Summary
Hypoxia-Inducible Factors (HIFs) are crucial for cellular and organismal adaptation to hypoxia. The mitochondrial respiratory chain is the largest consumer of oxygen in most mammalian cells; however, it is unknown whether the respiratory chain is necessary for in vivo activation of HIFs and organismal adaptation to hypoxia. HIF-1 activation in the epidermis has been shown to be a key regulator of the organismal response to hypoxic conditions, including renal production of erythropoietin (Epo). Therefore, we conditionally deleted expression of TFAM in mouse epidermal keratinocytes. TFAM is required for maintenance of the mitochondrial genome and TFAM-null cells are respiratory-deficient. TFAM loss in epidermal keratinocytes reduced epidermal levels of HIF-1α protein and diminished the hypoxic induction of HIF-dependent transcription in epidermis. Furthermore, epidermal TFAM deficiency impaired hypoxic induction of renal Epo expression. Our results demonstrate that the mitochondrial respiratory chain is essential for in vivo HIF activation and organismal adaptation to hypoxia.
Introduction
The importance of oxygen homeostasis for the survival of eukaryotes has necessitated the evolution of multiple mechanisms by which organisms maintain their oxygen supply. The cellular response to hypoxia is governed largely by a family of transcription factors termed Hypoxia-Inducible Factors (HIFs) which promote the activation of genes involved in glycolysis, angiogenesis, cell cycle regulation, and survival (Semenza, 2012). Under normoxic conditions, proline residues within the oxygen-dependent degradation domain of HIF-α subunits (HIF-1α, HIF-2α, and HIF-3α) are hydroxylated by a family of 2-oxoglutarate-dependent dioxygenases termed prolyl hydroxylases 1, 2, and 3 (PHD1–3) (Kaelin and Ratcliffe, 2008). This proline-directed hydroxylation targets HIF-α subunits for recognition by a ubiquitin ligase containing the von Hippel-Lindau tumor suppressor protein (VHL). The activity of PHD proteins is inhibited during hypoxia, stabilizing HIF-α subunits, leading to their dimerization with a common β-subunit (HIF-1β), and transactivation of HIF target genes.
Mitochondria metabolism has been implicated in regulating the hydroxylation reaction carried out by PHDs. Mitochondrial generation of TCA cycle intermediates, consumption of oxygen, and production of reactive oxygen species (ROS) have been shown to inhibit the hydroxylation of HIFs resulting in activation of HIF target genes (Bell et al., 2007; Brunelle et al., 2005; Guzy et al., 2005; Hagen et al., 2003; Isaacs et al., 2005; Mansfield et al., 2005; Pan et al., 2007; Selak et al., 2005; Sullivan et al., 2013). Despite evidence for the role of mitochondrial metabolism in regulating the cellular response to hypoxia, it remains to be demonstrated whether this phenomenon, heretofore observed only in cell culture, plays a physiological role in the mammalian systemic response to hypoxia in vivo.
Epidermal keratinocytes have been shown to play a critical role in regulating the systemic response to hypoxia (Boutin et al., 2008). The epidermis, which obtains oxygen directly from the atmosphere, responds to reductions in atmospheric oxygen by inducing vasodilation in the underlying dermis in a HIF- and nitric oxide-dependent manner (Boutin et al., 2008; Minson, 2003; Stucker et al., 2002). This hypoxic vasodilation in the skin results in reduced blood flow to the internal organs, increasing internal hypoxia, and promoting renal production of erythropoietin (Epo), a glycoprotein hormone which promotes proliferation and differentiation of erythroid progenitor cells into red blood cells, increasing the oxygen carrying capacity of the blood (Boutin et al., 2008; Bunn et al., 1998).
We recently reported on mice which lack expression of Transcription Factor A, Mitochondrial (TFAM) in epidermal keratinocytes (Hamanaka et al., 2013). TFAM is required for transcription and replication of the mitochondrial genome, and cells lacking TFAM are respiratory-deficient (Larsson et al., 1998). Loss of TFAM in epidermal keratinocytes resulted in epidermal barrier function defects and lethality 2 weeks post-birth. The epidermal defect was due to a decrease in the production of mitochondrial ROS, which is necessary for Notch-dependent epidermal differentiation (Hamanaka et al., 2013). Here we show that epidermal keratinocytes derived from these TFAM epidermal knockout (TFAM EpiKO) mice display impaired HIF activation upon exposure to hypoxia. Furthermore, neonatal mice displayed diminished HIF-1α protein and target gene induction in their epidermis after exposure to hypoxia. This resulted in an impaired hypoxic induction of Epo expression in the kidneys of these mice. Renal Epo expression could be induced in TFAM EpiKO mice by pharmacologic promotion of cutaneous vasodilation or by co-deletion of VHL, demonstrating conclusively the link between mitochondria and the HIF degradation machinery in vivo.
