Abstract
Objective
Studies suggest nerve growth factor inhibitors (NGFi) relieve pain but may accelerate disease progression in some patients with osteoarthritis (OA). We sought cost and toxicity thresholds that would make NGFi a cost-effective treatment for moderate-to-severe knee OA.
Design
We used the Osteoarthritis Policy (OAPol) model to estimate the cost-effectiveness of NGFi compared to standard of care (SOC) in OA, using Tanezumab as an example. Efficacy and rates of accelerated OA progression were based on published studies. We varied the price/dose from $200 to $1,000. We considered self-administered subcutaneous injections (no administration cost) vs. provider-administered IV infusion ($69-$433/dose). Strategies were defined as cost-effective if their incremental cost-effectiveness ratio (ICER) was less than $100,000/quality-adjusted life year (QALY). In sensitivity analyses we varied efficacy, toxicity, and costs.
Results
SOC in patients with high levels of pain led to an average discounted quality-adjusted life expectancy of 11.15 QALYs, a lifetime risk of TKR of 74%, and cumulative discounted direct medical costs of $148,700. Adding Tanezumab increased QALYs to 11.42, reduced primary TKR utilization to 63%, and increased costs to between $155,400 and $199,500. In the base-case analysis, Tanezumab at $600/dose was cost-effective when delivered outside of a hospital. At $1,000/dose, Tanezumab was not cost-effective in all but the most optimistic scenario. Only at rates of accelerated OA progression of 10% or more (10-fold higher than reported values) did Tanezumab decrease QALYs and fail to represent a viable option.
Conclusions
At $100,000/QALY, Tanezumab would be cost effective if priced ≤$400/dose in all settings except IV hospital delivery.
Keywords: osteoarthritis, Nerve Growth Factor Inhibitors, cost-effectiveness, Tanezumab
Introduction
Knee osteoarthritis (OA) is a painful debilitating disease that affects more than 9 million American adults1. Current medications for knee OA pain, such as non-steroidal anti-inflammatory drugs (NSAIDs) and opioids, are limited in their long-term efficacy and safety2–9. Consequently, over half of patients with knee OA elect to receive total knee replacement surgery (TKR) within their lifetimes10. With knee OA patients estimated to spend an average of 13.3 years without adequate pain relief prior to TKR11, additional pharmacologic therapies with increased efficacy and safety could improve quality of life (QOL) and reduce the number of TKRs in this population12.
Nerve growth factor (NGF) represents a potential target for treatment of pain, and several antibodies have been developed to inhibit NGF,13, 14 the most thoroughly studied of which was developed by Pfizer under the trade name Tanezumab. Clinical trials documented impressive relief of knee OA pain, but in 2010, the FDA suspended all trials for anti-NGF drugs in OA due to concerns about rapidly progressing OA leading to joint replacement in some patients15–22. In 2012, the FDA’s Arthritis Advisory Committee (AAC) approved continued testing of anti-NGF drugs provided that certain safety recommendations are met16.
Tanezumab is a biologic drug delivered via intravenous infusion or subcutaneous injection23. Biologics, such as those used in rheumatoid arthritis (RA), have high costs due to the resources needed to produce the drugs themselves and to their mode of administration23. Because OA is more prevalent than RA (12.1% vs 0.6% in the US), Tanezumab and other drugs in its class could conceivably be priced lower than biologics for RA1, 24.
Given the promising results surrounding the efficacy of Tanezumab, we sought to address several open questions: At what price might Tanezumab be cost-effective in the treatment of OA pain? How might the risk of accelerated OA progression affect the value of Tanezumab? Does Tanezumab have the potential to reduce primary and revision TKR utilization? Early clinical trials showed promising results regarding the attractiveness of Tanezumab for knee OA with some concerns about safety and no information about potential costs. Given the FDA’s most recent decision to continue testing of anti-NGF drugs, it makes sense at this point to ask what clinical outcomes, side-effect profiles, and costs might make Tanezumab a cost-effective option for the treatment of OA pain. Such information would provide practical guidance to practitioners, payers, and designers of future trials regarding performance benchmarks and standards of evidence for treatment and reimbursement decisions.
Methods
Analytic Overview
We used the Osteoarthritis Policy (OAPol) Model to project the clinical and economic implications of adding Tanezumab monotherapy to the current standard of care. Outcomes included lifetime medical costs, quality-adjusted life years (QALYs), incremental cost-effectiveness ratios (ICERs), and utilization of primary and revision TKR. We determined the efficacy, toxicity, and cost ranges for Tanezumab that would be required to satisfy accepted, societal willingness to pay (WTP) thresholds. To implement trial-reported data into the OAPol model, we generated a sample with pain scores based on the distribution reported in the trial (mean pain 67.1, standard deviation 12.7)17 and then grouped the generated values by the pain group categories used in the OAPol model. We stratified the change in pain score by the initial pain groups, assuming a correlation between the initial and the final pain scores of 0.39, obtained from a meta-analysis comparing the pain relief between NSAIDs and opioids25. We considered three willingness-to-pay (WTP) thresholds often used in the US: $50,000/QALY, $100,000/QALY, and $150,000/QALY26–28. Results are presented in 2014 USD with costs and QALYs discounted at 3% per year29.
The OAPol Model
The OAPol model is a validated, state transition, Monte Carlo simulation of the natural history and management of knee OA30–32. The model generates cohorts of hypothetical subjects and assigns them initial characteristics from pre-specified distributions of age, sex, race/ethnicity, obesity, comorbid conditions, knee OA severity, and pain severity. The OAPol model accounts for the inter-relationships among key variables. For example, quality of life is a function of pain, obesity and comorbidities; background medical costs are based on sex, age and comorbidities; and pain reduction depends on baseline pain.
