Abstract
The bromodomain and extraterminal (BET) protein BRD4 can physically interact with the Mediator complex, but the relevance of this association to the therapeutic effects of BET inhibitors in cancer is unclear. Here, we show that BET inhibition causes a rapid release of Mediator from a subset of cis-regulatory elements in the genome of acute myeloid leukemia (AML) cells. These sites of Mediator eviction were highly correlated with transcriptional suppression of neighboring genes, which are enriched for targets of the transcription factor MYB and for functions related to leukemogenesis. An shRNA screen of Mediator in AML cells identified the MED12, MED13, MED23, and MED24 subunits as performing a similar regulatory function to BRD4 in this context, including a shared role in sustaining a block in myeloid maturation. These findings suggest that the interaction between BRD4 and Mediator has functional importance for gene-specific transcriptional activation and for AML maintenance.
Introduction
The bromodomain and extraterminal (BET) protein BRD4 is a therapeutic target in several human malignancies, including acute myeloid leukemia (AML) (Shi and Vakoc, 2014). While the efficacy of BET inhibitors in mouse models has motivated ongoing clinical trials in hematologic malignancies (e.g. Clinicaltrials.gov: NCT01713582), the underlying molecular mechanism of BRD4 function in supporting cancer progression remains poorly understood. BRD4 uses tandem bromodomain modules to recognize acetyl-lysine side chains on histones and transcription factors (TFs), thereby localizing to hyper-acetylated promoter and enhancer regions of the genome (Dey et al., 2003; Roe et al., 2015). Moreover, chemical inhibitors of BET bromodomains (e.g. JQ1 and IBET) cause a global release of BRD4 from the genome (Filippakopoulos et al., 2010; Nicodeme et al., 2010). When bound to chromatin, BRD4 recruits various proteins, including P-TEFb, JMJD6, and NSD3, to activate its target genes (Jang et al., 2005; Liu et al., 2013; Rahman et al., 2011; Shen et al., 2015; Yang et al., 2005). Proteomic analyses of BRD4 complexes have revealed numerous other associated factors (Dawson et al., 2011; Jang et al., 2005; Rahman et al., 2011), however the relevance of such interactions to the cancer maintenance function of BRD4 is largely unstudied.
A physical association between the Mediator complex and BRD4 has been shown in several prior studies (Donner et al., 2010; Jang et al., 2005; Jiang et al., 1998; Wu and Chiang, 2007). Mediator is a ~30-subunit coactivator complex that interacts with TFs and participates in the recruitment and activation of RNA polymerase II (Pol II) (Allen and Taatjes, 2015; Malik and Roeder, 2010). Since the precise binding surface that links BRD4 and Mediator has yet to be defined, the functional importance of this physical interaction is currently unclear. In support of a functional link between BRD4 and Mediator, it has been observed that both factors colocalize at super-enhancers (clusters of highly active enhancers) and BET inhibition can perturb BRD4 and Mediator occupancy at such sites (Di Micco et al., 2014; Loven et al., 2013). In addition, embryonic stem cells require both BRD4 and Mediator to maintain Oct4 expression and the pluripotent cell state (Di Micco et al., 2014; Kagey et al., 2010; Wu et al., 2015). However, a recent study has shown that the kinase subunits of Mediator (CDK8 and CDK19) function in opposition to BRD4 to repress super-enhancer associated genes (Pelish et al., 2015). Taken together, these prior studies raise two key questions: 1) At what locations of the genome is Mediator released following BET inhibitor treatment? and 2) Does perturbation of Mediator contribute to the transcriptional effects and therapeutic activity of BET inhibition in cancer and other diseases?
Here, we show that JQ1 causes a dramatic loss of Mediator occupancy at a subset of cis elements in the genome of AML cells, which only partially overlaps with the location of super-enhancers. Notably, Mediator eviction tracked closely with the sensitivity of gene expression to JQ1-mediated suppression, which suggests that release of Mediator from the genome contributes to the transcriptional effects of BET inhibition. In support of this model, a Mediator-focused shRNA screen performed in AML cells revealed that BRD4 and Mediator coordinate a common gene regulatory network that maintains a blocked state of differentiation. Since Mediator is preferentially evicted by JQ1 near genes that promote leukemogenesis, our findings implicate release of Mediator from the genome as a contributor to the therapeutic activity of BET inhibition in AML.
Results
The Mediator complex is released from the leukemia genome in a variable manner following JQ1 exposure
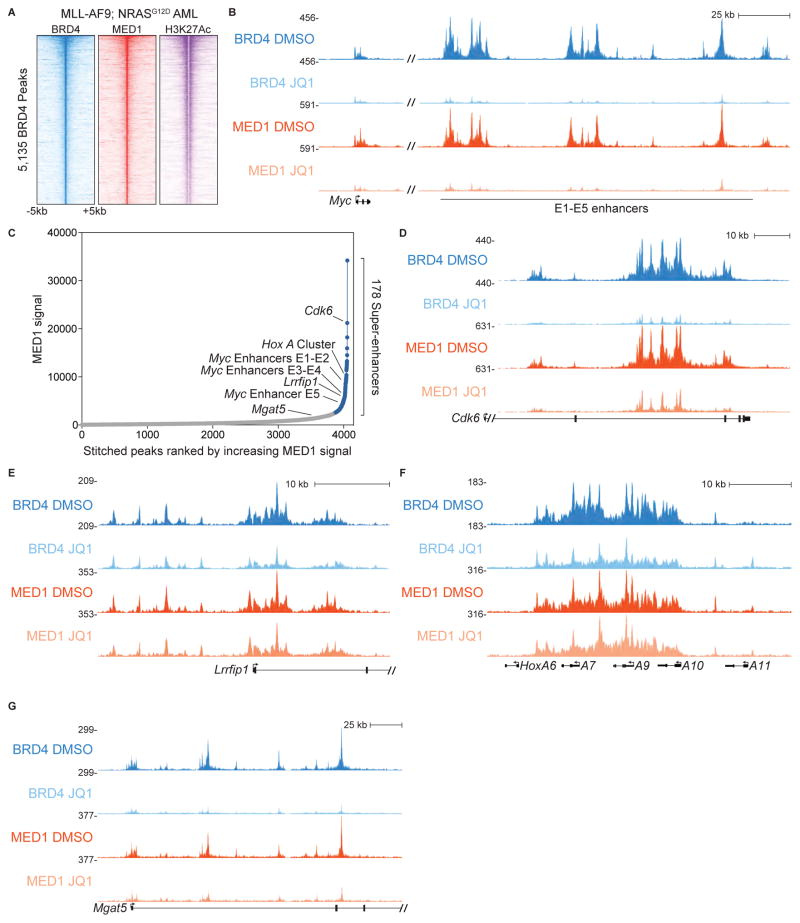
We tested the hypothesis that BET inhibition with JQ1 elicits anti-leukemia effects by interfering with the Mediator complex. To this end, we first performed ChIP-seq analysis comparing the chromatin occupancy profiles of BRD4 and MED1 (a Mediator subunit) in cells derived from a mouse model of MLL-AF9;NrasG12D AML (the RN2 cell line) (Zuber et al., 2011a). This revealed that BRD4 and MED1 co-localized across the AML genome in a pattern that overlapped with H3K27 hyper-acetylation (Figure 1A). Moreover, the tag counts of MED1 and BRD4 at each individual peak were highly correlated (R2=0.91, Figure S1A). The close correlation between BRD4 and MED1 across the AML genome is consistent with a physical interaction between these regulators occuring in this cell type.
