Abstract
Autophagy is a lysosomal degradative pathway that functions to promote cell survival by supplying energy in times of stress or by removing damaged organelles and proteins after injury. The involvement of autophagy in the pathogenesis of nonalcoholic fatty liver disease (NAFLD) was first suggested by the finding that this pathway mediates the breakdown of intracellular lipids in hepatocytes and therefore may regulate the development of hepatic steatosis. Subsequent studies have demonstrated additional critical functions for autophagy in hepatocytes and other hepatic cell types such as macrophages and stellate cells that regulate insulin sensitivity, hepatocellular injury, innate immunity, fibrosis and carcinogenesis. These findings suggest a number of possible mechanistic roles for autophagy in the development of NALD and progression to NASH and its complications. The functions of autophagy in the liver, together with findings of decreased hepatic autophagy in association with conditions that predispose to NAFLD such as obesity and aging, suggest that autophagy may be a novel therapeutic target in this disease.
Keywords: autophagy, insulin sensitivity, liver injury, nonalcoholic fatty liver disease, oxidative stress, steatosis
Introduction
Autophagy is an intracellular pathway that targets and transports cellular components such as organelles and proteins to lysosomes for degradation. There are three types of autophagy - macroautophagy, chaperone-mediated autophagy (CMA) and microautophagy - which differ from each other in many respects including regulation, substrate capability and mechanism of substrate translocation into the lysosome. With macroautophagy entire cellular organelles such as mitochondria or lipid droplets, or large protein aggregates, are sequestered in a double membrane structure of unclear origin termed an autophagosome. The autophagosome fuses to a lysosome resulting in the degradation of the contents of the autophagosome by the hydrolytic enzymes of the lysosome [1]. CMA degrades soluble proteins containing a specific pentapeptide motif through binding to a chaperone protein for translocation to the lysosome and internalization after binding to the lysosome-associated membrane protein type 2A (LAMP-2A) [2]. After degradation of the cellular components in the lysosome, the breakdown products are released into the cytosol for reuse or metabolism into energy. All cells have basal levels of macroautophagy and CMA that are generally increased to cope with a variety of cellular stresses. However, it is now recognized that basal levels of autophagy are also functionally important [3]. The third form of autophagy, microautophagy, is not inducible and differs from the other two forms of autophagy in that it involves the direct uptake of substrates by invaginations of the lysosomal membrane. The function of this form of autophagy in the liver has yet to be delineated.
Our understanding of the functions of autophagy have expanded greatly from the original concept that this pathway only performs the two basic functions of maintenance of cellular energy homeostasis in times of limited nutrients and removal of damaged or aged cellular constituents [4–6]. Interestingly many of the more recently described functions of autophagy are highly relevant to the pathogenesis of nonalcoholic fatty liver disease (NAFLD) including the ability of macroautophagy to regulate cellular insulin sensitivity, metabolize cellular lipid stores, mediate hepatocyte resistance to injurious stimuli such as oxidants and cytokines and prevent over activation of the innate immune response. These functions of autophagy in pathways considered important to the development of NAFLD, the current direct evidence of the pathophysiological involvement of hepatic autophagy in NAFLD and its complications, and the possible therapeutic targeting of autophagy in this disease will be discussed.
Autophagy is Down Regulated under Conditions that Predispose to NASH
One of the factors driving the concept that autophagy plays a role in NAFLD pathophysiology is that autophagic function in decreased in the liver under several conditions that predispose to NASH. The prevalence of NASH increases with age in both mice [7] and humans [8], and with aging there is a loss of autophagic function. High fat diet (HFD)-induced and genetic obesity result in decreased macroautophagy [9, 10] and CMA [11] in the liver. Although reports exist of decreased autophagy in human NAFLD [12–14], there are currently no methods to determine the levels of autophagy in human liver samples. Measures of the numbers of autophagic vacuoles (autophagosomes or autolysosomes which are autophagosomes fused to lysosomes) by various forms of microscopy, or of protein levels for the integral autophagosome membrane protein microtubule-associated protein light chain 3 (LC3), simply indicate that there are altered steady-state numbers of autophagosomes which may result from either an increase or decrease in the level of autophagy. Levels of the p62/sequestome 1, a protein degraded by autophagy, are also inaccurate in measuring autophagic function due to possible concomitant proteasomal degradation and increased expression of this protein. Thus, it remains unclear at this time whether levels of autophagy are decreased in human NAFLD as has been demonstrated in rodent models.
The effect of obesity on autophagy in the liver differs from that in adipose tissue where macroautophagy is increased with obesity [15, 16]. The mechanisms of these divergent organ-specific effects on autophagic function in response to excess lipid accumulation need to be examined, but the implication is that there are distinct cell-specific effects of lipids on the autophagic machinery of the hepatocyte. A number of possible mechanisms for the inhibition of autophagy in fatty livers have been suggested including decreased expression of autophagy genes and reduced levels of degradative lysosomal enzymes [17], but the most important cause is likely impaired fusion of the autophagosome with the lysosome [18, 19]. Hyperinsulinemia may also contribute to the hepatic defect in autophagy as the ability of insulin to suppress autophagy in mouse liver is retained despite the presence of hepatic insulin resistance, indicating that hyperinsulinemia might contribute to impaired autophagy in the diabetic/obese state [20]. In NAFLD a self-perpetuating cycle might therefore exist in which hepatic lipid accumulation promotes insulin resistance, and both of these events impair autophagy, further worsening steatosis and insulin insensitivity. Findings of decreased autophagy in conditions that predispose for NASH such as aging and obesity support the possibility that decreased autophagic function may contribute to the pathophysiology of NASH.
