Abstract
Chronic treatment with the monoamine releaser d-amphetamine has been consistently shown to decrease cocaine self-administration in laboratory studies and clinical trials. However, the abuse potential of d-amphetamine is an obstacle to widespread clinical use. Approaches are needed that exploit the efficacy of the agonist approach but avoid the abuse potential associated with dopamine releasers. The present study assessed the effectiveness of chronic oral administration of phendimetrazine (PDM), a pro-drug for the monoamine releaser phenmetrazine, to decrease cocaine self-administration in four rhesus monkeys. Each day, monkeys pressed a lever to receive food pellets under a 50-response fixed-ratio schedule of reinforcement and self-administered cocaine (0.005-0.56 mg/kg per injection, i.v.) under a progressive-ratio (PR) schedule in the evening. After completing a cocaine self-administration dose-response curve, sessions were suspended and PDM was administered (1.0-9.0 mg/kg, p.o., b.i.d.). Cocaine self-administration was assessed using the PR schedule once every 7 days while food-maintained responding was studied daily. When a persistent decrease in self-administration was observed, the cocaine dose-effect curve was re-determined. Daily PDM treatment decreased cocaine self-administration by 30-90% across monkeys for at least 4 weeks. In two monkeys, effects were completely selective for cocaine. Tolerance developed to initial decreases in food-maintained the third monkey, and in the fourth fluctuations were observed that were lower in magnitude than effects on cocaine self-administration. Cocaine dose-effect curves were shifted down and/or rightward in three monkeys. These data provide further support for the use of agonist medications for cocaine abuse, and indicate that the promising effects of d-amphetamine extend to a more clinically viable pharmacotherapy.
Keywords: agonist medication, medication development, pharmacotherapy, phendimetrazine, phenmetrazine, rhesus monkey
Cocaine abuse persists as a major public health problem for which no pharmacotherapy has proven to be sufficiently effective (Haile and Kosten, 2014). The success of methadone and nicotine replacement therapies in the treatment of opiate and nicotine addiction, respectively, has encouraged efforts to develop an indirect dopamine agonist medication to treat stimulant abuse (Grabowski et al., 2004a; Herin et al., 2010). Chronic treatment with d-amphetamine decreased cocaine self-administration in laboratory animals under several conditions, including progressive-ratio (PR) and second-order schedules of reinforcement, as well as food-cocaine choice procedures (Negus, 2003; Negus and Mello, 2003a,b; Chiodo et al., 2009; Chiodo and Roberts, 2009; Czoty et al., 2010, 2011; Thomsen 2013). Moreover, clinical studies have supported the safety and efficacy of amphetamine and its analogs as a treatment for cocaine use disorders, including multiple double-blind, placebo-controlled studies (Grabowski et al., 2001, 2004b; Shearer et al., 2003; Mooney et al., 2009; Levin et al., 2015).
Although ample evidence has accumulated for the suitability of d-amphetamine as a medication for substance use disorders, there are several obstacles to widespread clinical use of d-amphetamine and other monoamine-releasing drugs (cf. Negus and Henningfield, 2015). For example, their high abuse potential and corresponding Schedule II status presents a formidable barrier to clinical use, particularly in individuals with substance use disorders (e.g., Kollins, 2008). Moreover, although drugs that release dopamine and/or norepinephrine are currently clinically available to treat such disorders as attention-deficit hyperactivity disorder and narcolepsy and as appetite suppressants, development and evaluation of specific products remains to be conducted. Thus, to achieve FDA approval of a novel product, considerable work may be required which would involve an as-yet unidentified sponsor (cf. Negus and Henningfield, 2015).
One alternative to a novel rapid-acting monoamine releaser is a pro-drug which is itself relatively inert if injected or insufflated, but is converted to an active amphetamine-like compound when taken orally (Huttune et al., 2011; Rush and Stoops, 2012). One example, lisdexamfetamine (LDX, Vyvanse®), is a pro-drug that is converted to amphetamine in erythrocytes after oral ingestion (Pennick, 2010). In preclinical studies, LDX has demonstrated characteristics desirable of an agonist pharmacotherapy, including a relatively slow onset and long duration of action, a lack of reinforcing effects, generalization to the discriminative-stimulus effects of cocaine and the ability to reduce cocaine self-administration (Heal et al., 2013; Banks et al., 2015). Moreover, a recent retrospective analysis of data from U.S. poison centers indicated that exposure to supra-therapeutic doses of LDX was associated with fewer serious adverse outcomes than exposure to high doses of sustained- or immediate-release d-amphetamine (Kaland and Klein-Schwartz, 2015). Importantly, although a recent clinical study did not find statistically significant differences between LDX and placebo, a secondary analysis of those that completed the trial indicated that LDX significantly decreased craving and lowered rates of cocaine use (Mooney et al., 2015). The authors noted that this was a proof-of-concept that was limited in the maximum doses that could be used and suggested that higher doses should be explored in future studies.
Another alternative to d-amphetamine itself is phendimetrazine (PDM), available clinically as an anorectic for over 50 years (Cass, 1961). Unlike d-amphetamine and LDX, which are designated Schedule II drugs by the US Drug Enforcement Administration, PDM is categorized as Schedule III due to its lower abuse liability in humans and lack of reinforcing effects in laboratory animals under most conditions (Jain et al., 1979; Corwin et al., 1987). After oral administration PDM is converted in the liver to phenmetrazine (PM), an amphetamine-like releaser of dopamine and norepinephrine (Rothman et al., 2002; Negus et al., 2009; Banks et al., 2013b). Preclinical studies have demonstrated that the metabolite PM shares discriminative-stimulus effects with cocaine (Negus et al., 2009; Banks et al., 2011) and can decrease cocaine self-administration in rats and monkeys, as well as cocaine-primed reinstatement in rats, at doses that do not alter food-maintained responding (e.g., Negus et al., 2009; Banks et al., 2013d; Czoty et al., 2015). Orderly pharmacokinetics and behavioral effects have been observed over several weeks of repeated administration of the pro-drug PDM (Banks et al., 2013a, b, c), indicating a sustained conversion of PDM to PM. In the only study to examine effects of administering the pro-drug itself on cocaine self-administration, 14 days of treatment with PDM was shown to decrease choice of cocaine in the context of concurrently available food reinforcement (Banks et al., 2013b).
