Abstract
Non-alcoholic fatty liver disease (NAFLD) is a liver manifestation of metabolic syndrome. Overconsumption of high-fat diet (HFD) and increased intake of sugar sweetened beverages are major risk-factors for development of NAFLD. Today the most commonly consumed sugar is high fructose corn syrup. Hepatic lipids may be derived from dietary intake, esterification of plasma free fatty acids (FFA) or hepatic de novo lipogenesis (DNL). A central abnormality in NAFLD is enhanced de novo lipogenesis. Hepatic de novo lipogenesis is increased in individuals with NAFLD, while the contribution of dietary fat and plasma FFA to hepatic lipids is not significantly altered. The importance of DNL in NAFLD is further established in mouse studies with knockout of genes involved in this process. Dietary fructose increases levels of enzymes involved in DNL even more strongly than HFD. Several properties of fructose metabolism make it particularly lipogenic. Fructose is absorbed via portal vein and delivered to the liver in much higher concentrations as compared to other tissues. Fructose increases protein levels of all DNL enzymes during its conversion into triglycerides. Additionally, fructose supports lipogenesis in the setting of insulin resistance as fructose does not require insulin for its metabolism and it directly stimulates SREBP1c, a major transcriptional regulator of DNL. Fructose also leads to ATP depletion and suppression of mitochondrial fatty acid oxidation resulting in increased production of reactive oxygen species. Furthermore fructose promotes ER stress and uric acid formation, additional insulin independent pathways leading to DNL. In summary, fructose metabolism supports DNL more strongly than HFD and hepatic DNL is a central abnormality in NAFLD. Disrupting fructose metabolism in the liver may provide a new therapeutic option for the treatment of NAFLD.
Keywords: NAFLD, NASH, Fructose, HFD, Metabolism, De novo lipogenesis, Liver
High fat and high sugar intake correlate with obesity and NAFLD development
There is a worldwide epidemic of obesity, type 2 diabetes and metabolic syndrome (1; 2). Non-alcoholic fatty liver disease (NAFLD) is a liver manifestation of metabolic syndrome and it is estimated to affect 1 billion individuals worldwide (3). While host genetics, western style diet and components of the gut microbiome contribute to the development of obesity and NAFLD, positive energy balance, driven by increased caloric intake and decreased activity, is a prerequisite. Consumption of high fat diet was initially believed to be the primary driver of obesity epidemic, as its consumption has increased over the last century. As a product of intense research, public awareness and policy changes, total intake of fat has stabilized in the last 30 years, and the percentage of calories ingested from saturated fat has decreased (4). Notwithstanding, the prevalence of obesity and NAFLD has not improved pointing to the importance of other dietary or environmental drivers.
As low fat foods became more highly sought after, the food industry has replaced these calories by another highly palatable energy source, the refined sugar. Thus, even more dramatic than the increase in dietary fat, has been an increase in dietary fructose intake over the same time period. Before 1900, Americans consumed approximately 15 g of fructose per day (4% of total cal), mainly through consumption of fruits and vegetables. By 1940’s, fructose intake had increased to 24 g per day (5% of total cal); by 1977, it was 37 g per day (7% of total cal); and by 1994, 55 g per day (10% of total cal)(5). Adolescents today consume 15% of total calories from refined sugar and 10% of population consumes 25% or more of their total calories from refined sugar (6). Food consumption survey data from the US Department of Agriculture, reported by the National Health And Nutrition Examination Survey (NHANES) program between 1971 and 2004, indicated that the observed increase in total energy intake is accounted almost entirely by increased carbohydrate consumption (5). In spite of substantial increase in sugar and fructose in particular, there is a lack of in depth understanding of fructose metabolism and its consequences on health.
Non-alcoholic fatty liver disease (NAFLD) is defined as excessive hepatic lipid accumulation in individuals who have no apparent liver disease and whose alcohol intake is less than 30 g per day for men and 20 g per day for women (~10 g of alcohol per one drink unit) (7). As estimated histologically, the fat concentration in hepatocytes is normally less than 5%, with amounts exceeding this threshold being classified as steatosis (8). Whereas excessive accumulation of triglycerides (TG) in hepatocytes is a hallmark of NAFLD, this may not be pathogenic, as NAFLD may be reversible with weight loss and exercise. However, steatosis with elements of hepatocellular death, and inflammation is termed non-alcoholic steatohepatitis (NASH) which may progress to fibrosis, cirrhosis and liver failure.
NAFLD is the most common cause of elevated liver enzymes and is the third most common indication for liver transplantation (9). NAFLD correlates strongly with other parameters of metabolic syndrome such as insulin resistance and cardiovascular disease. In fact, cardiovascular disease represents the most common cause of death in patients with NAFLD and NASH ((10–13) reviewed in (14)). Increased consumption of sugar sweetened beverages is linked with development and progression of NAFLD but also it is associated with development of obesity (15), type 2 diabetes mellitus (16; 17), dyslipidemia (18) and cardiovascular disease (19)
Hepatic de novo lipogenesis
Hepatic lipid accumulation may be a product of hepatic de novo lipogenesis, esterification of plasma free fatty acids or increased dietary fat intake. Dietary lipids and esterification of plasma free fatty acids do play a role in development of NAFLD. Dietary intake of HFD is a risk factor for development of NAFLD in human subjects and most animal models utilize HFD to induce NAFLD. Increased uptake of plasma FFA derived from lipolysis also significantly contributes to NAFLD development, as studies of liver specific knockout of fatty acid transporters, FATP-2 and FATP-5 report protection from NAFLD development (20; 21), whereas liver specific overexpression of fatty acid translocase (CD36) exacerbates the condition (22). In this review, however, we will focus on hepatic de novo lipogenesis as a central abnormality in development of NAFLD.
De novo lipogenesis is a process by which lipids are endogenously synthesized from dietary sources, usually carbohydrates, or stored energy depots. The process may be divided into three sequential steps: fatty acid synthesis, fatty acid elongation/desaturation, and assembly into triglycerides. Dietary carbohydrates, mainly consumed either as starch (glucose polymer) or table sugar (sucrose, a glucose-fructose disaccharide) are broken down to six carbon monosaccharides, glucose or fructose, whose metabolism converges onto production of three carbon intermediates, glyceraldehyde-3 phosphate (GA3P) and dihydroxyacetone phosphate (DHAP). These intermediates may be interconverted or further metabolized to pyruvate, the end product of cytoplasmic carbohydrate metabolism (reviewed in (23).