Lipophagy Regulates Hepatocellular Fat Accumulation
A defining feature of NAFLD is steatosis which is the excessive accumulation of triglycerides (TGs) and cholesterol in lipid droplets in hepatocytes [21, 22]. Whether steatosis is a benign and distinct process, or a critical initial stage of disease that must occur prior to the development of inflammation, hepatocyte injury and the eventual fibrosis that characterizes nonalcoholic steatohepatitis (NASH), is uncertain [23–25]. The presence of peripheral insulin resistance in NAFLD increases the flow of serum free fatty acids (FFAs) to the liver where they are taken up and stored, but the other mechanisms that underlie the abnormal lipid accumulation in NAFLD remain unclear.
The initial finding that prompted interest in the involvement of autophagy in NAFLD was that macroautophagy mediates hepatocyte metabolism of intracellular lipids. Although endogenous lipids had not been described as a substrate for autophagy, the regulatory and functional similarities between lipolysis and macroautophagy prompted an examination for a potential role for autophagy in the degradation of hepatic lipids [26]. Both a pharmacological inhibition of autophagy and genetic knockdown of the autophagy gene Atg5 increased lipid content in cultured hepatocytes in response to lipid challenges such as FFA supplementation [9]. Fluorescence microscopy confirmed that with a decrease in autophagy lipid accumulated in lipid droplets, and that lipids trafficked through the autophagic pathway in hepatocytes. The inhibition of macroautophagy decreased the lipolytic breakdown of TGs and cholesterol from lipid droplets leading to increased steatosis after a lipid challenge. A reduction in hepatocyte mitochondrial fatty acid β-oxidation also occurred presumably secondary to the reduced supply of FFAs, a finding which has important implications for the maintenance of hepatic energy homeostasis during any form of stress.
Studies of mouse livers confirmed the in vivo association of the autophagosome-associated protein LC3 with lipid droplets, and the presence of lipid and lipid droplet proteins in autophagosomes [9]. In response to fasting the numbers of autophagic vacuoles containing lipid cargo increased, indicating the ability of autophagy to specifically target lipids, a process termed lipophagy. The ability of alterations in hepatocellular autophagic function to modulate the development of steatosis was demonstrated by findings in hepatocyte specific Atg7 knockout mice that inhibition of macroautophagy led to marked increases in hepatic TG and cholesterol content. Together these findings identified an essential function for macroautophagy in the regulation of hepatocellular lipid stores through the metabolism of stored lipids, and suggested that an impairment in this pathway may be a mechanism for the development of hepatic steatosis. Further support for this concept was provided by findings that autophagy also regulates steatosis in alcohol-induced fatty liver [27]. Other mechanisms by which macroautophagy may regulate lipid content likely exist as for example through the degradation of factors that mediate lipid metabolism as has been reported for apoprotein-B [28].
A number of subsequent studies have also supported the fact that an inhibition of hepatocyte macroautophagy results in excessive liver lipid accumulation. Knockdown of Atg14 increased hepatic TGs [29] as did knockout of the positive transcriptional regulator of autophagy transcription factor EB (TFEB) [30]. Adenoviral overexpression of Atg7 or Atg14 successfully reversed hepatic steatosis in genetic obesity models [10, 29]. Thus, considerable evidence among different autophagy genes and different rodent models of NAFLD support the concept that a decrease in autophagy promotes hepatic steatosis. However, other studies have failed to find increased steatosis, or even reported decreased steatosis, when hepatocyte macroautophagy is inhibited. These inconsistences may in part be due to different genetic models, diverse dietary manipulations, varying levels of macroautophagy inhibition or compensatory up regulation of CMA, or experimental conditions such as the age of the mice. Another report in mice with a hepatocyte-specific knockout of Atg7 reported decreased steatosis after an unspecified period of high fat diet (HFD) feeding, although quantitative lipid studies were not performed [31]. Hepatocyte deletion of focal adhesion family kinase-interacting protein of 200 kDa (FIP200), a core component of the complex initiating autophagosome formation, also reduced hepatic TG content after the induction of hepatic steatosis by starvation or HFD feeding [32]. These findings may be explained by the failure to sufficiently block lipophagy as FIP200 knockout mice actually had increased rather than decreased LC3-II levels. FIP200 deficiency affected many genes involved in lipid metabolism suggesting that loss of FIP200 may have altered lipid metabolism through off-target effects not on autophagy. Thus, considerable evidence supports the fact that factors that decrease hepatocyte macroautophagy promote steatosis in murine NAFLD and therefore potentially in the human disease, although controversy still exists and the area requires further study.
Alternative mechanisms by which autophagy may regulate hepatocyte lipid metabolism have been demonstrated for CMA. Mice with an inhibition of CMA due to a knockout of LAMP-2A developed hepatic steatosis in association with abnormalities in carbohydrate and lipid metabolism secondary to the failure to degrade certain metabolic enzymes by CMA [33]. Knockout mice did not develop the phenotype of the metabolic syndrome and in fact displayed decreased body and adipose mass. A second mechanism of CMA involvement in steatosis may be through effects on the degradation of proteins that coat the surface of the lipid droplet. Although the studies were all conducted in fibroblasts, two critical lipid droplet proteins, perilipin 2 (PLIN2) and perilipin 3 (PLIN3), were identified as substrates that are degraded by CMA prior to the start of lipolysis [34]. This degradation promoted the association of adipose triglyceride lipase and macroautophagy proteins to the lipid droplets for the purpose of initiating lipolysis. Blocking CMA in cultured cells and mouse liver led to reduced association of adipose triglyceride lipase and macroautophagy-related proteins with lipid droplets, a subsequent decrease in lipid oxidation and the accumulation of lipid droplets in cells. The overall contribution to this mechanism of CMA-dependent lipid metabolism versus the effects of CMA on the degradation of metabolic enzymes in CMA-knockout mice was not defined. These findings suggest that decreases in either macroautophagy or CMA have the potential to promote the development of steatosis.