The present study was designed to further characterize the effects of chronic PDM treatment on cocaine self-administration in rhesus monkeys using a procedure deemed to better reflect the clinical experience in several ways (see Czoty et al., 2011). Briefly, features of the model include: (1) suspension of cocaine access during drug administration to model a treatment scenario in which an addict is able to refrain from using cocaine during an initial brief period; (2) assessment of cocaine self-administration weekly rather than daily, permitting seven consecutive days of PDM treatment in the absence of cocaine; (3) daily monitoring of food-reinforced responding to identify drug effects that could indicate a likelihood of side effects in a clinical population; (4) individual-subject adjustment of PDM treatment based on the presence or absence of an effect on cocaine-reinforced responding, rather than a group design in which all monkeys received the same PDM doses for predetermined lengths of time; (5) the use of the use of cocaine-experienced (versus drug-naïve) subjects and (6) the use of a PR schedule to measure the reinforcing strength of cocaine rather than fixed-ratio (FR), fixed-interval or second-order schedules of reinforcement that assess the presence/absence of reinforcing effects. In general, data generated using this paradigm have shown good concordance with the results of clinical trials for several drugs including d-amphetamine, methylphenidate, varenicline and mecamylamine (Czoty et al., 2011, 2013; Gould et al., 2011). In addition, weekly blood samples were collected to provide an indication of circulating PM concentrations necessary to produce behavioral effects.
1.0. Experimental procedures
1.1. Subjects and Apparatus
Subjects were four adult male rhesus monkeys (Macaca mulatta), each prepared with a chronic indwelling venous catheter and subcutaneous vascular access port (Access Technologies, Skokie, IL) as described previously (Czoty et al. 2010). Two subjects (R-1714 and R-1610) had approximately nine months of experience self-administering cocaine at the outset of the present study and two subjects (R-1550 and R-1552) had self-administered cocaine for over three years. Monkeys were housed individually in sound-attenuating chambers (0.91 × 0.91 × 0.91 m; Plas Labs, Lansing, MI). The front wall of each cubicle was constructed of Plexiglas to allow the monkey visual access to the laboratory. Each cubicle was equipped with two response levers (BRS/LVE, Beltsville, MD). Four stimulus lights, alternating white and red, were located in a horizontal row above each lever. A receptacle located between the levers was connected via Tygon tubing to a pellet dispenser located outside the chamber for response-contingent delivery of food pellets. Each animal was fitted with a stainless-steel restraint harness and spring arm (Restorations Unlimited, Chicago, IL) that attached to the rear of the cubicle. A peristaltic infusion pump (Cole-Parmer Instrument Co., Vernon Hills, IL) was located on the top of the chamber for delivering injections at a rate of approximately 1.5 ml/10 sec. Monkeys received fresh fruit, peanuts and vegetables several days per week and water was available ad libitum. Animal housing and handling and all experimental procedures were performed in accordance with the 2011 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the Animal Care and Use Committee of Wake Forest University. Environmental enrichment was provided as outlined in the Animal Care and Use Committee of Wake Forest University Non-Human Primate Environmental Enrichment Plan.
1.2. Food-reinforced responding
Monkeys were trained under a 50-response fixed-ratio (FR 50) schedule. Under this schedule, white stimulus lights above the left lever were illuminated and 50 responses resulted in the food pellet delivery, extinguishing of white lights and illumination of red stimulus lights for 10 sec, followed by a 10-sec timeout (TO) period during which no lights were illuminated and responding had no scheduled consequences. Sessions began at approximately 8:30 a.m. each day and lasted until the maximum allowed number of food reinforcers was earned or 23 hours elapsed. Thus only food was available from 8:30 a.m. to 3:00 pm when the self-administration session began (see below). At that point, food and cocaine were concurrently available if the monkey had not yet received the maximum number of pellets, determined for each monkey as that required to provide enough food to maintain a healthy body weight as determined by visual inspection and periodic veterinary exams. When monkeys earned fewer than the maximum number of food pellets, supplementary food (Purina Monkey Chow) was given at approximately 8:00 a.m. in an amount calculated to raise the total grams of food to the desired level. Target food amounts for the monkeys in the present study were 125-150 g per day.