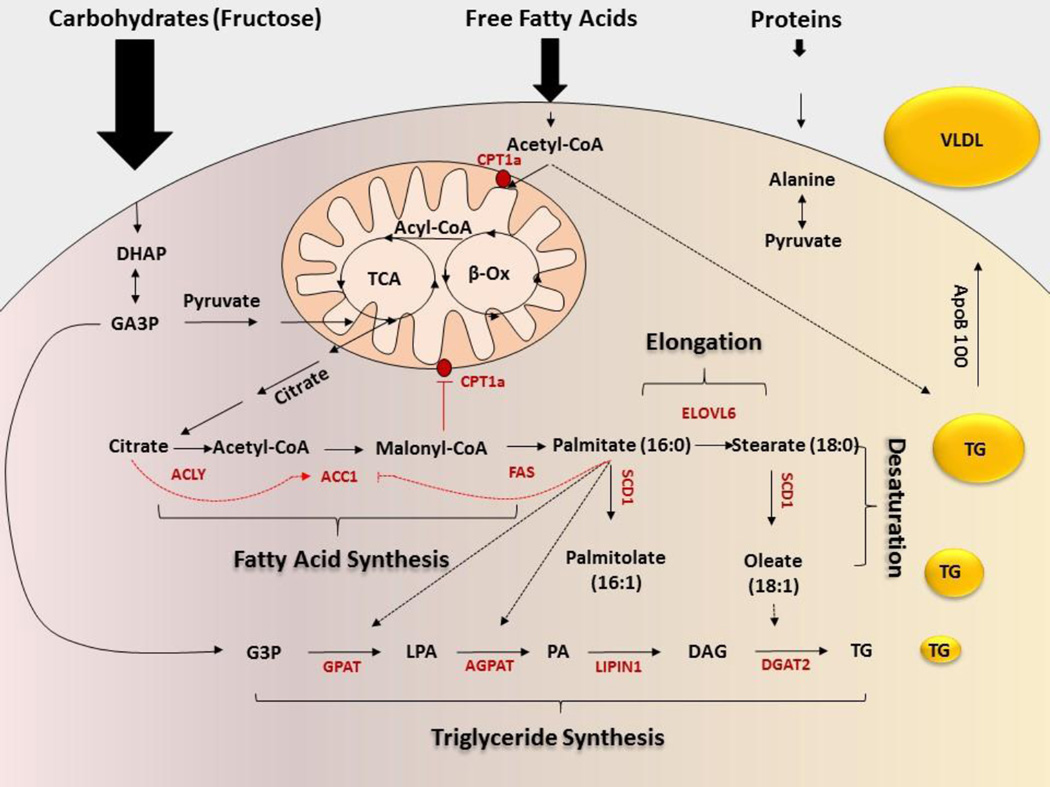
Pyruvate may enter mitochondria, where it is converted to acetyl-CoA, to be used in tricarboxylic acid (TCA) cycle, (also known as citric acid cycle), for energy production. When energy stores are plentiful, TCA intermediates accumulate, and citrate is transported back into the cytoplasm by mitochondrial tricarboxylate transport system (24). Citrate is converted to acetyl-CoA, by the action of adenosine triphosphate citrate lyase (ACL), which is the first step of endogenous fatty acid synthesis (Figure 1). Furthermore, citrate is an allosteric activator of cytoplasmic acetyl-CoA carboxylase (ACC), whose action is to convert acetyl-CoA to malonyl-CoA, thus initiating de novo lipogenesis (25; 26). Malonyl-CoA, is the primary carbon source utilized for endogenous fatty acid synthesis (27). Fatty acid synthase (FAS) sequentially utilizes malonyl-CoA to extend the growing fatty acyl chain by two carbons, forming a 16 carbon saturated fatty acid, palmitate, the major product of fatty acid synthesis. Enzymatic action of ACC is the key regulatory step of endogenous lipid synthesis. When rates of de novo lipogenesis are high, malonyl-CoA accumulates in the cytoplasm and inhibits carnitine palmitoyltransferase 1alpha (CTP1α), the rate limiting enzyme of fatty acid transport and utilization in mitochondria. This ensures that a futile cycle of fatty acid synthesis and degradation do not occur simultaneously. Furthermore, increased cytoplasmic concentrations of palmitate allosterically inhibit ACC activity and reduce rates of de novo lipogenesis. ACC may also be inhibited by cyclic adenosine monophosphate (AMP)-dependent phosphorylation induced by glucagon or by AMP activated protein kinase (AMPK) (reviewed in (28), providing further negative feedback on the process.
Figure 1. Hepatic de novo lipogenesis.
Dietary carbohydrates, lipids and proteins may be used as substrates for de novo lipogenesis. Carbohydrates are metabolized to three carbon intermediates dihydroxyacetone phosphate (DHAP) and glyceraldehyde three phosphate (GA3P) which are further metabolized to pyruvate. Pyruvate enters mitochondria to be used for energy production. When energy stores are plentiful citrate is transported to cytoplasm where by the action of ATP citrate lyase (ACL) it is converted to acetyl-CoA. Acetyl CoA carboxylase (ACC) converts acetyl-CoA to malonyl CoA. Fatty acid synthase (FAS) sequentially adds acetyl-CoA to growing fatty acid chain to form saturated fatty acids, mainly palmitate. Palmitate may be further elongated to stearate or longer fatty acids by the action of elongation of very long chain fatty acids (ELOVL6). Stearoyl CoA desaturase (SCD1) converts saturated fatty acids to monounsaturated fatty acids. Glycerol-3-phosphate acyltransferase (GPAT), adds acyl-CoA to glycerol-3 phosphate (G3P) to form lysophosphatidic acid (LPA). The enzymatic action of 1-acylglycerol-3-phosphate acyltransferase (AGPAT) adds second acyl-CoA to produce phosphatidic acid (PA), which is then dephosphorylated by Lipin1 (LPIN1) to form 1,2-diacylglycerol (DAG). Diacylglycerol acyltransferase (DGAT) converts diacylglycerols into triglycerides (TG) which may be stored in the liver or assembled into VLDL and exported to circulate in the blood. Dietary lipids or adipocyte lipolysis supplies free fatty acids which are converted in hepatocytes to acetyl-CoAs. They may be used in mitochondria for energy production or be exported back to cytosol as citrate and used for de novo lipogenesis similar to carbohydrates. Additionally, acetyl-CoA may be directly assembled into TGs by the action of DGAT bypassing the majority of enzymes involved in de novo lipogenesis. Proteins are degraded to amino acids, some of which may be used for gluconeogenesis and/or ketogenesis.
Palmitate (C16:0) is the primary fatty acid synthesized endogenously (reviewed in (29)), but it may be elongated to the 18-carbon fatty acid stearate or to longer fatty acids by the enzyme elongation of very long chain fatty acids protein 6 (ELOVL6). Both palmitate and stearate may be desaturated to form palmitolate (16:1) and oleate (18:1), respectively, by stearoyl-CoA desaturase (SCD1), which converts saturated to monounsaturated fatty acids. Once fatty acids are synthesized, elongated and desaturated, they may be esterified to the glycerophosphate backbone that is also supplied by carbohydrate metabolism to form more complex lipids, including triglycerides.
The first step of triglyceride assembly is catalyzed by glycerol-3-phosphate acyltransferase (GPAT), which adds acyl-CoA to glycerol-3 phosphate to form lysophosphatidic acid. The enzymatic action of 1-acylglycerol-3-phosphate acyltransferase (AGPAT) adds second acyl-CoA to produce phosphatidic acid, which is then dephosphorylated by Lipin1 (LPIN1) to form 1,2-diacylglycerol. Unsaturated fatty acids such as oleate are preferentially utilized for addition of the third fatty acyl-CoA because its cis double bond is oriented away from the triglyceride core. Some studies suggest that oleate thus promotes triglyceride synthesis and thereby protects against palmitate-induced lipotoxicity (30). This final step of triglyceride synthesis is catalyzed by diacylglycerol acyltransferase (DGAT), which converts diacylglycerols into triglycerides. This step is critical because the hepatic accumulation of diacylglycerol is associated with insulin resistance, while liver accumulation of biochemically inert triglycerides may be dissociated from insulin resistance We and others showed that 1,2-diacylglycerols induce insulin resistance by activating novel and atypical isoforms of protein kinase C (PKC), (31–34) (reviewed in (35)) and perhaps to a lesser extent classical PKCs (36). In support of this mechanism, the hepatic concentration of diacylglycerols is increased in patients with NALFD (37). In mice, liver-specific overexpression of DGATs does not result in insulin resistance in spite of augmented accumulation of triacylglycerol in the liver (38). Conversely, suppression of DGAT2 in mice decreases diacylglycerol concentrations and PKC activation, and may increase hepatic insulin sensitivity (39)
Evidence that de novo lipogenesis is prominent abnormality in NAFLD
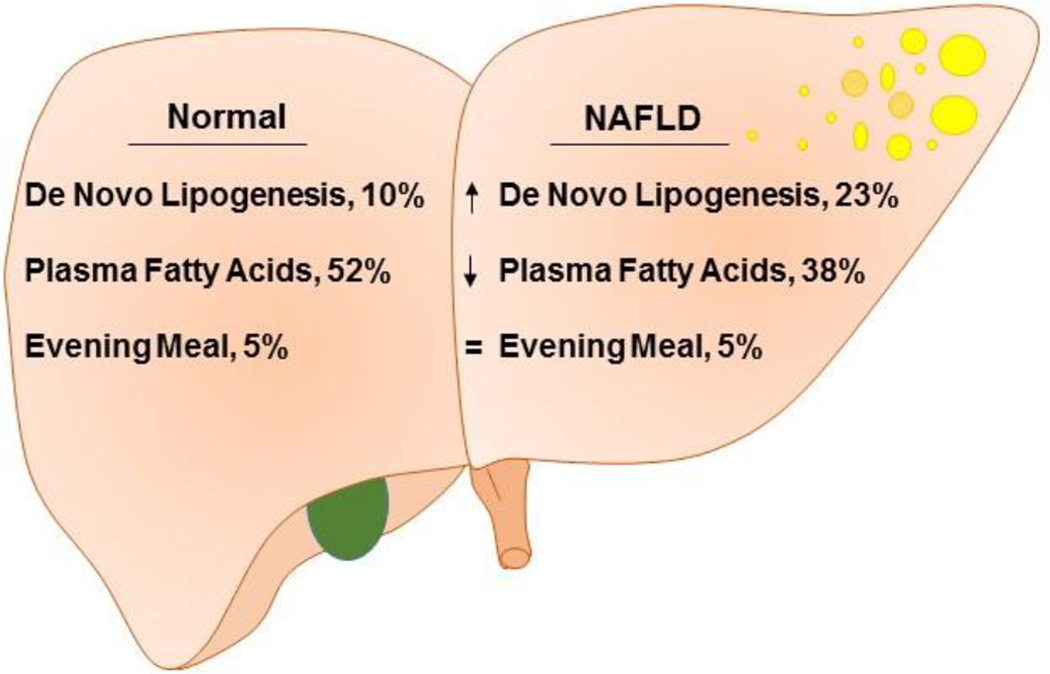