Macroautophagy Maintains Insulin Sensitivity
In humans insulin resistance is strongly associated with NAFLD development. Peripheral insulin resistance triggers an elevated rate of lipolysis in adipose tissue leading to increased serum FFAs that are taken up by the liver and incorporated into lipid droplet-stored TGs. As previously discussed, the increased inhibition of autophagy by hyperinsulinemia may worsen this effect because steatosis resulting from decreased autophagy may promote hepatic insulin resistance that increases hepatic glucose production.
In addition to the direct effects of lipophagy on hepatocyte steatosis, the decrease in macroautophagy that occurs in obesity has been mechanistically linked to hyperinsulinemia and insulin resistance [10, 20]. Yang et al. [10] demonstrated reduced hepatic autophagic function in hyperinsulinemic, obese HFD-fed and ob/ob mice as reflected in decreased amounts of LC3-II, increased levels of p62 and decreased numbers of autophagosomes/autolysosomes by electron microscopy [20]. A functional impairment of autophagy was demonstrated by a failure of the liver to up regulate macroautophagy with starvation. Hepatic expression of autophagy genes including Atg5 and Atg7 was decreased, suggesting that this effect was the mechanism of decreased autophagy. In cultured hepatocytes and mouse liver an inhibition of autophagy decreased insulin-induced cell signaling in association with increased endoplasmic reticulum (ER) stress. Importantly viral expression of the autophagy gene Atg7 reduced ER stress and improved insulin sensitivity [10]. ER stress has been implicated in the pathogenesis of NASH and these findings suggest that decreased hepatic autophagy in obesity may lead to ER stress due to a reduced ability to remove damaged proteins. Increased ER stress in the presence of decreased macroautophagy may promote insulin resistance as well as other events such as hepatocellular injury. However, direct evidence that ER stress mediates these effects in the setting of decreased autophagy is needed to fully prove this hypothesis. Moreover, other studies of the livers of HFD-induced obese mice have failed to reveal significant decreases in autophagy genes, and more recent studies attribute the defect in hepatic autophagy in obesity to defective autophagosome/lysosome fusion [18, 19], as previously discussed. Nonetheless, these studies provide convincing evidence that defects in autophagy with obesity promote steatohepatitis and reversing the defect in hepatic autophagy can have beneficial effects on the hepatic manifestations of the metabolic syndrome.
Involvement in Hepatocyte Injury
The mechanisms that underlie the progression from simple steatosis to NASH with hepatocyte injury and death, inflammation and fibrosis remain unclear, but may involve cellular injury from factors such as oxidative stress [35] and inflammatory cytokines [24]. Multiple functions of autophagy that mediate cellular resistance to death have been demonstrated that are relevant to NASH because they modulate injury from factors and pathways thought to be involved in hepatocyte injury in NASH. Autophagy is known to regulate cell death pathways including those triggered by oxidants and tumor necrosis factor (TNF) which may mediate NASH injury. In addition, autophagy may reduce injury in NASH by removing damaged organelles or proteins that contribute to cellular dysfunction. Finally, there is crosstalk between autophagy and the apoptotic cell death pathway, and components of the two pathways interact or have dual functions [36, 37]. Numerous potential mechanisms therefore exist by which autophagy may protect against cellular injury in NASH.
Evidence exists from a series of in vitro and in vivo studies that autophagy is a critical pathway for hepatocyte resistance to a number of death stimuli relevant to the pathogenesis of NASH. A hallmark of NASH is the accumulation of abnormal mitochondria suggesting that the inadequate removal of damaged mitochondria may contribute to this disease [38]. Mitophagy, a selective form of macroautophagy that degrades mitochondria, is particularly important in hepatocytes because their lack of proliferation results in a long life span that leads to the excessive accumulation of many cellular components including mitochondria. The impairment in hepatic autophagy that occurs in association with a fatty liver may lead to a failure to adequately remove damaged mitochondria, particularly ones in which activation of the mitochondrial death pathway has occurred, and cause oxidative stress or the release of mitochondrial factors that trigger apoptosis in NASH [39]. Cultured hepatocytes with a knockdown of Atg5 to inhibit macroautophagy are sensitized to death from superoxide-mediated oxidative stress [40]. In these studies knockout cells became depleted of ATP and released increased mounts of mitochondrial cytochrome c, consistent with the necessity for macroautophagy to remove damaged mitochondria and maintain mitochondrial ATP levels in response to oxidant stress. However, sensitization to death from oxidative stress was not mediated completely by direct effects on mitochondria as death was partly the result of over activation of the upstream c-Jun N-terminal kinase (JNK)/c-Jun signaling pathway. Increased JNK signaling from oxidative stress and impaired autophagy may promote disease in NAFLD as hepatic JNK over activation underlies NASH development and progression [41]. The mechanism by which macroautophagy selectively targets the JNK pathway remains to be determined. These investigations also demonstrated a role for CMA in hepatocyte resistance to oxidant stress as a knockdown of the CMA receptor LAMP-2A sensitized hepatocytes to death from menadione through a mechanism distinct from that for macroautophagy [40]. The mechanism of CMA’s protective effect was not determined but may be through the removal of damaging, oxidized proteins. Studies are needed to examine the specific role of autophagy in oxidant stress generated in the setting of hepatocyte steatosis.