1.3. Cocaine self-administration
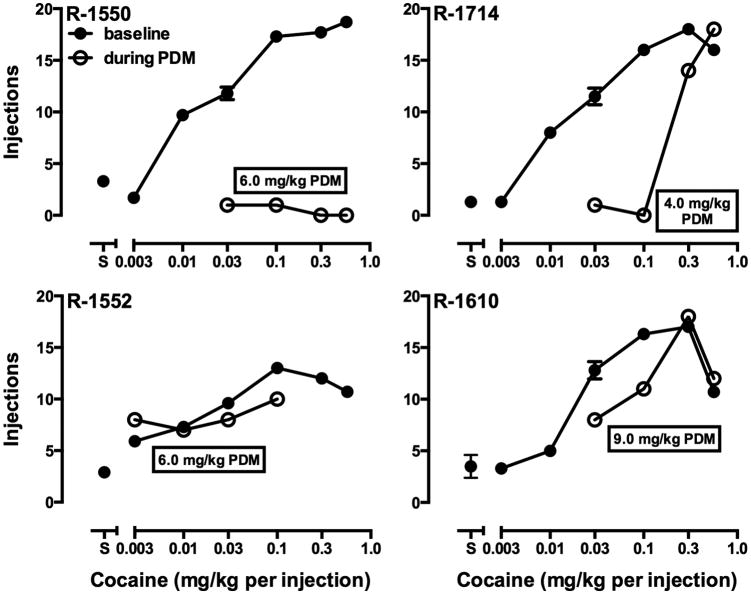
Monkeys self-administered (-)-cocaine HCl under a PR schedule of reinforcement in sessions that began at 3:00 p.m. each day. Under this schedule, white stimulus lights were illuminated above the right lever and 50 responses on that lever resulted in the first injection of the maintenance dose of cocaine (0.03 mg/kg per injection in approximately 1.5 ml over 10 seconds), extinguishing of white lights and illumination of red stimulus lights for 10 sec, followed by a 10-min TO. The response requirement for subsequent injections was determined by the equation used by Richardson and Roberts (1996): ratio = [5 × e(R × 0.2)] – 5, where e is the mathematical constant and R is equal to the reinforcer number. For the present studies, the first response requirement (50 responses) corresponds to the 12th value given by this equation and was followed by 62, 77, 95, 117, 144, 177, 218, 267, 328, 402, 492, 602, 737, 901, 1102, etc. Sessions ended when 2 hours elapsed without an injection. Food- and cocaine-maintained responding under the FR 50 and PR schedules, respectively, were similar to that observed in previous studies with this procedure (e.g., Czoty et al., 2011, 2013). Under baseline conditions, monkeys earned all available food pellets, typically within the first three hours of availability (data not shown). In cocaine self-administration sessions, the number of injections received increased significantly as a function of the available cocaine dose in all monkeys (Fig. 1, closed symbols). These reached a plateau in three subjects; in R-1610 a decrease in responding was observed when a higher cocaine doses (0.56 mg/kg per injection) was made available. The maintenance dose of cocaine (0.03 mg/kg per injection) was selected as one that maintained an intermediate amount of behavior so that either increases or decreases in self-administration of cocaine could be detected.
Fig. 1.
Dose-effect curves for cocaine self-administration before (closed symbols) and during (open symbols) PDM treatment in four monkeys. Each point is the mean of the last three days of availability of a cocaine dose or saline. When a dose or saline was made available on more than one occasion, error bars indicate the standard error of the mean. Ordinates: number of cocaine injections earned; abscissae, available cocaine dose.
1.4. Chronic phendimetrazine treatment
Initially, 0.03 mg/kg per injection cocaine was made available in evening PR sessions until responding stabilized (3 consecutive days on which the number of injections were within 2 of the 3-day mean, with no upward or downward trend). Subsequently, a complete cocaine dose-effect curve was generated by substituting other doses of cocaine for at least four days and until the number of injections delivered stabilized. Once responding was stable during availability of the maintenance dose, cocaine self-administration sessions were suspended and treatment with PDM was initiated by administering the drug orally at approximately 8:00 am each day. Food-reinforced responding was studied daily throughout treatment. On the seventh day, at 3:00 p.m., the maintenance dose of cocaine was again made available for self-administration under the PR schedule of reinforcement, signaled by the illumination of the white stimulus lights above the right lever. Each day during PDM treatment, laboratory personnel noted occurrences of any behaviors that were atypical for each monkey, with a focus on behaviors that have been observed during treatment with other stimulant drugs, such as locomotor activation, agitation, stereotypies or other unconditioned behavioral effects.
This procedure (availability of 0.03 mg/kg per injection cocaine for one session) was repeated on day 14 of treatment. If, at that time, the number of cocaine injections delivered was decreased from baseline by approximately 30% or more, treatment was continued another 7 to 14 days in order to examine whether tolerance or sensitization developed to the treatment. If no decrease in cocaine self-administration was observed, or if tolerance to initial decreases had developed, the dose of PDM was increased and the effects of the next PDM dose were similarly assessed for up to 28 days. The progression of PDM doses was 1.0, 2.0, 3.0, 4.0, 6.0 and 9.0 mg/kg, p.o., b.i.d. Not all monkeys started treatment with the same PDM dose; based on data in the first two monkeys to complete testing (see 2.1) and the resources required to generate the large quantities of PDM needed for these experiments, higher starting PDM doses were selected for R-1714 and R-1610. Regardless of starting dose, PDM doses were presented in ascending order for all monkeys with one exception. R-1714 was initially exposed to 6.0 mg/kg, b.i.d. Adverse unconditioned effects (leg biting and aggression towards a mirror that was hung in the cage) were noted on days 5, 6 and 7 of treatment with this dose, so the PDM dose was decreased to 4.0 mg/kg, b.i.d.
In all monkeys, when a PDM dose was reached at which the number of cocaine injections remained decreased at day 28, other cocaine doses (up to 0.56 mg/kg per injection) were made available for a single day at 3-day intervals while PDM treatment was continued. After the curve had been completed, PDM treatment was discontinued and, for R-1550, R-1714 and R-1610, self-administration of the maintenance dose of cocaine was again examined on post-treatment days 3, 7 and 14. R-1552 did not participate in this experiment due to loss of catheter patency.
1.5. Blood collection and analysis
Each week during PDM treatment, at approximately 3:00 pm, 24 or 48 hours before the start of the cocaine self-administration session, monkeys were anesthetized with 5-10 mg/kg ketamine and a 3-ml blood sample was collected from the saphenous vein. Samples were transferred into 3-ml vacutainer tubes containing K3-EDTA and stored on ice. Samples were then centrifuged for 15 min and the supernatant was transferred to a 1.5-ml storage tube and frozen at -80 C until shipped on dry ice to Research Triangle Institute for analysis. Plasma samples were analyzed as described in Banks et al., 2013b.