Traditionally, hepatic de novo lipogenesis has not been considered a major contributor to stored liver lipids. This notion was based on initial estimates showing that < 5% of stored triglycerides were derived from de novo lipogenesis (40). However, these initial studies were performed under fasting conditions, when lipid synthesis is at a nadir (41; 42) and on lean subjects who have much lower rates of lipogenesis than obese and insulin resistant patients (43). A more recent analysis of obese patients with NAFLD found that while 60% of liver triglycerides are derived from plasma free fatty acid, which themselves are derived from adipose tissue lipolysis, 26% of liver fat in these individual came from de novo lipogenesis and another 15% from dietary fat (44). However, it was not until a study comparing obese individuals with high versus low liver fat content that de novo lipogenesis emerged as a central factor in NAFLD (Figure 2) (45). In this study, Lambert at al. demonstrated that the contribution of fatty acids from de novo lipogenesis was 3.5-fold greater in the high liver fat group (0.14 ± 0.03 mmol/L) as compared to low liver fat group (0.04 ± 0.01 mmol/L). In these individuals, the proportion of hepatic triglycerides deriving from dietary source was not different, (5% in both groups) and the proportion of triglycerides synthesized from plasma FFA in low liver-fat group, was significantly higher than in high liver-fat group (52% vs. 38%). By contrast, triglycerides synthesized via de novo lipogenesis was significantly higher in high liver-fat group, compared to low liver-fat group (10% vs. 23%) (45). This study suggests that, while adipose derived free fatty acids contribute the largest proportion of liver triglycerides in subjects with normal liver-fat, the contribution of this pathway does not increase in NAFLD subjects, whereas hepatic de novo lipogenesis does increase in this patient population. When taken together, the data from this study indicate that the primary difference in nutrient homeostasis in individuals with and without NAFLD is attributed to hepatic de novo lipogenesis.
Figure 2. De novo lipogenesis is a central abnormality in NAFLD.
Lambert et al., used isotope analysis to compare de novo lipogenesis and fatty acid flux in obese subjects with and without NAFLD. The proportion of hepatic triglycerides deriving from the evening meal was not different, 5% versus 5%, at the end of the study. The proportion of triglycerides synthesized from plasma FFA in low liver-fat group (52%), was significantly higher than in high liver-fat group (38%). By contrast, triglycerides synthesized via de novo lipogenesis was significantly higher in high liver-fat group (23%), compared to low liver-fat group (10%).
Other studies have presented data that support the concept that increased DNL may be a dominant abnormality in NAFLD (46) and substantiated the claim that DNL is significantly increased in subjects with NAFLD compared with healthy controls (46; 47). In one study, short term carbohydrate overfeeding for 3 weeks increased liver fat by 27 percent, while total body weight increased by only 2 percent. Conversely, following six months of a hypocaloric diet, the same subjects lost 25% of liver fat and 4 % of body weight (48). Tracer studies have further demonstrated that fructose is acutely (during short 4 hours observation period) incorporated into both glycerol and free fatty acids, the two components of triglycerides, whereas glucose was not incorporated into triglycerides either (49). Thus, fructose strongly supports de novo lipogenesis, a dominant driver of NAFLD pathogenesis.
Enzymes of de novo lipogenesis and NAFLD
The importance of endogenous de novo lipogenesis to NAFLD development may also be inferred from studies utilizing genetically engineered mice with single enzyme perturbation in this pathway (reviewed in (50; 51). Enzymes involved in hepatic fatty acid synthesis pathway including ACL, ACC, FAS, SCD1 and ELOVL6 has been studied extensively in mice, both as global and liver-specific knockouts.
The first enzyme of de novo lipogenesis is adenosine triphosphate citrate lyase. Global knock out of ACL results in utero death, indicating the essential role of de novo lipogenesis in embryonic development (52). On the other hand, pharmacologic inhibition of ACL results in hypolipidemia and resistance to high fat diet (HFD)-induced obesity (53–55). Furthermore, knock out of ACL in the liver markedly reduces rate of hepatic de novo lipogenesis, resulting in protection against hepatic steatosis and insulin resistance in obese, hyperglycemic db/db mice. (56). Wang et al, found diet-specific effects, where knockdown of ACL resulted in increased liver triglycerides and increased protein levels of FAS and ACC1 on low fat diet, while on HFD, FAS was decreased with no changes in ACC1 (57). This suggests that a relatively high carbohydrate chow diet is sufficient to overcome the loss of ACL. Indeed, ACL is nutritionally regulated with relatively low expression in fasting and a dramatic upregulation upon refeeding, especially when feeding involves a high-carbohydrate, low-fat diet (58).
The second enzyme involved in hepatic de novo lipogenesis, acetyl-CoA carboxylase exists as two isoforms. ACC1 is cytosolic and produces malonyl-CoA required for lipogenesis, while ACC2 is mitochondrial and is thought to be involved in the negative regulation of mitochondrial β-oxidation by modulating local malonyl-CoA levels. Similar to ACL, global inactivation of ACC1 leads to embryonic lethality (59). On the other hand, global ACC2-knockout mice have normal lifespan and are leaner than controls due to increased mitochondrial fatty acid oxidation (60). In addition, some but not all studies have suggested that global ACC2 knockout mice are protected against the development of obesity, diabetes and NAFLD when fed high-fat high-carbohydrate (HFHC) diet (59; 61; 62).
To further elucidate the role of ACC1 in liver de novo lipogenesis, two liver-specific ACC1-knockout mouse models have been generated using Cre- lox/P system (63; 64). Mao et al. reported that on standard chow diet ACC1 knockout mice accumulate less triglycerides in their livers and have decreased malonyl-CoA levels. When fed a fat-free diet for 10 days, mice with liver specific ACC1 knockout had significant up-regulation of several enzymes in the fatty acid synthesis pathway, again indicating that dietary carbohydrates may compensate for a loss of single DNL enzyme (63). Surprisingly, in a study by Harada at al., liver specific ACC1 knockout did not alter hepatic DNL or malonyl-CoA levels, however, hepatic DNL was completely inhibited by the dual ACC1 and ACC2 inhibitor, 5-tetradecyloxyl-2-furancarboxylic acid (64). In both of these models compensatory upregulation in ACC2 expression was observed, making the interpretation of ACC1 knockout in these studies challenging.
An alternative approach to study hepatic DNL was achieved by liver specific knock down utilizing antisense oligonucleotide (ASO) inhibitors targeting ACC1 and/or ACC2 (65). This approach did not result in compensatory increase in the non-targeted isoform, perhaps due to limited knock down, which left 20% of residual ACC1 and ACC2 expression. In vitro suppression of ACC1 inhibited lipogenesis in primary rat hepatocytes, whereas a similar reduction in ACC2 had no effect. Furthermore, when rats were fed high fat diet, the synergic inhibition of ACC1 and ACC2 was required to significantly reduce hepatic malonyl-CoA concentrations, lower hepatic lipids, and improve hepatic insulin sensitivity (65). Similar to ACL regulation, carbohydrate-rich diets increase expression and activity of both ACC isoforms, while starvation and diabetes decrease it (66).