Results
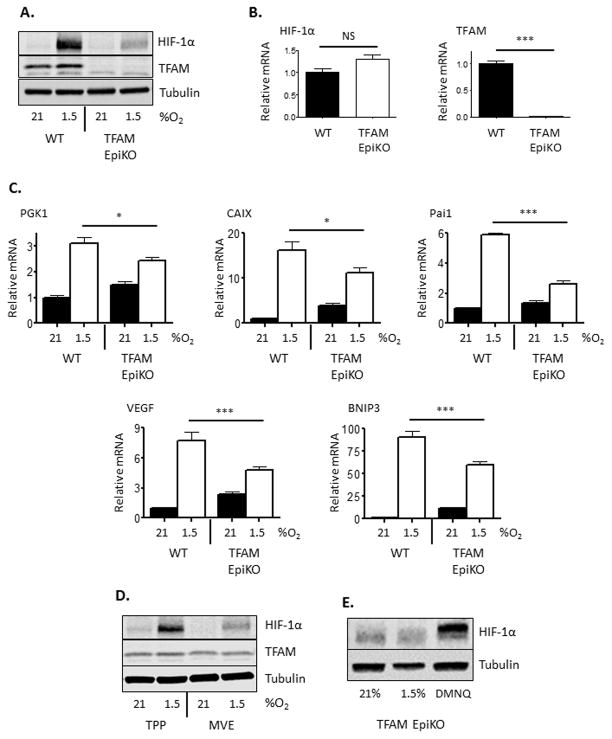
TFAM epidermal knockout (TFAM EpiKO) mice were generated by crossing mice bearing floxed alleles of TFAM to mice which express Cre recombinase under the control of the keratin-14 promoter (Dassule et al., 2000). We have previously demonstrated that primary epidermal keratinocytes isolated from TFAM EpiKO mice do not consume oxygen or produce mitochondrial ROS (Hamanaka et al., 2013). Consistent with their inability to initiate mitochondria-dependent signaling events, primary epidermal keratinocytes isolated from TFAM EpiKO mice displayed an impaired induction of HIF-1α protein after exposure to hypoxia (1.5% O2) (Figure 1A). Hypoxic accumulation of HIF-2α was similarly inhibited in TFAM EpiKO keratinocytes (Figure S1A). The failure of TFAM EpiKO keratinocytes to induce HIF-1α was not the result of decreased HIF-1α mRNA expression (Figure 1B), but was due to the inability of these cells to stabilize HIF-1α protein upon exposure to hypoxia. This resulted in impaired transcriptional induction of HIF-1α target genes that regulate metabolism (phosphoglycerate kinase-1; PGK1 and BCL2/adenovirus E1B 19kDa interacting protein 3; Bnip3), pH balance (carbonic anhydrase 9; CAIX), and blood flow (vascular endothelial growth factor; VEGF and plasminogen activator inhibitor 1; Pai1) (Figure 1C). The inability of TFAM-null cells to respond to hypoxia was not due to bioenergetic defects; keratinocytes treated with deferoxamine (DFO), an iron chelator which stabilizes HIF-1α protein under normoxic conditions (Wang and Semenza, 1993), resulted in HIF-1α protein accumulation as well as HIF-1α target gene expression in both wild-type and TFAM EpiKO cells (Figure S1B, S1C).
Figure 1.
Mitochondrial generation of ROS is required for hypoxic induction of HIF-1α protein and target gene expression in mouse epidermal keratinocytes. (A) Representative Western blot analysis of HIF-1α and TFAM protein levels in primary mouse keratinocytes isolated from wild-type and TFAM EpiKO mice. Cells were exposed to normoxia (21% O2) or hypoxia (1.5% O2) for 4 hours. (B) Real Time PCR analysis of HIF-1α and TFAM mRNA expression in primary mouse keratinocytes isolated from wild-type and TFAM EpiKO mice. (C) Real Time PCR analysis of PGK1, CAIX, Pai1, VEGF, and BNIP3 mRNA expression in primary mouse keratinocytes isolated from wild-type and TFAM EpiKO mice. Cells were exposed to normoxia or hypoxia for 4 hours. (D) Representative Western blot analysis of HIF-1α protein levels in primary wild-type mouse keratinocytes after exposure to normoxia or hypoxia for 4 hours. Cells were treated with mitochondria-targeted vitamin E (MVE) or the control compound methyl-triphenylphosphonium (TPP; mitochondria-targeting moiety lacking antioxidant activity). (E) Representative Western blot analysis of HIF-1α protein levels in TFAM EpiKO primary mouse keratinocytes after exposure to normoxia, hypoxia, or 2,3-dimethoxy-4-naphthoquinone (DMNQ) for 4 hours. Charts represent means +/− SEM. N=4 independent keratinocyte preparations per genotype. mRNA expression was normalized to RPL19 levels. Significance was determined by (B) Students T-test or (C) one way ANOVA using Bonferonni’s post-test.
