Abstract
Introduction
Obesity is the major risk factor for the development of osteoarthritis (OA); however, the mechanisms involved are not clearly understood. Obesity is associated with increased production of adipokine and elevated levels of circulating free fatty acids (FFA). A recent study has shown that saturated fatty acid palmitate induced pro-inflammatory and pro-apoptotic pathways in chondrocytes. Meniscus has been shown to be more susceptible than articular cartilage to catabolic stimuli. Thus, the aim of this study was to determine the effect of FFA (specifically, palmitate) on meniscus cells.
Methods
Cultured primary porcine meniscus cells were stimulated with 500 μM free fatty acids (palmitate and oleate) for 24 hours to induce endoplasmic reticulum (ER) stress. After treatment, cell lysates were prepared and immunoblotted for C/EBP homologous protein (CHOP). To determine the activation of unfolded protein response (UPR) signaling, cell lysates were probed for cJun n-terminal kinase (JNK), cleaved caspase -3 and Xbp-1s, an alternative mRNA splicing product generated due to Ire1α activation.
Results
Treatment of isolated primary meniscus cells with palmitate but not oleate induced expression of CHOP and Xbp-1s. Palmitate treatment of meniscus cells also activated JNK and increased expression of caspase-3, thus promoting apoptosis in meniscus cells.
Conclusions
Palmitate induces ER stress and promotes apoptotic pathways in meniscus cells. This is the first study to establish ER stress as a key metabolic mechanistic link between obesity and OA, in addition to (or operating with) biomechanical factors.
Keywords: Meniscus, endoplasmic reticulum stress, unfolded protein response (UPR), free fatty acids, apoptosis
Introduction
Obesity is one of the major risk factors for the development of osteoarthritis (OA) (1); however, the mechanisms involved are not clearly understood. Obesity is associated with elevated levels of free fatty acids (FFA) (2), resulting in lipid accumulation in non-adipose tissues. This process leads to lipotoxicity, characterized by cell dysfunction, inflammation, and cell death. Induction of endoplasmic reticulum (ER) stress is one mechanism proposed for lipid toxicity. ER is an important cell organelle involved in protein and lipid biosynthesis. Disruption of this highly synchronized process leads to accumulation of unfolded proteins, resulting in ER stress and triggering ER signaling or the unfolded protein response (UPR).The UPR pathway is activated to restore ER homeostasis by upregulating ER chaperones, attenuating protein translation, and degrading misfolded proteins (3). UPR is mediated by the ER stress transducers IRE1α (inositol requiring ER-to-nucleus signal kinase-1), ATF-6 (activating transcription factor-6), and PERK (PKR [double–stranded-RNA dependent protein kinase]-like ER kinase). Normally, these proteins are inactive and are bound to the ER chaperone, GRP 78/BiP. However, under stress conditions, BiP disassociates itself from these proteins and bind to unfolded proteins, thus activating the ER stress transducers and UPR signaling (4 and references therein).
Studies have shown that joint tissue and synovial fluid accumulates free fatty acids (FFAs) and OA severity correlates with FFA levels (4). A recent study showed that saturated fatty acid palmitate induced pro-apoptotic and pro-inflammatory pathways in chondrocytes (5). OA is a disease of the whole joint; multiple tissues (articular cartilage, bone, ligaments, and meniscus) are involved in its pathology. Meniscus tissue, like articular cartilage, is exposed to similar biomechanical forces and biochemical environment, yet its role in obesity-linked OA is not clearly understood. A recent study showed that increase in body mass index (BMI) negatively regulated gene transcripts associated with extracellular matrix in human meniscus (6). In addition, meniscus was more susceptible than articular cartilage to catabolic effects of adipokines (7). These studies suggest that meniscus could be a primary tissue affected in obesity-linked OA. Thus, in the current study we examined the effect of FFAs (specifically, palmitate) on meniscus cells. Palmitate induced ER stress and activated UPR signaling in meniscus cells, and activation of UPR (Ire1α) signaling by palmitate induced apoptotic pathways in these cells.
