T, Taylor EJ, Pantazaka E, Taylor CW. Regulation of inositol 1,4,5-trisphosphate receptors by cAMP independent of cAMP-dependent protein kinase. J Biol Chem. 2010;285:12979–12989. doi: 10.1074/jbc.M109.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner LE, 2nd, Joseph SK, Yule DI. Regulation of single inositol 1,4,5-trisphosphate receptor channel activity by protein kinase A phosphorylation. J Physiol. 2008;586:3577–3596. doi: 10.1113/jphysiol.2008.152314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JW, Feeney J, Trentham DR. Photolabile precursors of inositol phosphates. Preparation and properties of 1-(2-nitrophenyl)ethyl esters of myo-inositol 1,4,5-trisphosphate. Biochemistry. 1989;28:3272–3280. doi: 10.1021/bi00434a023. [DOI] [PubMed] [Google Scholar]
- Wang SS, Denk W, Hausser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Wilson CJ. Regulation of action-potential firing in spiny neurons of the rat neostriatum in vivo. J Neurophysiol. 1998;79:2358–2364. doi: 10.1152/jn.1998.79.5.2358. [DOI] [PubMed] [Google Scholar]
- Wieland S, Schindler S, Huber C, Kohr G, Oswald MJ, Kelsch W. Phasic Dopamine Modifies Sensory-Driven Output of Striatal Neurons through Synaptic Plasticity. J Neurosci. 2015;35:9946–9956. doi: 10.1523/JNEUROSCI.0127-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagishita S, Hayashi-Takagi A, Ellis-Davies GC, Urakubo H, Ishii S, Kasai H. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science. 2014;345:1616–1620. doi: 10.1126/science.1255514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.