Figure 1. The Mediator complex is released from the AML genome in a variable manner following JQ1 exposure.
(A) Density plot of BRD4, MED1, and H3K27Ac ChIP-seq datasets in murine MLL-AF9/NrasG12D AML. Data is centered on 5,135 high-confidence BRD4-occupied elements (Roe et al., 2015). Each row represents a 10-kilobase interval surrounding a single peak.
(B) ChIP-seq occupancy profiles of BRD4 and MED1 at the Myc locus following a 2 hr treatment with DMSO (vehicle) or 500 nM JQ1.
(C) 178 super-enhancers defined by MED1 occupancy using the ROSE algorithm.
(D–G) ChIP-seq occupancy profiles of BRD4 and MED1 at Cdk6, Mgat5, Lrrfip1, and the HoxA cluster following a 2 hr treatment with DMSO or 500 nM JQ1.
We next performed ChIP-seq analysis of BRD4 and MED1 in RN2 cells following a 2-hour exposure to 500 nM JQ1 or to vehicle control (DMSO). Transcription of the Myc proto-oncogene in RN2 cells is highly sensitive to JQ1 and is regulated by BRD4 via a super-enhancer (also known as E1–E5) located 1.7 megabases downstream of the Myc promoter (Shi et al., 2013; Zuber et al., 2011c). As expected, we found that BRD4 was completely evicted from this location by JQ1 (Figure 1B). In addition, we observed an equally dramatic reduction of MED1 occupancy at this same region (Figure 1B). To ensure that the entirety of the Mediator complex was released from this site, we complemented our ChIP-seq studies with ChIP-qPCR analysis of BRD4, MED1, MED12, and MED23, which revealed parallel reductions of all four factors at the Myc super-enhancer following JQ1 treatment (Figure S1B–S1G and Table S1). Since MED1, MED12, and MED23 reside in distinct modules of Mediator (middle, kinase, and tail, respectively), this result suggests that the entire Mediator complex was released following BET bromodomain inhibition.
Since prior studies have shown that BET inhibition reduces MED1 occupancy at several super-enhancers (Di Micco et al., 2014; Loven et al., 2013), we considered whether the release of Mediator from the genome in RN2 cells was restricted to this class of elements. For this purpose, we applied the Rank Ordering of Super-Enhancers (ROSE) algorithm to MED1 ChIP-seq data to define super-enhancers in RN2 cells and inspected the sensitivity of MED1 at these locations to JQ1-mediated eviction (Figure 1C). Cdk6 has previously been implicated as a BRD4 target gene in AML and is regulated by an intronic super-enhancer (Dawson et al., 2011; Roe et al., 2015). Similar to the Myc locus, we observed reductions of both BRD4 and MED1 at the Cdk6 super-enhancer following BET inhibition (Figure 1D). However, we unexpectedly observed that not all super-enhancers were susceptible to JQ1-mediated MED1 eviction. The Lrrfip1 gene harbors a promoter-proximal super-enhancer which displayed no apparent reduction of MED1 following exposure to JQ1 (Figure 1C and 1E). As another example, a large domain of MED1 occupancy at the HoxA locus qualifies as a super-enhancer (Figure 1C), and yet is entirely unaffected by JQ1 (Figure 1F). Conversely, we found that MED1 was released from several regions that fell below the threshold of being called as super-enhancers, such as intronic locations of Mgat5 (Figure 1C and 1G). For most genomic sites, we observed a modest correlation between the magnitude of MED1 and BRD4 release following JQ1 exposure (R2=0.13) (Supplemental Fig S1H). However, we also identified a small subset of sites (see below) that exhibit severe decreases of both MED1 and BRD4 following JQ1 treatment (Supplemental Fig S1H). Collectively, these findings show that JQ1 causes variable Mediator release at cis-regulatory elements in AML cells.
