Abstract
Background
People with cystic fibrosis are managed differently in the US and UK providing an opportunity to learn from differences in practice patterns.
Objectives
To compare cross-sectional demographics, practice patterns and clinical outcomes between US and UK cystic fibrosis patients.
Methods
This was a cross-sectional study using 2010 data from patients in the US Cystic Fibrosis Foundation and the UK Cystic Fibrosis patient registries. The a priori outcome measures of interest were lung function and nutritional status. Descriptive statistics and two sample comparisons were performed. Stratification and multivariable linear regression was used to adjust for confounding.
Results
The study cohort included 13,777 children and 11,058 adults from the US and 3,968 children and 3,965 adults from the UK. In children, mean body mass index percentiles were similar. Lung function (FEV1 and FVC% predicted) was significantly higher in US patients ages 6 through 25 years of age. In a regression model adjusted for only age, FEV1% predicted was on average 3.31% of predicted (95% CI: 2.65, 3.96) higher in the US compared to the UK. When adjusted for age, age at diagnosis, gender, pancreatic insufficiency and genotype, FEV1% predicted was on average 3.03% of predicted (95% CI: 2.37, 3.69) higher in the US compared to the UK These differences persisted despite adjustment for possible confounders. Hypertonic saline and dornase alfa were much more commonly prescribed in US children.
Conclusions
Children and young adults with cystic fibrosis have better lung function in the US compared to the UK despite similar nutritional status.
Keywords: cystic fibrosis, pediatric lung disease, clinical epidemiology
Introduction
A number of advances in the care and outcomes of people with cystic fibrosis (CF) have occurred over the last two decades. This time span has noted dramatic improvements in survival from a median predicted survival of 28 years in 1990 to 38 years in 2010 in the United States.[1, 2] This improvement has been highlighted in by Dodge and colleagues noting dramatic improvements in survival by birth cohorts [3] and in a recent analysis of the CF Foundation Patient Registry.[4] Improved survival is likely due to the introduction of new therapeutics,[5-8] multidisciplinary care, improved nutritional support and the liberal use of antibiotics. The impact of therapies that treat the basic defect,[9, 10] recently approved therapies[11-14] and eradication protocols for Pseudomonas[15, 16] are still unknown but likely to be significant.
International comparisons can be extremely informative when comparing how different treatment approaches or medications impact disease progression as in the comparisons of nutritional outcomes and survival between the Boston and Toronto CF care centers,[17] which demonstrated the benefits of a high fat, high calorie diet. This work was instrumental in unifying the dietary recommendations for CF across the world.[18] Other international comparisons conducted to date are more challenging to interpret due to differences in data collection between different nations.[19-21]
To further our understanding of the role of therapies early in disease and the role of different health care systems on outcomes in CF, we compared the CF populations of the United States(US) and the United Kingdom(UK). There were three objectives: 1) to compare age-specific demographic characteristics; 2) to compare cross-sectional clinical characteristics; 3) to determine the age-specific differences in lung function and nutritional status between the patient populations.
Methods
Study Population
This study is a cross-sectional analysis of two study populations. The study population included all patients enrolled in the CF Foundation(CFF) Patient Registry and the UK CF Registry in 2010 with clinical data inputed that year into the respective registries and a confirmed diagnosis of CF.[22] Both data sets included CF demographics, as well as clinical data(See online supplement for details). Each site involved in the US CFF Patient Registry obtained approval for human subjects participation in research based on local standards and all patients or legally authorized representatives provided informed consent to be included in the registry. National Health Service(NHS) research ethics approval was granted for the UK CF Registry and each patient or legally authorized representative provided written informed consent for data collection and research. Under the terms of the NHS ethics approval, the UK CF Trust steering committee approved this study. One of the major challenges and a unique aspect of this analysis was harmonizing the two data registries to address differences in data elements and differences in the seasonality of data entry by country. The data were de-identified and merged to ensure similar seasonality of clinical encounters and recoding of key variables (See the online supplement: Data Merging and Figure E1a, E1b and E2).
Primary outcome measure and predictor of interest
The primary outcome measure was forced expiratory volume in one second(FEV1) percent of predicted, the single best predictor of mortality in CF.[23-25] All percent predicted values were re-calculated for Caucasians in the merged data employing reference equations from Wang and Hankinson[26, 27] with a sensitivity analysis using the global lung function prediction equations.[28] We also performed sensitivity analyses restricting the population to those who were homozygotes for the F508del gene and evaluated the differences in FVC% predicted.