The importance of de novo lipogenesis in embryonic development was once again demonstrated with global inactivation of fatty acid synthase, resulting in embryonic lethality (67). By contrast, liver-specific FAS knockout did not result in noticeable metabolic derangements in mice on chow diet, but when challenged with low fat/high carbohydrate diet, these mice surprisingly developed hepatic steatosis. This was due to a reduction in β-oxidation, and was accompanied by a 3-fold increase in hepatic malonyl-CoA concentrations and a significant decrease in blood ketone bodies (68). These data suggest that palmitate, synthesized via the action of FAS may stimulate the nuclear receptor PPARα and lead to enhanced β-oxidation (69).
Mice with a global knockout of stearoyl-CoA desaturase are protected from a high-carbohydrate high-fat (HCHF) diet-induced or genetically-induced obesity and show decreased lipogenic gene expression coupled with increased β-oxidation (70; 71). Interestingly, liver-specific SCD1 knockout mice are protected from obesity and hepatic steatosis induced by high-carbohydrate diet, but not from hepatosteatosis induced by high-fat diet. Furthermore, oleate, but not stearate, supplementation can normalize adiposity, and hepatic lipogenesis in liver-specific SCD1 knockout mice. Others who inhibited or knocked out SCD1 in the liver reported prevention of many of the HFHC diet-induced metabolic complications, including hepatic steatosis and hyperglycemia (72–74). In these studies, the protective effect of SCD1 ablation on hepatic steatosis has been attributed to a decrease in lipogenic rates combined with activation of the β-oxidation pathway. SCD1 is also nutritionally regulated, especially by high carbohydrate diet (75).
Global Elovl6 knockout mice gain significantly less weight when fed either chow or HFHC diet, but they do become obese and develop hepatic steatosis when fed HFHC diet for 12 weeks. Despite the fatty liver, these mice showed marked protection from insulin resistance and have enhanced insulin sensitivity. This is associated with suppression of PKCε activity and restoration of insulin receptor substrate-2 protein in the liver. Improvement in insulin signaling occurred despite the accumulation of palmitate, which is a potent inducer of insulin resistance, at least in primary hepatocyte cultures (76). These results are in agreement with the above-mentioned studies of FAS knockout mice that identify palmitate as an activator of PPARα, which promotes fatty acid oxidation. Studies utilizing genetic or adenoviral overexpression of Elovl6, reported development of liver inflammation and progression to NASH (77). However, a more recent study of global Elovl6 knockout did not confirm these findings (78), perhaps explaining why liver specific Elovl6 mice have yet to be reported.
Taken together, single enzyme knockout studies in mice lend support to a key link between de novo lipogenesis and NAFLD development because perturbations in the expression of these genes, in most instances, altered the propensity to develop fatty liver. Moreover, enzymes of de novo lipogenesis are nutritionally regulated and carbohydrate-rich diets generally overcome the loss of a single enzyme and rescue liver lipogenesis. Studies in knockout mice are further intriguing because DNL was decreased in some instances and increased in others, and the concomitant effects on insulin responsiveness were also variable. These findings suggest that the hepatic fatty acid composition, rather than total hepatic triglyceride accumulation, is important for development and progression of NAFLD. Lipidomic analysis of NAFLD livers supports the assertion that specific types of hepatic lipid deposition may predict development of liver damage and NALFD progression better than the total liver fat content (37). Future studies are needed to investigate whether upregulation of specific enzymes of DNL and accumulation of different lipid species preferentially give rise to either macrovesicular or microvesicular steatosis, especially since some evidence suggests that microvesicular steatosis is associated with mitochondrial dysfunction and progression of NAFLD (79).
Fructose promotes robust de novo lipogenesis
The major risk-factor for development of NAFLD is excess calorie intake, which is mainly derived from overconsumption of high-fat foods and increased intake of sugar sweetened beverages. In mouse models and human studies, a HFD has long been recognized as a risk factor for development of NAFLD, while restriction of HFD intake leads to improvement in fatty liver. Furthermore, some studies have shown that HFD is sufficient to induce progression of NAFLD to NASH (80), whereas others claim that only certain fats, such as saturated trans-fats, are harmful (81).
In population-based studies, overconsumption of refined sugar is a risk factor for development of obesity, diabetes and NAFLD. In fact, countries that use high fructose corn syrup (HFCS) in their food supply have a 20% higher prevalence of diabetes compared to countries that did not use HFCS, independent of obesity (82). Today the most commonly consumed sugar in the United States is high fructose corn syrup (83). In research studies, consumption of fructose, but not glucose sweetened drinks, has been associated with increased visceral adiposity, insulin resistance and increased hepatic de novo lipogenesis (84–86). A study in obese patients undergoing bariatric surgery found that higher carbohydrate intake was associated with significantly higher odds of hepatic inflammation, whereas higher fat intake was associated with significantly lower risk of inflammation (87). Furthermore, increased fructose intake in humans has been linked with progression of liver disease to fibrosis (88), while fructose restriction leads to improvement in NAFLD (89–91). Additional studies have found that fructose consumption is increased nearly 2–3 fold in adults with biopsy proven NAFLD (92–94).
Similar observations have been made in a pediatric population, wherein increased sugar intake was observed in children with NAFLD compared to obese children without NAFLD (95). Children in particular may be at greater risk to experience negative metabolic effects linked to fructose consumption because their sugar intake tends to be greater than that of adults. A recent analysis of NHANES III data, showed that adolescents consume 17% of their calories from sugar, similar to sugar consumption of young adults 18–34 years of age, but considerably more compared to older adults, who consumed only 11% of total energy from sugar (96). Furthermore, children with NAFLD may be more sensitive to the adverse metabolic effects of fructose than children without NAFLD as they more effectively metabolize fructose (97). Fructose potentiates its own metabolism through upregulation of ketohexokinase (KHK), the first step of fructose metabolism. KHK expression is increased in patients with NAFLD, and inhibition of KHK, leads to decreased fatty liver and decreased liver inflammation in mice fed high-fat high-sucrose diet (98). Dietary fructose can also contribute to development of liver fibrosis in some murine models of NASH (99–101).
Whereas diets rich in both fat and fructose contribute to the development of NAFLD, fructose itself more strongly upregulates enzymes of de novo lipogenesis than does fat. In our own studies of mice fed chow or HFD diet, supplementation with 30% fructose in the drinking water more robustly increased levels of ACC1, FAS and SCD1 than HFD. Maratos-Flier and colleagues observed that mice fed a ketogenic diet (>90% k calories from fat) actually lost weight and exhibited reduced rates of hepatic de novo lipogenesis and increased fatty acid oxidation (102). Ketogenic diets also decreased expression of ACC, FAS, and SCD1 in liver, but these mice eventually developed NAFLD after 12 weeks (103). The development of NAFLD in this setting may have been related to the fact that the ketogenic diet used in these studies (Bio-Serv F3666) was deficient in protein, as well as methionine and choline (104), both factors which promote development of NAFLD in mice. In human studies, a high carbohydrate, eucaloric diet supports DNL more than an equivalent diet containing less carbohydrates and more fat (105). Hellerstein at al. observed that normal weight subjects on weight-maintaining low fat, high carbohydrate diet (10% fat, 70% CHO) for 3 weeks had higher rates of DNL than subjects on diet with a typical carbohydrate to fat ratio (105).