1.6. Drugs
(-)Cocaine HCl (National Institute on Drug Abuse, Bethesda, MD) was dissolved in sterile 0.9% saline for self-administration studies. Changing the self-administered cocaine dose was accomplished by changing the concentration of cocaine delivered over 10 seconds (∼1.5 ml). (+)-PDM fumarate was synthesized at Research Triangle Institute, Research Triangle Park, NC. PDM was administered orally by dissolving it in sterile water at a concentration of 50 mg/ml and mixing a calculated volume with banana and granola in a small cup which was given to the monkey; monkeys were observed to ensure the monkey consumed the drug. PDM was administered at approximately 8:00 am and 5:00 pm.
1.7. Data Analysis
The dependent variable of primary interest was the number of cocaine injections earned under the PR schedule of reinforcement. In addition, the number food reinforcers received was recorded in hourly bins. Because individual differences in sensitivity to PDM resulted in different regimens of PDM treatment across subjects, individual subjects' data are shown.
2.0. Results
2.1. Effects of PDM on food- and cocaine-reinforced responding
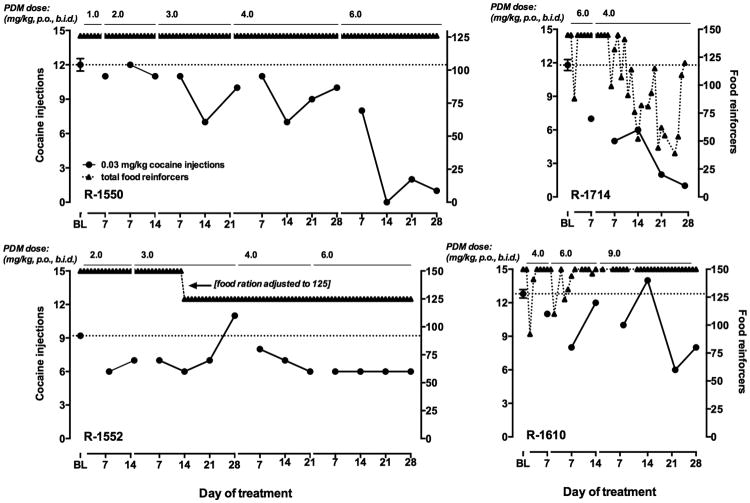
In subject R-1550, food-reinforced responding was not affected during 14 weeks of treatment with increasing doses of PDM (Fig. 2, upper left panel). No effects on cocaine self-administration were observed during treatment with 1.0 or 2.0 mg/kg PDM, b.i.d. During treatment with 3.0 and, subsequently, 4.0 mg/kg PDM, b.i.d., the number of injections of 0.03 mg/kg cocaine earned was decreased from baseline by 40-50% on day 14, but in both cases tolerance had developed to this effect by day 21. The PDM dose was then increased to 6.0 mg/kg, b.i.d. Although the number of cocaine injections delivered was only slightly decreased on day 7 of treatment, on day 14 self-administration of 0.03 mg/kg cocaine was decreased substantially. In contrast to the tolerance that developed to effects of lower PDM doses on cocaine self-administration, the effect of 6.0 mg/kg, b.i.d. persisted for four weeks.
Fig 2.
Cocaine self-administration and food-maintained responding during PDM treatment in four monkeys. Left ordinates: number of cocaine injections received; right ordinates: number of food reinforcers earned; abscissae: day of exposure to each condition, which is indicated across the top of each panel. BL represents average (±SEM) number of injections earned at baseline prior to phendimetrazine treatment. The dashed line in each graph indicates the mean number of injections received under baseline conditions (i.e., prior to PDM treatment).
Effects of PDM on food-reinforced responding were more prominent in R-1714 (Fig. 2, upper right panel). PDM treatment was initiated at a dose of 6.0 mg/kg, b.i.d. As described above, exposure to this dose resulted in adverse effects over the first week, and the dose was decreased to 4.0 mg/kg, b.i.d. No adverse effects were observed in this monkey after the change in dose, or in any other monkey at any dose. During the second week of treatment with 4.0 mg/kg, b.i.d., food-maintained responding was disrupted; on some days the monkey earned only two-thirds of available food pellets. However, the monkey ate supplemental chow given on the following morning before the start of the session. Effects on food-maintained responding were greater during the third and fourth weeks of treatment, although the number of food pellets delivered returned to baseline on occasion. This fluctuation continued during the redetermination of the cocaine dose-response curve (not shown), but within three days of termination of PDM treatment, the maximum number of food pellets were delivered daily. The 4.0 mg/kg, b.i.d. dose of PDM produced a progressive decrease in cocaine self-administration that persisted for four weeks.
PDM treatment was without effect on food-reinforced responding in R-1552 (Fig. 2, lower left panel). In this monkey, treatment with 2.0 mg/kg PDM, b.i.d. decreased cocaine self-administration by approximately 25%. Increasing the PDM dose to that which was effective in R-1550 and R-1714 resulted only in a small increase in this effect.
In R-1610, twice-daily PDM at 4.0 and 6.0 mg/kg produced small, transient disruptions of food-maintained responding (Fig. 2, lower right panel). When the dose was increased to 6.0 mg/kg, b.i.d. a suppression of cocaine self-administration was observed on day 7 to which tolerance had developed by day 14. In this monkey, the PDM dose was increased to 9.0 mg/kg without adverse effects. On day 21 and 28, cocaine self-administration was decreased by approximately 40%. Thus, in all four monkeys, PDM treatment decreased cocaine self-administration; in three of these subjects the decreases were selective for cocaine relative to food-maintained responding.