Materials and Methods
Collagenase-P was purchased from Roche Applied Science. Pronase was purchased from Calbiochem. Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 (1:1), antibiotics, fetal bovine serum and BCA reagent were obtained from Thermo Fisher (Rockford, IL). Nitrocellulose membranes and ECL chemiluminescence detection kits were purchased from GE Life Sciences (Pittsburgh, PA). 4-phenyl butyric acid (PBA), low-endotoxin, fatty acid-free bovine serum albumin, FFA (palmitate & oleate), tunicamycin, and thapsigargin were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies to CHOP, Xbp1s, cleaved caspase-3, phospho-JNK, total JNK, and GAPDH were from Cell Signaling Technology (Danvers, MA). 4μ8c, an Ire1α inhibitor, was purchased from Axon Medchem (Reston, VA).
Methods
Meniscus cell isolation and culture conditions
Meniscus tissue was harvested from the knee joints of 3-month-old pigs (n=16), donated by the Departments of Cardiovascular Surgery and Plastic and Reconstructive Surgery at Wake Forest School of Medicine. Meniscus cells were isolated under aseptic conditions by sequential enzymatic digestion at 37°C using pronase 2 mg/ml in serum-free DMEM/F-12/antibiotics for 1 hour, followed by overnight digestion with collagenase-P at 0.25 mg/ml in DMEM/F-12 (5% fetal bovine serum). Viability of isolated cells was determined using trypan blue and cells were counted using a hemocytometer. Monolayer cultures were established by plating cells in six-well plates at 2 × 106 cells/ml in DMEM/F-12 medium supplemented with 10% fetal bovine serum. Cells were maintained for approximately 3 to 5 days with feedings every 2 days until they reached 100% confluency prior to experimental use.
BSA-free fatty acid conjugates
FFA (palmitate and oleate) were conjugated to low-endotoxin, fatty acid-free bovine serum albumin as described previously (8). For experiments, BSA-FFA conjugate was diluted in culture media.
Meniscus cell stimulation and immunoblotting
Confluent monolayers of porcine meniscus cells were made serum-free overnight before treating with FFA (500 μM) or 2 μM of thapsigargin and 1μg/ml tunicamycin overnight. In some experiments, cells were pretreated with 1mM of phenyl butyric acid (PBA) or Ire1α inhibitor (4μ8c) for 30 minutes followed by treatment with palmitate or oleate. Treatment of cells with inhibitor did not affect meniscus cell viability (data not shown). After incubation, cells were washed with PBS and lysed with lysis buffer that contained 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM tetrapyrophosphate, 1 mM glycerol phosphate, 1 mM Na3VO4, 1 μl/ml leupeptin, and 1 mM phenyl methyl sulfonyl fluoride. Lysates were centrifuged to remove insoluble material, and the soluble protein concentration was determined with BCA reagent. Samples containing equal amounts of total protein were separated by SDS-PAGE, transferred to nitrocellulose, and probed for signaling proteins. Immunoreactive bands were detected using the ECL system. All immunoblotting experiments were repeated at least three times with similar results.
Statistics
The data presented in study are representative of at least three independent experiments performed with cells obtained from different pigs.
Results
Palmitate-induced ER stress in meniscus cells
Meniscus cells responded to palmitate, but not to oleate treatment. Treatment of meniscus cells with palmitate increased the expression of C/EBP homologous protein (CHOP), a marker for ER stress (Fig 1A). Tunicamycin and thapsigargin are known ER stress inducers, which we have used as positive controls in our experiments. Pretreatment of cells with 4-phenylbutyric acid (PBA), a small molecule chemical chaperone, reduced the palmitate-induced expression of CHOP (Fig 1B). These data suggest that palmitate induced ER stress in meniscus cells.
Figure 1.