Additional reasons why fructose may be lipogenic
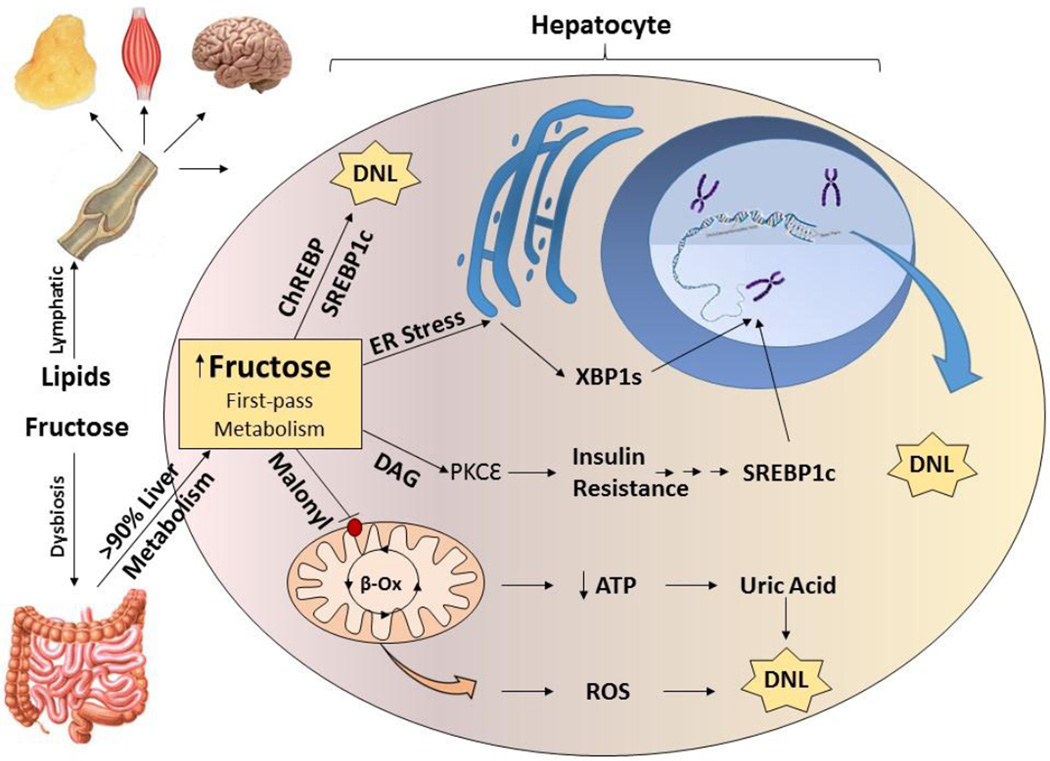
There are additional theoretical explanations as to why fructose may be a more potent stimulator of de novo lipogenesis than HFD (Figure 3). First, fructose, as all other carbohydrates, is absorbed from the intestine into the portal vein and delivered directly to the liver. It is only after the first pass metabolism through the liver that dietary carbohydrates may enter systemic circulation. Macronutrient and insulin concentration in the portal circulation may be 10 times higher than in the systemic circulation (106). Thus the liver is exposed to much higher carbohydrate load than the rest of the metabolically active tissues. By contrast long chain fatty acids absorbed from the intestine as chylomicron particles first enter the lymphatic circulation from which they eventually enter systemic circulation via thoracic duct. Thus the concentration of dietary fat that the liver is exposed to is the same as any other metabolically active tissue. This taken together with the distribution of lipoprotein lipase in capillaries of muscle and fat tissue, results in chylomicrons being largely metabolized by non-hepatic tissue (107). The second reason why fructose robustly stimulates DNL is that carbohydrates are metabolized to the two carbon intermediate acetyl-CoA and activate the lipogenic transcriptional factors SREBP1c and ChREBP in the liver, stimulating every step of de novo lipogenesis which converts the acetyl-CoA into triglycerides. By contrast, fatty acids may be directly incorporated into triglycerides through the action of a single enzyme, DGAT, thus bypassing the vast majority of enzymes involved in DNL and failing to activate the same lipogenic transcriptional factors.
Figure 3. Lipogenic potential of fructose.
Dietary lipids are absorbed from the intestine via lymphatic system and are equally available to all metabolically active tissue. Dietary carbohydrates are absorbed from the intestine via portal vein and directly reach the liver. More than 90% of fructose is metabolized by the liver via first pass metabolism. Fructose may directly upregulate transcriptional factors regulating de novo lipogenesis or may do so indirectly by inducing ER stress, insulin resistance and decreased mitochondrial metabolism leading to the production of uric acid and reactive oxygen species.
Fructose metabolism and DNL are similar in regards to energy balance as both processes require hepatic ATP expenditure. DNL requires ATP in order to synthesize lipids from dietary carbohydrates, while rapid phosphorylation of fructose to fructose-1 phosphate depletes hepatic ATP levels and leads to uric acid production (108). This step is catalyzed by ketohexokinase (KHK), the first enzyme of fructose metabolism. The phosphorylation of fructose by KHK in liver is 10 times faster than phosphorylation of glucose by glucokinase (GCK) (109). Therefore, serum fructose levels following ingestion of 1 g/kg oral fructose reache only 0.5 mM, whereas glucose levels rise to 10 mM in response to a similar oral glucose challenge (110). Following an IV fructose load in healthy human subjects, hepatic ATP levels drop within 5 minutes with concomitant increase in inorganic phosphate and phosphate monoesters (111). While inorganic phosphate and phosphate monoester levels return to normal within 10 minutes, ATP levels continue to be suppressed for at least 60 minutes. This decrease in ATP is entirely dependent on KHK activity. A study of diabetic subjects revealed that high dietary fructose intake leads to lower hepatic ATP levels as long as 50 minutes after a fructose load (112). This prolonged reduction in ATP levels is puzzling because fructose blood levels in mice peak at 5 minutes following an IP fructose load and decreased 4-fold by 30 minutes (113). Thus the decrease in ATP and phosphorylation of fructose to fructose-1 phosphate should also peak at 5 minutes since there is less substrate to metabolize after that point. Prolonged ATP depletion lasting much longer than peak blood fructose levels indicates that other reactions in addition to phosphorylation of fructose must contribute to decreased ATP levels in the liver. One of these processes may be fructose-induced DNL, which decreases mitochondrial ATP production by inhibiting mitochondrial beta-oxidation via increased malonyl-CoA levels. Patients with NAFLD exhibit reduced hepatic ATP levels (114; 115), perhaps owing to increased rates of DNL.
The role of insulin in hepatic DNL
Insulin strongly promotes hepatic DNL through activation of the major transcriptional regulator of lipogenesis, sterol regulatory element binding protein (SREBP)-1c, which induces the entire cascade of genes necessary for the synthesis of fatty acids (116; 117). Insulin in a concentration dependent manner, also stimulates hepatic uptake of FFA, which further supports DNL (118). The importance of insulin signaling in hepatic lipogenesis is indicated by the fact that mice with liver specific insulin receptor knockout exhibit decreased hepatic expression of SREBP1c and its transcriptional targets (119; 120) and do not develop steatosis in spite of developing hyperglycemia and hyperinsulinemia (121). In states of insulin resistance such as obesity and NALFD, insulin continues to support DNL, while its capacity to reduce hepatic gluconeogenesis is impaired. This has been referred to as selective insulin resistance. Insulin signaling is mediated by a complex, highly integrated network, which controls many cellular processes (122; 123), so it is quite possible that insulin resistance impairs some pathways more than others. However, others have proposed that dietary macronutrients, especially fructose, may mediate lipogenesis in the setting of complete insulin resistance (124).
Fructose, unlike glucose, does not require insulin for its metabolism and acute fructose feedings do not stimulate significant insulin secretion. Chronic fructose ingestion, on the other hand, can lead to hyperinsulinemia and insulin resistance. SREBP1c nuclear translocation, as well as concomitant increases in ACC, FAS and SCD1 can be induced by fructose feedings even in mice with liver specific knockout of insulin receptor, indicating that fructose can stimulate lipogenesis in the setting of a complete lack of insulin signaling (124). A second mechanism by which fructose may stimulate lipogenesis is by promoting ER stress. ER stress leads to activation of the transcription factor XBP1s (125), which upregulates lipogenic enzymes. Liver-specific XBP1s knockout mice exhibit decreased expression of ACC, FAS, and SCD1 (126). Likewise, XBP1s has been shown to be elevated in liver biopsies from obese subjects undergoing bariatric surgery and declines following weight loss (127). Lee et al. have demonstrated a marked induction of hepatic XBP1s in mice fed a 60% fructose diet (125). A third insulin independent mechanism by which fructose may support lipogenesis is through induction of uric acid synthesis. As noted above, fructose leads to ATP depletion, and increases in ADP and IMP, which is converted to uric acid. It has been proposed that uric acid can promote hepatic steatosis through generation of mitochondrial oxidative stress (128; 129). Fructose metabolism also generates reactive oxygen species (5), and nutrient derived ROS may augment hepatic steatosis through insulin independent activation of phosphoinositide 3-kinase pathway (130).