We have previously demonstrated that mitochondrial reactive oxygen species (mROS) are critical signaling regulators of hypoxic HIF activation (Bell et al., 2007; Brunelle et al., 2005; Chandel et al., 2000). Consistent with this, the effects of TFAM knockout on HIF-1α stabilization were mimicked by the treatment of wild-type keratinocytes with antioxidants such as N-acetylcysteine and mitochondria-targeted Vitamin E (Figure 1D, S1D). To determine if elevation of cellular ROS levels could rescue HIF-1α stabilization in the absence of mROS production, we treated TFAM EpiKO cells with the redox cycling agent 2,3-dimethoxy-4-naphthoquinone (DMNQ). DNMQ has previously been demonstrated to increase cellular ROS levels leading to HIF-1α stabilization in transformed cells (Kohl et al., 2006). Treatment with DMNQ induced HIF-1α protein accumulation in TFAM EpiKO keratinocytes (Figure 1E). Mitochondrial respiratory complex III has been shown to be the major site of mROS production necessary for HIF-1α protein stabilization during hypoxia (Bell et al., 2007; Orr et al., 2015). Thus, acute knockdown of the complex III subunit Reiske Iron Sulfur Protein (RISP) diminished HIF-1α protein stabilization to a similar extent as TFAM knockout (Figure S1E). Collectively, these results demonstrate that TFAM EpiKO keratinocytes exhibit diminished HIF-1α protein stabilization after exposure to hypoxia due to their inability to produce mROS.
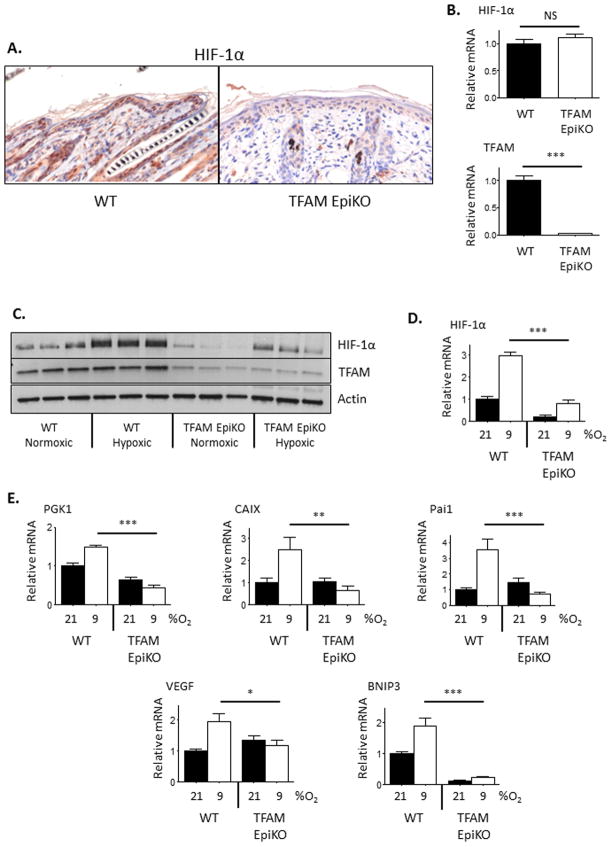
Elimination of TFAM expression in the epidermal keratinocytes of mice leads to premature follicular catagen and development of a hyperplastic interfollicular epidermis ((Hamanaka et al., 2013), Figure S2A). TFAM EpiKO mice develop an epidermal barrier defect at day P14.5 and die soon thereafter (Hamanaka et al., 2013), therefore all in vivo experiments on these mice were conducted at day P9.5. Consistent with previous reports (Bedogni et al., 2005; Boutin et al., 2008; Wong et al., 2014), the epidermis of wild-type mice displayed constitutive expression of HIF-1α protein, even under normoxic conditions (Figure 2A). Reduced levels of HIF-1α protein were observed in the epidermal cells of TFAM EpiKO mice when compared to wild-type mice. As observed in cultured keratinocytes, the diminished HIF-1α protein level was not due to a decrease in HIF-1α mRNA expression in the TFAM EpiKO epidermis as epidermal HIF-1α mRNA levels in wild-type and TFAM EpiKO mice were similar (Figure 2).
Figure 2.
Hypoxic induction of HIF-1α protein and target gene expression is inhibited in the epidermis of TFAM EpiKO mice. (A) Representative immunohistochemical analysis of HIF-1α protein expression in the epidermis of wild-type and TFAM EpiKO mice. (B) Real Time PCR analysis of HIF-1α and TFAM mRNA expression in the epidermis of wild-type and TFAM EpiKO mice. (C) Representative Western blot analysis of HIF-1α and TFAM protein levels in epidermal lysates prepared from wild-type and TFAM EpiKO mice. Mice were exposed to normoxia (21% O2) or hypoxia (9% O2) for 16 hours. (D) Densitometric quantification of (C). HIF-1α protein levels were normalized to β-actin levels. (E) Real Time PCR analysis of PGK1, CAIX, Pai1, VEGF, and BNIP3 mRNA expression in epidermal lysates prepared from wild-type and TFAM EpiKO mice. Mice were exposed to normoxia or hypoxia for 16 hours. Charts represent means +/− SEM. N=5 mice per genotype per condition. mRNA expression was normalized to RPL19 levels. Significance was determined by one way ANOVA using Bonferonni’s post-test.