2.2. Redetermination of dose-effect curves
Once a dose of PDM was reached that produced a decrease in self-administration of the maintenance dose of cocaine that lasted until day 28, other doses of cocaine were tested in three-day intervals to determine whether the effect of PDM treatment could be overcome. In three of four monkeys, the reinforcing potency of cocaine was attenuated by PDM treatment across multiple doses (Fig. 1, open symbols). The ascending portion of the cocaine dose-response curve was shifted downward in R-1550 and leftward in R-1714 and R-1610. In the latter two monkeys, PDM-induced decreases in cocaine self-administration were overcome by increasing the available cocaine dose. In R-1552, the monkey in whom modest effects of PDM were observed that were not dose-related, there was a decrease in self-administration of 0.1 mg/kg, the highest dose that was tested in this monkey, but the dose-effect curves were otherwise similar. As described above, a loss of catheter patency prevented higher cocaine doses from being tested in R-1552. Thus a conclusive determination of the effects of PDM on a the full dose-effect curve cn not be made in this subject. Food-reinforced responding remained at baseline levels in R-1550, R-1552 and R-1610 (data not shown) indicating that the effect of PDM was selective for cocaine. In R-1714, fluctuations in food-maintained responding depicted in Fig. 2 persisted into the period of time that the dose-effect curve was being determined. Thus, for this monkey, PDM-induced decreases in responding were less selective for cocaine, although decreases in cocaine-maintained responding were larger.
2.3. Recovery of cocaine self-administration after termination of PDM treatment
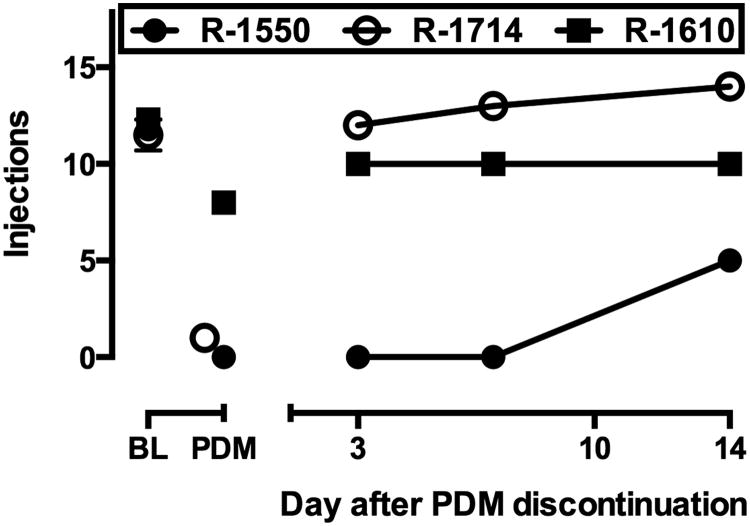
In three monkeys (R-1550, R-1714 and R-1610), after the cocaine dose-effect curve was re-determined, PDM treatment was discontinued and self-administration of the maintenance dose of cocaine under the PR schedule was examined 3, 7 and 14 days later (Fig. 3). In R-1550 and R-1714, self-administration was near baseline levels on all three days. In R-1550, the monkey most affected by PDM treatment, cocaine self-administration had only partially recovered by day 14 after PDM was discontinued. Over the next 5 days, on which 0.03 mg/kg per injection cocaine was self-administered daily, the number of injections delivered returned to baseline levels in R-1550 as well (not shown).
Fig. 3.
Cocaine self-administration after discontinuation of PDM treatment in individual monkeys. Ordinate: number of cocaine injections; abscissae: days after discontinuation of d-amphetamine treatment. Points above BL and PDM indicate mean (± SEM) number of injections delivered under baseline conditions and the number of injections delivered on the last test session during PDM treatment.
2.4. Concentrations of phendimetrazine and phenmetrazine in blood
Blood samples were collected within 48 hours prior to each self-administration test and concentrations of PDM and PM were determined. Table 1 shows the blood concentrations of PDM and PM measured in the last sample taken during treatment with each dose of PDM in each monkeys, along with the effect on cocaine self-administration that was observed during the session that occurred 24-28 hours later. For R-1552, data for the third week of treatment with 3.0 mg/kg, b.i.d. dose is shown, because a spurious increase in self-administration occurred in week 4 that may not have been related to the circulating PM concentration (see Fig. 2). For R-1550 and R-1610, circulating PM concentrations increased as a function of oral PDM dose. Whereas 4.0 mg/kg, b.i.d. produced similar PM concentrations (∼110 ng/ml) and similar behavioral effects (∼15% decrease in cocaine self-administration) in the two monkeys, a higher dose (6.0 mg/kg, b.i.d.) resulted in higher PM concentrations in R-1550 (233 ng/ml) compared to R-1610 (168 ng/ml). In R-1714, in whom the lower PDM dose was effective (4.0 mg/kg, b.i.d.), associated PM concentrations were correspondingly lower. PM concentrations in R-1610 approached those reached in R-1550 when the dose was increased to 9.0 mg/kg, b.i.d. (208 ng/ml). In R-1552, in whom the PDM dose-effect curve was flat, increasing the administered PDM dose from 3.0 to 6.0 mg/kg, b.i.d. did not appreciably increase circulating PM concentrations.
Table 1.