Another potential mechanism by which autophagy may protect against liver injury in NAFLD is through inhibition of hepatocyte death receptor pathways. Macroautophagy prevents death receptor-mediated apoptosis from TNF and Fas, two factors implicated in hepatocellular injury in NASH [42, 43]. Mouse fibroblasts lacking Atg5 are sensitized to death from both Fas and TNF [44]. Mice with a hepatocyte-specific knockout of Atg5 have increased TNF-dependent liver injury in the setting of hepatotoxin exposure [45]. Finally, as previously discussed, ER stress is associated with insulin resistance and NASH, and macroautophagy has been reported to protect against cell death from ER stress [10, 46].
Whether these mechanisms of autophagy-induced protection against cellular injury function in NAFLD is unclear as limited investigations have been conducted on the effects of hepatocyte autophagy on liver injury in rodent NAFLD models. When mice with a knockout of the autophagy gene Fip200 were fed a HFD, increased liver injury and fibrosis developed as compared to control mice [32]. These findings support the concept that hepatocyte autophagy provides resistance to liver injury in NAFLD and may protect against the progression to NASH. However, hepatocyte autophagy gene knockout mice develop profound, spontaneous liver injury with a normal diet because of the excessive accumulation of damaged and toxic cellular components in these non-dividing cells. Whether steatotic hepatocytes are any more sensitive to injury and death with a loss of macroautophagy is therefore still uncertain and requires further study with inducible hepatocyte autophagy knockout models.
Macrophage Autophagy Regulates the Innate Immune Response in NAFLD
Although initial studies of autophagy in the liver focused on the role of this pathway in hepatocytes, recent investigations have expanded our understanding of the functions of this pathway into other hepatic cell types such as macrophages. A central mechanism of many of the manifestations of the metabolic syndrome is a systemic over activation of the innate immune response that results in multi-organ inflammation [47]. Macroautophagy was initially implicated in the regulation of innate immunity through the ability of autophagy to sequester microorganisms and prevent them from initiating an inflammatory response. Subsequently macroautophagy was found to directly regulate the innate immune response induced by pathogen recognition receptors such as the toll-like receptors (TLRs) [48]. A number of TLR ligands are thought to be involved in the pathogenesis of NASH including saturated fatty acids and lipopolysaccharide (LPS). Atg16L1, an autophagy gene associated with susceptibility to inflammatory bowel disease, has been demonstrated to regulate LPS-induced intestinal inflammation. With a reduction in autophagy, macrophages have increased activation of the inflammasome, an intracellular structure that cleaves the proforms of the proinflammatory cytokines IL-1β and IL-18 into their active, secreted forms [49]. Mice lacking hematopoietic cell Atg16L1 develop increased dextran sulphate-induced inflammatory colitis mediated by these two cytokines [49]. LPS is a TLR4 ligand and both LPS and TLR4 have been implicated in the pathogenesis of NASH [50, 51]. These findings suggested the possibility that defects in macrophage macroautophagy may therefore amplify hepatic steatosis and/or liver injury. Interestingly LPS-induced TLR signaling stimulates the autophagic response [52], suggesting that the role for TLR signaling in NASH may be complex with both beneficial and detrimental effects.
Recent investigations have demonstrated an important anti-inflammatory role for macrophage autophagy in nonalcoholic fatty liver disease, but through a different mechanism than reported in the intestine [53]. Initially the studies demonstrated that in HFD-induced obesity macroautophagy was decreased in macrophages, indicating the relevance of studies of impaired macrophage autophagy to the pathophysiology of the end organ manifestations of obesity such as NASH. Mice with a myeloid cell specific knockout of Atg5 fed a HFD and injected with low-dose LPS for 2 weeks developed increased systemic and hepatic inflammation in the absence of any change in the numbers of inflammatory cells in the liver. Surprisingly the knockout mice had systemic increases in proinflammatory cytokines such as TNF and IL-6, but not in IL-1β as had been reported in the intestine. The mechanism of this increase in inflammation was that hepatic macrophages with decreased macroautophagy had both increased polarization into proinflammatory M1 macrophages and decreased polarization into anti-inflammatory M2 macrophages [53]. Decreased macrophage autophagy, as occurs with obesity, led therefore to over activation of the hepatic innate immune response through effects on macrophage polarization pathways. This effect promoted liver injury as HFD-fed, LPS-treated knockout mice had increased liver injury although the amount of steatosis was unaffected. This mechanism of increased inflammation was specific for the liver as the decrease in macrophage autophagy failed to affect the extent of inflammation in adipose tissue. In addition, in a toxin-induced model of acute liver injury decreased macrophage autophagy also led to increased liver injury but secondary to toxicity from elevated amounts of IL-1β generated by increased inflammasome activation [54], similar to what had been reported in the intestinal inflammatory model [49]. Therefore in the fatty liver distinct mechanisms lead to over activation of the innate immune response in macrophages with defective autophagy. Individual variation in the levels of macrophage macroautophagy may be a determinant of whether progression from simple steatosis to steatohepatitis occurs in patients with NAFLD.