Despite the constitutive levels of HIF-1α protein observed in normoxic wild-type mice, exposure of wild-type mice to hypoxia (9% O2) for 16 hours led to an additional accumulation of HIF-1α protein in epidermal cells as well as increased expression of HIF-1α target genes (Figure 2C–E). Exposure of TFAM EpiKO mice to hypoxia resulted in an attenuated induction of HIF-1α protein and no increase in the expression of HIF-1α target genes (Figure 2C–E).
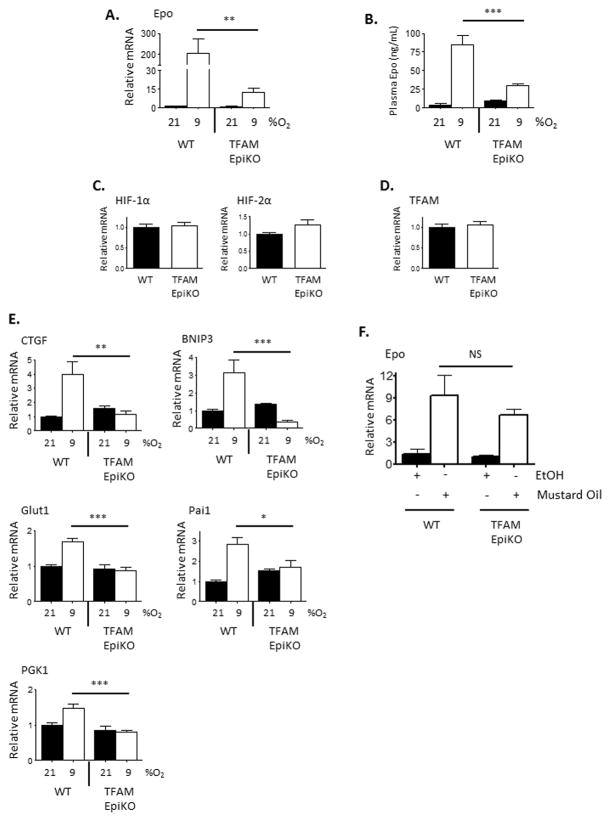
Activation of the HIF response in epidermal keratinocytes promotes the systemic response to hypoxia by promoting nitric oxide (NO)-dependent vasodilation in the underlying dermis, shunting blood away from the internal organs and causing them to encounter greater levels of hypoxia than they would in the absence of the epidermal response (Boutin et al., 2008). Kidneys respond to this increased level of hypoxia by producing the glycoprotein hormone erythropoietin (Epo), a HIF-2α target gene (Gruber et al., 2007; Rankin et al., 2007). After exposure to hypoxia for 16 hours, expression of Epo mRNA was highly induced in the kidneys of wild-type mice, an effect that was inhibited in TFAM EpiKO mice (Figure 3A). This led to reduced levels of Epo protein in the serum of TFAM EpiKO mice after exposure to hypoxia (Figure 3B). Hypoxic induction of HIF-1α target genes such as PGK1, BNIP3, Pai1, CTGF (Connective Tissue Growth Factor), and Glut1 (Glucose Transporter 1) was also inhibited in the kidneys of TFAM EpiKO mice (Figure 3E). As in the epidermis, the renal mRNA expression of both HIF-1α and HIF-2α was similar between wild-type and TFAM epiKO mice (Figure 3C). Furthermore, the levels of renal TFAM expression were not affected in TFAM EpiKO mice, indicating the specificity of the keratin 14-driven Cre for epidermal cells (Figure 3D).
Figure 3.
Hypoxic induction of HIF-1α target genes and erythropoietin is inhibited in the kidneys of TFAM EpiKO mice. (A) Real Time PCR analysis of Epo mRNA expression in renal lysates prepared from wild-type and TFAM EpiKO mice. Mice were exposed to normoxia or hypoxia for 16 hours. (B) Epo protein concentrations in the serum of wild-type and TFAM EpiKO mice as determined by ELISA. (C, D) Real Time PCR analysis of (C) HIF-1α, HIF-2α, or (D) TFAM mRNA expression in the kidney of wild-type and TFAM EpiKO mice. (E) Real Time PCR analysis of CTGF, Bnip3, Glut1, Pai1, and PGK1 mRNA expression in the kidneys of wild-type and TFAM EpiKO mice exposed to normoxia (21% O2) or hypoxia (9% O2) for 16 hours. (F) Real Time PCR analysis of Epo mRNA expression in the kidneys of wild-type and TFAM EpiKO mice exposed to epidermal application of either mustard seed oil (Allyl isothiocyanate) or EtOH as vehicle control. Charts represent means +/− SEM. mRNA expression was normalized to RPL19 levels. (A–E) N=5, (F) N=3 mice per genotype per condition. Significance was determined by one way ANOVA using Bonferonni’s post-test.