JQ1-induced Mediator eviction correlates with JQ1-induced transcriptional suppression
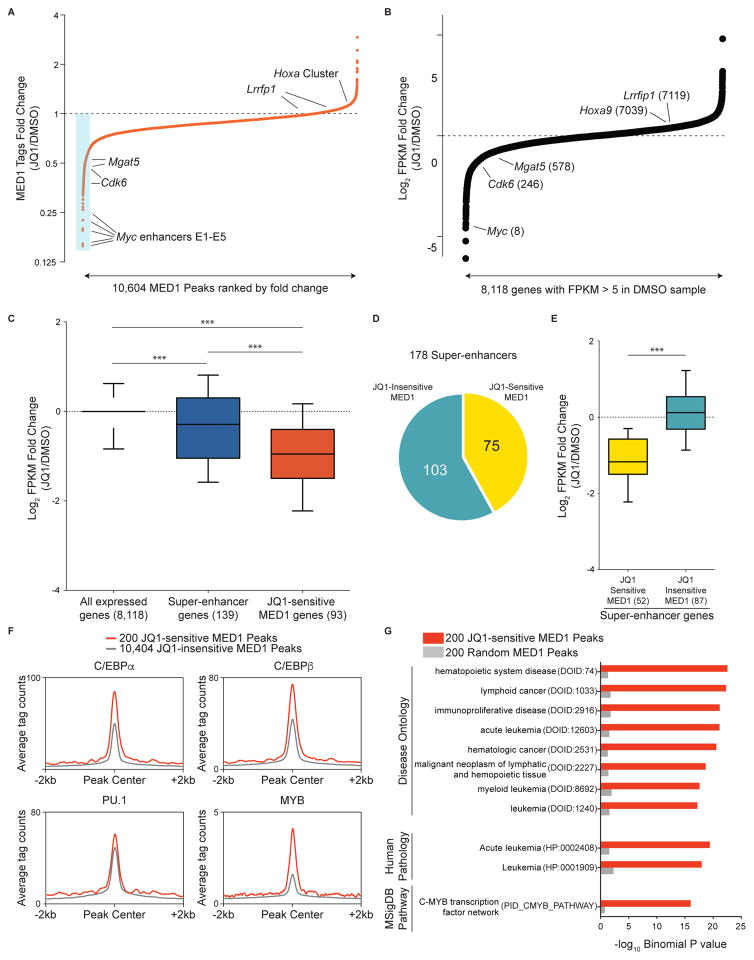
We next sought to determine whether MED1 eviction was correlated with the gene expression changes caused by JQ1. 10,604 reproducible MED1 peaks were defined using Model-based Analysis of ChIP-seq (MACS) software and rank-ordered based on the average fold-change of MED1 tag counts following exposure to JQ1 in two biological replicates (Zhang et al., 2008). This revealed that the constituent MED1 peaks within the Myc super-enhancer were outliers in the RN2 genome with regard to the severity of MED1 loss (Figure 2A). This result was striking, since Myc is also among the most down-regulated mRNAs in AML cells after JQ1 treatment (Dawson et al., 2011; Zuber et al., 2011c) (Figure 2B). Furthermore, the magnitude of MED1 loss at peaks located near Cdk6, Lrrfip1, Hoxa9, and Mgat5 correlated well with the relative effect of JQ1 on expression of these genes (Figure 2A and 2B). This analysis raised the possibility that release of Mediator from the genome may contribute to the effect of BET inhibition on transcription.
Figure 2. JQ1-induced Mediator eviction correlates with JQ1-induced transcriptional suppression.
(A) Fold-change in occupancy of MED1 at 10,604 individual MED1 peaks in AML following 2 h treatment with 500 nM JQ1. The peaks are ranked in order of increasing fold change (JQ1/DMSO). The blue box highlights the 200 most JQ1-sensitive MED1 elements. Fold changes presented are the average of two independent biological replicates.
(B) Fold-change in FPKM (fragments per kilobase of transcript per million) for 8,118 expressed genes in AML (defined by FPKM>5 in DMSO sample) following 6 h of 500 nM JQ1 treatment. The genes are ranked in order of increasing fold change. The numbers in parentheses are the fold-change expression rank of the indicated genes.
(C) Average fold-change in FPKM after JQ1 treatment for all expressed genes (left), for genes associated with super-enhancers (center), and for genes associated with JQ1-sensitive MED1 peaks (right). The numbers in parentheses represent the number of genes matched to the class of peaks indicated. *** represents a p value < 0.0001, the result of a Mann-Whitney test.
(D) Stratification of 178 MED1 super-enhancers based on whether or not they overlap with at least one of the 200 JQ1-sensitive MED1 peaks (minimum overlap 1 bp).
(E) Average fold change in FPKM for genes associated with JQ1-sensitive MED1 super-enhancers and for genes associated with JQ1-insensitive MED1 super enhancers (as delineated in (D)). The numbers in parentheses represent the number of genes matched to the subclass of super-enhancer indicated. *** represents a p value < 0.0001, the result of a Mann-Whitney test.
(F) ChIP-seq meta-profiles for hematopoietic TF occupancy at 200 JQ1-sensitive MED1 peaks or the remaining 10,404 MED1 peaks.
(G) GREAT ontology analysis Binomial P values for 200 JQ1-sensitive MED1 peaks versus 200 random MED1 peaks. Top-ranking ontology terms for the JQ1-sensitive peaks are displayed alongside values for the same ontology terms in the random peaks. Terms in parentheses represent the ontology identifiers in the GREAT database.
To test this hypothesis in a more systematic manner, we defined 200 MED1 peaks in RN2 cells that exhibited the greatest sensitivity to JQ1 (Figure 2A, Table S1, and Figure S2A). We next applied the Genomic Regions Enrichment of Annotations Tool (GREAT) to link each of these peaks to the nearest expressed gene as its presumed target (McLean et al., 2010) (Table S2). We performed an analogous GREAT analysis to link each super-enhancer in RN2 cells to the nearest expressed gene (Figure 1B and Table S2). These two gene sets were then independently evaluated in RNA-seq data derived from RN2 cells treated with 500 nM JQ1 for 6 hours (Roe et al., 2015). Consistent with prior observations, we found that super-enhancer genes were more suppressed by JQ1 than randomly chosen expressed genes (Figure 2C) (Loven et al., 2013). However, we found that genes located near JQ1-sensitive MED1 peaks were suppressed to a significantly greater extent than genes located in proximity to super-enhancers (Figure 2C and Figure S2B). This result prompted us to consider that super-enhancers encompass two distinct classes of cis elements that differ with regard to their sensitivity to JQ1-mediated perturbation. Indeed, we found that 75 of the 178 super-enhancers in RN2 cells overlapped with at least one JQ1-sensitive MED1 peak (Figure 2D), and only this subset of super-enhancers was associated with transcriptional suppression by JQ1 (Figure 2E). Importantly, this result suggests that a large fraction of super-enhancers (103 out of 178) are not perturbed by JQ1 and appear to be unrelated to the transcriptional effects of BET inhibition.
