Abstract
With the obesity epidemic, nonalcoholic fatty liver disease (NAFLD) has become a public health problem with increasing prevalence. The mechanism of disease progression remains obscure and effective therapy is lacking. Therefore, there is a need to understand the pathogenic mechanisms responsible for disease development and progression in order to develop innovative therapies. To accomplish this goal, experimental animal models that recapitulate the human disease are necessary, especially, since causative mechanistic studies of NAFLD are more difficult or unethical to perform in humans. A large number of studies regarding the pathophysiology and treatment of NASH have been undertaken in mice to model human NAFLD and nonalcoholic steatohepatitis (NASH). This review discusses the known dietary, genetic and inflammation based animal models of NASH described in recent years, with a focus on the major advances made in this field.
Keywords: animal model, fibrosis, hepatocellular ballooning, inflammation, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, steatosis
Introduction
Nonalcoholic fatty liver disease (NAFLD) has emerged as a growing public health problem linked to the increased incidence of obesity. It is currently the most common cause of chronic liver disease [1]. NAFLD is defined by excess fat accumulation in the liver of a patient without a history of excessive alcohol intake. NAFLD is classified into simple steatosis and nonalcoholic steatohepatitis (NASH). NASH is characterized by lobular inflammation and hepatocellular ballooning, and often associated with fibrosis [2]. In the context of the metabolic syndrome and obesity, insulin resistance plays an important role in the pathogenesis of NAFLD [3]. There is increasing body of evidence suggesting that the relationship between NAFLD and insulin resistance is bidirectional: Liver fat content is significantly increased in subjects with insulin resistance due to increased free fatty acid flux from adipose tissue and uptake by the liver, impaired fatty acid beta oxidation, and increased de novo hepatic lipogenesis. Hepatic triglyceride accumulation in turn contributes to impaired glucose metabolism and insulin sensitivity in muscle and in the liver [4]. NAFLD is now recognized as a predictor of future risk of type 2 diabetes mellitus [5,6]. Long-term follow-up studies in NASH suggest that the disease is usually slowly progressive, but can ultimately lead to cirrhosis in a subset of patients with its sequelae of portal hypertension, end-stage liver disease and hepatocellular carcinoma and an increase in overall mortality [7–9]. The molecular and cellular mechanisms involved in the pathogenesis of NASH have not been completely elucidated, and NASH is still lacking regulatory agency-approved pharmacotherapy. With the inherent limitations in acquiring human tissue, and conducting experimental drug studies in human, NASH animal models are necessary to test the different pathogenic mechanisms, and therapeutic targets. Here we discuss the known dietary murine model of NASH, with an emphasis on the different dietary components and their impact on the histological readout and the metabolic profile of the disease. We also highlight the commonly used genetic mouse models of NASH and their importance in studying the lipid metabolism. We finally discuss the importance of the sterile inflammatory response associated with NASH, and the different inflammatory models that have been recently reported.
Histopathology of NAFLD
Hepatocytes steatosis is the histological hallmark of NAFLD, and it is often predominant in zone 3 of the acinus. Steatosis is further classified into macrovesicular steatosis characterized by large vacuoles occupying most of the cytoplasm and pushing the nucleus to the periphery of the cell (Fig. 1 A), and microvesicular steatosis characterized by multiple small lipid vacuoles, with a central nucleus. In NASH, steatosis is associated with lobular inflammation with mixed type of inflammatory cells, which includes polymorphonuclear leukocytes, macrophages and invariant Natural Killer T cells. Other common features are hepatocellular ballooning, a degenerative, swollen hepatocyte devoid of cytokeratin 18 [10]. Poorly formed Mallory's hyaline (an eosinophilic and amorphous structure in the cytoplasm of hepatocytes) (Fig. 1B) and glycogenated nuclei are often described histological features in patients with NASH [11]. Perisinusoidal fibrosis (Fig. 1C) mainly in zone 3 of the acinus is an important feature of NASH. Periportal and portal fibrosis are mild in NASH in contrast to other forms of liver disease. As the disease progresses, bridging fibrosis from the portal tract to the central vein occurs (Fig. 1 D) and liver cirrhosis may develop; steatosis and active inflammation may actually resolve, resulting in what is known as burn-out NASH, and presents as cryptogenic cirrhosis. To be noted, in pediatric NAFLD, steatosis is not predominant in zone 3, lobular inflammation is milder and portal inflammation is more prominent when compared to adult NAFLD [12,13]. The grading and staging system of NASH was initially developed by Brunt et al [11]. The Pathology Committee of the NASH Clinical Research Network designed and validated a histological feature scoring system referred to NAFLD activity score (NAS) for use in clinical trials [14]. These systems are currently used in human studies. The NAS has not been validated in animal models.