Pharmacologic induction of cutaneous NO is sufficient to induce renal Epo expression in the absence of hypoxic stimuli (Boutin et al., 2008). We found that the dermal vascular density of TFAM EpiKO mice was similar to that of wild-type mice when assessed using the endothelial marker CD31 (Figure S2B). Thus, we sought to determine whether TFAM EpiKO mice remained sensitive to cutaneous application of mustard seed oil (Allyl isothiocyanate), which promotes NO synthesis. Indeed, application of mustard seed oil to the skin of wild-type or TFAM EpiKO mice induced renal Epo mRNA expression to a similar extent (Figure 3F). These results suggest that the impaired hypoxic Epo induction observed in TFAM EpiKO mice is the result of an oxygen sensing defect within the epidermis.
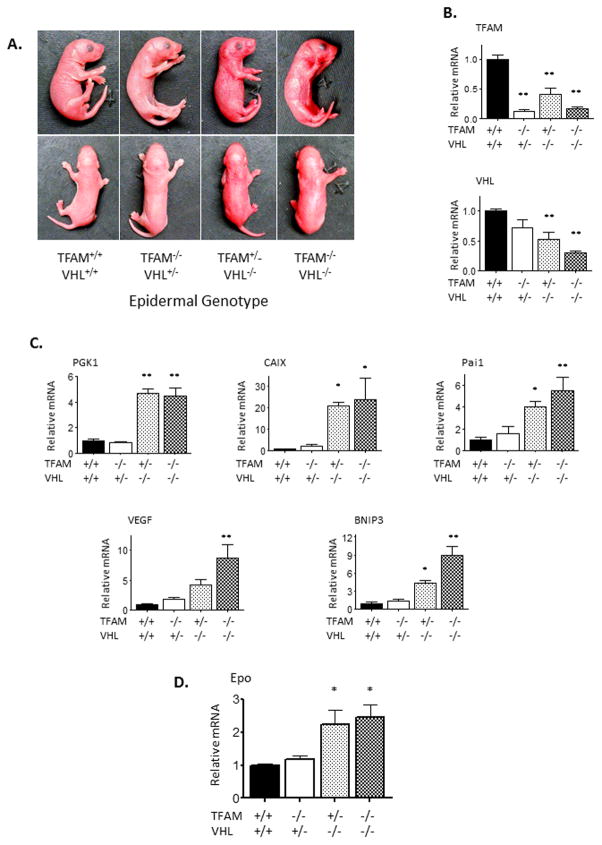
Since mitochondrial metabolism and mROS production regulate HIF activity upstream of recognition by the VHL-containing ubiquitin ligase, we sought to determine if epidermal deletion of VHL could rescue HIF-1α activity in the epidermal keratinocytes of TFAM EpiKO mice. To do this, we generated a mouse expressing floxed alleles of both TFAM and VHL and knocked out expression of both genes in the epidermis of mice using the keratin-14 Cre allele. Mice lacking expression of VHL in the epidermis were notable at birth as they were red in color due to increased cutaneous blood flow (Figure 4A–B). Both VHL EpiKO and TFAM+VHL EpiKO mice exhibited perinatal mortality, dying by day P5.5. At day P3.5, however, increased levels of HIF target mRNA were detected in epidermal lysates from both VHL EpiKO and TFAM+VHL EpiKO mice (Figures 4C). Furthermore, epidermal deletion of VHL elevated renal Epo expression to a similar extent in mice either heterozygous or homozygous for the TFAM floxed allele (Figure 4D).
Figure 4.
Deletion of VHL is sufficient to induce HIF-1α target gene expression in the epidermis of TFAM EpiKO mice. (A) Representative images of wild-type (KRT14-Cre−), TFAM EpiKO, VHL EpiKO, and TFAM+VHL EpiKO mice. (B–C) Real Time PCR analysis of (B) TFAM and VHL and (C) PGK1, CAIX, Pai1, VEGF, and BNIP3 mRNA expression in the epidermis of wild-type (KRT14-Cre−), TFAM EpiKO, VHL EpiKO, and TFAM+VHL EpiKO mice. (D) Real Time PCR analysis of Epo mRNA expression in the kidneys of wild-type (KRT14-Cre−), TFAM EpiKO, VHL EpiKO, and TFAM+VHL EpiKO mice. Charts represent means +/− SEM. mRNA expression was normalized to RPL19 levels. N=5 mice per genotype. Significance was determined by one way ANOVA using Dunnett’s post-test.