Concentrations of phendimetrazine (PDM) and phenmetrazine (PM) in blood at the end of treatment with each PDM dose (mg/kg p.o., b.i.d.), and the percent decrease in cocaine self-administration observed at that time.
| Monkey | PDM (mg/kg, b.i.d.) | PDM (ng/ml) | PM (ng/ml) | Effect (% decrease) |
|---|---|---|---|---|
| R-1550 | 2.0 | 7.5 | 70.5 | 8.3 |
| 3.0 | 19.6 | 69.0 | 16.7 | |
| 4.0 | 18.5 | 109.0 | 16.7 | |
| 6.0 | 31.8 | 233.0 | 91.7 | |
| R-1714 | 4.0 | 43.4 | 116.0 | 83.1 |
| R-1552 | 2.0 | 10.3 | 58.0 | 23.9 |
| 3.0* | 12.3 | 81.2 | 23.9 | |
| 4.0 | 9.8 | 72.7 | 34.8 | |
| 6.0 | 15.2 | 80.8 | 34.8 | |
| R-1610 | 4.0 | 9.8 | 110.0 | 14.1 |
| 6.0 | 25.9 | 168.0 | 6.3 | |
| 9.0 | 30.2 | 208.0 | 37.5 |
data derived from third rather than fourth week of treatment
3.0. Discussion
Accumulating evidence from laboratory studies and clinical trials indicates that various formulations of d-amphetamine possess therapeutic potential in the treatment of cocaine use disorder. The results of the present study compliment those of a previous study of monkeys self-administering cocaine under a food-drug choice procedure (Banks et al., 2013a,c) in extending these findings to PDM, a pro-drug for the amphetamine-like monoamine releaser PM. As observed previously for d-amphetamine (Czoty et al., 2010, 2011), PDM decreased cocaine-maintained responding under the PR schedule at doses that did not produce persistent disruption of food-maintained responding or other adverse effects. Moreover, in three of four monkeys, cocaine self-administration dose-effect curves generated during PDM treatment were shifted to the right and/or down compared to curves generated before PDM exposure. Finally, although PDM-induced decreases in cocaine self-administration did not persist once treatment was terminated, there was no evidence that chronic treatment produced dependence or that discontinuation had any other adverse effects.
During PDM administration, mild disruption of food-maintained responding was observed in two subjects. Interestingly, effects on food-maintained responding were not observed in the two monkeys in whom treatment was initiated at relatively low doses (R-1550 and R-1552), but did occur in monkeys whose PDM treatment started at higher doses. In R-1610, tolerance developed to the initial, modest decreases in food-reinforced responding, limiting the effect to the first two weeks of PDM treatment. In R-1714, effects were larger in magnitude but fluctuated and were generally lower in magnitude than the effects on cocaine self-administration. The data suggest that chronic treatment with a low, ineffective dose may have facilitated development of tolerance to adverse effects of PDM in R-1550 and R-1552. This hypothesis remains to be explored systematically.
When decreases in food pellet deliveries were observed, monkeys always ate supplemental food given on the following morning. It is possible that this measure—providing chow within an hour of the start of the food self-administration session—played a role in maintaining the disrupted pattern of food-maintained responding over days, particularly in R-1714. Further study will be required to determine the relative importance of the effects of PDM and supplemental feeding in these effects. Taken together, to the extent that disruption of food-maintained responding may predict side effects in a clinical population, these results suggest a low likelihood of serious adverse effects during PDM treatment, a conclusion similar to that made for d-amphetamine (Negus and Mello, 2003b; Czoty et al., 2010, 2011). Effects of PDM on food-maintained responding were less prominent than those observed with d-amphetamine under the same procedure (Czoty et al., 2011). This difference may be due to the slower onset inherent in a pro-drug as well as the fact that PDM was administered orally whereas d-amphetamine was given as a continuous intravenous infusion. Taken together, the data suggest that treatment with monoamine releasers or their pro-drugs would result in only minor side effects in some individuals that could likely be managed to encourage compliance.
Importantly, the pattern of effects of increasing the PDM dose was similar to that observed with d-amphetamine. With both drugs, lower doses that were tested initially were without effect, or produced decreases in cocaine self-administration that were not sustained. Eventually a PDM dose was reached that produced prolonged decreases in cocaine self-administration. The number of cocaine injections delivered during treatment with higher PDM doses decreased to near zero in two monkeys and decreased by 30-50% in the other two subjects. Although success in clinical trials has typically been measured by total abstinence, it is intuitive that a large reduction in drug use is likely to have beneficial consequences (see Falk et al., 2010). Reflecting this understanding, the US Food and Drug Administration recently released a draft guidance that endorses “percent subjects with no heavy drinking days” as a meaningful endpoint in trials for medications for alcohol use disorder (FDA, 2015); researchers have called for a similar approach for cocaine use disorders (Winchell et al., 2012; McCann et al., 2015). From this point of view, the results with PDM in the present experiment are encouraging.
When self-administration of other cocaine doses was assessed during PDM treatment, cocaine dose-effect curves were shifted downward and/or rightward in three monkeys. The experiment cold not be completed in the fourth subject (R-1552) due to loss of patency of the intravenous catheter. Finally, when PDM treatment was discontinued, cocaine self-administration returned to baseline levels. The lack of a rebound increase in cocaine self-administration or disruption in food-maintained responding after termination of PDM treatment suggests that chronic PDM treatment did not result in dependence, which may have been revealed by hypersensitivity to cocaine and/or a decrease in food-maintained responding if monkeys were experiencing withdrawal. This recovery of cocaine self-administration was observed on the first test day, 3 days after PDM discontinuation, in two monkeys. Although it remains possible that hypersensitivity to cocaine may have been present within 72 hours of the termination of PDM treatment, the fact that it took more than 14 days for cocaine self-administration to recover in the third monkey (R-1550) suggests that this effect of chronic PDM treatment dissipated at different rates across subjects.