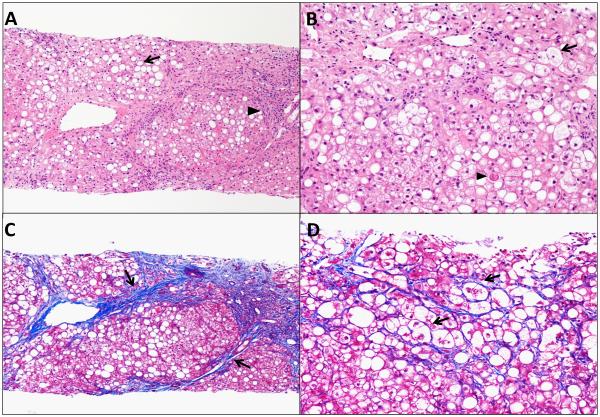
Figure 1. Histological sections of a liver biopsy of a patient with nonalcoholic steatohepatitis.
(A) Hematoxylin and Eosin (H & E) stain taken with 10 × objective, shows steatosis (arrow) with a cirrhotic nodule and inflammatory cells infiltration (arrow head). (B) H & E stain taken with 20 × objective, shows ballooned hepatocytes (arrow) with an acidophil body (arrowhead). (C) Trichrome stain taken with 10× objective, shows bridging fibrosis (arrows) with a cirrhotic nodule. (D) Trichrome stain taken with 20 × objective, shows pericellular fibrosis (arrows).
Pathogenesis of NAFLD
The development and progression of NAFLD to NASH remains unclear [15]. A substantial body of evidence suggest that NASH pathogenesis encompasses multiple interrelated processes, including hepatotoxicity and apoptosis mediated by free fatty acids [16], cytokine-mediated liver inflammation and injury [17], insulin resistance and adipose tissue dysfunction [18] which collectively lead to hepatic steatosis, inflammation and ultimately fibrosis. It is to be noted that various histological features of NAFLD are not only consequences of overnutrition, obesity, or insulin resistance, but are based on the balance between biological mechanisms for host hepatic susceptibility and the physiological consequences of overnutrition. Human studies of the pathophysiology of NAFLD have been limited, partially, by difficulties in distinguishing primary cause(s) from secondary effects and epiphenomena related to obesity and liver disease [19].
An ideal NASH animal model
The ideal NASH animal model encompasses all the defining features of the human condition, including obesity, insulin resistance, steatohepatitis, and fibrosis. Such a model replicates the pathophysiology of the human disease, as confirmed by the histological readout. The model if based on genetic loci should be based on aberrations found in humans, such as PNPLA3 polymorphism [20]. Ideally, the transcriptome profile of the animal model should replicate identical changes in humans. Likely, human NASH is heterogeneous in pathogenesis, and no single animal model recapitulates all subsets of human NASH. To date, a perfect mouse model of NASH has not been developed. The availability of an “accurate” mouse model of NASH would facilitate studying the pathophysiology of NAFLD and NASH and screening for potential therapeutic targets.
Dietary animal models of NASH
Over the last decade we have seen an increasing number of animal models of NAFLD, with various degrees of fidelity to the metabolic profile and the histological patterns seen in human NASH [21–27]. More recent overnutrition-based models have demonstrated substantial metabolic similarity to humans with NAFLD and NASH with reproducibility of the histological features of NASH, more importantly progressive hepatic fibrosis [19,28] and hepatocellular ballooning [19,29], in a context of high fidelity to the physiological profile seen in humans with fibrosing NASH. The severity of diet induced-NASH may depend on the species (rats appear to be more sensitive than mice [30]), gender (males appear to be more sensitive than females as estrogen is protective against NASH, and estrogen replacement in estrogen-deficient mice improves steatosis [31]), and strain of the animal (C57BL/6J mice appear to be more susceptible to NASH [32]).