Secondary Outcomes, Confounders and Effect Modifiers
Weight and height were converted to the metric system. BMI was calculated using a standard equation(kg/m2). Weight-for-length and BMI percentiles were recalculated using US Centers for Disease Control data reference values.[29] Use of pancreatic enzymes was deemed synonymous with pancreatic insufficiency. Sputum microbiology results were categorized as negative, positive(≥ 2 sputum samples positive in one year) or intermittent for each CF pathogen(see online supplement). The use of chronic nebulized antibiotics was defined as the use of any one of several inhaled antimicrobial agents. In a post-hoc analysis to understand differences in treatments, we employed a modified treatment intensity score(see online supplement), an additive index of the following treatments: hypertonic saline, aerosolized tobramycin, rhDNase, macrolides, aerosolized colistin and other aminoglycosides.[30] National-level treatment differences may not be reflected at the individual center level. Because of this concern, we created center-level metrics(median and IQR) for both the US and the UK to perform additional sensitivity analyses. The following covariates were treated as confounders: age at encounter, gender, age at diagnosis, pancreatic sufficiency, genotype, chronic Pseudomonas infection, chronic MSSA infection, B. cepacia infection and MRSA infection. Age and age strata were treated as effect modifiers along with genotype. Age was handled as a categorical variable grouped by 4 age increments (<12 years, 12-17 years, 18-23 years, ≥24 years) for regression models. For comparisons of microbiology and pulmonary therapy, we performed stratified analysis of people under 18 years and those above 18 years.
Statistical Analysis
The primary analyses were performed based on an a priori statistical analysis plan. Descriptive analyses of the characteristics of patients according to country (US vs UK) were conducted. Linear regression models with robust standard errors were used to model the association of FEV1 % adjusted for key covariates. The primary predictor of interest was country (US vs UK). Multivariate models were adjusted for possible confounders by forcing covariates into the model based on known demographic and clinical confounders. Our pre-specified multivariable regression models were restricted to data in white patients. We first assessed whether differences in lung function could be explained by differences in age, gender, age at diagnosis, pancreatic insufficiency and CFTR mutation. An interaction term was used for age and country of origin (UK vs US) in the analyses. Stratified analyses and statistical measures of interaction were used to analyze the relationship of country and the following covariates (gender, age, CFTR mutation classification). In multivariate models, we assessed only first order interactions and tested for significance using the likelihood ratio test. All of our models assumed statistical independence of every subject within the study population. Since specific centers have been shown to be linked to better outcomes and presumably better care, we repeated analyses with clustering on center to allow correlation of lung function in subjects within the same center. Two-tailed α < 0.05 was considered statistically significant for all study analyses. Analyses were conducted using Stata version 13 (College Station, Texas, USA).
Results
Demographics
The study cohort included 13,777 children and 11,058 adults from the US and 3,968 children and 3,965 adults from the UK. A number of key differences were found when comparing the populations from 2010 (Table 1). The median age of the overall population was significantly higher in the UK [17.9 years compared to 16.2 years, difference: 1.2(95% CI: 0.90-1.50), p<0.001] with a higher proportion of males [53.1 vs 51.6%, difference: 1.5% (95% CI: 0.2% -2.8%), p = 0.02]. Median age of diagnosis was earlier in the UK (95% CI: 0.3 vs 0.4 years, p<0.001). The racial/ethnic distribution also differed in the two populations, with significantly more Asians and fewer Blacks in the UK compared to the US CF population, but overall, the vast majority of the CF populations in both countries were white.
Table 1.
Comparisons of demographic characteristics between UK and US data. Results are presented as means (SD), medians (interquartile range, IQR) or n(%). [n] refers to the number of non-missing observations for each given variable in each country/subgroup
| USA | UK | USA-UK Difference (95% CI) * | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| N | 24835 | 7933 | ||||
|
| ||||||
| Number of centers providing data in 2010 | 237 | 132 | ||||
|
| ||||||
| [n] | [n] | |||||
|
| ||||||
| Age (years) | 24835 | 7933 | ||||
| Median (IQR) | 16.2 (8.3-25.6) | 17.9 (9.3-27.0) | -1.2 (-1.50, -0.90) | <0.001 | ||
| ≥16 years; n(%) | 12631 (50.9) | 4421 (55.7) | -4.9% (-6.1%, -3.6%) | <0.001 | ||
| ≥18 years; n(%) | 11058 (44.5) | 3965 (50.0) | -5.5% (-6.7%,-4.2%) | <0.001 | ||
|
| ||||||
| Sex | 24835 | 7933 | ||||
| Male; n(%) | 12819 (51.6) | 4214 (53.1) | -1.5% (-0.2% - 2.8%) | 0.020 | ||
|
| ||||||
| Race/ethnicity | 24835 | 7837 | <0.001 | |||
| Asian; n(%) | 92 (0.4%) | 198 (2.5%) | ||||
| Black; n(%) | 842 (3.4%) | 29 (0.4%) | ||||
| White†; n(%) | 21594 (87.0%) | 7580 (96.7%) | ||||
| Other††; n (%) | 2307 (9.3%) | 30 (0.4%) | ||||
|
| ||||||
| Age at diagnosis (years) | 24727 | 7856 | ||||
| Median (minmax) | 0.4 (0-78.5) | 0.3 (0-79.2) | <0.001 | |||
| < 3 mo; n(%) | 10209 (41.3) | 3682 (46.9) | <0.001 | |||
| 3-6 mo; n(%) | 2743 (11.1) | 862 (11.0) | (trend) | |||
| 6-12 mo; n(%) | 2842 (11.5) | 649 (8.3) | ||||
| 12 mo – 3 y; n(%) | 3251 (13.2) | 1047 (13.3) | ||||
| ≥3 y; n(%) | 5682 (23.0) | 1616 (20.6) | ||||
| ≥18 y; n(%) | 1280 (5.2) | 469 (6.0) | -0.8% (0.2%, 1.4%) | 0.007 | ||
Where medians are used, differences between the groups are presented as the median difference between values sampled from two groups. This difference is not strictly equal to the difference between the two medians.