We next evaluated whether JQ1-sensitive MED1 peaks harbor unique sequences features. A set of hematopoietic TFs (C/EBPα, C/EBPβ, ERG, FLI1, MYB, and PU.1) have been shown to facilitate BRD4 recruitment in AML cells (Roe et al., 2015). When compared to all MED1 peaks, JQ1-sensitivity was associated with a higher motif density and genomic occupancy of the TF MYB, and to a lesser extent C/EBPα and C/EBPβ (Figure 2F and S2C–S2D). In contrast, sequence motifs and overall occupancy for PU.1, ERG, and FLI1 were not dramatically elevated at JQ1-sensitive versus JQ1-insenstive MED1 peaks. We also evaluated whether the genes located near JQ1-sensitive MED1 peaks were enriched for any particular biological or molecular pathways. For this purpose, we used GREAT to compare the ontology of genes located near 200 JQ1-sensitive MED1 peaks with genes located near 200 random MED1 peaks. This revealed that Mediator was released disproportionately near genes involved in leukemia pathogenesis (Figure 2G). In this same analysis, MYB was the most enriched transcription factor network among the JQ1-sensitive MED1 peaks, in agreement with the higher relative level of MYB occupancy at this class of cis elements (Figure 2F and 2G). These results suggest that Mediator is preferentially evicted by JQ1 near MYB targets genes and near genes involved in the pathogenesis of leukemia.
Knockdown of select Mediator tail and kinase module subunits triggers differentiation of leukemic blasts
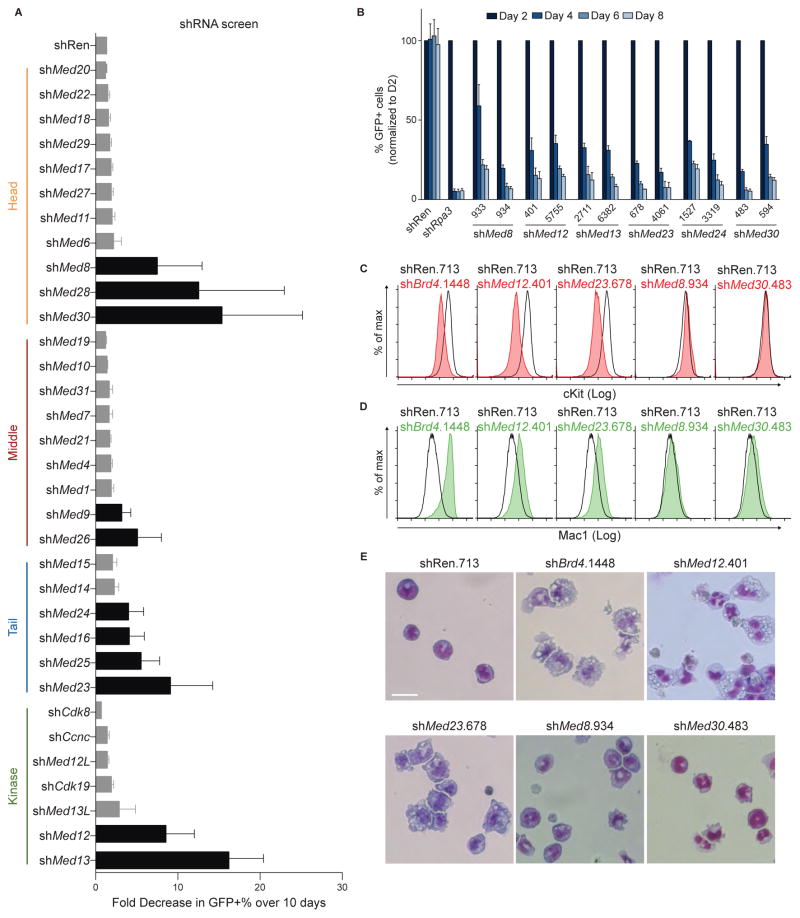
The genomic correlations described above support a model in which BRD4 and Mediator function as linked coactivators for a common set of target genes in AML cells. One prediction of this model is that targeting of specific subunits of Mediator may lead to similar cellular phenotypes and transcriptional changes as observed when targeting BRD4. Inhibition of BRD4 with either JQ1 or shRNA impairs the proliferation of RN2 cells and triggers differentiation of leukemic blasts into macrophage-like cells, which express higher levels of Mac1 and lower levels of cKit on their cell surface (Zuber et al., 2011c). To evaluate the phenotypic consequences of Mediator perturbation in this cell type, we constructed a custom library of 190 short hairpin RNAs (shRNAs) targeting all of the known Mediator subunits (~6 shRNAs/gene, Table S3) and carried out a negative-selection screen to identify essential subunits for cell proliferation and/or viability. The relative growth-arrest observed over 10 days in culture was quantified using GFP reporters in a competition-based assay (Figure 3A). This screen revealed that RN2 cell proliferation was hypersensitive to targeting of select subunits of the Mediator complex. This includes components of the head (MED8, MED28, and MED30), middle (MED9 and MED26), tail (MED16, MED23, MED24, and MED25), and kinase (MED12, MED13) modules (Figure 3A and 3B). On-target knockdown of the intended target gene of each scoring shRNA was validated using RT-qPCR (Figure S3A). These findings confirm that several Mediator subunits are essential for AML proliferation.
Figure 3. Genetic knockdown of select Mediator subunits triggers differentiation of AML blasts.
(A) Summary of negative-selection shRNA screen targeting the indicated Mediator subunits. Bars represent the average of all hairpins for each gene. Black bars highlight subunits having at least 3-fold loss of GFP-positive cells with at least two independent hairpins. shRNAs are expressed using the LMN vector. Data are represented as the mean of all hairpins for the corresponding gene ± SEM.
(B) Negative-selection experiments using the indicated shRNAs chosen from the screen in (A). GFP+/shRNA+ percentages were normalized to values taken on day 2 and tracked for 8 days. Data are presented as mean ± SEM and n=3.
(C–D) Flow cytometric analysis of cell-surface cKit and Mac1 following 96 h of doxycycline-induced expression of the indicated shRNAs. shRNAs were expressed using the TRMPV-Neo vector. Gating was performed on GFP+/shRNA+ cell populations.
(E) Light microscopy analysis of May-Grünwald-Giemsa-stained RN2 cells after 96 h of doxycycline-induced expression of the indicated shRNAs. The images were taken with 40X objective.
To evaluate the specificity of these growth-arrest phenotypes, we transduced immortalized MEF cells (iMEFs) with shRNAs targeting each of the essential Mediator subunits identified in the screen above, and performed competition-based proliferation assays. We found that the knockdown of MED12, MED13, MED23, and MED24 led to no apparent growth defect in iMEFs, whereas MED8 and MED30 were each found to be essential for iMEF growth (Figure S3B and S3C). This heterogeneity of growth-arrest phenotypes following perturbation of individual Mediator subunits was unrelated to the level of expression of these subunits (data not shown). Collectively, our findings suggest that AML cell proliferation is uniquely hypersensitive to targeting of Mediator subunits MED12, MED13, MED23, and MED24. Interestingly, MED12 and MED23 have each been implicated previously in the physical interaction with BRD4 (Jang et al., 2005; Wang et al., 2013).