Summary and future perspective
In summary, dietary fructose consumption has dramatically increased over the last century, and this has been associated with increased prevalence of obesity, insulin resistance and fatty liver disease. Fructose intake has also been linked with progression of liver disease, whereas fructose avoidance leads to improvement in NAFLD. Fructose metabolism has many unique aspects, the most important of which is its ability to support enhanced hepatic DNL. Studies in both rodents and humans indicate that hepatic DNL is a central abnormality in development of NAFLD. Fructose stimulates lipogenesis in insulin independent manner, and it induces insulin resistance by activating novel PKC isoforms and by generating lipid intermediates that themselves may promote progression of fatty liver disease. Hepatic DNL, dietary intake and adipocyte lipolysis are all important contributors to NALFD. Because dietary interventions generally fail to achieve sustained responses and targeting adipocyte lipolysis is technically more challenging, targeting hepatic DNL or fructose metabolism may offer an attractive approach to the pharmacologic management of NAFLD.
Key Findings.
Population-wide increases in total energy intake are accounted in large part by increased carbohydrate consumption.
Hepatic de novo lipogenesis is a prominent abnormality in NAFD.
Dietary fructose robustly supports de novo lipogenesis, even in the setting of insulin resistance.
Inhibition of a single enzyme regulating de novo lipogenesis may result in the resolution of experimental NAFLD in mice. However, carbohydrate feeding may overcome a loss of a single lipogenic enzyme.
Inhibition of fructose metabolism and de novo lipogenesis may offer an attractive approach to the pharmacologic management of NAFLD.
Acknowledgments
This work was supported in part by NIH grants (R01 DK031036, and R01 DK033201) to C.R.K, (R37 DK048873, R01 DK056626 and R01 DK103046) to D.E.C and K12 HD000850 to S.S.
REFERENCES
- 1.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 2.Gotto AM, Jr, Blackburn GL, Dailey GE, III, Garber AJ, Grundy SM, Sobel BE, Weir MR. The metabolic syndrome: a call to action. Coron. Artery. Dis. 2006;17:77–80. doi: 10.1097/00019501-200602000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 4.Chanmugam P, Guthrie JF, Cecilio S, Morton JF, Basiotis PP, Anand R. Did fat intake in the United States really decline between 1989–1991 and 1994–1996? J Am Diet Assoc. 2003;103:867–872. doi: 10.1016/s0002-8223(03)00381-x. [DOI] [PubMed] [Google Scholar]
- 5.Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7:251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 6.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014;174:516–524. doi: 10.1001/jamainternmed.2013.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ, American Gastroenterological A, American Association for the Study of Liver D, American College of G. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 9.Zezos P, Renner EL. Liver transplantation and non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:15532–15538. doi: 10.3748/wjg.v20.i42.15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soderberg C, Stal P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 11.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 14.Cohen DE, Fisher EA. Lipoprotein metabolism, dyslipidemia, and nonalcoholic fatty liver disease. Semin Liver Dis. 2013;33:380–388. doi: 10.1055/s-0033-1358519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, Ludwig DS. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367:1407–1416. doi: 10.1056/NEJMoa1203388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: reanalyses of a meta-analysis. Am J Clin Nutr. 2009;89:438–439. doi: 10.3945/ajcn.2008.26980. author reply 439–440. [DOI] [PubMed] [Google Scholar]
- 17.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367:1397–1406. doi: 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- 18.Welsh JA, Sharma A, Cunningham SA, Vos MB. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation. 2011;123:249–257. doi: 10.1161/CIRCULATIONAHA.110.972166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation. 2012;125:1735–1741. S1731. doi: 10.1161/CIRCULATIONAHA.111.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falcon A, Doege H, Fluitt A, Tsang B, Watson N, Kay MA, Stahl A. FATP2 is a hepatic fatty acid transporter and peroxisomal very long-chain acyl-CoA synthetase. Am J Physiol Endocrinol Metab. 2010;299:E384–E393. doi: 10.1152/ajpendo.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doege H, Grimm D, Falcon A, Tsang B, Storm TA, Xu H, Ortegon AM, Kazantzis M, Kay MA, Stahl A. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J Biol Chem. 2008;283:22186–22192. doi: 10.1074/jbc.M803510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koonen DP, Jacobs RL, Febbraio M, Young ME, Soltys CL, Ong H, Vance DE, Dyck JR. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 2007;56:2863–2871. doi: 10.2337/db07-0907. [DOI] [PubMed] [Google Scholar]
- 23.Nomura K, Yamanouchi T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J Nutr Biochem. 2012;23:203–208. doi: 10.1016/j.jnutbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan RS, Mayor JA, Johnston N, Oliveira DL. Purification and characterization of the reconstitutively active tricarboxylate transporter from rat liver mitochondria. J Biol Chem. 1990;265:13379–13385. [PubMed] [Google Scholar]
- 25.Brownsey RW, Boone AN, Elliott JE, Kulpa JE, Lee WM. Regulation of acetyl-CoA carboxylase. Biochem Soc Trans. 2006;34:223–227. doi: 10.1042/BST20060223. [DOI] [PubMed] [Google Scholar]
- 26.Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O’Neill HM, Ford RJ, Palanivel R, O’Brien M, Hardie DG, Macaulay SL, Schertzer JD, Dyck JR, van Denderen BJ, Kemp BE, Steinberg GR. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leavens KF, Birnbaum MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Crit Rev Biochem Mol Biol. 2011;46:200–215. doi: 10.3109/10409238.2011.562481. [DOI] [PubMed] [Google Scholar]
- 28.Hillgartner FB, Salati LM, Goodridge AG. Physiological and molecular mechanisms involved in nutritional regulation of fatty acid synthesis. Physiol Rev. 1995;75:47–76. doi: 10.1152/physrev.1995.75.1.47. [DOI] [PubMed] [Google Scholar]
- 29.Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. 2013;48:434–441. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shmueli E, Alberti KG, Record CO. Diacylglycerol/protein kinase C signalling: a mechanism for insulin resistance? J Intern Med. 1993;234:397–400. doi: 10.1111/j.1365-2796.1993.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O’Brien WR, Littman DR, Shulman GI. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bezy O, Tran TT, Pihlajamaki J, Suzuki R, Emanuelli B, Winnay J, Mori MA, Haas J, Biddinger SB, Leitges M, Goldfine AB, Patti ME, King GL, Kahn CR. PKCdelta regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans. J Clin Invest. 2011;121:2504–2517. doi: 10.1172/JCI46045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, Farese R, Cantley LC, Kahn CR. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cepsilon and hepatic insulin resistance. Cell Metab. 2012;15:574–584. doi: 10.1016/j.cmet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takayama S, White MF, Kahn CR. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem. 1988;263:3440–3447. [PubMed] [Google Scholar]
- 37.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 38.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, Hevener AL, Farese RV., Jr Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, Zhang D, Wang A, Zhang XM, Kahn M, Cline GW, Pandey SK, Geisler JG, Bhanot S, Monia BP, Shulman GI. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282:22678–22688. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 40.Diraison F, Beylot M. Role of human liver lipogenesis and reesterification in triglycerides secretion and in FFA reesterification. Am J Physiol. 1998;274:E321–E327. doi: 10.1152/ajpendo.1998.274.2.E321. [DOI] [PubMed] [Google Scholar]
- 41.Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J Clin Invest. 1999;104:1087–1096. doi: 10.1172/JCI6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hellerstein MK. De novo lipogenesis in humans: metabolic and regulatory aspects. Eur J Clin Nutr. 1999;53(Suppl 1):S53–S65. doi: 10.1038/sj.ejcn.1600744. [DOI] [PubMed] [Google Scholar]