In the model, subjects progress through health states in 1-year intervals, during which they may develop comorbidities, increase body mass index (BMI), progress in OA severity, change in pain severity, and/or die. Five comorbidities were considered: cancer, cardiovascular disease (CVD), chronic obstructive pulmonary disease, diabetes mellitus, and musculoskeletal conditions other than OA. Prevalence and incidence rates for these diseases were stratified by age, sex, race/ethnicity, and obesity. We used underlying mortality rates derived from the 2010 CDC life tables, accounting for increased mortality due to specific comorbidities33–37. The initial BMI distribution was stratified by sex and race/ethnicity with obesity defined as a BMI ≥ 30 kg/m2. Progression in OA severity was defined as an increase in Kellgren-Lawrence (K-L) radiographic grade and was stratified by sex and obesity31, 38. Pain severity in the OAPol model is measured on a 0–100 scale and is assigned to one of five pain groups. There are no well-established cut-offs for defining mild, moderate, and severe OA pain. Several lines of inquiry guided our effort. Kapstad et al defined thresholds between mild/moderate and moderate/severe at 4 and 7 out of 10 on the Body Pain Index (BPI)39. Since most of our data come from clinical trials that use the WOMAC Pain scale, we transformed the WOMAC Pain scale to a 0–100 scale with 100=worst. We did a similar transformation with BPI, and established thresholds of 40 and 70 for moderate and severe pain. To distinguish mild from moderate pain we drew upon studies of TKR efficacy showing WOMAC<15 reflects mild pain40. This designation has face validity in that pain scores between 0 and 1 (none and mild) across 5 items correspond to the 1–15 group, scores between 1 and 2 (mild and moderate) correspond roughly to the 16–40 group, and pain scores in the 3–4 (severe, extreme) range correspond to the >70 group. Downgrading by one group level corresponds to a clinically meaningful difference in pain41, 42. QOL decrements corresponding to each pain group were derived using data from the Osteoarthritis Initiative43, 44. Table 1 contains select cohort and treatment characteristics.
Table 1.
Select Model Inputs
| Parameter | Estimate | Data Source Used in Derivations | ||||
|---|---|---|---|---|---|---|
| Cohort Characteristics
| ||||||
| Demographics | Mean (SD) or Percent | Lane et al. 201017 | ||||
| Mean Age | 58.7 (7.9) | |||||
| Percent Female | 59% | |||||
| Percent White | 88% | |||||
| Percent K-L 2 | 30% | |||||
| Percent K-L 3 | 53% | |||||
| WOMAC Pain | 67(13) | |||||
| Starting WOMAC Pain | ||||||
| 15–40 | 41–70 | 71+ | ||||
|
|
||||||
| Percent of Cohort | 2% | 58% | 41% | |||
|
| ||||||
| Quality of Life Utilities (Nonobese/Obese) | WOMAC Pain (0–100) | Osteoarthritis Initiative38 | ||||
| 0 Comorbidities | Brazier et al. 200439 | |||||
|
|
||||||
| Age Group | 0 | 1–15 | 16 – 40 | 41 – 70 | 71 – 100 | |
|
|
||||||
| 25–44 | 0.865/0.845 | 0.840/0.820 | 0.781/0.761 | 0.699/0.679 | 0.609/0.589 | |
| 45–54 | 0.841/0.830 | 0.816/0.806 | 0.780/0.769 | 0.714/0.703 | 0.656/0.645 | |
| 55–64 | 0.847/0.836 | 0.822/0.812 | 0.786/0.775 | 0.720/0.709 | 0.662/0.651 | |
| 65–74 | 0.871/0.860 | 0.846/0.835 | 0.810/0.799 | 0.744/0.733 | 0.685/0.675 | |
| 75+ | 0.854/0.843 | 0.829/0.818 | 0.793/0.782 | 0.727/0.716 | 0.669/0.658 | |
| 1 Comorbidity | ||||||
|
|
||||||
| Age Group | 0 | 1–15 | 16 – 40 | 41 – 70 | 71 – 100 | |
|
|
||||||
| 25–44 | 0.845/0.825 | 0.820/0.800 | 0.761/0.741 | 0.679/0.659 | 0.589/0.569 | |
| 45–54 | 0.818/0.807 | 0.791/0.780 | 0.755/0.744 | 0.679/0.668 | 0.645/0.634 | |
| 55–64 | 0.824/0.813 | 0.797/0.786 | 0.761/0.750 | 0.685/0.674 | 0.651/0.640 | |
| 65–74 | 0.848/0.837 | 0.821/0.810 | 0.785/0.774 | 0.708/0.698 | 0.674/0.664 | |
| 75+ | 0.831/0.820 | 0.804/0.793 | 0.768/0.757 | 0.692/0.681 | 0.658/0.647 | |
| 2+ Comorbidities | ||||||
|
|
||||||
| Age Group | 0 | 1–15 | 16 – 40 | 41 – 70 | 71 – 100 | |
|
|
||||||
| 25–44 | 0.825/0.805 | 0.800/0.780 | 0.741/0.721 | 0.659/0.639 | 0.569/0.549 | |
| 45–54 | 0.806/0.795 | 0.794/0.783 | 0.732/0.721 | 0.635/0.624 | 0.500/0.489 | |
| 55–64 | 0.812/0.801 | 0.800/0.789 | 0.738/0.727 | 0.641/0.630 | 0.506/0.495 | |
| 65–74 | 0.836/0.825 | 0.824/0.813 | 0.762/0.751 | 0.665/0.654 | 0.530/0.519 | |
| 75+ | 0.819/0.808 | 0.807/0.796 | 0.745/0.734 | 0.648/0.637 | 0.513/0.502 | |
|
| ||||||
| Underlying Medical Costs | Comorbidities | Pope et al 201441 NHANES 2009–201233 MCBS 200943 Red Book Online53 CPI63 |
||||
| Age group | 0–1 | 2–3 | 4+ | |||
|
|
||||||
| 25–34 | $1,400 | $7,500 | $14,300 | |||
| 35–44 | $2,000 | $8,000 | $14,300 | |||
| 45–49 | $2,700 | $8,200 | $14,300 | |||
| 50–54 | $2,700 | $8,200 | $14,300 | |||
| 55–59 | $3,500 | $8,800 | $14,700 | |||
| 60–64 | $4,300 | $9,600 | $15,500 | |||
| 65–69 | $4,600 | $9,900 | $15,500 | |||
| 70–74 | $5,300 | $10,700 | $16,200 | |||
| 75–79 | $6,200 | $11,600 | $17,100 | |||
| 80+ | $8,200 | $13,500 | $19,100 | |||
|
| ||||||
|
Tanezumab Treatment Characteristics
| ||||||
| Annual Cost (2014 USD) | Drug Cost | Administrative Cost | Monitoring Cost | |||
| First Year | 200, 400, 600, 800, 1000 | 61, 69, 133, 241, 433 | 277, 495 | |||
| Subsequent Years | 200, 400, 600, 800, 1000 | 0, 69, 133, 241, 433 | 277, 495 | Medicare Fee Schedules49 | ||
|
| ||||||
| Pain Relief | Starting Pain | |||||
|
|
||||||
| 15–40 | 41–70 | 71+ | ||||
|
|
||||||
| Mean (SD) Pain Reduction | 13 (16) | 29 (18) | 41 (18) | Lane et al 201017 | ||
| Late Failure Rate (%) | 10 | - | - | Klareskog et al. 200645 | ||
| Max Years Efficacy | 15 | - | - | Assumption | ||
|
| ||||||
| Adverse Effects | Minor Toxicity | Major Toxicity | ||||
| First Year Rate (%) | 60 | 1 | Hochberg et al. 201548 | |||
| Subsequent Years Rate (%) | 30 | 0.5 | ||||
| QOL Multiplier | 0.997 | 0.59 | ||||
| Cost (2014 USD) | 52 | 137 | ||||
In 2014 USD. Values exclude as needed pain management. An annual cost of $102 was added for patients with symptomatic knee OA
Minor toxicity disutility and cost based on NSAID toxicity characteristics. Rate of minor toxicity was assumed. Major toxicity cost based on additional physician office visits and x-rays to diagnose rapid OA progression. Major toxicity disutility calibrated to yield QOL of 0.5 immediately prior to TKR following rapid OA progression.