For the purposes of this analysis (to facilitate comparisons between the US and the UK), white was defined as non-Hispanic whites.
For the purposes of this analysis, subjects with Hispanic ethnicity were included in the “other” category
Pulmonary and Nutritional Outcomes
Given the differences noted in the two populations, age and gender stratified comparisons were performed for lung function and nutritional status. As noted in Table E1 and Figure 1a-d, BMI percentile was significantly higher in US male children ages 10-17 years and lung function was significantly higher in US children ages 6 to 25 years. Interestingly, FEV1% predicted was higher in the UK population that was over the age of 50 years (+5.47% predicted, 95% CI; 1.03-9.92). Figure 2a clearly denotes the differences between the UK and the US of FEV1% predicted by age in years (a cross-sectional comparison). The differences in lung function persists when: 1) comparing homozygotes for the c.1521_1523delCTT allele in the CFTR gene(F508del); and 2) employing the global lung function prediction equations[28] (see online supplement Table E2 and Figure E3). Additionally, similar patterns were observed when examining FVC% predicted(See Figure 2b).
Figure 1. BMI by age and country.

Top left: Mean BMI percentile (95% CI) among male children aged 2 to 17 years. Top right: Mean BMI percentile (95% CI) among female children aged 2 to 17 years. Bottom left: Median BMI (IQR) among adult males aged 18 years and older. Bottom right: Median BMI (IQR) among adult females aged 18 years and older.
Figure 2a. Mean (95% CI) FEV1 % predicted among Caucasian patients by age at clinical encounter and country.
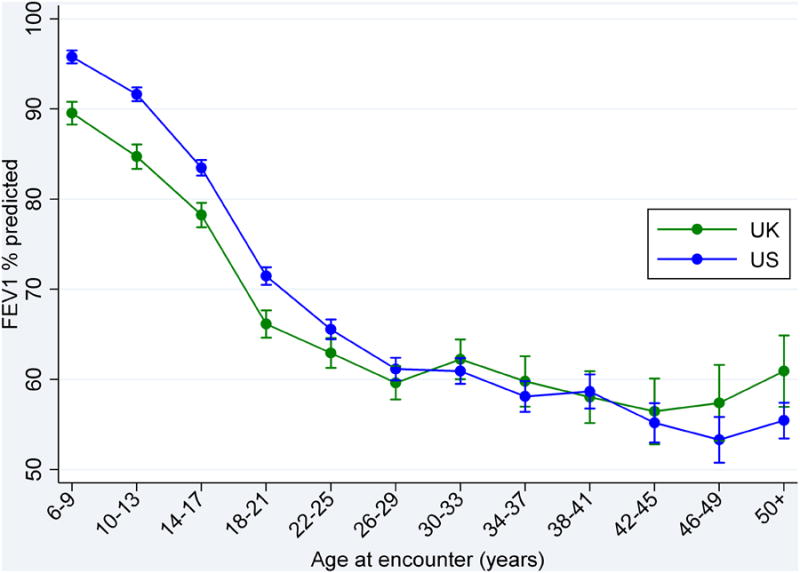
Figure 2b. Mean (95% CI) FVC % predicted among Caucasian patients by age at clinical encounter and country.

Microbiology
There were modestly higher rates of chronic Pseudomonas aeruginosa infection and markedly lower rates of methicillin-resistant Staphylococcus aureus(MRSA) and methicillin-sensitive Staphylococcus aureus(MSSA) in the UK compared to the US(Table 2). The higher rate of P. aeruginosa in the UK compared to the US might be due in part to the age difference. The low rates of MRSA in the UK may be due to differences of antibiotic use in the general population and mirrors MRSA rates in other parts of Europe.[31]
Table 2. Characteristics of chronic* airway infections in the two countries.