- 43.Marques-Lopes I, Ansorena D, Astiasaran I, Forga L, Martinez JA. Postprandial de novo lipogenesis and metabolic changes induced by a high-carbohydrate, low-fat meal in lean and overweight men. Am J Clin Nutr. 2001;73:253–261. doi: 10.1093/ajcn/73.2.253. [DOI] [PubMed] [Google Scholar]
- 44.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 47.Timlin MT, Parks EJ. Temporal pattern of de novo lipogenesis in the postprandial state in healthy men. Am J Clin Nutr. 2005;81:35–42. doi: 10.1093/ajcn/81.1.35. [DOI] [PubMed] [Google Scholar]
- 48.Sevastianova K, Santos A, Kotronen A, Hakkarainen A, Makkonen J, Silander K, Peltonen M, Romeo S, Lundbom J, Lundbom N, Olkkonen VM, Gylling H, Fielding BA, Rissanen A, Yki-Jarvinen H. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr. 2012;96:727–734. doi: 10.3945/ajcn.112.038695. [DOI] [PubMed] [Google Scholar]
- 49.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85:1511–1520. doi: 10.1093/ajcn/85.6.1511. [DOI] [PubMed] [Google Scholar]
- 50.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Postic C, Girard J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 2008;34:643–648. doi: 10.1016/S1262-3636(08)74599-3. [DOI] [PubMed] [Google Scholar]
- 52.Beigneux AP, Kosinski C, Gavino B, Horton JD, Skarnes WC, Young SG. ATP-citrate lyase deficiency in the mouse. J Biol Chem. 2004;279:9557–9564. doi: 10.1074/jbc.M310512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearce NJ, Yates JW, Berkhout TA, Jackson B, Tew D, Boyd H, Camilleri P, Sweeney P, Gribble AD, Shaw A, Groot PH. The role of ATP citrate-lyase in the metabolic regulation of plasma lipids. Hypolipidaemic effects of SB-204990, a lactone prodrug of the potent ATP citrate-lyase inhibitor SB-201076. Biochem J. 1998;334(Pt 1):113–119. doi: 10.1042/bj3340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Preuss HG, Rao CV, Garis R, Bramble JD, Ohia SE, Bagchi M, Bagchi D. An overview of the safety and efficacy of a novel, natural(−)-hydroxycitric acid extract (HCA-SX) for weight management. J Med. 2004;35:33–48. [PubMed] [Google Scholar]
- 55.Li JJ, Wang H, Tino JA, Robl JA, Herpin TF, Lawrence RM, Biller S, Jamil H, Ponticiello R, Chen L, Chu CH, Flynn N, Cheng D, Zhao R, Chen B, Schnur D, Obermeier MT, Sasseville V, Padmanabha R, Pike K, Harrity T. 2-hydroxy-N-arylbenzenesulfonamides as ATP-citrate lyase inhibitors. Bioorg Med Chem Lett. 2007;17:3208–3211. doi: 10.1016/j.bmcl.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q, Jiang L, Wang J, Li S, Yu Y, You J, Zeng R, Gao X, Rui L, Li W, Liu Y. Abrogation of hepatic ATP-citrate lyase protects against fatty liver and ameliorates hyperglycemia in leptin receptor-deficient mice. Hepatology. 2009;49:1166–1175. doi: 10.1002/hep.22774. [DOI] [PubMed] [Google Scholar]
- 57.Wang Q, Li S, Jiang L, Zhou Y, Li Z, Shao M, Li W, Liu Y. Deficiency in hepatic ATP-citrate lyase affects VLDL-triglyceride mobilization and liver fatty acid composition in mice. J Lipid Res. 2010;51:2516–2526. doi: 10.1194/jlr.M003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kornacker MS, Lowenstein JM. Citrate and the Conversion of Carbohydrate into Fat. The Activities of Citrate-Cleavage Enzyme and Acetate Thiokinase in Livers of Starved and Re-Fed Rats. Biochem J. 1965;94:209–215. doi: 10.1042/bj0940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abu-Elheiga L, Matzuk MM, Kordari P, Oh W, Shaikenov T, Gu Z, Wakil SJ. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proc Natl Acad Sci U S A. 2005;102:12011–12016. doi: 10.1073/pnas.0505714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 61.Abu-Elheiga L, Wu H, Gu Z, Bressler R, Wakil SJ. Acetyl-CoA carboxylase 2−/− mutant mice are protected against fatty liver under high-fat, high-carbohydrate dietary and de novo lipogenic conditions. J Biol Chem. 2012;287:12578–12588. doi: 10.1074/jbc.M111.309559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olson DP, Pulinilkunnil T, Cline GW, Shulman GI, Lowell BB. Gene knockout of Acc2 has little effect on body weight, fat mass, or food intake. Proc Natl Acad Sci U S A. 2010;107:7598–7603. doi: 10.1073/pnas.0913492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, Kordari P, Chirala SS, Heird WC, Wakil SJ. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci U S A. 2006;103:8552–8557. doi: 10.1073/pnas.0603115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harada N, Oda Z, Hara Y, Fujinami K, Okawa M, Ohbuchi K, Yonemoto M, Ikeda Y, Ohwaki K, Aragane K, Tamai Y, Kusunoki J. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol Cell Biol. 2007;27:1881–1888. doi: 10.1128/MCB.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, Bhanot S, Monia BP, Shulman GI. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50(Suppl):S138–S143. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chirala SS, Chang H, Matzuk M, Abu-Elheiga L, Mao J, Mahon K, Finegold M, Wakil SJ. Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc Natl Acad Sci U S A. 2003;100:6358–6363. doi: 10.1073/pnas.0931394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 71.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, Ronan J, Esau C, Murphy C, Szalkowski D, Bergeron R, Doebber T, Zhang BB. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest. 2005;115:1030–1038. doi: 10.1172/JCI23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gutierrez-Juarez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006;116:1686–1695. doi: 10.1172/JCI26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 75.Mauvoisin D, Mounier C. Hormonal and nutritional regulation of SCD1 gene expression. Biochimie. 2011;93:78–86. doi: 10.1016/j.biochi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 76.Mordier S, Iynedjian PB. Activation of mammalian target of rapamycin complex 1 and insulin resistance induced by palmitate in hepatocytes. Biochem Biophys Res Commun. 2007;362:206–211. doi: 10.1016/j.bbrc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Matsuzaka T, Atsumi A, Matsumori R, Nie T, Shinozaki H, Suzuki-Kemuriyama N, Kuba M, Nakagawa Y, Ishii K, Shimada M, Kobayashi K, Yatoh S, Takahashi A, Takekoshi K, Sone H, Yahagi N, Suzuki H, Murata S, Nakamuta M, Yamada N, Shimano H. Elovl6 promotes nonalcoholic steatohepatitis. Hepatology. 2012;56:2199–2208. doi: 10.1002/hep.25932. [DOI] [PubMed] [Google Scholar]
- 78.Moon YA, Ochoa CR, Mitsche MA, Hammer RE, Horton JD. Deletion of ELOVL6 blocks the synthesis of oleic acid but does not prevent the development of fatty liver or insulin resistance. J Lipid Res. 2014;55:2597–2605. doi: 10.1194/jlr.M054353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tandra S, Yeh MM, Brunt EM, Vuppalanchi R, Cummings OW, Unalp-Arida A, Wilson LA, Chalasani N. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J Hepatol. 2011;55:654–659. doi: 10.1016/j.jhep.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vilar L, Oliveira CP, Faintuch J, Mello ES, Nogueira MA, Santos TE, Alves VA, Carrilho FJ. High-fat diet: a trigger of non-alcoholic steatohepatitis? Preliminary findings in obese subjects. Nutrition. 2008;24:1097–1102. doi: 10.1016/j.nut.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 81.Machado RM, Stefano JT, Oliveira CP, Mello ES, Ferreira FD, Nunes VS, de Lima VM, Quintao EC, Catanozi S, Nakandakare ER, Lottenberg AM. Intake of trans fatty acids causes nonalcoholic steatohepatitis and reduces adipose tissue fat content. J Nutr. 2010;140:1127–1132. doi: 10.3945/jn.109.117937. [DOI] [PubMed] [Google Scholar]
- 82.Goran MI, Ulijaszek SJ, Ventura EE. High fructose corn syrup and diabetes prevalence: a global perspective. Glob Public Health. 2013;8:55–64. doi: 10.1080/17441692.2012.736257. [DOI] [PubMed] [Google Scholar]
- 83.Collino M. High dietary fructose intake: Sweet or bitter life? World J Diabetes. 2011;2:77–81. doi: 10.4239/wjd.v2.i6.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Silbernagel G, Machann J, Unmuth S, Schick F, Stefan N, Haring HU, Fritsche A. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: an exploratory trial. Br J Nutr. 2011;106:79–86. doi: 10.1017/S000711451000574X. [DOI] [PubMed] [Google Scholar]
- 86.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Keim NL, Havel PJ. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr Metab (Lond) 2012;9:68. doi: 10.1186/1743-7075-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Solga S, Alkhuraishe AR, Clark JM, Torbenson M, Greenwald A, Diehl AM, Magnuson T. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004;49:1578–1583. doi: 10.1023/b:ddas.0000043367.69470.b7. [DOI] [PubMed] [Google Scholar]