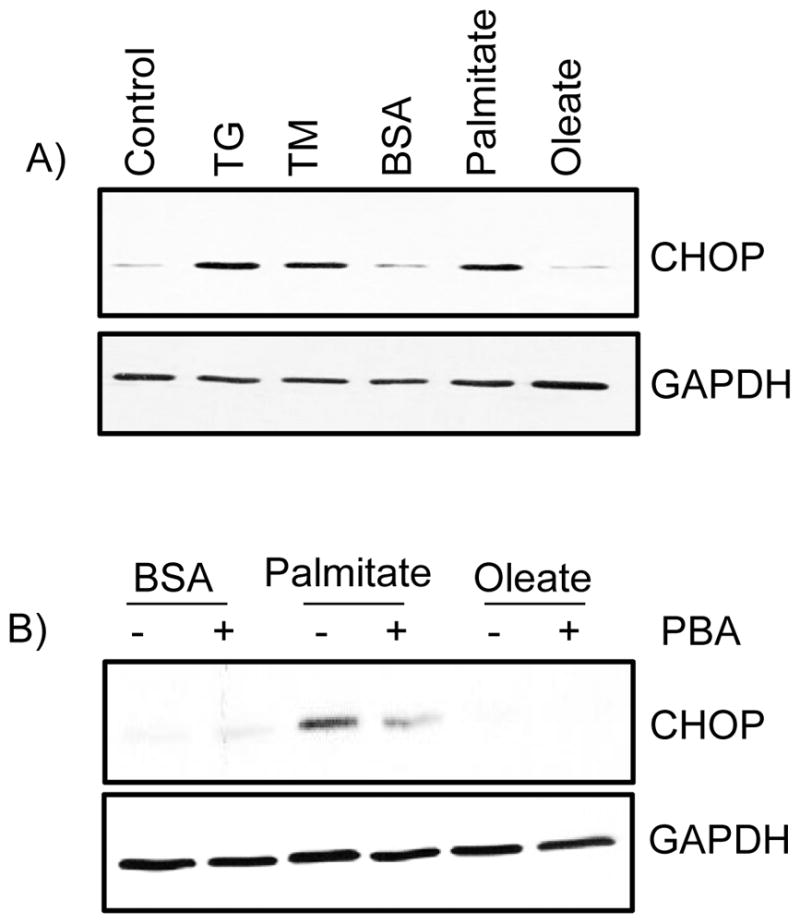
The free fatty acid palmitate induces endoplasmic reticulum (ER) stress. A) Porcine primary meniscus cells were stimulated with 500 μM free fatty acids (palmitate and oleate), 2 μM of thapsigargin, and 1μg/ml tunicamycin overnight. B) In other experiments, cells were pretreated with or without 1mM phenyl butyric acid (PBA) for 30 minutes followed by treatment with BSA-conjugatedpalmitate or oleate (500μM) overnight, After incubation, cell lysates were immunoblotted with antibodies to CHOP. Blots were stripped and reprobed with GAPDH as a loading control. Data are representative of at least three independent experiments performed with cells obtained from different pigs.
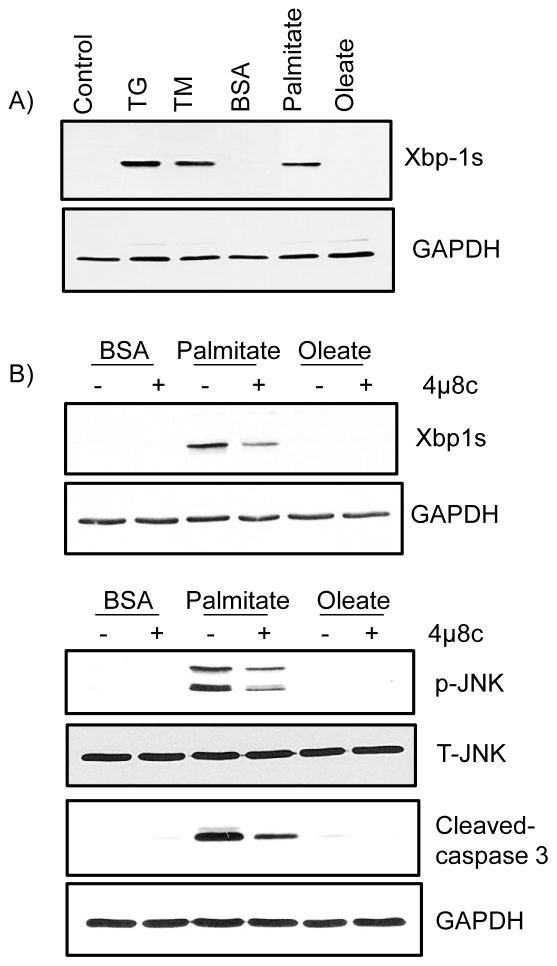
Palmitate promotes apoptosis via the Ire1α UPR pathway
UPR is mediated by the ER resident proteins ATF6, PERK, and Ire1α. Ire1α plays an important role in ER stress-induced apoptosis. Hence, we were interested in examining if palmitate activates Ire1α signaling. Palmitate treatment of meniscus cells induced expression of Xbp1s, an alternative mRNA splicing product generated due to Ire1α activation (Fig 2A). In addition, stimulation of cells with palmitate also induced both increased cJun n-terminal kinase (JNK) phosphorylation and cleaved caspase-3 expression (Fig 2B). Treating cells with an Ire1α inhibitor before palmitate stimulation blocked palmitate-induced expression of Xbp1s, cleaved caspase-3, and JNK phosphorylation (Fig 2B). These data suggest that palmitate activates Ire1α signaling and promotes apoptotic pathways in meniscus cells.