We next evaluated whether knockdown of specific Mediator subunits in RN2 cells caused myeloid maturation in an analogous manner to BRD4 inhibition (Zuber et al., 2011c). When expressed conditionally via a doxycycline(dox)-regulated promoter, we found that MED12, MED13, MED23, and MED24 shRNAs, but not MED8 and MED30 shRNAs, altered the cell surface expression of cKit and Mac1 in a manner that resembled the phenotype provoked by BRD4 knockdown (Figure 3C, 3D). To confirm that differentiation was induced following Mediator subunit knockdown, we imaged May-Grünwald/Giemsa-stained RN2 cells using light microscopy. Consistent with the flow cytometry analysis, knockdown of MED12, MED13, MED23, or MED24, but not of MED8 or MED30, led to similar morphologic changes towards mature macrophages as observed with BRD4 knockdown (Figure 3E). These findings suggest that targeting select Mediator subunits can trigger differentiation of AML blasts.
shRNA-based knockdown of the homologous kinase subunits CDK8 and CDK19 of Mediator failed to elicit proliferation arrest of RN2 cells (Figure 3A and Figure S3D–S3E). However, we found that dual shRNA-based targeting of these two kinases led to an arrest in the proliferation of RN2 cells (Figure S3E). This result suggests a redundant function for these two kinase subunits, which is consistent with a recent study showing that dual CDK8/CDK19 inhibition with Cortistatin A suppressed AML cell growth (Pelish et al., 2015).
Mediator subunits are required to sustain expression of BRD4 target genes in AML cells
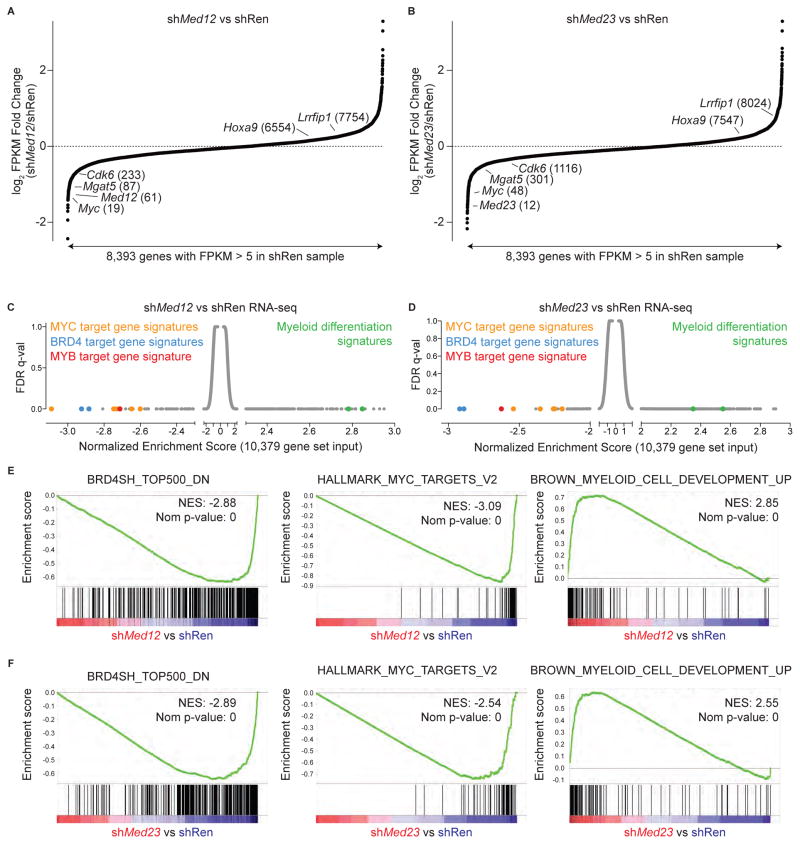
We next considered whether a common gene regulatory network depends on BRD4 and Mediator for expression in RN2 cells using RNA-seq analysis. In an analogous manner to JQ1 treatment, knockdown of MED12 or MED23 resulted in reduced mRNA levels of Myc, Mgat5, and Cdk6, but not of Lrrfip1 or Hoxa9 (Figure 4A and 4B). To provide an unbiased evaluation of gene signatures altered by knockdown, we performed gene set enrichment analysis (GSEA) to interrogate a database of 10,379 gene sets (Subramanian et al., 2005). Remarkably, several of the most down-regulated gene signatures in MED12 or MED23-deficient RN2 cells corresponded to the target genes of MYB, MYC, and BRD4 (Figure 4C–4F). Among the positively enriched gene sets were those related to myeloid cell maturation, consistent with the phenotypic changes observed following MED12 and MED23 knockdown (Figure 4C–4F). Knockdown of MED8 also led to suppression of BRD4 target genes such as Myc, however the global pattern of gene expression was different from what was observed following MED12 or MED23 knockdown, consistent with the differing phenotypes upon knockdown of these subunits (Figure S4A–S4C). GSEA revealed that while BRD4 and MYC signatures were affected by MED8 knockdown, a number of transcriptional pathways not suppressed by JQ1 treatment or by MED12 or MED23 shRNA were suppressed in MED8-deficient cells (Figure S4B and S4D). While it appears that individual Mediator subunits perform distinct transcriptional functions, our findings support a role for the Mediator complex in supporting BRD4-dependent gene activation.
Figure 4. MED12 and MED23 are required to sustain expression of BRD4, MYC, and MYB target gene signatures in AML cells.
(A–B) Fold-change in FPKM for 8,393 expressed genes in AML (defined by FPKM>5 in shRen sample) following 48 h of doxycycline treatment to induce two independent shRNAs targeting Med12 (401 and 5755) or Med23 (678 and 4061) versus shRen.713. Fold change values for each gene are the average value of the independent hairpins targeting the indicated Mediator subunit. The genes are ranked in order of increasing fold change. The numbers in parentheses represent the fold change expression rank of the indicated genes.
(C–D) Gene Set Enrichment Analysis (GSEA) of shMed12 and shMed23 versus shRen RNA-seq using 10,379 gene sets, including all gene sets in the Molecular Signatures Database. Signatures are plotted by their Normalized Enrichment Scores and FDR q-values according to GSEA.