Autophagy in Hepatic Stellate Cells Modulates Hepatic Fibrogenesis
Another hepatic cell type in which recent investigations have demonstrated an important function for autophagy in liver disease is the hepatic stellate cell. The most important determinant of the clinical outcome in patients with NASH is the extent of fibrosis that results from activated hepatic myofibroblasts producing excessive extracellular matrix. Quiescent stellate cell’s lipid stores, primarily in the form of vitamin A, are lost during activation which suggested the possibility that lipophagy may function in this lipid break down to mediate the transdifferentiation process. Recent studies have confirmed that macroautophagy mediates hepatic stellate cell activation and the development of hepatic fibrosis [55, 56]. Toxin-induced hepatic fibrosis led to increased levels of macroautophagy in stellate cells [55]. A knockdown of autophagy decreased stellate cell activation which was restored by supplementation with the fatty acid oleate. This effect of fatty acid supplementation indicates that it is specifically lipophagy that mediates stellate cell activation by autophagy, probably by supplying substrates for the energy needed to drive activation [55]. A stellate cell-specific inhibition of autophagy decreased the development of fibrosis in vivo [55]. In contrast to the detrimental effects that occur from the decrease in autophagy in hepatocytes and macrophages in obesity, it is possible that increases in hepatic stellate cell autophagy may promote the complication of fibrosis in NASH. Levels of hepatic stellate cell autophagy in NAFLD models, and the role of autophagy in NASH fibrosis, have to be examined to determine whether autophagy functions in this manner in fatty livers, but these findings raise the possibility that therapies to increase autophagy may promote fibrosis in this disease.
Decreased autophagy in macrophages has also been shown to promote fibrosis in the chronic carbon tetrachloride model [57]. In response to this toxin, the macrophage autophagy deficient mice discussed above had a greater inflammatory response with amplified IL-1α and IL-1β generation that resulted in an increase in the numbers of activated myofibroblasts and fibrosis. Knockout macrophages promoted myofibroblast activation in vitro by an IL-1-dependent mechanism, and in knockout mice fibrosis was reduced by IL-1 neutralization. These studies provide additional evidence of the importance of macrophage autophagy in regulating hepatic inflammation, and may be relevant to fibrosis in NASH in which inflammasome activation and IL-1β are known to have important biological effects.
Autophagy and Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is a well-recognized late complication of NASH, but the factors that promote malignant transformation are unclear [58]. Macroautophagy has been reported to have dual effects on carcinogenesis. Current evidence suggests that autophagy prevents tumor initiation by blocking cellular injury or promoting the removal of damaged cells, but that autophagy in cancer cells promotes tumor progression by supporting their high metabolic needs [59]. A number of animal studies in which hepatocyte macroautophagy has been inhibited have resulted in the development of hepatic tumors. Haploinsufficiency for the autophagy gene Beclin 1 in mice leads to the spontaneous development of liver tumors [60, 61]. Consistent with the mouse studies, loss of Beclin 1 is frequent in human HCC and correlates with a bad prognosis [62]. Mouse hepatocyte knockouts of Atg5 and Atg7 also lead to the development of large numbers of hepatic adenomas but not HCCs [63], supporting the importance for macroautophagy in preventing hepatic tumor initiation. However these findings must be interpreted with caution as a knockout of Atg5 or Atg7 in hepatocytes leads to the development of marked liver injury and compensatory proliferation, either of which by themselves may trigger malignant transformation. A mechanism of this tumor promotion is an increase in the autophagy-degraded adaptor protein p62 which leads to over activation of nuclear factor erythroid 2-related factor 2 (Nrf2) that up regulates antioxidant enzymes and drug efflux pumps that promote tumor survival and growth [64, 65]. None of the current animal studies have specifically addressed the development of HCC in NAFLD. With the exception of contrasts of the levels of autophagy genes in tumors versus normal liver tissue, little is known about autophagy in human HCC. Current evidence therefore suggests that the decrease in hepatocyte autophagy that occurs with steatosis may promote tumor initiation, but other factors in this disease likely mediate progression to HCC. Completely unstudied is the potential importance of autophagy in hepatic tumor macrophages that may modulate HCC development.
Targeting Autophagy as a Therapy for NASH
The involvement of autophagy in many cellular pathways relevant to NAFLD pathophysiology, and the decrease in autophagic function that occurs with a fatty liver, suggest that agents to increase hepatic autophagy may be effective in the treatment of this disease. In addition, the hepatic effects of autophagy may explain how some current interventions or established prognostic factors for NAFLD affect the outcome in this disease. The potential therapeutic effects of an increase in autophagy include: (1) improved insulin sensitivity; (2) reduced hepatocyte lipid accumulation; (3) cytoprotection against cytokine- and oxidant-induced injury; and (4) decreased macrophage activation that limits inflammation. These beneficial effects must be weighed against some suggestions that a global increase in autophagy might have negative effects as well by promoting hepatic stellate cell activation and fibrosis. However, the effects of reducing liver injury and inflammation would likely have a more potent anti-fibrotic effect than any direct effect that increasing hepatic stellate cell autophagy has on myofibroblast activation. Another consideration is that depending on the mechanism of action of the therapeutic agent, it may not effectively overcome the blockage in autophagic function. For example, if the defect in autophagy in fatty livers exists at the level of autophagosome/lysosome fusion, then agents that target upstream factors may not be able to overcome the block and effectively up regulate autophagic function.
The effects of several interventions already employed in NAFLD therapy, or known to influence the incidence of NASH, have been demonstrated to alter levels of autophagy. Weight loss is a treatment for obese patients with NASH, and modest weight reduction from dieting can significantly improve liver transaminases. Nutrient deprivation is the prime inducer of autophagy so it is possible that part of the effect of caloric restriction is through an increase in autophagy. Extended periods of fasting may be particularly effective to induce autophagy. Increased exercise is usually part of any weight loss regiment and exercise itself induces autophagy [66]. Coffee drinking is associated with a reduced prevalence of NASH [67], and caffeine protects against NASH in mice by increasing lipophagy and mitochondrial β-oxidation [68]. Thus, it is possible that the induction of autophagy by caffeine mediates coffee’s beneficial effect in NASH. Thyroid hormone induces hepatic lipophagy [69], which may be the mechanism for the association of increased NAFLD in hypothyroidism [70]. Similarly the association of hypovitaminosis D with NAFLD [71] might be explained by the ability of vitamin D to induce autophagy [72]. Changes in autophagy might therefore be the mechanism of the effects of a number of factors that have already been established to treat NAFLD or affect its prevalence.