Rigorous pharmacokinetic studies in nonhuman primates have convincingly demonstrated that the behavioral effects of PDM are due to in vivo conversion to its metabolite, PM (Banks et al., 2013b), which has effects on cocaine self-administration similar to d-amphetamine in monkeys and rodents (Negus et al. 2009; Banks et al. 2011; Banks et al. 2013d; Czoty et al., 2015). In the present studies, blood samples were collected during PDM treatment in an effort to determine how PDM and PM concentrations change during long-term treatment with ascending doses of oral PDM, as well as to provide some information relating circulating PM concentrations to the efficacy of PDM to decrease cocaine self-administration. For example, it was hypothesized that this analysis could reveal that significant behavioral effects of PDM would be associated with exceeding a threshold concentration of circulating PDM and/or PM. In two monkeys (R-1550 and R-1610), increasing the dose of oral PDM was associated with increases in circulating PM concentrations. In these two subjects, the PDM dose that produced the largest effect on cocaine self-administration resulted in PM concentrations of approximately 210-230 ng/ml. That this effect was produced by different PDM doses and resulted in different magnitudes of maximal effect suggests that the relationship between administered dose, resultant metabolite levels and behavioral efficacy is not a straightforward one. Moreover, the dose that produced the maximal effect in a third subject (R-1714) was associated with a much lower PM concentration. Doses that generated a similar PM concentration in R-1550 and R-1610 produced effects on cocaine self-administration that were small and/or were not sustained during chronic treatment. Data from the fourth subject (R-1714) are also informative in that circulating concentrations of PDM and PM remained relatively stable as oral PDM dose increased. This may explain why the effect of oral PDM on cocaine self-administration was consistent throughout the period of dose escalation. On the whole, although there was evidence in the present study that increasing oral PDM doses were associated with increases in both circulating PDM concentrations and in behavioral effects, clear quantitative relationships between these variables were not revealed. One caveat to this conclusion is that blood samples were collected once per treatment dose. A more thorough characterization of the pharmacokinetics of chronic oral PDM treatment, similar to that conducted by Banks et al (2013b) might reveal relationships that were not apparent in the present study.
The procedures used to predict pharmacotherapeutic efficacy in this study were designed to incorporate features of human cocaine use and treatment approaches that have not been used in prior models. Several features known or recommended to increase predictive validity were implemented, including chronic treatment with a putative pharmacotherapy, assessment of selectivity of medication effects through concurrent assessment of non-drug maintained behaviors, single-subject designs and examination of treatment drug effects on a range of self-administered cocaine doses (cf. Mello and Negus, 1996; Ator and Griffiths, 2003; Haney and Spealman, 2008). The proposed merits of this approach have been enumerated previously (see Czoty et al., 2011). Although increasing face validity does not guarantee enhanced predictive validity, and validation of any behavioral model is a gradual and ongoing process, the effects of several drugs on cocaine self-administration in this model have demonstrated good concordance with clinical data (Czoty et al., 2011, 2013; Gould et al., 2011). Regarding PDM, although clinical trials have not been conducted, recent findings suggest that oral PDM produces only minimal increases in positive subjective measures in cocaine users (Stoops et al., 2015). Taken together, the data provide support for testing the effectiveness of PDM in patients with cocaine use disorder.
Highlights.
Phendimetrazine (PDM) is a pro-drug for the amphetamine-like monoamine releaser phenmetrazine (PM).
Chronic oral PDM treatment decreased cocaine self-administration in rhesus monkeys for several weeks.
Modest, transient effects on food self-administration were seen in some monkeys; tolerance developed to these effects.
The relationships between oral PDM dose and blood PM levels varied across monkeys.
Plasma PM concentrations were generally consistent with behavioral effects.
Acknowledgments
The authors thank Tonya Calhoun, Michelle Bell and Heather Green for their assistance in completing these studies. This research was supported by NIDA grants DA 06634 and DA 12970; the sponsor had no role in the design of the experiments; the collection, analysis or interpretation of data; the writing of the manuscript or the decision to submit the article for publication.
Footnotes
The authors have no conflicts of interest to disclose.
Authors' contributions. PWC, MAN and BEB were responsible for the study concept and design. PWC contributed to the acquisition of behavioral data. BEB synthesized phendimetrazine. The analysis of phendimetrazine was designed and conducted by RF and RWS, who also provided interpretation of the concentration measurements. PWC and MAN performed analysis of the behavioral data and interpreted findings. PWC drafted the manuscript. BEB and MAN provided critical revision of the manuscript for intellectual content. All authors critically reviewed content and approved final version for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;5:S55–S72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Effects of phendimetrazine treatment on cocaine vs. food choice and extended-access cocaine consumption in rhesus monkeys. Neuropsychopharmacology. 2013a;38:2698–2707. doi: 10.1038/npp.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Role of phenmetrazine as an active metabolite of phendimetrazine: evidence from studies of drug discrimination and pharmacokinetics in rhesus monkeys. Drug Alcohol Depend. 2013b;130:158–166. doi: 10.1016/j.drugalcdep.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of 14-day treatment with the schedule III anorectic phendimetrazine on choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2013c;131:204–213. doi: 10.1016/j.drugalcdep.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Interaction between behavioral and pharmacological treatment strategies to decrease cocaine choice in rhesus monkeys. Neuropsychopharmacology. 2013d;38:395–404. doi: 10.1038/npp.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys. Behav Pharmacol. 2011;22:824–836. doi: 10.1097/FBP.0b013e32834d63ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Hutsell BA, Blough BE, Poklis JL, Negus SS. Preclinical assessment of lisdexamfetamine as an agonist medication candidate for cocaine addiction: effects in rhesus monkeys trained to discriminate cocaine or self-administer cocaine in a cocaine versus food choice procedure. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyv009. pyv009 10.1093/ijnp/pyv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass LJ. Evaluation of phendimetrazine bitartarate as an appetite suppressant. Can Med Assoc J. 1961;84:1114–1116. [PMC free article] [PubMed] [Google Scholar]
- Chiodo KA, Lack CM, Roberts DC. Cocaine self-administration reinforced on a progressive ratio schedule decreases with continuous D-amphetamine treatment in rats. Psychopharmacology. 2008;200:465–473. doi: 10.1007/s00213-008-1222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo KA, Roberts DC. Decreased reinforcing effects of cocaine following 2 weeks of continuous d-amphetamine treatment in rats. Psychopharmacology. 2009;206:447–456. doi: 10.1007/s00213-009-1622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Woolverton WL, Schuster CR, Johanson CE. Anorectics: effects on food intake and self-administration in rhesus monkeys. Alcohol Drug Res. 1987;7:351–361. [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Martelle JL, Nader MA. Prolonged attenuation of the reinforcing strength of cocaine by chronic d-amphetamine in rhesus monkeys. Neuropsychopharmacology. 2011;36:539–547. doi: 10.1038/npp.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Nader MA. Effects of chronic d-amphetamine on the reinforcing strength of cocaine in rhesus monkeys. Psychopharmacology. 2010;209:375–382. doi: 10.1007/s00213-010-1807-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Martelle SE, Gould RW, Nader MA. Effects of chronic methylphenidate on cocaine self-administration under a progressive-ratio schedule of reinforcement in rhesus monkeys. J Pharmacol Exp Ther. 2013;345:374–382. doi: 10.1124/jpet.113.204321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Tran P, Thomas LN, Martin TJ, Grigg A, Blough BE, Beveridge TJ. Effects of the norepinephrine releaser phenmetrazine on cocaine self-administration and cocaine-primed reinstatement in rats. Psychopharmacology. 2015;232:2404–2415. doi: 10.1007/s00213-015-3875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk D, Wang XQ, Liu Lei, Fertig J, Mattson M, Ryan M, Johnson B, Stout R, Litten RZ. Percentage of subjects with no heavy drinking days: evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res. 2010;34:2022–2034. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- FDA. Alcoholism: Developing drugs for treatment. [Accessed 10 September 2015];2015 Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm433618.pdf.