(E–J) Example GSEA plots from the indicated RNA-seq. Normalized Enrichment Scores (NES) and Nominal p-values (Nom p-value), as calculated by GSEA, are provided.
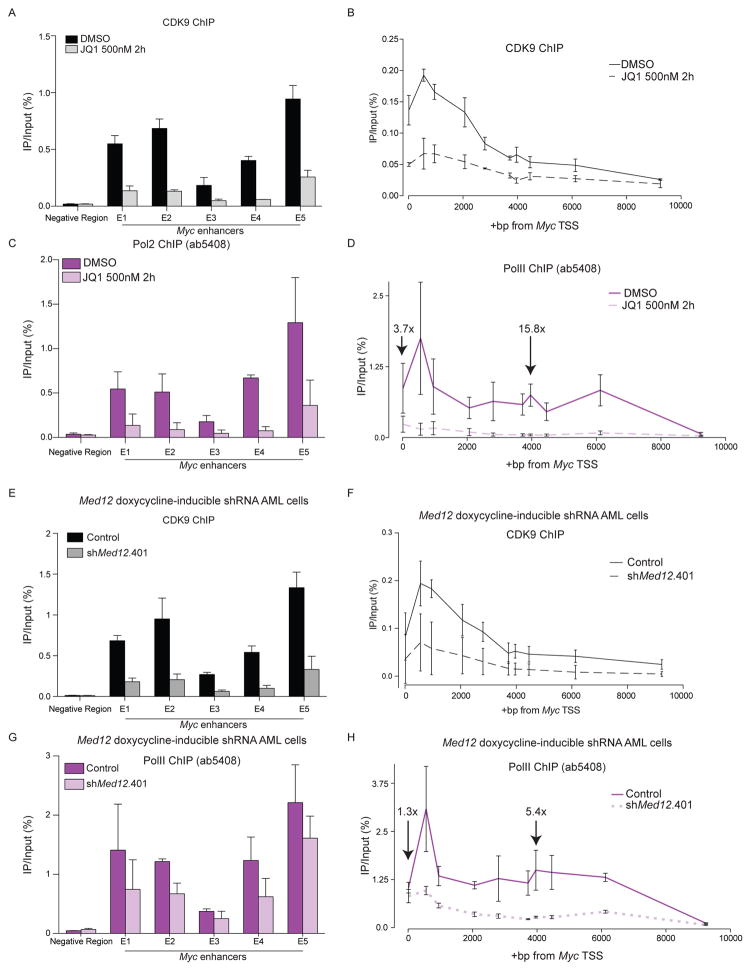
We next explored the mechanism by which BRD4 and Mediator regulate Pol II activity at their co-regulated target genes. Mediator and BRD4 have each been shown to interact with the kinase P-TEFb, comprised of CDK9 and Cyclin T, which can stimulate the release of paused Pol II near the transcriptional start site (TSS) (Donner et al., 2010; Jang et al., 2005; Takahashi et al., 2011; Yang et al., 2005). Using ChIP-qPCR, we found that BET inhibition and MED12 knockdown each led to a loss of CDK9 and Pol II from the Myc super-enhancer and gene body (Figure 5A–5H, Table S4). Notably, the loss of Pol II near the Myc TSS was less severe (3.7-fold for JQ1, 1.3 fold for shMED12) relative to the gene body (15.8-fold for JQ1, 5.4-fold for shMED12) (Figure 5D and 5H). This result is consistent with BRD4 and Mediator promoting the transition of Pol II from initiation to elongation by facilitating P-TEFb recruitment.
Figure 5. BET inhibition or MED12 knockdown cause loss of CDK9 occupancy and reduced Pol II elongation at the Myc locus.
(A) ChIP-qPCR analysis of CDK9 at the Myc super-enhancer following a 2 hr treatment with DMSO or with 500 nM JQ1.
(B) ChIP-qPCR analysis of CDK9 across the Myc gene body following a 2 hr treatment with DMSO or 500 nM JQ1.
(C) ChIP-qPCR analysis of Pol II at the Myc super-enhancer following a 2 hr treatment with DMSO or with 500 nM JQ1.
(D) ChIP-qPCR analysis of Pol II at the Myc gene body following a 2 hr treatment with DMSO or with 500 nM JQ1. Numbers above +14 bp and +3965 bp regions indicate fold change between DMSO and JQ1 ChIP-qPCR enrichment.
(E) ChIP-qPCR analysis of CDK9 at the Myc super-enhancer locus following 0- or 48-hour induction of shMed12 with doxycycline (dox). TRMPV-Neo vector was used.
(F) ChIP-qPCR analysis of CDK9 across the Myc gene body following 0- or 48-hour induction of shMed12 with dox. TRMPV-Neo vector was used.
(G) ChIP-qPCR analysis of Pol II at the Myc enhancer locus following 0- or 48-hour induction of shMed12 with dox. TRMPV-Neo vector was used.
(H) ChIP-qPCR analysis of Pol II across the Myc gene body following 0- or 48-hour induction of shMed12 with dox. Numbers above +14bp and +3965bp data indicate fold change between control and shMed12 ChIPs at these regions. TRMPV-Neo vector was used. All experiments were performed in RN2 cells. All data are presented as mean ± SEM and n=3.
A recent study has shown that Mediator can facilitate BRD4 chromatin localization in the setting of acquired resistance to BET inhibitors in breast cancer (Shu et al., 2016). We therefore considered whether Mediator plays a reciprocal role in stabilizing BRD4 occupancy in RN2 cells. Since MED12 and MED23 knockdown each mimicked the phenotypic and transcriptional effects of BRD4 inhibition, we performed BRD4 ChIP-seq following knockdown of these subunits. This revealed that MED12 and MED23 knockdown resulted in a modest reduction in BRD4 chromatin occupancy at the same cis elements at which MED1 is perturbed following BET inhibition (Figure S5A–S5E). This result suggests that BRD4 and Mediator can mutually stabilize one another’s chromatin occupancy.