Methionine and choline deficient (MCD) diet
The MCD is a widely employed diet in NASH animal studies. MCD diet is high in sucrose and fat (40% sucrose, 10% fat), and is deficient in methionine and choline, which are essential for hepatic β oxidation and the production of very low density lipoprotein (VLDL) [25]. In addition, choline deficiency impairs hepatic VLDL secretion [33], resulting in increased fat accumulation in the liver and decreased VLDL synthesis. On the other hand, oxidative stress [34] and changes in cytokines and adipokines occur in this model, culminating to liver injury [35]. Although this model replicates the histological features of steatohepatitis and fibrosis observed in human NASH, its metabolic context is distinct from human NASH, since animal fed the MCD diet lose weight (up to 40% in 10 weeks), have low fasting blood sugar, peripheral insulin sensitivity, low serum insulin and leptin levels, and unchanged or increased serum adiponectin levels [23,35], and decreased blood triglyceride and cholesterol, creating a metabolic profile opposite to the human disease. Therefore, genetically obese mice, such as ob/ob and db/db mice, are occasionally used as the MCD-fed animal [36]. The main advantages of the MCD diet are that it is widely available and replicates NASH histological phenotype within a relatively shorter feeding time than other dietary models of NASH. Serum alanine aminotransferase (ALT) levels are increased, and histological evidence of steatohepatitis is seen by day 10 [37], while perisinusoidal fibrosis develop by 8 to 10 weeks in mice on the MCD diet [38]. The MCD model causes more severe inflammation, oxidative stress, apoptosis, and fibrosis than other animal nutritional models of NASH [39]. Therefore the MCD diet is far from optimal to examine the metabolic parameters of NAFLD, and its use should be discouraged.
Choline-deficient L-amino-defined (CDAA) diet
CDAA diet has the same overall composition as the choline deficient (CD) diet, but proteins are replaced with an equivalent and corresponding mixture of L-amino acids [40]. Like the MCD diet, it inhibits fatty acid oxidation in hepatocytes, increases lipid synthesis, oxidative stress and inflammation, resulting in liver fibrosis [41], but requires a longer time frame than MCD diet for these histological changes to develop [40]. Mice on the CDAA diet do not display hepatic insulin resistance [39], nor do they gain weight or have changes in peripheral insulin sensitivity. Hence like the MCD diet the metabolic profile displayed is opposite of the human disease [39], and this diet should not be used to examine the metabolic profile of the disease.
High fat diet (HFD)
The first described high fat diet was in the form of a liquid diet (71% of energy from fat, 11% from carbohydrates, 18% from protein). Panlobular steatosis developed after 3 weeks in male Sprague-Dawley rats on the diet. Rats developed insulin resistance, increased hepatic molecular markers of inflammation, oxidative stress and indices of fibrogenesis [30]. C57BL/6 mice fed via an implanted gastrostomy tube a HFD (up to 85% in excess of the standard intake) for 9 weeks, developed obesity, insulin resistance, and the histopathological features of human NASH, with inflammatory infiltrate and perisinusoidal fibrosis, but no ballooning [21]. Ad-lib HFD with 60 % energy from fat (lard), 20% from carbohydrate and 20% from protein, caused steatohepatitis in male C57BL/6J mice; after 10-week exposure to the high fat diet, obesity and hyperinsulinemia developed. Furthermore, glucose intolerance was observed after 12 weeks. Despite the presence of hepatic steatosis, hepatic inflammation was not induced until 19 weeks on the HFD [22]. HFD seems to have a more pronounced effect in rats when compared to mice as a shorter duration up to 6 weeks was required and the pathology better resembles that seen in human NASH [42]. Thus, in rodents, high fat diets can replicate the altered metabolic parameters observed in human fatty liver disease, but the hepatic pathological outcome is not as severe.
Cholesterol and Cholate
High cholesterol diet (21% milk butter, 0.2% cholesterol), is an important risk factor for the progression of hepatic inflammation in high fat diet-induced NASH [43]. C57BL/6J mice fed the atherogenic diet consisting of cholesterol (1.25%), 7.5% cocoa butter, and cholate (0.5%) over 3 weeks display expansion of acute inflammation genes by the cholesterol component in the diet, and fibrogenesis genes by the cholate component [44]. The atherogenic diet consisting of 1.25% cholesterol and 0.5% cholate induces progressive steatosis, inflammation, and fibrosis over 6 to 24 weeks. Hepatocellular ballooning was observed at 24 weeks. The addition of 60% fat (cocoa butter) accelerated the development of histopathological changes of NASH, and hepatocellular ballooning was observed at 12 weeks. The atherogenic diet induces oxidative stress, dyslipidemia, lipid peroxidation, and stellate cell activation in the liver. Mice fed the atherogenic diet are remarkably sensitive to insulin compared to control. This ameliorating effect on the glucose tolerance and insulin sensitivity was attributed to decreased adipose tissue. On the other hand, mice on the atherogenic diet develop hepatic insulin resistance, as assessed by the pyruvate challenge test [45]. Recently, Sprague-Dawley rats were found to be more sensitive to a high fat and cholesterol diet. These rats developed hepatic features of NASH and progressed to cirrhosis within 9 weeks [46]. Therefore, the atherogenic diet replicates the human disease pathology, but the metabolic changes differ from human NASH; adding a high fat component accelerates the development of the histological changes of NASH.