Several drugs already in use in humans for other indications increase autophagy and have the potential to treat NASH through this mechanism. Recently carbamazepine, a commonly used anticonvulsant in humans, was demonstrated to be beneficial in the treatment of a murine model of α1-antitrypsin deficiency, a disease in which defects in autophagy are postulated to promote the progression to liver disease [73]. This drug was subsequently shown to significantly limit both rodent NAFLD and alcoholic fatty liver disease [74]. The potential anti-diabetic agent glucagon-like peptide 1 (GLP-1) may be effective in NASH in part due to its promotion of macroautophagy [75]. Calcium channel blockers have been reported to reverse murine NASH by restoring autophagosome-lysosome fusion which is disrupted by altered calcium homeostasis [19], although human studies on the effects of these agents on diabetes have led to mixed conclusions about the effectiveness of these drugs on the metabolic syndrome. With the significant interest in developing agents to increase autophagy in many diseases, additional agents may soon be available that are even more effective inducers of autophagy and potentially useful for the treatment of NAFLD.
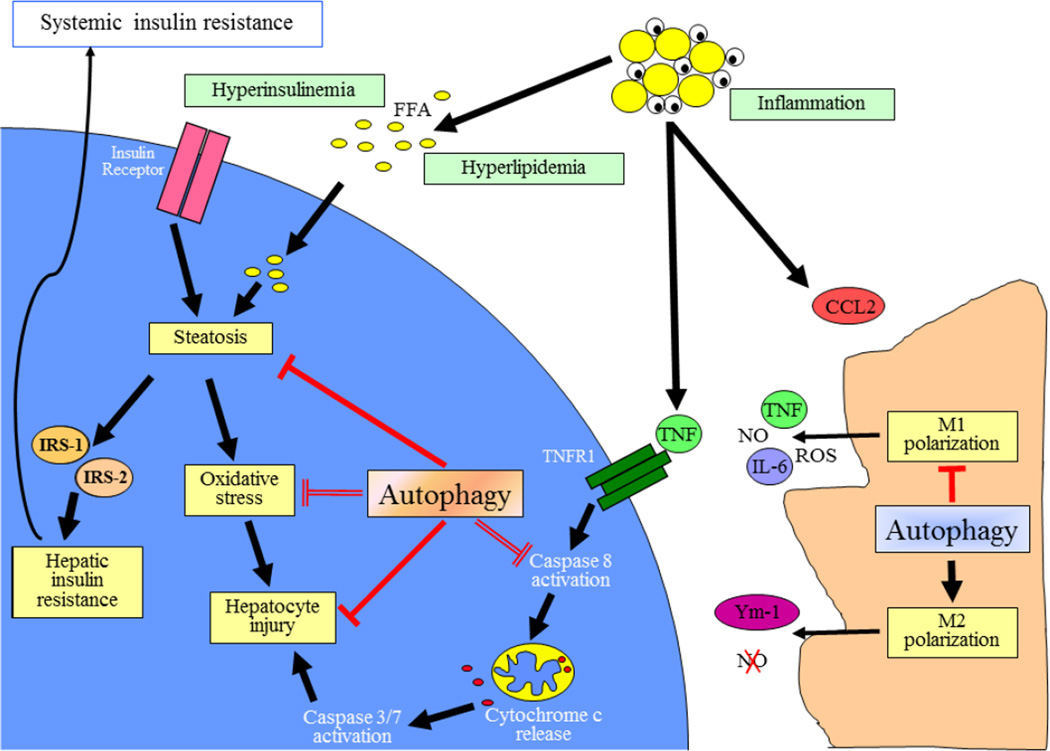
Fig. 1.
Potential roles of autophagy in NAFLD pathogenesis. In the setting of peripheral insulin resistance, increased lipolysis in adipose tissue elevates levels of serum FFAs that are then taken up and stored by hepatocytes leading to steatosis. This excessive lipid accumulation promotes hepatic insulin resistance and oxidative stress. Adipose tissue inflammation produces cytokines such as TNF and chemokine (C-C motif) ligand 2 (CCL2) that can cause hepatocyte toxicity and trigger a hepatic innate immune response, respectively. Activated M1 macrophages produce additional cytokines and other cytotoxic factors such as nitric oxide (NO) and reactive oxygen species (ROS), whereas the products of anti-inflammatory M2 macrophages mediate injury resolution. In hepatocytes autophagy may function to limit steatosis through the effects of lipophagy, and limit cellular injury from factors such as oxidant stress and TNF cytotoxicity. In macrophages autophagy functions to limit inflammation by decreasing M1 and increasing M2 macrophage polarization. With the decrease in autophagy that occurs in both hepatocytes and macrophages with obesity, reduced levels of autophagic function may lead to increased steatosis, hepatocellular injury and inflammation in NAFLD. Inhibitory pathways are shown in red.
Key Findings.
Autophagy is a lysosomal degradative pathway of which two types, macroautophagy and CMA, regulate a number of critical cellular functions that are relevant to the pathophysiological events underlying NAFLD.