Figure 2.
Palmitate activates the Ire1α arm of UPR signaling and promotes apoptosis in meniscus cells. A) Cells were stimulated with 500 μM free fatty acids (palmitate and oleate), 2 μM of thapsigargin, and 1μg/ml tunicamycin overnight. B) In other experiments, cells were pretreated with or without 60 nM Ire1α inhibitor (4μ8c) for 30 minutes followed by treatment with BSA-conjugated palmitate or oleate (500μM) overnight. After incubation, cell lysates were immunoblotted with antibodies specific for Xbp1s, cleaved caspase-3 and phosphorylated JNK (Thr183/Tyr185). Blots were stripped and reprobed with GAPDH and total JNK as a loading control. Data are representative of at least three independent experiments performed with cells obtained from different pigs.
Discussion
The pathogenesis of OA is complex; obesity-associated metabolic factors are known to promote OA. However, OA is now considered as a disease in which the individual tissues in a joint (e.g. articular cartilage, meniscus, synovium, and ligament) contribute to its overall pathogenesis. Emerging evidence shows that apoptosis plays a key role in OA pathology (9). Mice consuming a high-fat diet showed apoptosis in chondrocytes (10), suggesting that metabolic factors promote cell death; however, the mechanisms involved are not clearly understood.
In this study, we showed that the FFA palmitate induced apoptotic pathways in meniscus cells. Palmitate treatment of meniscus cells increased expression of CHOP, a pro-apoptotic molecule and marker for ER stress. In addition, palmitate also activated JNK and induced expression of cleaved caspase-3 via Ire1α signaling. Treatment of cells with PBA, a small molecule chemical chaperone or a chemical inhibitor that blocks Ire1α signaling (4μ8c), inhibited the effects of palmitate.
ER stress plays an important role in several human diseases, but its role in OA pathology is not clearly understood. One of the downstream effects of severe ER stress is cell death (3). Studies have shown that ER stress in chondrocytes induce expression of Cox2, MMP-13, mRNA and promote cell death/apoptosis; however, the mechanism involved is not clear. In this study, we found that palmitate induced CHOP expression. CHOP a pro-apoptotic molecule that is expressed at all levels during ER stress, and its expression is regulated by all 3 arms of UPR signaling. The primary function of CHOP is to aid in alleviating ER stress by restoring ER homeostasis, via upregulating genes involved in protein folding and ER-associated degradation (ERAD) of misfolded proteins (3). However, under prolonged and sustained ER stress, CHOP promotes apoptosis. CHOP induces apoptosis by suppressing the expression of Bcl-2, a pro-survival protein (11). CHOP promotes apoptosis by activating the genes that promote an oxidative environment in the ER and excessive protein load (12). Thus, CHOP plays an important role in ER stress–mediated cell death. Forced expression of CHOP in chondrocytes promoted apoptosis and enhanced the catabolic effect of IL-1β (13). Mice lacking CHOP showed less apoptosis in response to ER stress and also showed less cartilage degeneration and severity of OA (14). Taken together, these data show that CHOP could play an important role in the development of OA.
Another apoptotic pathway that is activated during ER stress is via Ire1α. Under severe ER stress, Ire1α interacts with an adaptor molecule tumor necrosis factor receptor (TNFR)-associated factor 2 (TRAF2) and then recruits apoptosis signal-regulating kinase (ASK1) a mitogen activated protein kinase kinase kinase (MAPKKK) that activates JNK. Activation of JNK promotes apoptosis via the caspase system (15). In our current study, treatment of meniscus cells with palmitate induced phosphorylation of JNK and increased the expression of cleaved caspase- 3. Knocking down the activity of Ire1α inhibited palmitate- induced phosphorylation of JNK and expression of cleaved caspase-3. Taken together, these studies demonstrate that activation of the Ire1α arm of UPR signaling induced apoptosis in meniscus cells.