Subjects in the model undergo OA treatments that reduce pain severity, incur a cost, and may be associated with toxicity. Each year subjects eligible for treatment have the opportunity to accept or reject it. Pain reduction is drawn from published data and its magnitude depends on pain severity at the start of treatment. Success of treatment is defined as reduction from a higher to a lower pain group. In subsequent years, pain relief may end based on a defined probability (late failure) at which point the subject’s pain severity is set to an estimate of what their pain severity would have been had they not received treatment. Subjects are removed from non-surgical regimens when their pain severity worsens to pre-treatment levels. Treatment regimens carry a risk of major (e.g. myocardial infarction) and minor (e.g. rash) toxicity, each with an associated decrease in QOL and increase in cost. Major toxicities lead to regimen discontinuation and may carry a risk of death. TKR eligibility criteria of pain severity >40 was defined based on published literature45.
Cohort Characteristics
Initial age, sex, race/ethnicity, pain severity, and K-L distributions were derived from Lane et al. (2010; Table 1)17. Subjects’ age at baseline was drawn from the normal distribution with a mean age of 59 (standard deviation (SD) 8 years). The cohort was 59% female. Initial pain severity was 67 (SD 13) on the WOMAC Pain scale (0–100, with 100=worst). Cohort and treatment characteristics can be found in Table 131, 38.
BMI distribution of persons with OA was derived from the National Health Interview Survey (NHIS) 2012 and stratified by sex and race/ethnicity46. Annual medical costs unrelated to OA ranged from $1,400 in young patients with at most one comorbidity to $19,100 in elderly patients with > 3 comorbidities. Subjects had an additional $102 in yearly costs associated with management of OA pain outside of specific treatment regimens34, 47–49.
Standard of Care
Subjects entered this analysis upon failure of non-invasive treatments for OA pain (NSAIDs and corticosteroid injections), based on inclusion criteria for past Tanezumab trials50. The standard of care (SOC) consisted of TKR for those whose pain severity exceeded 40 (current thresholds for those undergoing TKR) with K-L grades 3 or 4.
Tanezumab
Efficacy and Toxicity
Tanezumab efficacy (reduction in pain severity) was derived from Lane et al. (2010) and stratified by initial pain group17. Subjects experienced mean pain decreases in WOMAC pain scale of 13, 23 and 49 points in pain groups 3, 4, and 5, respectively. We estimated a late failure rate of 10% using discontinuation rates due to lack of efficacy for etanercept in RA51. Incidence, cost, and QOL decrease of minor toxicities were assumed to correspond with those found in NSAID therapy52, 53. ‘Late failure’ is defined as failure of a regimen that provided initial relief to provide pain relief in subsequent periods. The subjects remain on the regimen until the failure is ‘observed’ by a clinician. Subjects observed to fail (pain returned to pre-treatment levels) are removed from the regimen. For the base case, we assumed a late failure rate of 10% per year (analogizing from data on biologics for rheumatoid arthritis)51
We conducted these analyses with a validated model (OAPol) of the natural history and management of knee OA that has been used to examine the cost-effectiveness of opioids in OA, for a premarket evaluation of DMOADs, and to project lifetime costs in persons with knee OA11, 54, 55. We adapted the existing model to capture the essential clinical and economic performance attributes of Tanezumab. We added one structural feature, which provided the capacity to identify those who experienced rapid joint destruction, an important Tanezumab-related complication. We estimated a 1% chance of accelerated OA progression (major toxicity) in the first year and 0.5% in subsequent years based on findings from an independent adjudication committee18, 56. Accelerated OA progression was characterized by termination of Tanezumab treatment and immediate TKR. We assumed a worst-case scenario, and we reduced the durability and efficacy of TKR by 50% among those with joint destruction in order to reflect the bone destruction associated with this complication. TKR acceptance rates were based on data from the Multicenter Osteoarthritis Study (MOST) and the Osteoarthritis Initiative (OAI) and were calibrated so that all cause TKR rate in the first year of treatment matched those observed in large Tanezumab trials (~5%)18 For revision TKR, we used data from Paxton et al, since revision data were not reported due to short trial duration57
To assure the model output is concordant with trial-based input data, we present the results of the internal model validation. The model estimated the pain reduction due to Tanezumab at 37.8 WOMAC points, which is similar to the 33.7 (SD 19.5) point reduction seen in the clinical trial (an average across dosages ranging from 10 μg/kg to 100 μg/kg)17. Further, the trial reported that 5% of those on Tanezumab received TKR by the end of one year with 1% having TKR due to joint destruction. The model derived values were 4% and 1% respectively.
Costs
Tanezumab costs were broken into three categories: administration, drug, and monitoring. Administration costs refer to the cost associated with delivery of the drug and varied depending on the setting (self-administered subcutaneous (SC) vs intravenous (IV); non-hospital vs IV outpatient) as well as the type of procedure billed (non-chemotherapeutic IV vs chemotheraputic IV)58, 59. While published trials of Tanezumab for knee OA have focused on IV delivery, Tanezumab has been delivered via SC injection in other diseases, so both of these modes of delivery were included in this analysis60, 61. All SC injections were assumed to be self-administered, while IV infusions were delivered by a healthcare provider. Administration costs varied from $0/injection (self-administered SC) to $433/injection. For the purposes of this analysis, drug cost refers to the price of one dose of Tanezumab and, in the absence of current pricing, was varied from $200 to $1000, consistent with costs of other biologic regimens for other conditions62. Based on published studies, we assumed that Tanezumab doses were delivered once every 8 weeks15, 17, 19. Monitoring costs for IV infusions were fixed at $277 and included semi-annual physician’s visits, yearly blood tests, and x-rays to check for OA progression every other year58. Subjects receiving self-administered SC injections had a monitoring cost of $495, because their monitoring included two additional physician visits per year.