Levels of autophagy in the liver are decreased in conditions that predispose to the development of NAFLD such as obesity and aging, suggesting that the loss of autophagic function may be involved in the pathogenesis of NAFLD.
Autophagy has important metabolic effects including the promotion of insulin sensitivity and the degradation of intracellular lipids that may regulate the development of steatosis.
Macroautophagy and CMA mediate cellular resistance to death from many types of injurious stimuli such as oxidants and TNF suggesting a role for autophagy in preventing the progression to liver injury in NAFLD.
The decrease in macrophage autophagy that occurs with obesity promotes a more proinflammatory phenotype through effects on macrophage polarization that may be a mechanism for the over activation of innate immunity in NASH.
The existence of impaired autophagic function in NAFLD, and the potential beneficial effects of autophagy in preventing steatosis, hepatocyte injury and inflammation, suggest that therapies designed to increase autophagy may be an effective treatment for this disease.
Acknowledgments
Supported by NIH grants R01DK061498 and R01AA022601.
Abbreviations
- CMA
chaperone-mediated autophagy
- FFA
free fatty acid
- HCC
hepatocellular carcinoma
- HFD
high fat diet
- FIP200
focal adhesion family kinase-interacting protein of 200 kDa
- JNK
c-Jun N-terminal kinase
- LAMP-2A
lysosome-associated membrane protein type 2A
- LC3
microtubule-associated protein light chain 3
- LPS
lipopolysaccharide
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- TFEB
transcription factor EB
- TG
triglyceride
- TLR
toll-like receptor
- TNF
tumor necrosis factor
References
- 1.Mehrpour M, Esclatine A, Beau I, Codogno P. Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview. Am J Physiol Cell Physiol. 2010;298:C776–C785. doi: 10.1152/ajpcell.00507.2009. [DOI] [PubMed] [Google Scholar]
- 2.Orenstein SJ, Cuervo AM. Chaperone-mediated autophagy: molecular mechanisms and physiological relevance. Semin Cell Dev Biol. 2010;21:719–726. doi: 10.1016/j.semcdb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 4.Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Nutrition. 2006;22:830–844. doi: 10.1016/j.nut.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Iwata J, Ezaki J, Komatsu M, et al. Excess peroxisomes are degraded by autophagic machinery in mammals. J Biol Chem. 2006;281:4035–4041. doi: 10.1074/jbc.M512283200. [DOI] [PubMed] [Google Scholar]
- 6.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontana L, Zhao E, Amir M, Dong H, Tanaka K, Czaja MJ. Aging promotes the development of diet-induced murine steatohepatitis but not steatosis. Hepatology. 2013;57:995–1004. doi: 10.1002/hep.26099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noureddin M, Yates KP, Vaughn IA, et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58:1644–1654. doi: 10.1002/hep.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Navarro JA, Kaushik S, Koga H, et al. Inhibitory effect of dietary lipids on chaperone-mediated autophagy. Proc Natl Acad Sci U S A. 2012;109:E705–E714. doi: 10.1073/pnas.1113036109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuo Y, Yamashina S, Sonoue H, et al. Abnormality of autophagic function and cathepsin expression in the liver from patients with non-alcoholic fatty liver disease. Hepatol Res. 2014;44:1026–1036. doi: 10.1111/hepr.12282. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Rodriguez A, Mayoral R, Agra N, et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014;5:e1179. doi: 10.1038/cddis.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashima J, Shintani-Ishida K, Nakajima M, et al. Immunohistochemical study of the autophagy marker microtubule-associated protein 1 light chain 3 in normal and steatotic human livers. Hepatol Res. 2014;44:779–787. doi: 10.1111/hepr.12183. [DOI] [PubMed] [Google Scholar]
- 15.Jansen HJ, van Essen P, Koenen T, et al. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology. 2012;153:5866–5874. doi: 10.1210/en.2012-1625. [DOI] [PubMed] [Google Scholar]
- 16.Kovsan J, Bluher M, Tarnovscki T, et al. Altered autophagy in human adipose tissues in obesity. J Clin Endocrinol Metab. 2011;96:E268–E277. doi: 10.1210/jc.2010-1681. [DOI] [PubMed] [Google Scholar]
- 17.Czaja MJ. Functions of autophagy in hepatic and pancreatic physiology and disease. Gastroenterology. 2011;140:1895–1908. doi: 10.1053/j.gastro.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koga H, Kaushik S, Cuervo AM. Altered lipid content inhibits autophagic vesicular fusion. FASEB J. 2010;24:3052–3065. doi: 10.1096/fj.09-144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park HW, Park H, Semple IA, et al. Pharmacological correction of obesity-induced autophagy arrest using calcium channel blockers. Nature Comm. 2014;5:4834. doi: 10.1038/ncomms5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu HY, Han J, Cao SY, et al. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem. 2009;284:31484–31492. doi: 10.1074/jbc.M109.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung O, Sanyal AJ. Abnormalities of lipid metabolism in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:351–359. doi: 10.1055/s-0028-1091979. [DOI] [PubMed] [Google Scholar]
- 22.Cusi K. Role of insulin resistance and lipotoxicity in non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:545–563. doi: 10.1016/j.cld.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Day CP, James OF. Steatohepatitis: a tale of two"hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 24.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalor PF, Faint J, Aarbodem Y, Hubscher SG, Adams DH. The role of cytokines and chemokines in the development of steatohepatitis. Semin Liver Dis. 2007;27:173–193. doi: 10.1055/s-2007-979470. [DOI] [PubMed] [Google Scholar]