- 88.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin R, Welsh JA, Le NA, Holzberg J, Sharma P, Martin DR, Vos MB. Dietary fructose reduction improves markers of cardiovascular disease risk in Hispanic-American adolescents with NAFLD. Nutrients. 2014;6:3187–3201. doi: 10.3390/nu6083187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mager DR, Iniguez IR, Gilmour S, Yap J. The effect of a low fructose and low glycemic index/load (FRAGILE) dietary intervention on indices of liver function, cardiometabolic risk factors, and body composition in children and adolescents with nonalcoholic fatty liver disease (NAFLD) JPEN J Parenter Enteral Nutr. 2015;39:73–84. doi: 10.1177/0148607113501201. [DOI] [PubMed] [Google Scholar]
- 91.Lustig RH, Mulligan K, Noworolski SM, Tai VW, Wen MJ, Erkin-Cakmak A, Gugliucci A, Schwarz JM. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity (Silver Spring) 2015 doi: 10.1002/oby.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, Oren R. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol. 2007;47:711–717. doi: 10.1016/j.jhep.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 94.Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Konigsrainer A, Maier KP, Bischoff SC, Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138:1452–1455. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 95.Papandreou D, Karabouta Z, Pantoleon A, Rousso I. Investigation of anthropometric, biochemical and dietary parameters of obese children with and without non-alcoholic fatty liver disease. Appetite. 2012;59:939–944. doi: 10.1016/j.appet.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 96.Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr. 2011;94:726–734. doi: 10.3945/ajcn.111.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jin R, Le NA, Liu S, Farkas Epperson M, Ziegler TR, Welsh JA, Jones DP, McClain CJ, Vos MB. Children with NAFLD are more sensitive to the adverse metabolic effects of fructose beverages than children without NAFLD. J Clin Endocrinol Metab. 2012;97:E1088–E1098. doi: 10.1210/jc.2012-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ishimoto T, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Orlicky DJ, Cicerchi C, McMahan RH, Abdelmalek MF, Rosen HR, Jackman MR, MacLean PS, Diggle CP, Asipu A, Inaba S, Kosugi T, Sato W, Maruyama S, Sanchez-Lozada LG, Sautin YY, Hill JO, Bonthron DT, Johnson RJ. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology. 2013;58:1632–1643. doi: 10.1002/hep.26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, Tang PH, Miles L, Miles MV, Balistreri WF, Woods SC, Seeley RJ. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52:934–944. doi: 10.1002/hep.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, Masuoko H, Gores G. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011;301:G825–G834. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsuchiya H, Ebata Y, Sakabe T, Hama S, Kogure K, Shiota G. High-fat, high-fructose diet induces hepatic iron overload via a hepcidin-independent mechanism prior to the onset of liver steatosis and insulin resistance in mice. Metabolism. 2013;62:62–69. doi: 10.1016/j.metabol.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 102.Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, Libermann TA, Maratos-Flier E. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007;292:E1724–E1739. doi: 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- 103.Garbow JR, Doherty JM, Schugar RC, Travers S, Weber ML, Wentz AE, Ezenwajiaku N, Cotter DG, Brunt EM, Crawford PA. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol. 2011;300:G956–G967. doi: 10.1152/ajpgi.00539.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schugar RC, Crawford PA. Low-carbohydrate ketogenic diets, glucose homeostasis, and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2012;15:374–380. doi: 10.1097/MCO.0b013e3283547157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hellerstein MK, Schwarz JM, Neese RA. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr. 1996;16:523–557. doi: 10.1146/annurev.nu.16.070196.002515. [DOI] [PubMed] [Google Scholar]
- 106.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 2005;54:1649–1656. doi: 10.2337/diabetes.54.6.1649. [DOI] [PubMed] [Google Scholar]
- 107.Nestel PJ, Havel RJ, Bezman A. Sites of Initial Removal of Chylomicron Triglyceride Fatty Acids from the Blood. J Clin Invest. 1962;41:1915–1921. doi: 10.1172/JCI104648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, Patel JM, Johnson RJ. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–F631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 109.Asipu A, Hayward BE, O’Reilly J, Bonthron DT. Properties of normal and mutant recombinant human ketohexokinases and implications for the pathogenesis of essential fructosuria. Diabetes. 2003;52:2426–2432. doi: 10.2337/diabetes.52.9.2426. [DOI] [PubMed] [Google Scholar]
- 110.Gaby AR. Adverse effects of dietary fructose. Altern Med Rev. 2005;10:294–306. [PubMed] [Google Scholar]
- 111.Boesiger P, Buchli R, Meier D, Steinmann B, Gitzelmann R. Changes of liver metabolite concentrations in adults with disorders of fructose metabolism after intravenous fructose by 31P magnetic resonance spectroscopy. Pediatr Res. 1994;36:436–440. doi: 10.1203/00006450-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 112.Abdelmalek MF, Lazo M, Horska A, Bonekamp S, Lipkin EW, Balasubramanyam A, Bantle JP, Johnson RJ, Diehl AM, Clark JM. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology. 2012;56:952–960. doi: 10.1002/hep.25741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cha SH, Wolfgang M, Tokutake Y, Chohnan S, Lane MD. Differential effects of central fructose and glucose on hypothalamic malonyl-CoA and food intake. Proc Natl Acad Sci U S A. 2008;105:16871–16875. doi: 10.1073/pnas.0809255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schmid AI, Szendroedi J, Chmelik M, Krssak M, Moser E, Roden M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care. 2011;34:448–453. doi: 10.2337/dc10-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA. 1999;282:1659–1664. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- 116.Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci U S A. 1998;95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci U S A. 1999;96:13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Softic S, Kirby M, Berger NG, Shroyer NF, Woods SC, Kohli R. Insulin concentration modulates hepatic lipid accumulation in mice in part via transcriptional regulation of fatty acid transport proteins. PLOS One. 2012;7:e38952. doi: 10.1371/journal.pone.0038952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Emanuelli B, Vienberg SG, Smyth G, Cheng C, Stanford KI, Arumugam M, Michael MD, Adams AC, Kharitonenkov A, Kahn CR. Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest. 2014;124:515–527. doi: 10.1172/JCI67353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 122.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 123.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haas JT, Miao J, Chanda D, Wang Y, Zhao E, Haas ME, Hirschey M, Vaitheesvaran B, Farese RV, Jr, Kurland IJ, Graham M, Crooke R, Foufelle F, Biddinger SB. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab. 2012;15:873–884. doi: 10.1016/j.cmet.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jurczak MJ, Lee AH, Jornayvaz FR, Lee HY, Birkenfeld AL, Guigni BA, Kahn M, Samuel VT, Glimcher LH, Shulman GI. Dissociation of inositol-requiring enzyme (IRE1alpha)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J Biol Chem. 2012;287:2558–2567. doi: 10.1074/jbc.M111.316760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay M, Schreiner G, Rodriguez-Iturbe B, Nakagawa T, Kang DH, Sautin YY, Johnson RJ. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287:40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, Ishimoto T, Sautin YY, Lanaspa MA. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62:3307–3315. doi: 10.2337/db12-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kohli R, Pan X, Malladi P, Wainwright MS, Whitington PF. Mitochondrial reactive oxygen species signal hepatocyte steatosis by regulating the phosphatidylinositol 3-kinase cell survival pathway. J Biol Chem. 2007;282:21327–21336. doi: 10.1074/jbc.M701759200. [DOI] [PubMed] [Google Scholar]