Sensitivity Analysis
We varied early efficacy and late failure rate in two-way sensitivity analyses. Recognizing the paucity of reliable input data for many of the parameters in our model, we chose very wide ranges for sensitivity analysis to identify the data values and combinations that would (or would not) support Tanezumab as a cost-effective option.
Across a range of costs and major toxicity rates, we examined the sensitivity of Tanezumab cost-effectiveness to changes in efficacy (−30%—+30% of base case) and late failure rate (2.5%—20%). We preformed additional sensitivity analyses in which we examined cohorts with a lower starting pain (35, 45).
Results
Base case analysis (high pain group)
Subjects treated with the SOC had an average discounted quality-adjusted life expectancy of 11.15 QALYs (16.26 QALYs non-discounted). Seventy four percent of subjects initiating treatment with high pain in SOC underwent a primary TKR in their lifetime and 13% received a revision TKR. The average age at the time of primary TKR was estimated at 65.7 years. The cumulative discounted direct medical costs were estimated at $148,700.
Adding Tanezumab increased the average life expectancy to 11.42 QALYs (16.56 QALYs non-discounted) and reduced primary TKR utilization to 63% (revision TKRs to 9%). Subjects spent an average of 6.6 years (SD 5.15) on Tanezumab. In patients who received Tanezumab treatment, the mean age at the time of primary TKR increased to 68.9.
By five years, 69% of those in the standard of care strategy had severe or extreme pain, compared to 49% of those in the Tanezumab strategy. Further, by the end of five years, 32% of those in SOC strategy, and alive, had TKR, compared to 12% among those in Tanezumab strategy. By 10 years, the rates of TKR were 51% and 29% respectively in the SOC and Tenazumab groups, and the revision rates were 3.0% and 2.6% respectively. Costs ranged from $155,400 for self-administered Tanezumab at $200/dose to $199,500 for hospital-administered Tanezumab at $1000/dose.
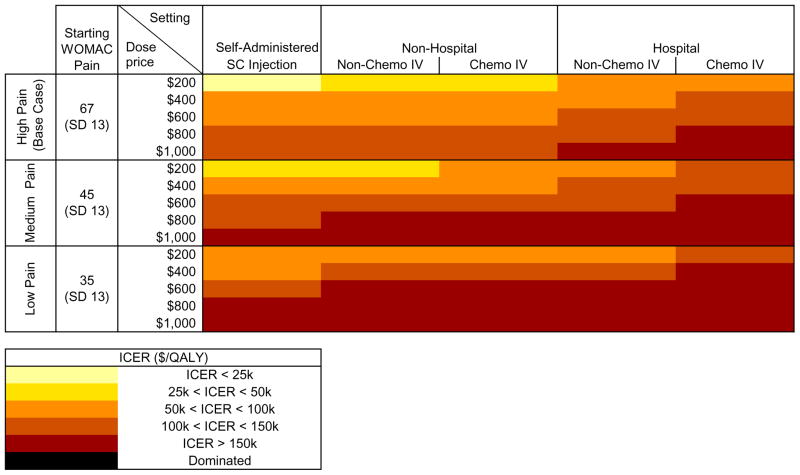
The incremental cost-effectiveness of adding Tanezumab to the SOC is presented in Figure 2. ICERs ranged from $24,400/QALY at $200/dose when self-administered as an SC injection to $189,000/QALY at $1,000/dose when delivered as a chemotherapeutic IV in an outpatient hospital setting. At a WTP of $50,000/QALY, Tanezumab was cost-effective when priced at $200/dose and delivered outside of a hospital. At a WTP of $100,000/QALY, Tanezumab was cost-effective in all settings except hospital delivery of a chemotherapeutic IV when priced at $400/dose and in all non-hospital settings when priced at $600/dose. At a WTP of $150,000/QALY, Tanezumab was cost-effective in all settings except hospital chemotherapeutic IV when priced at $800/dose and in all non-hospital settings at $1,000/dose.
Figure 2. Cost-effectiveness of Adding Tanezumab by Starting Pain, Cost, and Mode of Administration.
This figure shows the incremental cost-effectiveness ratios (ICERs) associated with adding Tanezumab to the standard of care. Lighter yellows indicate higher value, while darker reds indicate lower value. The results are stratified by starting WOMAC pain severity (0 – 100 scale, 100 = worst) and the cost of the drug and its delivery, assuming doses are delivered every 8 weeks. Mean WOMAC pain severity was 67 for high pain, 45 for medium pain, and 35 for low pain. These results all follow the base case assumption of a 1% incidence of rapid OA progression in the first year of treatment and 0.5% in subsequent years, where rapid OA progression requires immediate joint replacement with a lower than normal efficacy.
Sensitivity Analyses
Initial Pain Severity
Medium pain
Subjects with an average starting pain of 45 had a QALE of 11.88 QALYs when treated with SOC. Sixty-three percent of subjects underwent primary TKR and 8.5% received revision TKR. Adding Tanezumab before primary TKR increased average QALE to 12.06 QALY in the base case. Tanezumab decreased the number of primary and revision TKRs by 16% and 21%, respectively. We examined the cost-effectiveness of Tanezumab across the same four settings as in the base case and drug costs of $200 to $1000/dose as shown in the “Medium Pain” section of Figure 2. At a WTP of $50,000/QALY, Tanezumab was only cost-effective in subjects with a starting pain of 45 when priced at $200/dose and either self-administered or delivered in a non-hospital setting as a non-chemo IV. Given a WTP of $100,000/QALY, Tanezumab was cost-effective at a price of $400/dose when delivered in a non-hospital setting. At $150,000/QALY, Tanezumab was cost-effective in all settings except hospital chemotherapy IV at $600/dose and when self-administered at $800/dose. It was never cost-effective at $1000/dose for subjects with starting pain of 45.
Low pain
Subjects undergoing the SOC with a baseline pain of 35 had an average QALE of 12.20 QALY. Sixty percent underwent primary TKR and 5.5% had revision TKR. Tanezumab added to the SOC increased average QALE to 12.33 QALY, reduced the number of primary TKRs by 14%, and decreased the number of revision TKRs by 7%. Tanezumab was never cost-effective in this cohort given a WTP of $50,000/QALY. At WTP of $100,000/QALY, Tanezumab was only cost-effective at $400/dose when self-administered. Given a WTP of $150,000/QALY, Tanezumab was cost-effective at $600/dose when self-administered. Tanezumab was never cost-effective at or above $800/dose in subjects with a mean starting pain of 35. These results are presented in the low pain section of Figure 2.