- Gould RW, Czoty PW, Nader SH, Nader MA. Effects of varenicline on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2011;339:678–686. doi: 10.1124/jpet.111.185538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacology. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004a;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004b;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Haile CN, Kosten TR. Pharmacotherapy for stimulant-related disorders. Curr Psychiatry Rep. 2013;15:415. doi: 10.1007/s11920-013-0415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Buckley NW, Gosden J, Slater N, France CP, Hackett D. A preclinical evaluation of the discriminative and reinforcing properties of lisdexamfetamine in comparison to D-amfetamine, methylphenidate and modafinil. Neuropharmacology. 2013;73:348–358. doi: 10.1016/j.neuropharm.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Ann N Y Acad Sci. 2010;1187:76–100. doi: 10.1111/j.1749-6632.2009.05145.x. [DOI] [PubMed] [Google Scholar]
- Huttune KM, Raunio H, Rautio J. Prodrugs—from serendipity to rational design. Pharmacol Rev. 2011;63:750–771. doi: 10.1124/pr.110.003459. [DOI] [PubMed] [Google Scholar]
- Jain NC, Budd RD, Sneath TC. Frequency of use or abuse of amphetamine-related drugs. Am J Drug Alcohol Abuse. 1979;6:53–57. doi: 10.3109/00952997909007032. [DOI] [PubMed] [Google Scholar]
- Kaland ME, Klein-Schwartz W. Comparison of lisdexamfetamine and dextroamphetamine exposures reported to U.S. poison centers. Clin Toxicol. 2015;53:477–485. doi: 10.3109/15563650.2015.1027903. [DOI] [PubMed] [Google Scholar]
- Kollins SH. A qualitative review of issues arising in the use of psychostimulant medications in patients with ADHD and co-morbid substance abuse disorders. Curr Med Res Opin. 2008;24:1345–1357. doi: 10.1185/030079908x280707. [DOI] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Specker S, Mooney M, Mahony A, Brooks DJ, Babb D, Bai Y, Eberly LE, Nunes EV, Grabowski J. Extended-release mixed amphetamine salts vs placebo for comorbid adult attention-deficit/hyperactivity disorder and cocaine use disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72:593–602. doi: 10.1001/jamapsychiatry.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann DJ, Ramey T, Skolnick P. Outcome measures in medication trials for substance use disorders. Curr Treat Options Psych. 2015 in press. [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Specker S, Babb D, Levin FR, Grabowski J. Pilot study of the effects of lisdexamfetamine on cocaine use: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2015;153:91–103. doi: 10.1016/j.drugalcdep.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:909–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS, Baumann MH, Rothman RB, Mello NK, Blough BE. Selective suppression of cocaine- versus food-maintained responding by monoamine releasers in rhesus monkeys: benzylpiperazine, (+)phenmetrazine, and 4-benzylpiperidine. J Pharmacol Exp Ther. 2009;329:272–281. doi: 10.1124/jpet.108.143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Henningfield J. Agonist medications for the treatment of cocaine use disorder. Neuropsychopharmacology. 2015;40:1815–1825. doi: 10.1038/npp.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology. 2003a;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003b;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317–327. doi: 10.2147/ndt.s9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Katsnelson M, Vu N, Partilla JS, Dersch CM, Blough BE, Baumann MH. Interaction of the anorectic medication, phendimetrazine, and its metabolites with monoamine transporters in rat brain. Eur J Pharmacol. 2002;447:51–57. doi: 10.1016/s0014-2999(02)01830-7. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW. Agonist replacement therapy for cocaine dependence: a translational review. Future Med Chem. 2012;4:245–265. doi: 10.4155/fmc.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98:1137–1141. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Bolin BL, Sites JP, Rush CR. Abuse potential of oral phendimetrazine in cocaine-dependent individuals. College on Problems of Drug Dependence 77th Annual Meeting Abstracts. 2015;152 [Google Scholar]
- Thomsen M, Barrett AC, Negus SS, Caine SB. Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J Exp Anal Behav. 2013;99:211–233. doi: 10.1002/jeab.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchell C, Rappaport BA, Roca R, Rosebraugh CJ. Reanalysis of methamphetamine dependence treatment trial. CNS Neurosci Ther. 2012;18:367–368. doi: 10.1111/j.1755-5949.2011.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]