Discussion
The findings presented in this study support a model in which BRD4 regulates its downstream target genes, at least in part, via a physical interaction with the Mediator complex. We have previously shown that BRD4 directly interacts with the short isoform of NSD3, which acts as a bridge to recruit the CHD8 chromatin remodeler (Shen et al., 2015). In addition, BRD4 is known to recruit P-TEFb to promote transcription elongation of its target genes (Jang et al., 2005; Yang et al., 2005). Notably, Mediator has also been shown to recruit P-TEFb to promote transcription elongation (Donner et al., 2010; Takahashi et al., 2011). Taken together, these observations reinforce a model in which BRD4 acts as a scaffold that recruits multiple regulatory machineries to cis elements bound by acetylated TFs and nucleosomes to promote transcription of genes that sustain the aberrant growth and self-renewal properties of AML cells.
Our dual shRNA targeting experiments are in agreement with a recent study, which found that AML cells are sensitive to chemical inhibition of CDK8/CDK19 with Cortistatin A (Pelish et al., 2015). In this study, it was shown that these kinases act to restrain super-enhancer activity, thereby antagonizing the activation function of BRD4 (Pelish et al., 2015). Importantly, our shRNA screening results suggest that this repressive function is likely to be confined to the CDK8/CDK19 subunits of Mediator, whereas other subunits, including the kinase-associated subunit MED12, are involved in supporting BRD4-dependent transcriptional activation. While it is surprising to observe opposing effects of targeting individual subunits within the same module of the Mediator complex, other studies have also observed contrasting phenotypic and transcriptional consequences after knockdown of MED12 versus CDK8 subunits (Gobert et al., 2010; Kuuluvainen et al., 2014). Nevertheless, our study and Pelish et al reinforce how distinct perturbations of Mediator can destabilize the AML cell state.
Our study provides insights into how JQ1 exerts disproportionate effects on cancer-relevant genes while sparing housekeeping gene expression, a property that likely underlies the therapeutic efficacy of this agent in AML and other malignancies. It has previously been proposed that the location of super-enhancers harboring exceptional levels of BRD4 and Mediator provides the molecular basis for the hypersensitivity of specific genes to JQ1-mediated transcriptional suppression (Loven et al., 2013). We have shown here that the hypersensitivity of Mediator to JQ1-mediated displacement can be uncoupled from the pre-existing levels of Mediator occupancy, which is, in turn, reflected in the heterogeneous sensitivity of super-enhancer-linked genes to JQ1-mediated suppression. Our study shows that less than half of all super-enhancers in AML cells exhibit JQ1-mediated displacement of the Mediator complex (Loven et al., 2013). Importantly, our findings suggest that the degree of Mediator eviction can serve as a useful biomarker for revealing the critical cis elements that are functionally suppressed by BET inhibition. Measurements of MED1 eviction following JQ1 exposure could be applied more broadly to reveal the critical downstream genes that underlie the therapeutic effects BET inhibition in various cellular contexts.
While BRD4 occupancy correlates genomewide with the occupancy of several hematopoietic TFs (Roe et al., 2015), the sensitivity of Mediator to JQ1-mediated eviction correlates with cis elements specifically harboring higher levels of the TF MYB. In addition, our ontology analysis shows that genes associated with JQ1-sensitive MED1 also enriched for the MYB target gene network. Notably, a somatic mutation has been described in T-cell acute lymphoblastic leukemia that produces a novel MYB binding motif near the TAL1 gene, which leads to the formation of a MED1-occupied super-enhancer (Mansour et al., 2014). Interestingly, AML cells are known to be more sensitive to MYB knockdown than normal myeloblasts (Zuber et al., 2011b). Since MYB physically associates with BRD4 (Roe et al., 2015), our results suggest that MYB function might place unique demands on BRD4-Mediator complexes to sustain expression of genes with leukemogenic functions, which may underlie the hypersensitivity of AML cell growth to BET bromodomain inhibition.
Experimental Procedures
ChIP-Seq
120 million RN2 cells were crosslinked with 1% formaldehyde for 20 minutes and quenched for 10 minutes with 0.125M glycine. Nuclear lysates were sonicated in 20-million cell batches using a BioRuptor water bath sonicator. Sonicated chromatin was pre-cleared using rabbit IgG and agarose beads or was incubated with the BRD4 antibody for two hours followed by addition of magnetic beads (BRD4 ChIP-seq in MED12/23 knockdown conditions). IP with the relevant antibodies was performed overnight at 4°C with rotation. After extensive washing as previously described (Steger et al., 2008), crosslinks were reversed overnight at 65°C. DNA was treated with RNase A and Proteinase K and purified using the QIAGEN PCR purification kit. Libraries were constructed using the TruSeq ChIP Sample Prep kit from lllumina. Libraries underwent a final amplification step of 15 PCR cycles and were analyzed using a Bioanalyzer with a High Sensitivity chip (Agilent). Libraries were single-end sequenced on a HiSeq2000 with reads of 50 bp.
A full description can be found in the supplemental materials section.
Supplementary Material
Acknowledgments
We thank James Bradner and Jun Qi for providing JQ1. We thank R. Wysocki for assistance with microscopy. This work was supported by Cold Spring Harbor Laboratory NCI Cancer Center Support grant CA455087 for developmental funds and shared resource support. Additional funding was provided by the Alex’s Lemonade Stand Foundation and the V Foundation. J.S.R. is supported by the Martin Sass Foundation and the Lauri Strauss Leukemia Foundation. A.S.B. is supported by NIH T32 GM008444 and NCI F30 CA186632. C.R.V. is supported by a Burroughs-Wellcome Fund Career Award, National Institutes of Health grant NCI RO1 CA174793, and a Leukemia & Lymphoma Society Scholar Award.
Footnotes
Author Contributions
A.S.B., J.S.R., J.S., C.R.V designed experiments and analyzed results; A.S.B., J.S.R., B.A.M., and A.F.H. carried out experiments; C.R.V. supervised the research; A.S.B. and C.R.V wrote the manuscript.