Efficacy
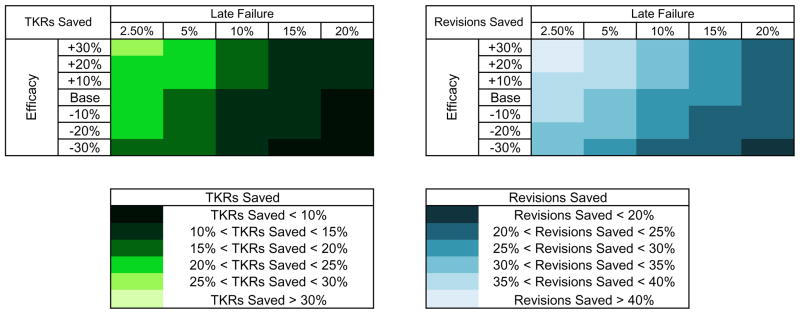
We performed two-way sensitivity analyses on efficacy and late failure of Tanezumab. Average QALE in sensitivity analyses ranged from 11.25 QALYs given an efficacy 30% lower than base case and a failure rate of 20% (vs. 10% in the base case) to 11.72 QALYs given an efficacy 30% greater than base case and a failure rate of 2.5%. The proportion of subjects receiving primary and revision TKR ranged from 54.8% to 68.3% and 7.3% to 10.5%, respectively. Figure 3 shows the reduction in surgeries when Tanezumab was added to SOC across these ranges of efficacy and failure.
Figure 3. Reduction in Primary and Revision TKR Utilization: Base Case Pain Analysis.
This figure shows the percent reduction in primary and revision total knee replacement (TKR) utilization when Tanezumab is added to the standard of care given base case pain severity (67) and rapid OA progression rate (1%). Sensitivity analyses around base case first year pain relief and subsequent year late failure rate are presented, with the numerical value of overall base case highlighted in white. Lighter colors indicate more joint replacements saved than darker colors.
Figure 4 depicts the sensitivity of the ICER for Tanezumab to broad, simultaneous variation in four important parameters. At $400/dose, Tanezumab was almost always cost-effective, given a WTP of $100,000/QALY; the only exceptions were scenarios involving the most pessimistic assumptions regarding administration costs, early efficacy and late failure rate. By contrast, with drug costs of $800 or more, Tanezumab was only cost-effective under the most optimistic assumptions.
Figure 4. Cost-effectiveness of Tanezumab: Sensitivity to Cost, Efficacy, and Late Failure.
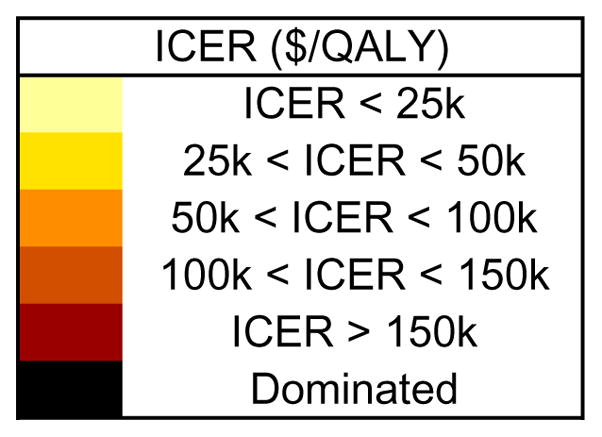
This figure shows the incremental cost-effectiveness ratios (ICERs) when Tanezumab is added to the standard of care across a range of sensitivity analyses. Lighter yellows indicate higher value, while darker reds indicate lower value. The figure shows the impact of drug cost ($200 – $1,000 per dose), mode and setting of administration (SC vs IV, self-administered vs non-hospital vs hospital), first year pain relief (percent difference from base case value), and subsequent year late failure rate (2.5% – 20% per year). These analyses all consider Tanezumab given to subjects with base case pain and base case incidence of rapidly progressing OA due to Tanezumab.
Toxicity: rapidly progressing OA
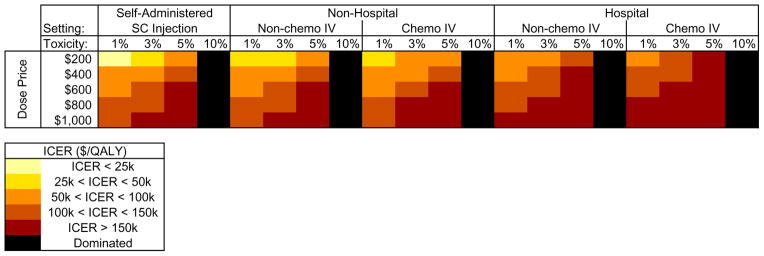
In order to address the potential consequences of rapidly progressing OA, we varied the rate at which rapidly progressing OA occurred. Eliminating the risk of rapid OA progression increased QALE to 11.46 QALY, decreased the percent of subjects undergoing TKRs to 61.6% and the percent of subjects undergoing revision to 7.2%. When the rate of rapidly progressing OA occurrence was increased to 3%, utilization of primary TKR still decreased relative to SOC by 20.1% but revisions increased by 25.1%. The ICER for Tanezumab remained below $100,000/QALY, at $400/dose when delivered outside a hospital setting (Figure 5). Further increasing the rate of rapid OA progression to 10% decreased QALE to 11.14 QALY; under this scenario, Tanezumab was dominated by SOC. At rapidly progressing OA rates of 14% or more, Tanezumab also increased the number of primary TKRs compared with SOC.
Figure 5. Cost-effectiveness of Tanezumab: Sensitivity to Cost and Rapid OA Progression.
This figure shows the sensitivity of incremental cost-effectiveness ratios to rapid OA progression for Tanezumab added to the standard of care. The figure shows the impact of drug cost ($200 – $1000 per dose), mode and setting of delivery (SC vs IV, self-administered vs non-hospital vs hospital), and rate of rapid OA progression, indicated as ‘toxicity’ (1%, 3%, 5%, 10%). All other variables in this analysis were set to base case values.
Discussion
We conducted a premarket evaluation of Tanezumab for knee OA to provide guidance for future research on efficacy, toxicity, and costs that result in acceptable ICERs based on societal norms. Our analysis showed that Tanezumab could be cost-effective across a range of WTP thresholds depending on its price and the setting in which it is delivered. Adding Tanezumab to standard treatment options could substantially decrease utilization of TKRs. Tanezumab was more cost-effective in patients with more severe pain. The value of Tanezumab was sensitive to costs associated with its administration and rates of rapid OA progression.