Discussion
At the cellular level, HIFs are crucial for metabolic adaptation to hypoxia (Semenza, 2010). Mitochondria are the primary cellular consumers of oxygen, and mitochondrial generation of TCA cycle intermediates, oxygen consumption, and production of ROS have all been demonstrated to regulate the activity of HIF prolyl hydroxylases (Bell et al., 2007; Brunelle et al., 2005; Guzy et al., 2005; Hagen et al., 2003; Isaacs et al., 2005; Mansfield et al., 2005; Pan et al., 2007; Selak et al., 2005; Sullivan et al., 2013). Despite accumulating evidence for mitochondrial regulation of HIF, this requirement had yet to be demonstrated in vivo. In this report, we demonstrate that the mitochondrial respiratory chain is an essential regulator of HIF-1 in vivo. The present findings are consistent with a recent study demonstrating that mitochondria regulate another adaptive response to hypoxia- contraction of the pulmonary artery (Waypa et al., 2013).
Our results demonstrate that although cells which lack TFAM expression are unable to conduct mitochondrial oxidative phosphorylation, they are energetically capable of inducing HIF-1α when PHD proteins or VHL are inhibited; it is the signaling upstream of the PHD proteins which is inhibited in the absence of TFAM. Thus, inhibition of PHD proteins with DFO or pharmacological elevation of cellular ROS levels with DMNQ was sufficient to induce HIF activity in TFAM EpiKO keratinocytes. In addition, our results support a role for mROS as crucial regulators of hypoxia signaling as treatment of wild-type keratinocytes with N-acetylcysteine or mitochondria-targeted vitamin E inhibited HIF accumulation to a similar extent as TFAM deletion in vitro. To conclusively demonstrate the role that mROS generation plays in promoting HIF activity in vivo, it will be critical to develop genetic tools which impair mROS production without simultaneously affecting oxidative phosphorylation.
Our results support previous findings that demonstrate that HIF activation in the epidermis promotes Epo production in the kidney. While it was previously demonstrated that deletion of epidermal HIF-1α expression impaired the renal response to hypoxia (Boutin et al., 2008), we now demonstrate that deletion of TFAM in epidermal keratinocytes recapitulates the effect of epidermal HIF-1α deletion. Although deletion of TFAM in epidermal cells inhibited hypoxic sensation and renal induction of Epo, these mice remained sensitive to the effects of cutaneous nitric oxide elevation or to co-deletion of epidermal VHL, supporting a crucial role for mitochondrial signaling upstream of VHL. These results demonstrate that mitochondria act as oxygen sensors in vivo, promoting HIF activation and adaptation to hypoxic conditions. While it is unclear why deletion of VHL in the epidermis resulted in early mortality, it was recently shown that in addition to regulating hypoxic adaptation, epidermal HIF levels regulate systemic arterial pressure and thermoregulation in mice (Cowburn et al., 2013). Thus, our results add to the increasing evidence for the importance of oxygen sensing and HIF activation within the epidermis for organismal homeostasis.
The importance of mitochondria as regulators of HIF signaling is highlighted by the fact that in a chemical screen of over 600,000 compounds, the 200 most effective inhibitors of hypoxic HIF activation were determined to be mitochondrial inhibitors (Lin et al., 2008). While the toxicities of mitochondrial inhibitors such as rotenone and cyanide are well known, metformin, an inhibitor of complex I of the respiratory chain is well tolerated in patients receiving it as a diabetes treatment. We recently demonstrated that metformin inhibits mROS generation and hypoxic HIF activation in cancer cells (Wheaton et al., 2014). Recently, Brand and colleagues identified multiple classes of compounds that inhibit mROS production from respiratory complex III without affecting mitochondrial energy metabolism (Orr et al., 2015). Although the in vivo efficacy of these compounds remains to be determined, these selective suppressors of complex III superoxide generation inhibited hypoxic induction of HIF-1α in cultured cells. Since hypoxia and aberrant HIF levels are associated with human pathologies such as pulmonary hypertension and cancer, it is of great importance to continue studying the mechanisms of mitochondrial HIF regulation.
Experimental Procedures
Mice and Keratinocyte Culture
TFAM floxed mice (TFAMfl/fl) mice were generated as described (Larsson et al., 1998) and were crossed with KRT14-Cre mice (Jackson Laboratory Stock No. 004782) (Dassule et al., 2000). Heterozygous TFAMfl/+;K14Cre+/− mice were mated back to TFAMfl/fl mice to obtain control (K14Cre−/−) and TFAM EpiKO mice. To create TFAM+VHL EpiKO mice, TFAMfl/fl mice were crossed with VHLfl/fl mice (Jackson Laboratory Stock No. 004081) and the progeny were mated to produce TFAMfl/fl;VHLfl/fl mice. These mice were crossed with KRT14-Cre mice and the heterozygous progeny were crossed back to the TFAMfl/fl;VHLfl/fl line.