Fructose
High fructose consumption from corn syrup is a typical feature of the diet in humans with NASH [47,48]. The addition of the high fructose content to a diet high in saturated fat and cholesterol has been found to reproduce all the features of NASH, including ballooning in large animals [49]. When C57BL/6 mice were housed to promote the sedentary status, and fed a high-fat [45% calories from fat, with 30% of the fat in the form of partially hydrogenated vegetable oil (28% saturated, 57% monounsaturated, 13% polyunsaturated fatty acids)], high fructose (55% fructose, 45% glucose in drinking water) diet for 16 weeks, obesity with insulin resistance, severe hepatic steatosis with associated necroinflammatory changes developed. Although mice displayed molecular markers of fibrogenesis, overt fibrosis was not striking on histology [26]. Given the importance of fibrosis as an indicator of severity and disease progression in NASH, Kohli et al used a high fat high carbohydrate diet (58% of calories from fat), and drinking water with high fructose (55% fructose); C57BL/6 mice fed the diet over a 16-week period developed a phenotype of obesity, insulin resistance, and fibrosing NASH. To be noted, when compared to mice fed the high fat diet only; the high fat high carbohydrate diet-fed mice had increased hepatic oxidative stress, macrophage infiltration and fibrosis [28]. A similar paradigm to obtain hepatocellular injury, inflammation, and fibrosis in NASH using fructose was subsequently reported in a rat model as well [50]. We developed a model of fibrosing NASH by reproducing the physiological milieu seen in humans with NASH, i.e., physical inactivity and chronic overnutrition with a high caloric intake rich in saturated fats and fructose. Because fibrosis in patients with NASH typically develops over prolonged periods of time, we continued overnutrition for 6 months, which is a longer interval than dietary overnutrition in previous studies of rodent models of NASH. Our diet consisted of 40% energy as fat (12% saturated fatty acid, 0.2% cholesterol), and a high-fructose corn syrup (42 g/l final concentration) in drinking water. This diet is also known as high fat, fructose and cholesterol (FFC) diet. FFC diet was compared to the high fat (HF) diet (60% energy as fat (1% saturated fatty acid); both diets recapitulated the features of the metabolic syndrome with obesity, insulin resistance, and steatosis. However, HF diet was associated with minimal inflammation, and no increase in fibrosis. The FFC diet demonstrated steatohepatitis with pronounced hepatocellular ballooning and progressive fibrosis (stage 2) (Fig. 2), with a high fidelity to the human NASH histological features, and increased expression of genes involved with fibrosis, inflammation, endoplasmic reticulum stress and lipoapoptosis [19]. The FFC diet highlights the contribution of the fructose and cholesterol dietary composition in addition to the high fat to the development of NAFLD and NASH [19]. Recently the same histological and metabolic features were reproduced in C57BL/6J mice over a 20 week feeding period of a high fat, high fructose diet consisting of 44.6% of kcal derived from fat (of which 61% saturated fatty acids) and 40.6% of kcal derived from carbohydrates (primarily sucrose 340 g/kg diet). This diet was termed high fat high sugar diet (HF-HSD) [29]. Adipose tissue dysregulation is a key component of NASH and the inflammation in the two tissues is interrelated [51]. Thus the FFC diet mimics human NASH in this perspective [52]. Therefore, the addition of the fructose to the nutrient excess diet promotes the development of fibrosis, hepatocellular ballooning and adipose tissue inflammation in the mouse models of NASH.
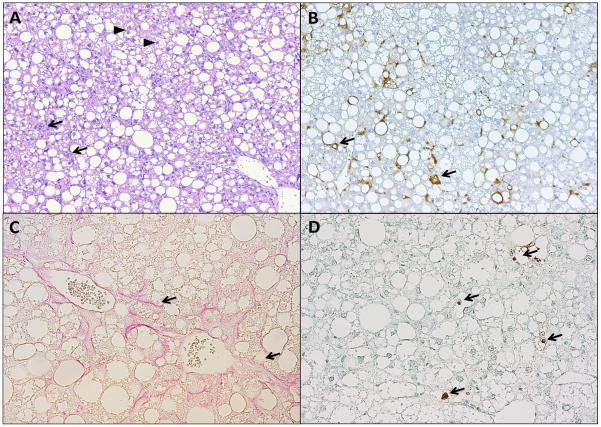
Figure 2. Histological sections of a mouse liver fed a high fat, fructose and cholesterol (FFC) diet for 6 months.