In a recent, widely-cited article, Neumann and colleagues argue that $50,000/QALY is too low in the US and recommend that analysts use $50,000, $100,000, and $200,000/QALY instead63. They suggest that if a single threshold had to be chosen, either $100,000 or $150,000 would be most reasonable. Accordingly, we present analyses across WTP thresholds ranging from $50K/QALY to $150K/QALY.
Biologics are widely used in the treatment of RA64. Common biologic drugs for RA range in price from $800/dose of etanercept to nearly $4,000/dose of infliximab with higher prices in drugs delivered less frequently62, 65, 66. However, our analysis suggests that Tanezumab is unlikely to be cost-effective treatment if priced at ≥$1,000/ dose even at a WTP of $150,000/QALY. The cost-effectiveness of more expensive RA treatment likely reflects the fact that RA is a systemic disease associated with markedly decreased quality-adjusted life expectancy, which biologics attenuate67–69. Furthermore, TKR is a very efficacious treatment option for knee OA70, and society’s willingness to pay for Tanezumab may be limited by the existence of a very efficacious and cost-effective surgical alternative.
Tanezumab provides an additional pharmacological regimen that can delay the need for surgical treatment and improve QALE. In the base case, Tanezumab increased the mean age for primary TKR by 3.2 years. This finding is important, as younger age is associated with decreased implant survivorship57. Delaying the need for surgery could improve primary TKR outcomes and greatly reduce the need for revision TKR, a more expensive and less efficacious surgery58,70. Increasing the age at which patients receive primary TKR diminishes their lifetime risk of revision. Additionally, Tanezumab reduces the number of primary TKRs performed, leaving fewer TKRs to revise.
Paxton et al showed that the risk of revision among those receiving TKR earlier (i.e., in their fifties) is greater than among those who receive TKR later. These data suggest that activity level may be higher in those who are younger, leading to greater implant failure and utilization of revision TKR. However, interventions are generally less cost-effective in older than in younger persons as older persons have lower survival71.
Our analysis demonstrated that even in the case of high attrition rates, Tanezumab had the potential to significantly reduce primary and revision TKR utilization while improving QALE in a cohort of subjects with advanced OA and high pain severity.
Rapid progression of OA remains a key concern surrounding NGF inhibitors. We assumed a worst-case scenario approach to the impact of rapid OA progression --that patients who experienced this toxicity would achieve greatly reduced primary and revision TKR efficacy. Even under this pessimistic assumption, a 1% rate of rapid OA progression (0.5% after the first year of treatment) yielded a reduction in QALE of just 0.04 QALYs compared to Tanezumab with no risk of rapid OA progression. Accelerated OA progression had a significant impact on cost-effectiveness and revision TKR utilization at rates as low as 3%, but only at rates of rapid OA progression of 10% or more did Tanezumab cause a net reduction in QALE. This falls well outside the range observed in large Tanezumab trials, even if one attributes every joint replacement to Tanezumab treatment and considers only Tanezumab plus NSAID combination therapy, which carries a substantially higher risk of joint toxicity18. In our analyses we included the cost of radiographic monitoring to determine rapid progression. It is feasible that monitoring should be performed on multiple joints since rapid OA progression often occurred in non-index joint. The balance between the frequency of x-ray exposure and additional benefit of identifying rapid progression early are likely factors that will guide monitoring strategies. Due to low cost of radiographs, they are unlikely to alter cost-effectiveness results.
Our analysis had several limitations. Since no studies have investigated long term use of Tanezumab, we had to estimate discontinuation rates using data from biologics drugs in RA. Additionally, our data on the toxicity, pain efficacy, and structural efficacy of TKR were derived from multiple sources.
Results of this evaluation suggest that rapid OA progression at rates observed in clinical trials does not lead to an overall decrease in quality-adjusted life expectancy. Therefore, continued research is vital to determining the appropriate role for Tanezumab in the treatment of OA. Tanezumab has the potential to improve QALE and decrease utilization of TKR, a surgery performed over 600,000 times/year in the US72; however, the cost-effectiveness will depend heavily on how the drug is priced and appropriate selection of patients. These insights could help project budgetary implications upon approval of NGF inhibitors making society more prepared to implement these potent analgesics into general clinical practice.
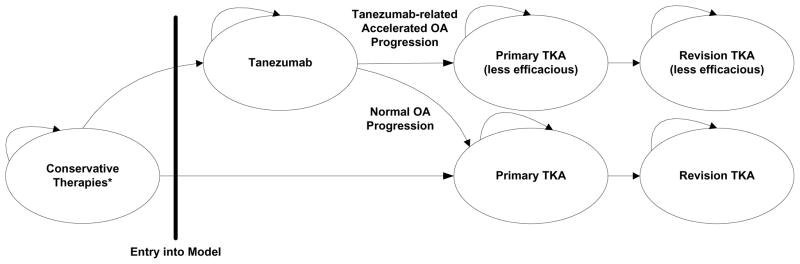
Figure 1.
Model Structure
This figure provides a visual description of where Tanezumab enters the model and some key features of how subjects progress through the model. Conservative therapies include physical therapy, NSAIDs, and corticosteroid injections. Between each stage of the model, subjects can take acetaminophen for pain. Death can occur at any stage. Subjects may stay at each model phase for multiple years before progressing to the next phase.
Acknowledgments
Role of Funding
Supported by: National Institute of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases: R01 AR064320, K24 AR057827. The funding source had no role in the study design, collection, analysis and interpretation of the data, drafting of the manuscript, or decision to submit the manuscript for publication
Footnotes
Author Contributions
Dr Losina had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Obtaining of Funding: Losina
Conception and design: Losina, Katz
Collections and assembly of data: Losina, Michl
Statistical expertise: Losina, Collins
Analysis and interpretation of the data: Losina, Michl, Collins, Hunter, Jordan, Yelin, Paltiel, Katz
Drafting of article: Losina, Michl
Critical revision of the article for important intellectual content: Losina, Michl, Collins, Hunter, Jordan, Yelin, Paltiel, Katz
Final approval of the article: Losina, Michl, Collins, Hunter, Jordan, Yelin, Paltiel, Katz
Competing Interests Statement
None of the authors received any money or any other compensation from Pfizer for the matters related to this paper. Dr. Jordan had received grant support from Johnson and Johnson to evaluate prevalence of osteonecrosis in Johnston County OA Project data. The rest of the authors do not have any relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elena Losina, Email: elosina@partners.org.
Griffin Michl, Email: griffinmichl@gmail.com.
Jamie E. Collins, Email: jcollins13@partners.org.