To obtain primary mouse keratinocytes, pups were skinned at P0.5. Epidermis was separated from dermis by overnight incubation in dispase (5mg/mL) and disrupted using TrypLE Select (Invitrogen). Cells were cultured in CnT07 media (CELLnTEC) supplemented with 100μg/mL Uridine and 1mM Sodium Pyruvate. Methyl-triphenylphosphonium, N-acetylcysteine, 2,3-dimethoxy-4-naphthoquinone, Deferoxamine, and mustard seed oil were purchased from Sigma. Mitochondria-targeted vitamin E was a kind gift of Dr. Balaraman Kalyanaraman (Medical College of Wisconsin).
Human neonatal foreskin keratinocyte cultures were obtained from the Northwestern University Skin Disease Research Center (NU-SDRC) and grown in Medium 153 (Cascade) supplemented with Human Keratinocyte Growth Supplement (Thermo Fisher). For knockdown experiments, cells were electroporated with 250nmol siRNA using a Lonza Nucleofector IIb. siRNAs were purchased from Dharmacon stock number D-001810-01 (non-targeting), and L-020100-00-0005 (RISP).
Western Blots
Keratinocytes or epidermal preparations were lysed in Cell Lysis Buffer (Cell Signaling) or Urea Sample Buffer respectively. Urea Sample Buffer-8M deionized urea, 1% SDS, 10% glycerol, 60mM Tris pH6.8, 0.1% pyronin-Y, 5% β-mercaptoethanol. Lysates were resolved on 4–20% Criterion gels (Biorad) and transferred to nitrocellulose. Antibodies used were: HIF-1α (Cayman), TFAM (Gift of Gerald Shadel, Yale University), α-Tubulin (Sigma), and β-Actin (Sigma).
Real Time RT-PCR
Total RNA was extracted from cells or epidermal preparations using TRIzol reagent (Invitrogen) and 1μg was reverse transcribed using RETROscript first strand synthesis kit (Ambion). Real-time PCR was performed on a Bio-Rad CFX using iQ SYBR green Supermix (Bio-Rad). Primers used were: TFAM (5′-CCAAAAAGACCTCGTTCAGC-3′, 5′-ATGTCTCCGGATCGTTTCAC-3′), VHL (5′-TCCACAGCTACCGAGGTCAT-3′, 5′-CGACATTGAGGGATGGCACA -3′), HIF-1α (5′-GCGAGAACGAGAAGAAAAAGATGA-3′, 5′-ACTCTTTGCTTCGCCGAGAT-3′), HIF-2α (5′-GAACATGGCCCCCGATGAA-3′, 5′-AACCCAGTCTTGGCGTTCTC-3′), Epo (5′-AATGGAGGTGGAAGAACAGGCCAT-3′, 5′-CGAAGCAGTGAAGTGAGGCTACGTA-3′), BNIP3 (5′-GAAGCGCACAGCTACTCTCA-3′, 5′-TCCAATGTAGATCCCCAAGCC-3′), CAIX (5′-GCGCTAAGCAGCTCCATACT-3′, 5′-GCAGGGAAGGAAGCCTCAAT-3′), CTGF (5′-AGAACTGTGTACGGAGCGTG-3′, 5′-GTGCACCATCTTTGGCAGTG-3′), GLUT1 (5′-CGGCCTGACTACTGGCTTTG-3′, 5′-GCCAAACACCTGGGCAATAAG-3′), PAI1 (5′-GTAAACGAGAGCGGCACAGT-3′, 5′-GAGGATTGTCTCTGTCGGGT-3′), PGK1 (5′-TCTTGGGAGGCGCTAAAGTT-3′, 5′-AAGGCCATTCCACCACCAAT-3′), VEGF (5′-GGCCTCCGAAACCATGAACT-3′, 5′-CTGGGACCACTTGGCATGG-3′), mRPL19 (5′-GAAGGTCAAAGGGAATGTGTTCAA-3′, 5′-TTTCGTGCTTCCTTGGTCTTAGA-3′).
Epo ELISA
Mouse blood serum was collected using Serum Separator Tubes (BD). Serum Epo levels were determined using the Mouse Erythropoietin Quantikine ELISA kit (R&D Systems).
Histology and Immunostaining
Excised dorsal epidermal tissues were fixed in 10% buffered formalin and embedded in paraffin. 4μm sections were placed on charged slides. For automated HIF-1α immunohistochemistry, slides were prepared using the Bond Polymer Refine Detection kit (Leica Biosystems) and staining was performed using a Leica BOND-MAX fully automated immunohistochemistry machine. Primary HIF-1α antibody (ab2185; Abcam) was diluted 1:200 in Bond Primary Antibody Diluent before use. Imaging was performed using a Zeiss Axioplan 2 microscope and high resolution AxioCam digital color camera. Image analysis was performed with Zeiss AxioVision software.