Accession Numbers
The accession number for the raw and processed sequencing data reported in this paper is GEO: GSE74651, with the subseries accession numbers GEO: GSE74536 and GSE78221 (ChIP-seq), and GSE74650 and GSE78224 (RNA-seq).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen BL, Taatjes DJ. The Mediator complex: a central integrator of transcription. Nature reviews Molecular cell biology. 2015;16:155–166. doi: 10.1038/nrm3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung CW, Hopf C, Savitski MM, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Chitsaz F, Abbasi A, Misteli T, Ozato K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8758–8763. doi: 10.1073/pnas.1433065100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Micco R, Fontanals-Cirera B, Low V, Ntziachristos P, Yuen SK, Lovell CD, Dolgalev I, Yonekubo Y, Zhang G, Rusinova E, et al. Control of embryonic stem cell identity by BRD4-dependent transcriptional elongation of super-enhancer-associated pluripotency genes. Cell reports. 2014;9:234–247. doi: 10.1016/j.celrep.2014.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner AJ, Ebmeier CC, Taatjes DJ, Espinosa JM. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nature structural & molecular biology. 2010;17:194–201. doi: 10.1038/nsmb.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert V, Osman D, Bras S, Auge B, Boube M, Bourbon HM, Horn T, Boutros M, Haenlin M, Waltzer L. A genome-wide RNA interference screen identifies a differential role of the mediator CDK8 module subunits for GATA/ RUNX-activated transcription in Drosophila. Mol Cell Biol. 2010;30:2837–2848. doi: 10.1128/MCB.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Molecular cell. 2005;19:523–534. doi: 10.1016/j.molcel.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Jiang YW, Veschambre P, Erdjument-Bromage H, Tempst P, Conaway JW, Conaway RC, Kornberg RD. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8538–8543. doi: 10.1073/pnas.95.15.8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuuluvainen E, Hakala H, Havula E, Sahal Estime M, Ramet M, Hietakangas V, Makela TP. Cyclin-dependent kinase 8 module expression profiling reveals requirement of mediator subunits 12 and 13 for transcription of Serpent-dependent innate immunity genes in Drosophila. J Biol Chem. 2014;289:16252–16261. doi: 10.1074/jbc.M113.541904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ma Q, Wong K, Li W, Ohgi K, Zhang J, Aggarwal AK, Rosenfeld MG. Brd4 and JMJD6-associated anti-pause enhancers in regulation of transcriptional pause release. Cell. 2013;155:1581–1595. doi: 10.1016/j.cell.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, Bradner JE, Lee TI, Young RA. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Roeder RG. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nature reviews Genetics. 2010;11:761–772. doi: 10.1038/nrg2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour MR, Abraham BJ, Anders L, Berezovskaya A, Gutierrez A, Durbin AD, Etchin J, Lawton L, Sallan SE, Silverman LB, et al. Oncogene regulation. An oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element. Science. 2014;346:1373–1377. doi: 10.1126/science.1259037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, Wenger AM, Bejerano G. GREAT improves functional interpretation of cis-regulatory regions. Nature biotechnology. 2010;28:495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, Chandwani R, Marazzi I, Wilson P, Coste H, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelish HE, Liau BB, Nitulescu II, Tangpeerachaikul A, Poss ZC, Da Silva DH, Caruso BT, Arefolov A, Fadeyi O, Christie AL, et al. Mediator kinase inhibition further activates super-enhancer-associated genes in AML. Nature. 2015;526:273–276. doi: 10.1038/nature14904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Sowa ME, Ottinger M, Smith JA, Shi Y, Harper JW, Howley PM. The Brd4 extraterminal domain confers transcription activation independent of pTEFb by recruiting multiple proteins, including NSD3. Molecular and cellular biology. 2011;31:2641–2652. doi: 10.1128/MCB.01341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe JS, Mercan F, Rivera K, Pappin DJ, Vakoc CR. BET Bromodomain Inhibition Suppresses the Function of Hematopoietic Transcription Factors in Acute Myeloid Leukemia. Molecular cell. 2015;58:1028–1039. doi: 10.1016/j.molcel.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Ipsaro JJ, Shi J, Milazzo JP, Wang E, Roe JS, Suzuki Y, Pappin DJ, Joshua-Tor L, Vakoc CR. NSD3-Short Is an Adaptor Protein that Couples BRD4 to the CHD8 Chromatin Remodeler. Molecular cell. 2015;60:847–859. doi: 10.1016/j.molcel.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Vakoc CR. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Molecular cell. 2014;54:728–736. doi: 10.1016/j.molcel.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Whyte WA, Zepeda-Mendoza CJ, Milazzo JP, Shen C, Roe JS, Minder JL, Mercan F, Wang E, Eckersley-Maslin MA, et al. Role of SWI/SNF in acute leukemia maintenance and enhancer-mediated Myc regulation. Genes & development. 2013;27:2648–2662. doi: 10.1101/gad.232710.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu S, Lin CY, He HH, Witwicki RM, Tabassum DP, Roberts JM, Janiszewska M, Jin Huh S, Liang Y, Ryan J, et al. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature. 2016 doi: 10.1038/nature16508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger DJ, Lefterova MI, Ying L, Stonestrom AJ, Schupp M, Zhuo D, Vakoc AL, Kim JE, Chen J, Lazar MA, et al. DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells. Molecular and cellular biology. 2008;28:2825–2839. doi: 10.1128/MCB.02076-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Parmely TJ, Sato S, Tomomori-Sato C, Banks CA, Kong SE, Szutorisz H, Swanson SK, Martin-Brown S, Washburn MP, et al. Human mediator subunit MED26 functions as a docking site for transcription elongation factors. Cell. 2011;146:92–104. doi: 10.1016/j.cell.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yao X, Huang Y, Hu X, Liu R, Hou D, Chen R, Wang G. Mediator MED23 regulates basal transcription in vivo via an interaction with P-TEFb. Transcription. 2013;4:39–51. doi: 10.4161/trns.22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. The Journal of biological chemistry. 2007;282:13141–13145. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]
- Wu T, Pinto HB, Kamikawa YF, Donohoe ME. The BET family member BRD4 interacts with OCT4 and regulates pluripotency gene expression. Stem cell reports. 2015;4:390–403. doi: 10.1016/j.stemcr.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, Zhou Q. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Molecular cell. 2005;19:535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, McJunkin K, Fellmann C, Dow LE, Taylor MJ, Hannon GJ, Lowe SW. Toolkit for evaluating genes required for proliferation and survival using tetracycline-regulated RNAi. Nature biotechnology. 2011a;29:79–83. doi: 10.1038/nbt.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Rappaport AR, Luo W, Wang E, Chen C, Vaseva AV, Shi J, Weissmueller S, Fellmann C, Taylor MJ, et al. An integrated approach to dissecting oncogene addiction implicates a Myb-coordinated self-renewal program as essential for leukemia maintenance. Genes & development. 2011b;25:1628–1640. doi: 10.1101/gad.17269211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011c;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.