David J. Hunter, Email: david.hunter@sydney.edu.au.
Joanne M. Jordan, Email: joanne_jordan@med.unc.edu.
Edward Yelin, Email: ed.yelin@ucsf.edu.
A. David Paltiel, Email: david.paltiel@yale.edu.
Jeffrey N. Katz, Email: jnkatz@partners.org.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–79. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjordal JM, Klovning A, Ljunggren AE, Slordal L. Short-term efficacy of pharmacotherapeutic interventions in osteoarthritic knee pain: A meta-analysis of randomised placebo-controlled trials. Eur J Pain. 2007;11:125–38. doi: 10.1016/j.ejpain.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Bjordal JM, Ljunggren AE, Klovning A, Slordal L. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials. BMJ. 2004;329:1317. doi: 10.1136/bmj.38273.626655.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnsen SP, Larsson H, Tarone RE, McLaughlin JK, Norgard B, Friis S, et al. Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case-control study. Arch Intern Med. 2005;165:978–84. doi: 10.1001/archinte.165.9.978. [DOI] [PubMed] [Google Scholar]
- 6.Scholes D, Stergachis A, Penna PM, Normand EH, Hansten PD. Nonsteroidal antiinflammatory drug discontinuation in patients with osteoarthritis. J Rheumatol. 1995;22:708–12. [PubMed] [Google Scholar]
- 7.Whelton A. Renal and related cardiovascular effects of conventional and COX-2-specific NSAIDs and non-NSAID analgesics. Am J Ther. 2000;7:63–74. doi: 10.1097/00045391-200007020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther. 2005;7:R1046–51. doi: 10.1186/ar1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170:1968–76. doi: 10.1001/archinternmed.2010.391. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein AM, Rome BN, Reichmann WM, Collins JE, Burbine SA, Thornhill TS, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95:385–92. doi: 10.2106/JBJS.L.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Losina E, Paltiel AD, Weinstein AM, Yelin E, Hunter DJ, Chen SP, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken) 2015;67:203–15. doi: 10.1002/acr.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz N. The impact of pain management on quality of life. J Pain Symptom Manage. 2002;24:S38–47. doi: 10.1016/s0885-3924(02)00411-6. [DOI] [PubMed] [Google Scholar]
- 13.McMahon SB, Bennett DL, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med. 1995;1:774–80. doi: 10.1038/nm0895-774. [DOI] [PubMed] [Google Scholar]
- 14.Hefti FF, Rosenthal A, Walicke PA, Wyatt S, Vergara G, Shelton DL, et al. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci. 2006;27:85–91. doi: 10.1016/j.tips.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Balanescu AR, Feist E, Wolfram G, Davignon I, Smith MD, Brown MT, et al. Efficacy and safety of tanezumab added on to diclofenac sustained release in patients with knee or hip osteoarthritis: a double-blind, placebo-controlled, parallel-group, multicentre phase III randomised clinical trial. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-203164. [DOI] [PubMed] [Google Scholar]
- 16.Holmes D. Anti-NGF painkillers back on track? Nat Rev Drug Discov. 2012;11:337–8. doi: 10.1038/nrd3732. [DOI] [PubMed] [Google Scholar]
- 17.Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363:1521–31. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnitzer TJ, Ekman EF, Spierings EL, Greenberg HS, Smith MD, Brown MT, et al. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204905. [DOI] [PubMed] [Google Scholar]
- 19.Schnitzer TJ, Lane NE, Birbara C, Smith MD, Simpson SL, Brown MT. Long-term open-label study of tanezumab for moderate to severe osteoarthritic knee pain. Osteoarthritis Cartilage. 2011;19:639–46. doi: 10.1016/j.joca.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain. 2012;13:790–8. doi: 10.1016/j.jpain.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Nagashima H, Suzuki M, Araki S, Yamabe T, Muto C. Preliminary assessment of the safety and efficacy of tanezumab in Japanese patients with moderate to severe osteoarthritis of the knee: a randomized, double-blind, dose-escalation, placebo-controlled study. Osteoarthritis Cartilage. 2011;19:1405–12. doi: 10.1016/j.joca.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Spierings EL, Fidelholtz J, Wolfram G, Smith MD, Brown MT, West CR. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain. 2013;154:1603–12. doi: 10.1016/j.pain.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 23.Wong BJ, Cifaldi MA, Roy S, Skonieczny DC, Stavrakas S. Analysis of drug and administrative costs allowed by U.S. Private and public third-party payers for 3 intravenous biologic agents for rheumatoid arthritis. J Manag Care Pharm. 2011;17:313–20. doi: 10.18553/jmcp.2011.17.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 25.Smith S, Deshpande B, Collins J, Katz J, Losina E. Comparative efficacy of oral non-steroidal anti-inflammatory drugs and opioids for osteoarthritis: Systematic review and meta-analysis. Osteoarthritis and Cartilage. 2015:A355–A6. doi: 10.1016/j.joca.2016.01.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–56. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 27.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163:1637–41. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 28.Ryen L, Svensson M. The Willingness to Pay for a Quality Adjusted Life Year: A Review of the Empirical Literature. Health Econ. 2014 doi: 10.1002/hec.3085. [DOI] [PubMed] [Google Scholar]
- 29.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339–41. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 30.Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94:201–7. doi: 10.2106/JBJS.J.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holt HL, Katz JN, Reichmann WM, Gerlovin H, Wright EA, Hunter DJ, et al. Forecasting the burden of advanced knee osteoarthritis over a 10-year period in a cohort of 60–64 year-old US adults. Osteoarthritis Cartilage. 2011;19:44–50. doi: 10.1016/j.joca.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, Wright EA, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169:1113–21. doi: 10.1001/archinternmed.2009.136. discussion 21–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Health Interview Survey (NHIS). Centers for Disease Control and Prevention (CDC), and National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services (DHHS) [Accessed March 13, 2014];2010 http://www.cdc.gov/nchs/nhis.htm.
- 34.Centers for Disease Control and Prevention (CDC) 2009–2012 National Health and Nutrition Examinations Survey (NHANES) Data. Hyattsville, MD: National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services; 2011. [Google Scholar]
- 35.National Vital Statistics System (NVSS) (accessed from Health Data Interactive). Centers for Disease Control and Prevention (CDC), and National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services (DHHS) [Accessed March 13, 2014];2010 http://www.cdc.gov/nchs/hdi.htm.