| US | UK | US-UK Difference (95% CI) | p-value | |
|---|---|---|---|---|
|
| ||||
| N | 24835 | 7933 | ||
|
| ||||
| P. aeruginosa | ||||
| <18 years; n(%) | 4907 (35.6) | 1516 (41.2) | -5.6% (-7.4%, -3.8%) | <0.001 |
| ≥18 years; n(%) | 7348 (66.5) | 2703 (71.1) | -4.6% (-6.3%, -2.9%) | <0.001 |
|
| ||||
| B.cepacia | ||||
| <18 years; n(%) | 196 (1.4) | 57 (1.5) | -0.1% (-0.5%, 0.4%) | 0.718 |
| ≥18 years; n(%) | 399 (3.6) | 186 (4.9) | -1.3% (-2.1%, -0.5%) | <0.001 |
|
| ||||
| MRSA | ||||
| <18 years; n(%) | 3372 (24.5) | 65 (1.7) | 22.8% (21.9%, 23.6%) | <0.001 |
| ≥18 years; n(%) | 2700 (24.4) | 133 (3.5) | 20.9% (19.9%, 21.9%) | <0.001 |
|
| ||||
| MSSA | ||||
| <18 years; n(%) | 7902 (57.4) | 1059 (29.2) | 28.2% (26.5%, 29.9%) | <0.001 |
| ≥18 years; n(%) | 4108 (37.2) | 1443 (38.3) | -1.2% (-3.0%, -0.6%) | 0.201 |
In this table, chronic is defined as at least two positive sputum samples in 2010.
Pulmonary Therapies
When we evaluated treatments recorded at annual visits for routine care, we found that a number of chronic pulmonary therapies were used much less frequently in the UK compared to the US (Table 3). The most striking differences were noted in the use of hypertonic saline and rhDNase in both children and adults. Chronic macrolide antibiotics were also used less frequently in the UK but the magnitude of the difference was much less than for hypertonic saline and rhDNase. The overall use of chronic inhaled antibiotics (grouped, given the different antibiotics used in each nation) was similar in the two countries. Because of the stark differences noted in both children and adults, additional analyses were performed stratified by lung function to assess whether these therapies were being preferentially used for those with more advanced disease in the UK. The differences remained in all strata with a general trend of more common use of all medications in both countries in patients with more advanced disease (see online supplement Table E3). To capture how medications are used in combination, we employed a modified treatment intensity score.[30] In all categories of treatment intensity, mean FEV1% predicted was higher in the US than in the UK (see online supplement Table E4).
Table 3. Treatment comparisons between the US and the UK.
| US | UK | US-UK Difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Treatment | Total n | n(%) | Total n | n(%) | ||
|
| ||||||
| Hypertonic saline; n(%) | ||||||
| <18 years | 13412 | 5489 (40.9) | 39073 | 327 (8.4) | 32.6 (31.4, 33.8) | <0.001 |
| ≥18 years | 10803 | 5594 (51.8) | 870 | 571 (14.8) | 37.0 (35.6, 38.5) | <0.001 |
|
| ||||||
| Any nebulised antibiotic, n(%) | ||||||
| <18 years | 13412 | 6000 (44.7) | 3907 | 1674 (42.9) | 1.9 (0.1, 3.7) | 0.036 |
| ≥18 years | 10803 | 7079 (65.5) | 3870 | 2396 (61.9) | 3.6 (1.8, 5.4) | <0.001 |
|
| ||||||
| rhDNase; n(%) | ||||||
| <18 years | 13412 | 10360 (77.2) | 3907 | 1382 (35.4) | 41.9 (40.2, 43.5) | <0.001 |
| ≥18 years | 10803 | 8126 (75.2) | 3870 | 2009 (51.9) | 23.3 (21.5, 25.1) | <0.001 |
|
| ||||||
| Macrolides, n(%) | ||||||
| <18 years | 13412 | 4505 (33.6) | 3907 | 942 (24.1) | 9.5 (7.9, 11.0) | <0.001 |
| ≥18 years | 10803 | 7061 (65.4) | 3870 | 2373 (61.3) | 4.0 (2.3, 5.8) | <0.001 |
When evaluating our center-level metrics(median and IQR) for both the US and the UK, we found that the distribution of treatment rates had a fairly normal distribution in the US, and that treatment rates at UK centers were significantly skewed to lower treatment rates (Figure 3 and online supplement Table E5). This distribution reflects the aggressiveness of care in the US versus the UK. For children under age 12 years treated with 4 or more therapies, the mean FEV1% predicted in the US was 85.8% compared to a mean FEV1% predicted of 74.0% in the UK. This suggests that in the US children with milder lung impairment are treated with more therapies than their counterparts in the UK. Treatments were much less commonly used at a large number of UK CF centers. However, the distribution of use of inhaled antibiotics appeared to be much more similar between the two countries.
Figure 3. Distribution of center-level treatment rates by treatment and country.