For CD31 immunofluorescence, paraffin sections were baked overnight at 50°C and dewaxed followed by rehydration through Sub-X and graded alcohols, respectively. Sections were blocked in 5% donkey serum followed by overnight incubation in primary antibody (sc-1506; Santa Cruz Biotechnology). A donkey anti-goat Cy3 secondary antibody (Jackson ImmunoResearch Laboratories, 1:250 dilution) was then used followed by DAPI counterstaining.
Mustard Seed Oil Treatment
Hair was removed from the backs of nine day old pups using Nair (Church & Dwight Co., Inc.). The mice were rinsed with water and dried. The backs of the mice were then painted with either 100% EtOH or 1% mustard seed oil (Allyl isothiocyanate, diluted in 100% EtOH). Solutions were applied every 80 minutes for 7 hours.
Acknowledgments
Primary epidermal keratinocyte cultures were obtained from the Northwestern University Skin Disease Research Center (NU-SDRC), Skin Tissue Engineering Core (Paul Hoover). The NU-SDRC Morphology and Phenotyping Core (Shuangni Yang) assisted in morphological analyses. We are grateful to Dr. Gerald Shadel for the mouse TFAM antibody. This research is supported by National Institutes of Health Grants 1F32HL099007-01 to R.B.H, T32 T32HL076139 to SEW, T32 HL076139-11 to CRR, 5R21AR061174-02 (N.S.C) and 521HL112329-02 (N.S.C.). The NU-SDRC is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases grant 5P30AR057216.
Footnotes
Author Contributions
RBH and SEW performed mouse experiments and gene expression analysis. RBH and CRR performed western blot. RBH and NSC wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bedogni B, Welford SM, Cassarino DS, Nickoloff BJ, Giaccia AJ, Powell MB. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cell. 2005;8:443–454. doi: 10.1016/j.ccr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, Wiesener M, Eckardt KU, Koch CJ, Ellies LG, et al. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell. 2008;133:223–234. doi: 10.1016/j.cell.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Gu J, Huang LE, Park JW, Zhu H. Erythropoietin: a model system for studying oxygen-dependent gene regulation. J Exp Biol. 1998;201:1197–1201. doi: 10.1242/jeb.201.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- Cowburn AS, Takeda N, Boutin AT, Kim JW, Sterling JC, Nakasaki M, Southwood M, Goldrath AW, Jamora C, Nizet V, et al. HIF isoforms in the skin differentially regulate systemic arterial pressure. Proc Natl Acad Sci U S A. 2013;110:17570–17575. doi: 10.1073/pnas.1306942110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- Hamanaka RB, Glasauer A, Hoover P, Yang S, Blatt H, Mullen AR, Getsios S, Gottardi CJ, DeBerardinis RJ, Lavker RM, et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci Signal. 2013;6:ra8. doi: 10.1126/scisignal.2003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kohl R, Zhou J, Brune B. Reactive oxygen species attenuate nitric-oxide-mediated hypoxia-inducible factor-1alpha stabilization. Free Radic Biol Med. 2006;40:1430–1442. doi: 10.1016/j.freeradbiomed.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- Lin X, David CA, Donnelly JB, Michaelides M, Chandel NS, Huang X, Warrior U, Weinberg F, Tormos KV, Fesik SW, et al. A chemical genomics screen highlights the essential role of mitochondria in HIF-1 regulation. Proc Natl Acad Sci U S A. 2008;105:174–179. doi: 10.1073/pnas.0706585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT. Hypoxic regulation of blood flow in humans. Skin blood flow and temperature regulation. Adv Exp Med Biol. 2003;543:249–262. doi: 10.1007/978-1-4419-8997-0_18. [DOI] [PubMed] [Google Scholar]
- Orr AL, Vargas L, Turk CN, Baaten JE, Matzen JT, Dardov VJ, Attle SJ, Li J, Quackenbush DC, Goncalves RL, et al. Suppressors of superoxide production from mitochondrial complex III. Nat Chem Biol. 2015;11:834–836. doi: 10.1038/nchembio.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Mansfield KD, Bertozzi CC, Rudenko V, Chan DA, Giaccia AJ, Simon MC. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol Cell Biol. 2007;27:912–925. doi: 10.1128/MCB.01223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucker M, Struk A, Altmeyer P, Herde M, Baumgartl H, Lubbers DW. The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. J Physiol. 2002;538:985–994. doi: 10.1113/jphysiol.2001.013067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LB, Martinez-Garcia E, Nguyen H, Mullen AR, Dufour E, Sudarshan S, Licht JD, Deberardinis RJ, Chandel NS. The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol Cell. 2013;51:236–248. doi: 10.1016/j.molcel.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82:3610–3615. [PubMed] [Google Scholar]
- Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, Schumacker PT. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med. 2013;187:424–432. doi: 10.1164/rccm.201207-1294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM, Budigner GS, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WJ, Richardson T, Seykora JT, Cotsarelis G, Simon MC. Hypoxia-Inducible Factors Regulate Filaggrin Expression and Epidermal Barrier Function. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.283. [DOI] [PMC free article] [PubMed] [Google Scholar]