- 36.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1. 46 million white adults. N Engl J Med. 2010;363:2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.United States Life Tables. Centers for Disease Control, US Department of Health and Human Services; 2010. [Google Scholar]
- 38.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–80. [PubMed] [Google Scholar]
- 39.Kapstad H, Hanestad BR, Langeland N, Rustoen T, Stavem K. Cutpoints for mild, moderate and severe pain in patients with osteoarthritis of the hip or knee ready for joint replacement surgery. BMC Musculoskelet Disord. 2008;9:55. doi: 10.1186/1471-2474-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bourne RB, Chesworth B, Davis A, Mahomed N, Charron K. Comparing patient outcomes after THA and TKA: is there a difference? Clin Orthop Relat Res. 2010;468:542–6. doi: 10.1007/s11999-009-1046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol. 2002;29:131–8. [PubMed] [Google Scholar]
- 42.Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64:29–33. doi: 10.1136/ard.2004.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osteoarthritis Initiative (OAI) University of California; San Francisco: 2013. [Google Scholar]
- 44.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42:851–9. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 45.Hawker GA, Wright JG, Coyte PC, Williams JI, Harvey B, Glazier R, et al. Determining the need for hip and knee arthroplasty: the role of clinical severity and patients’ preferences. Med Care. 2001;39:206–16. doi: 10.1097/00005650-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health Interview Survey (NHIS) 2012. [Google Scholar]
- 47.Pope GC, Kautter J, Ellis RP, Ash AS, Ayanian JZ, Lezzoni LI, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–41. [PMC free article] [PubMed] [Google Scholar]
- 48.Red Book: Pharmacy’s Fundamental Reference: 2012 Edition. Montvale, NJ: PDR Network, LLC; 2012. [Google Scholar]
- 49.Medicare Current Beneficiary Survey. Centers for Medicare &Medicaid Services; 2009. [Google Scholar]
- 50.Tanezumab Arthritis Advisory Committee Briefing Document. Pfizer; 2012. [accessed 03.17.14]. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM295205.pdf. [Google Scholar]
- 51.Klareskog L, Gaubitz M, Rodriguez-Valverde V, Malaise M, Dougados M, Wajdula J. A long-term, open-label trial of the safety and efficacy of etanercept (Enbrel) in patients with rheumatoid arthritis not treated with other disease-modifying antirheumatic drugs. Ann Rheum Dis. 2006;65:1578–84. doi: 10.1136/ard.2005.038349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jansen JP, Pellissier J, Choy EH, Ostor A, Nash JT, Bacon P, et al. Economic evaluation of etoricoxib versus non-selective NSAIDs in the treatment of ankylosing spondylitis in the UK. Curr Med Res Opin. 2007;23:3069–78. doi: 10.1185/030079907X242575. [DOI] [PubMed] [Google Scholar]
- 53.Kamath CC, Kremers HM, Vanness DJ, O’Fallon WM, Cabanela RL, Gabriel SE. The cost-effectiveness of acetaminophen, NSAIDs, and selective COX-2 inhibitors in the treatment of symptomatic knee osteoarthritis. Value Health. 2003;6:144–57. doi: 10.1046/j.1524-4733.2003.00215.x. [DOI] [PubMed] [Google Scholar]
- 54.Losina E, Burbine SA, Suter LG, Hunter DJ, Solomon DH, Daigle ME, et al. Pharmacologic regimens for knee osteoarthritis prevention: can they be cost-effective? Osteoarthritis Cartilage. 2014;22:415–30. doi: 10.1016/j.joca.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katz JN, Smith SR, Collins JE, Solomon DH, Jordan JM, Hunter DJ, et al. Cost-effectiveness of nonsteroidal anti-inflammatory drugs and opioids in the treatment of knee osteoarthritis in older patients with multiple comorbidities. Osteoarthritis Cartilage. 2015 doi: 10.1016/j.joca.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hochberg MC. Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage. 2015;23(Suppl 1):S18–21. doi: 10.1016/j.joca.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Paxton EW, Namba RS, Maletis GB, Khatod M, Yue EJ, Davies M, et al. A prospective study of 80,000 total joint and 5000 anterior cruciate ligament reconstruction procedures in a community-based registry in the United States. J Bone Joint Surg Am. 2010;92(Suppl 2):117–32. doi: 10.2106/JBJS.J.00807. [DOI] [PubMed] [Google Scholar]
- 58.Medicare Fee Schedules. Centers for Medicare & Medicaid Services; 2014. [Google Scholar]
- 59.Medicare Hospital Outpatient Prospective Payment System. Centers for Medicare & Medicaid Services; 2014. [Google Scholar]
- 60.Gimbel JS, Kivitz AJ, Bramson C, Nemeth MA, Keller DS, Brown MT, et al. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain. 2014;155:1793–801. doi: 10.1016/j.pain.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Bramson C, Herrmann DN, Carey W, Keller D, Brown MT, West CR, et al. Exploring the Role of Tanezumab as a Novel Treatment for the Relief of Neuropathic Pain. Pain Med. 2015 doi: 10.1111/pme.12677. [DOI] [PubMed] [Google Scholar]
- 62.Red Book Online®. Truven Health Analytics Inc; 2015. [Google Scholar]
- 63.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–7. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 64.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–9. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 66.Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130:478–86. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- 67.Watson DJ, Rhodes T, Guess HA. All-cause mortality and vascular events among patients with rheumatoid arthritis, osteoarthritis, or no arthritis in the UK General Practice Research Database. J Rheumatol. 2003;30:1196–202. [PubMed] [Google Scholar]
- 68.Listing J, Kekow J, Manger B, Burmester GR, Pattloch D, Zink A, et al. Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFalpha inhibitors and rituximab. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-204021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nuesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Juni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. 2011;342:d1165. doi: 10.1136/bmj.d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katz JN, Mahomed NN, Baron JA, Barrett JA, Fossel AH, Creel AH, et al. Association of hospital and surgeon procedure volume with patient-centered outcomes of total knee replacement in a population-based cohort of patients age 65 years and older. Arthritis Rheum. 2007;56:568–74. doi: 10.1002/art.22333. [DOI] [PubMed] [Google Scholar]
- 71.Jenkins PJ, Clement ND, Hamilton DF, Gaston P, Patton JT, Howie CR. Predicting the cost-effectiveness of total hip and knee replacement: a health economic analysis. Bone Joint J. 2013;95-B:115–21. doi: 10.1302/0301-620X.95B1.29835. [DOI] [PubMed] [Google Scholar]
- 72.Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) Agency for Healthcare Research and Quality; 2012. [Accessed 2015 March 23]. http://hcupnet.ahrq.gov/ [Google Scholar]
- 73.Consumer Price Index (CPI) National Bureau of Labor Statistics; 2014. [Google Scholar]