Multivariable Statistical Models
In a regression model adjusted for only age at encounter, FEV1% predicted was on average 3.31% of predicted (95% CI: 2.65, 3.96) higher in the US compared to the UK (Model 1) (Table 4). When we adjusted for the impact of age, age at diagnosis, gender, pancreatic insufficiency (based on pancreatic enzyme use) and genotype, the FEV1% predicted was on average 3.03% of predicted (95% CI: 2.37, 3.69) higher in the US compared to the UK (Model 2). Because sputum microbiology could relate to the local environment, we created an additional regression model adjusting for the above noted variables and sputum microbiology. The results showed a 3.85% predicted (95% CI: 3.17, 4.53) difference in FEV1% predicted between countries (Model 3). In each of these regression models, the effects of the other adjusted covariates mirrored effects seen in other studies.[32, 33] In a post-hoc analysis, we employed an interaction term of age versus country; this analysis was driven by prior analyses showing that the majority of the effect between countries was in children. As there was statistically significant evidence of interaction, we ran model 3 stratified by age group and noted that the difference between UK and US patients was statistically significant only in those under 24 years (Table 4). We also conducted a number of analyses first restricting models to only those who were homozygous for the F508del mutation. Differences persisted in these analyses. We also reran the regression models employing raw FEV1 adjusting for height, age and gender and demonstrated clinically significant differences between the US and the UK in lung function(see online supplement Table E6 and E7).
Table 4.
Linear regression models of FEV1 % predicted among white patients. Results are presented as difference between the US-UK of FEV1 % predicted (95% CI).
| Number of patients in model | Adjusted US-UK effect (95% CI) | |
|---|---|---|
|
| ||
| Model 1: Adjusted for age at encounter | N=22867 | 3.31 (2.65, 3.96) |
|
| ||
| Model 2: Adjusted for age at encounter, gender, age at diagnosis, pancreatic sufficiency and genotype | N=22591 | 3.03 (2.37, 3.69) |
|
| ||
| Model 3: Adjusted for age at encounter, gender, age at diagnosis, pancreatic sufficiency, genotype, chronic Pseudomonas infection, chronic MSSA infection, B. cepacia infection and MRSA infection | N=22276 | 3.85 (3.17, 4.53) |
|
| ||
| Model 3 stratified by age group: | ||
| <12 years | N=4681 | 7.62(6.24, 9.00) |
| 12-17 years | N=5078 | 6.65 (5.21, 8.08) |
| 18-23 years | N=4578 | 5.20 (3.64, 6.75) |
| 24 years + | N=7939 | 0.26 (-0.88, 1.39) |
An additional set of sensitivity analyses were performed to allow correlation of lung function in subjects within the same center. These models did not differ significantly from any of the earlier models.
Discussion
In a cross-sectional analysis of data from two National CF registries, we have demonstrated stark differences in lung function in children with CF in two countries with well developed yet different healthcare systems. Lung function as measured by FEV1% predicted was higher in children in the US compared to those in the UK, and these differences persisted up to the early 20's. The differences were not associated with accompanying differences in nutritional status and persisted in both stratified analyses and in multiple variable adjusted regression models. The most striking differences between the two populations was the low rate of MRSA and MSSA infection in the UK in children and adults and the modestly higher rate of Pseudomonas infection in children in the UK. The low rates of MSSA noted in the UK could be due to the common practice of Staphylococcus prophylaxis used in the first three years of life. The results noting higher MRSA in the US but higher lung function appear to contradict earlier analyses regarding the role of MRSA on survival and lung function by Dasonbrook and colleagues noting worse lung function and higher mortality in those with persistent MRSA. [34, 35] However, Sawicki and colleagues found the opposite results – that incident MRSA had no impact on lung function decline but was a marker of more intensive treatement.[36] The differences noted between the two countries were not due to different or over-representation of milder mutations, given the concordant findings in those analyses restricted to those patients who were homozygous for the F508del mutation. The differences were also not due to differences in age distribution of the populations or age of diagnosis. The striking difference in practice patterns between the two populations was in the rate of use of several pulmonary therapies. US centers had on average a higher intensity of therapy compared with the UK.
Comparing clinical outcomes between countries can be very informative particularly where clear differences in care models and treatment approaches occur. Survival differences and their association with nutritional approach noted between Toronto and Boston led to a complete revision of the nutritional model of care throughout the world.[17] Recent analyses of the European CF Registry noted marked differences in the median age in the European Union(EU) compared to non-EU countries with far fewer CF patients over age 40 living in non-EU countries.[20] One recent analysis that addressed treatment differences focused on differences between the US and Australia.[19] This analysis noted the benefit of newborn screening on lung function was significantly less in Australian children compared with US children, and mean FEV1% predicted adjusted for age, gender, and genotype did not differ between the two countries. These studies have demonstrated the potential benefit of international comparisons of clinical outcomes in CF.