In conclusion, to the best of our knowledge, this is the first study to show that palmitate induced ER stress in meniscus cell. Induction of ER stress by palmitate activated UPR (Ire1α) signaling, which then promoted apoptotic pathways in meniscus cells. Our data suggest that ER stress/UPR signaling mediates apoptosis and may play a major role in obesity–linked OA.
Acknowledgments
We thank Dr. James Jordan from the Departments of Cardiothoracic Surgery, and Plastic and Reconstructive Surgery at Wake Forest School of Medicine for providing the porcine meniscus tissue.
Funding
This work was supported by the National Institute of Arthritis, Musculoskeletal and Skin Diseases (R01 AR550699 and R21 AR 558535) to R.R.Y. The funding agency played no role in the study design, data collection, analysis and interpretation of data, writing of the report, or decision to submit the paper for publication.
Footnotes
Author contributions
Conception and Design: RRY, JH. Acquisition of data: JH. Analysis and Interpretation of data: RRY, JH. Both authors revised the article for important intellectual content and approved the final version of the manuscript.
Statement of competing interests:
No conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jamie Haywood, Email: jhaywood@wakehealth.edu.
Raghunatha R. Yammani, Email: ryammani@wakehealth.edu.
References
- 1.Kluzek S, Newton JL, Arden NK. Is osteoarthritis a metabolic disorder? Br Med Bull. 2015;115:111–121. doi: 10.1093/bmb/ldv028. [DOI] [PubMed] [Google Scholar]
- 2.Opie LH, Walfish PG. Plasma free fatty acid concentrations in obesity. N Engl J Med. 1963;268:757–760. doi: 10.1056/NEJM196304042681404. [DOI] [PubMed] [Google Scholar]
- 3.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 4.Lippiello L, Walsh T, Fienhold M. The association of lipid abnormalities with tissue pathology in human osteoarthritic articular cartilage. Metabolism. 1991;40:571–576. doi: 10.1016/0026-0495(91)90046-y. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Garcia O, Rogers NH, Smith RG, Lotz MK. Palmitate has proapoptotic and pro-inflammatory effects on articular cartilage and synergizes with interleukin-1. Arthritis Rheumatol. 2014;66:1779–88. doi: 10.1002/art.38399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rai MF, Patra D, Sandell LJ, Brophy RH. Relationship of gene expression in the injured human meniscus to body mass index: a biologic connection between obesity and osteoarthritis. Arthritis Rheumatol. 2014;66:2152–64. doi: 10.1002/art.38643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimuta JF, Levenston ME. Meniscus is more susceptible than cartilage to catabolic and anti-anabolic effects of adipokines. Osteoarthritis Cartilage. 2015;23:1551–62. doi: 10.1016/j.joca.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das SK, Chu WS, Mondal AK, Sharma NK, Kern PA, Rasouli N, Elbein SC. Effect of pioglitazone treatment on endoplasmic reticulum stress response in human adipose and in palmitate-induced stress in human liver and adipose cell lines. Am J Physiol Endocrinol Metab. 2008;295:E393–400. doi: 10.1152/ajpendo.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamli Z, Sharif M. Chondrocyte apoptosis: a cause or consequence of osteoarthritis? Int J Rheum Dis. 2011;14:159–66. doi: 10.1111/j.1756-185X.2011.01618.x. [DOI] [PubMed] [Google Scholar]
- 10.Iwata M, Ochi H, Hara Y, Tagawa M, Koga D, Okawa A, Asou Y. Initial responses of articular tissues in a murine high-fat diet-induced osteoarthritis model: pivotal role of the IPFP as a cytokine fountain. PLoS One. 2013;8:e60706. doi: 10.1371/journal.pone.0060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husa M, Petursson F, Lotz M, Terkeltaub R, Liu-Bryan R. C/EBP homologous protein drives pro-catabolic responses in chondrocytes. Arthritis Res Ther. 2013;15:R218. doi: 10.1186/ar4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takada K, Hirose J, Senba K, Yamabe S, Oike Y, Gotoh T, Mizuta H. Enhanced apoptotic and reduced protective response in chondrocytes following endoplasmic reticulum stress in osteoarthritic cartilage. Int J Exp Pathol. 2011;92:232–42. doi: 10.1111/j.1365-2613.2010.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhanasekaran DN, Reddy PE. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]