- 26.Czaja MJ. Autophagy in health and disease. 2. Regulation of lipid metabolism and storage by autophagy: pathophysiological implications. Am J Physiol Cell Physiol. 2010;298:C973–C978. doi: 10.1152/ajpcell.00527.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding WX, Li M, Chen X, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan M, Maitin V, Parathath S, et al. Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proc Natl Acad Sci U S A. 2008;105:5862–5867. doi: 10.1073/pnas.0707460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong X, Tao R, DePinho RA, Dong XC. The autophagy-related gene 14 (Atg14) is regulated by forkhead box O transcription factors and circadian rhythms and plays a critical role in hepatic autophagy and lipid metabolism. J Biol Chem. 2012;287:39107–39114. doi: 10.1074/jbc.M112.412569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Settembre C, De Cegli R, Mansueto G, et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013;15:647–658. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim KH, Jeong YT, Oh H, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19:83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 32.Ma D, Molusky MM, Song J, et al. Autophagy deficiency by hepatic FIP200 deletion uncouples steatosis from liver injury in NAFLD. Mol Endocrinol. 2013;27:1643–1654. doi: 10.1210/me.2013-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider JL, Suh Y, Cuervo AM. Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation. Cell Metab. 2014;20:417–432. doi: 10.1016/j.cmet.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015;17:759–770. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497–1502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 36.Yousefi S, Perozzo R, Schmid I, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 37.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Caldwell SH, Swerdlow RH, Khan EM, et al. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31:430–434. doi: 10.1016/s0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 39.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Singh R, Xiang Y, Czaja MJ. Macroautophagy and chaperone-mediated autophagy are required for hepatocyte resistance to oxidant stress. Hepatology. 2010;52:266–277. doi: 10.1002/hep.23645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 43.Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Singh R, Massey AC, et al. Loss of macroautophagy promotes or prevents fibroblast apoptosis depending on the death stimulus. J Biol Chem. 2008;283:4766–4777. doi: 10.1074/jbc.M706666200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amir M, Zhao E, Fontana L, et al. Inhibition of hepatocyte autophagy increases tumor necrosis factor-dependent liver injury by promoting caspase-8 activation. Cell Death Differ. 2013;20:878–887. doi: 10.1038/cdd.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogata M, Hino S, Saito A, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maher JJ, Leon P, Ryan JC. Beyond insulin resistance: Innate immunity in nonalcoholic steatohepatitis. Hepatology. 2008;48:670–678. doi: 10.1002/hep.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saitoh T, Akira S. Regulation of innate immune responses by autophagy-related proteins. J Cell Biol. 2010;189:925–935. doi: 10.1083/jcb.201002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saitoh T, Fujita N, Jang MH, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 50.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 52.Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–144. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu K, Zhao E, Ilyas G, et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy. 2015;11:271–284. doi: 10.1080/15548627.2015.1009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ilyas G, Zhao E, Liu K, et al. Macrophage autophagy limits acute toxic liver injury in mice through down regulation of interleukin-1beta. J Hepatol. 2016;64:118–127. doi: 10.1016/j.jhep.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142:938–946. doi: 10.1053/j.gastro.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thoen LF, Guimaraes EL, Dolle L, et al. A role for autophagy during hepatic stellate cell activation. J Hepatol. 2011;55:1353–1360. doi: 10.1016/j.jhep.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Lodder J, Denaes T, Chobert MN, et al. Macrophage autophagy protects against liver fibrosis in mice. Autophagy. 2015;11:1280–1292. doi: 10.1080/15548627.2015.1058473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 59.White E. The role for autophagy in cancer. J Clin Invest. 2015;125:42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qu X, Yu J, Bhagat G, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding ZB, Shi YH, Zhou J, et al. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. 2008;68:9167–9175. doi: 10.1158/0008-5472.CAN-08-1573. [DOI] [PubMed] [Google Scholar]
- 63.Takamura A, Komatsu M, Hara T, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inami Y, Waguri S, Sakamoto A, et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol. 2011;193:275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ni HM, Woolbright BL, Williams J, et al. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J Hepatol. 2014;61:617–625. doi: 10.1016/j.jhep.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molloy JW, Calcagno CJ, Williams CD, Jones FJ, Torres DM, Harrison SA. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology. 2012;55:429–436. doi: 10.1002/hep.24731. [DOI] [PubMed] [Google Scholar]
- 68.Sinha RA, Farah BL, Singh BK, et al. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology. 2014;59:1366–1380. doi: 10.1002/hep.26667. [DOI] [PubMed] [Google Scholar]
- 69.Sinha RA, You SH, Zhou J, et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J Clin Invest. 2012;122:2428–2438. doi: 10.1172/JCI60580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liangpunsakul S, Chalasani N. Is hypothyroidism a risk factor for non-alcoholic steatohepatitis? J Clin Gastroenterol. 2003;37:340–343. doi: 10.1097/00004836-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 71.Barchetta I, Angelico F, Del Ben M, et al. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med. 2011;9:85. doi: 10.1186/1741-7015-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campbell GR, Spector SA. Hormonally active vitamin D3 (1α,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J Biol Chem. 2011;286:18890–18902. doi: 10.1074/jbc.M110.206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hidvegi T, Ewing M, Hale P, et al. An autophagy-enhancing drug promotes degradation of mutant α1-antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329:229–232. doi: 10.1126/science.1190354. [DOI] [PubMed] [Google Scholar]
- 74.Lin CW, Zhang H, Li M, et al. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol. 2013;58:993–999. doi: 10.1016/j.jhep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One. 2011;6:e25269. doi: 10.1371/journal.pone.0025269. [DOI] [PMC free article] [PubMed] [Google Scholar]