A number of national comparisons have been done primarily to compare survival.[21, 37] Much of this work has focused on issues related to comparing survival metrics between countries and addressing some of those challenges. While these initial demographic and survival comparisons are interesting, to improve our understanding of how to best manage CF patients, we need to assess more proximal outcomes like lung function and nutrition with accompanying analysis of treatments. Differences in treatment strategies and treatment approaches may lead to changes in intermediate outcomes such as lung function and nutrition. Our analysis has clearly demonstrated significant lung function differences between the two countries seen primarily in children. Although the two patient registries use similar software for data entry, the countries differed in frequency of data collection. We paid special attention to this key difference by using random sampling methodology in the US data to match seasonal differences in data entry. Earlier comparisons have not addressed this important potential confounder.[19] A number of earlier studies have demonstrated the role of seasons on exacerbation frequency in relation to seasonal variation in respiratory viruses and in relation to pseudomonal acquisition.[38-40] Our analysis is the first to carefully ensure that differences in timing of clinical assessment do not confound potential associations.
Our results demonstrate striking differences in the use of CF therapies in the two countries, particularly in children. In the UK, universal access to care is available while in the US that is not the case. Because of universal access to care, the UK employs a reimbursement system for CF specialized care based on tariffs linked to disease severity[41] in addition to careful review of the cost effectiveness of medications via the UK's National Institute for Health and Clinical Excellence(NICE).[42] In the US, medications are much more likely to be used outside of the confines of the study populations defined in the pivotal clinical trials in CF leading to dramatically increases in cost between 2001 and 2007.[43] While such use of therapeutics is emblematic of US healthcare and likely has not yielded improved outcomes,[44] in the case of CF, this intensity of use of therapeutics may confer benefit, particularly in children with the disease.[45] While use of therapeutics was more common in adults in the US compared with the UK, these differences did not translate into improved lung function. One could argue that prescribing patterns in the UK are more efficient to achieve similar lung function with fewer therapies. This pattern however, was not seen in children.
Our analyses have a number of limitations. The first limitation is the fact that this analysis is a cross-sectional analysis, a weaker study design that limits causal inference. Both temporal changes and cohort effects can conflate the results, particularly if sicker subjects are dying leaving a healthier population. Give the very low death rate in children in CF, this is unlikely to account for our findings. This potential weakness however does not diminish the significance of our findings. Our results point to stark differences in lung function over many years of age, and these differences are coupled with very different treatment patterns. The direction of the bias for treatment intensity should have been opposite of our findings – the US should have had lower lung function if treatment was directed at more severe patients. We found the reverse association. An additional limitation that deserves mention is neither registry is likely to capture every CF patient residing in each nation. If nations have differential sampling of the CF population, our results may merely reflect that differential sampling. Prior data however support the finding that those not captured in the US CFF Patient Registry are more likely to be post lung transplant, thus not impacting the findings of this analysis.[46, 47] An additional potential bias could be due to differential capture of atypical or mild CF in the US compared to the UK. This is extremely unlikely given that the subgroup analysis in patients who were homozygous for the F508del mutation replicated the main analysis. Of note, data related to adherence to therapies is not available. Thus when patients are noted in the respective registries of being on a therapy, we do not know if they merely trialed the therapy and then stopped it. This limitation cannot be overcome in the current registry data, however this potential misclassification is unlikely to be differential. Lastly, the differences that we found could be due to differences in socioeconomic status.[48, 49] Unfortunately, measures are not available that can easily compare the socioeconomic status of those CF patients living in the US and the UK.
Conclusion
We have clearly demonstrated stark differences in lung function as measured by FEV1% predicted between the US and the UK in children and young CF adults. These differences in lung function persisted with a number of sensitivity analyses and in multivariable adjustment for confounders. The differences were associated with very significant differences in the aggressiveness of care particularly in CF children, which may have long term implications to outcome in this disease. Further longitudinal comparisons of national data are needed to unravel the causal implications of earlier and more aggressive treatment of CF children.
Supplementary Material
What is the key question?
Does lung function differ between patients with cystic fibrosis in the United Kingdom and the United States?
What is the bottom line?
We have demonstrated important and significant differences in lung function in CF children and young adults in the US and the UK with children in the US having better lung function.
Why read on?
Our findings suggest that earlier and more aggressive use of chronic pulmonary therapies may be beneficial.
Acknowledgments
We would like to acknowledge the support of the US CF Foundation and the UK CF Trust that made this analysis possible. We would also like to acknowledge all the CF patients in both the US and the UK that consent to be part of their respective National Patient Registries. Last we would like to acknowledge Ase Sewall of Sewall, Inc., Bethesda, for her work on the US CF Foundation Patient Registry.
Sources of support: Dr. Goss receives funding from the Cystic Fibrosis Foundation, the NIH (R01HL103965, R01HL113382, R01AI101307, U M1HL119073, P30DK089507) and the FDA (R01FD003704).
Dr. Bilton is supported by NIHR funding to the Respiratory Biomedical Research Unit at Royal Brompton Hospital and Imperial College London, UK.
US CF Foundation Patient Registry is funded by the US CF Foundation.
UK CF Registry is funded by the UK CF Trust.
Footnotes
Author Contributions: CHG, SJM, HQ, BCM, AE, EAK, KP, EG, JO and DB contributed to the conception and design of the study. AE merged the study data. CHG, SJM, HQ, BCM, AE, EAK, KP, EG, JO and DB contributed to the analysis and interpretation of the data. CHG drafted the article. CHG, SJM, HQ, BCM, AE, EAK, KP, EG, JO and DB revised the article critically for important intellectual content. All authors contributed to the final version of the article and approve of the final version to be published.
This article has an online data supplement, which is accessible from this issue's table of content online.
References
- 1.Cystic Fibrosis Foundation Patient Registry 2012 Annual Data Report to the Center Directors. Bethesda, MD: Cystic Fibrosis Foundation; 2013. [Google Scholar]
- 2.Kulich M, Rosenfeld M, Goss CH, et al. Improved survival among young patients with cystic fibrosis. J Pediatr. 2003;142(6):631–6. doi: 10.1067/mpd.2003.197. [DOI] [PubMed] [Google Scholar]
- 3.Dodge JA, Lewis PA, Stanton M, et al. Cystic fibrosis mortality and survival in the UK: 1947-2003. Eur Respir J. 2007;29(3):522–6. doi: 10.1183/09031936.00099506. [DOI] [PubMed] [Google Scholar]
- 4.MacKenzie T, Gifford AH, Sabadosa KA, et al. Lifetime of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation Patient Registry. Ann Intern Med. 2014;161:233–41. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331(10):637–42. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 6.Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340(1):23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 7.Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290(13):1749–56. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 8.Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354(3):229–40. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 9.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363(21):1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663–72. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCoy KS, Quittner AL, Oermann CM, et al. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med. 2008;178(9):921–8. doi: 10.1164/rccm.200712-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Retsch-Bogart GZ, Quittner AL, Gibson RL, et al. Efficacy and safety of inhaled aztreonam lysine for airway pseudomonas in cystic fibrosis. Chest. 2009;135(5):1223–32. doi: 10.1378/chest.08-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aitken ML, Bellon G, De Boeck K, et al. Long-term inhaled dry powder mannitol in cystic fibrosis: an international randomized study. Am J Respir Crit Care Med. 2012;185(6):645–52. doi: 10.1164/rccm.201109-1666OC. [DOI] [PubMed] [Google Scholar]
- 14.Bilton D, Robinson P, Cooper P, et al. Inhaled dry powder mannitol in cystic fibrosis: an efficacy and safety study. Eur Respir J. 2011;38(5):1071–80. doi: 10.1183/09031936.00187510. [DOI] [PubMed] [Google Scholar]
- 15.Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, et al. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2011;165(9):847–56. doi: 10.1001/archpediatrics.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratjen F, Munck A, Kho P, et al. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: the ELITE trial. Thorax. 2010;65(4):286–91. doi: 10.1136/thx.2009.121657. [DOI] [PubMed] [Google Scholar]
- 17.Corey M, McLaughlin FJ, Williams M, et al. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol. 1988;41(6):583–91. doi: 10.1016/0895-4356(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 18.Stallings VA, Stark LJ, Robinson KA, et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832–9. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Hunter RC, Klepac-Ceraj V, Lorenzi MM, et al. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am J Respir Cell Mol Biol. 2012;47(6):738–45. doi: 10.1165/rcmb.2012-0088OC. [DOI] [PubMed] [Google Scholar]
- 20.McCormick J, Mehta G, Olesen HV, et al. Comparative demographics of the European cystic fibrosis population: a cross-sectional database analysis. Lancet. 2010;375(9719):1007–13. doi: 10.1016/S0140-6736(09)62161-9. [DOI] [PubMed] [Google Scholar]
- 21.Jackson AD, Daly L, Jackson AL, et al. Validation and use of a parametric model for projecting cystic fibrosis survivorship beyond observed data: a birth cohort analysis. Thorax. 2011;66(8):674–9. doi: 10.1136/thoraxjnl-2011-200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrell PM, Rosenstein BJ, White TB, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153(2):S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerem E, Reisman J, Corey M, et al. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992;326(18):1187–91. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 24.Mayer-Hamblett N, Rosenfeld M, Emerson J, et al. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002;166(12 Pt 1):1550–5. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 25.Liou TG, Adler FR, Cahill BC, et al. Priorities for lung transplantation among patients with cystic fibrosis. JAMA. 2002;287(12):1523–4. doi: 10.1001/jama.287.12.1523. author reply 4-5. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Dockery DW, Wypij D, et al. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15(2):75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 27.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 28.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention, N. C. f. H. S. CDC growth charts: United States. Centers for Disease Control and Prevention; 2000. [cited 2013 June 1]. Available from: http://www.cdc.gov/growthcharts/html_charts/bmiagerev.htm. [Google Scholar]
- 30.Sawicki GS, Ren CL, Konstan MW, et al. Treatment complexity in cystic fibrosis: trends over time and associations with site-specific outcomes. J Cyst Fibros. 2013;12(5):461–7. doi: 10.1016/j.jcf.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goss CH, Muhlebach MS. Review: Staphylococcus aureus and MRSA in cystic fibrosis. J Cyst Fibros. 2011;10(5):298–306. doi: 10.1016/j.jcf.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Goss CH, Mayer-Hamblett N, Aitken ML, et al. Association between Stenotrophomonas maltophilia and lung function in cystic fibrosis. Thorax. 2004;59(11):955–9. doi: 10.1136/thx.2003.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders DB, Bittner RC, Rosenfeld M, et al. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol. 2011;46(4):393–400. doi: 10.1002/ppul.21374. [DOI] [PubMed] [Google Scholar]
- 34.Dasenbrook EC, Checkley W, Merlo CA, et al. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303(23):2386–92. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 35.Dasenbrook EC, Merlo CA, Diener-West M, et al. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med. 2008;178(8):814–21. doi: 10.1164/rccm.200802-327OC. [DOI] [PubMed] [Google Scholar]
- 36.Sawicki GS, Rasouliyan L, Pasta DJ, et al. The impact of incident methicillin resistant Staphylococcus aureus detection on pulmonary function in cystic fibrosis. Pediatr Pulmonol. 2008;43(11):1117–23. doi: 10.1002/ppul.20914. [DOI] [PubMed] [Google Scholar]
- 37.Jackson AD, Daly L, Kelleher C, et al. The application of current lifetable methods to compare cystic fibrosis median survival internationally is limited. J Cyst Fibros. 2011;10(1):62–5. doi: 10.1016/j.jcf.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Ortiz JR, Neuzil KM, Victor JC, et al. Influenza-associated cystic fibrosis pulmonary exacerbations. Chest. 2010;137(4):852–60. doi: 10.1378/chest.09-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collaco JM, McGready J, Green DM, et al. Effect of temperature on cystic fibrosis lung disease and infections: a replicated cohort study. PLoS One. 2011;6(11):e27784. doi: 10.1371/journal.pone.0027784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Psoter KJ, De Roos AJ, Wakefield J, et al. Season is associated with Pseudomonas aeruginosa acquisition in young children with cystic fibrosis. Clin Microbiol Infect. 2013;19(11):E483–9. doi: 10.1111/1469-0691.12272. [DOI] [PubMed] [Google Scholar]
- 41.Webb AK, Dudley-Southern R, Jones AM. Development of a modern adult cystic fibrosis centre in Manchester. J R Soc Med. 2010;103(Suppl 1):S15–9. doi: 10.1258/jrsm.2010.s11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appleby J, Devlin N, Parkin D, et al. Searching for cost effectiveness thresholds in the NHS. Health Policy. 2009;91(3):239–45. doi: 10.1016/j.healthpol.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Briesacher BA, Quittner AL, Fouayzi H, et al. Nationwide trends in the medical care costs of privately insured patients with cystic fibrosis (CF), 2001-2007. Pediatr Pulmonol. 2011;46(8):770–6. doi: 10.1002/ppul.21441. [DOI] [PubMed] [Google Scholar]
- 44.The Organisation for Economic Co-operation and Development (OECD) 2013 [cited 2014 January 1]. Available from: http://www.oecd.org/els/health-systems/oecdhealthdata2013-frequentlyrequesteddata.htm.
- 45.Johnson C, Butler SM, Konstan MW, et al. Factors influencing outcomes in cystic fibrosis: a center-based analysis. Chest. 2003;123(1):20–7. doi: 10.1378/chest.123.1.20. [DOI] [PubMed] [Google Scholar]
- 46.Rodman DM, Polis JM, Heltshe SL, et al. Late diagnosis defines a unique population of long-term survivors of cystic fibrosis. Am J Respir Crit Care Med. 2005;171(6):621–6. doi: 10.1164/rccm.200403-404OC. [DOI] [PubMed] [Google Scholar]
- 47.Nick JA, Chacon CS, Brayshaw SJ, et al. Effects of gender and age at diagnosis on disease progression in long-term survivors of cystic fibrosis. Am J Respir Crit Care Med. 2010;182(5):614–26. doi: 10.1164/rccm.201001-0092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schechter MS, Shelton BJ, Margolis PA, et al. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331–7. doi: 10.1164/ajrccm.163.6.9912100. [DOI] [PubMed] [Google Scholar]
- 49.Taylor-Robinson DC, Smyth R, Diggle PJ, et al. A longitudinal study of the impact of social deprivation and disease severity on employment status in the UK cystic fibrosis population. PLoS One. 2013;8(8):e73322. doi: 10.1371/journal.pone.0073322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