(A) H& E stain, taken with 10 × objective, shows steatosis with inflammatory cells infiltration (arrows), and ballooned hepatocytes (arrow heads). (B) Macrophage galactose-specific lectin (Mac-2) immunohistochemical stain, taken with 10 × objective, shows macrophage infiltration (arrows). (C) Sirius red stain taken with 20 × objective, shows pericellular and bridging fibrosis (arrows), (D) Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay, taken with 20 × objective, shows labeled DNA strand breaks in apoptotic nuclei (arrows).
Genetic animal models of NASH
A great variety of genetically modified mice have been developed to better understand NASH. To date, most of these models have in common that the genetic alterations result in a significant hepatic lipid accumulation, with absent marked inflammation and fibrosis. An additional stimulus, such as a modified diet, is often required to induce histopathological features of NASH. NASH induced by a genetic manipulation has a distinct etiology from human NASH associated with metabolic syndrome, which represents the major limitation of these models. Nevertheless, NAFLD/NASH genetic models have provided us with many important insights into the mechanisms by which lipid metabolism promotes fatty liver disease.
Leptin deficiency (the ob/ob mice)
Ob/ob mice lack functional leptin due to a spontaneous point mutation in the leptin gene. Leptin, which is secreted as a peptide hormone predominantly by white adipose tissue, negatively regulates food intake and increases energy expenditure. Ob/ob mice are hyperphagic, inactive and develop severe obesity, hyperlipidemia, hyperglycemia, hyperinsulinemia, and insulin resistance [53]. Although hyperphagia contributes to the obesity, ob/ob mice gain excess weight even when restricted to a diet sufficient for normal weight maintenance in lean mice. In ob/ob mice fat is redistributed from adipose tissue to the liver and other non-adipose tissues. Fat accumulation in the liver induces hepatocyte lipotoxicity and lipoapoptosis. However, ob/ob mice rarely develop severe liver damage or spontaneous steatohepatitis, which may be due to alterations in inflammatory responses. For induction of “true” NASH, an additional stimulus is required. This additional impetus may involve administration of small doses of lipopolysaccharide endotoxin, ischemia-reperfusion injury, high fat diet or MCD diet feeding. [54,55] Interestingly, ob/ob mice are protected against MCD diet-induced fibrosis despite developing similar necroinflammatory lesions as their genetic controls [56]. The fibrosis in these mice can be induced by supplying exogenous leptin [56]. Leptin mutations have been reported in humans, yet serum leptin levels in NASH population are normal or elevated, which represents a shortcoming of this model [57–59].
Leptin receptor deficiency (the db/db mice)
Db/db mice carry a spontaneous mutation in the leptin receptor gene. Although these mice have normal or elevated levels of leptin, the receptor mutation confers resistance to the effects of leptin. As predicted, the phenotype of db/db mice is similar to one of ob/ob mice, which are deficient in leptin. The db/db mice are hyperphagic and develop obesity, hyperglycemia, hyperinsulinemia, insulin resistance and hepatic steatosis [60]. They also progress to NASH only when an additional stimulus is added, for example on the MCD diet. In contrast to ob/ob mice, db/db mice on MCD diet develop significant fibrosis [61].
Sterol regulatory element-binding protein 1 (SREBP-1) c transgenic mice
SREBP-1c is one of two transcript variants encoded by SREBP-1. SREBP-1c is an insulin-regulated transcription factor regulating genes required for glucose metabolism and fatty acid and lipid production. SREBP-1c transgenic mice that overexpress SREBP-1c selectively in their fatty tissue have impaired adipose differentiation and develop severe insulin resistance and marked hepatic steatosis [62]. Besides steatosis, they also spontaneously develop lobular inflammation, perivenular and pericellular fibrosis and ballooned hepatocytes [63]. Although the liver histology recapitulates many features of human NASH, this model has decreased adipose tissue mass, which is in sharp contrast to the human condition. These mice represent a suitable model of congenital lipodystrophy and lipodystrophy associated steatohepatitis model.
KK-Ay/a mice
KK-Ay/a mice possess a heterozygous mutation in the agouti gene. These mice are hyperphagic as a result of impaired hypothalamic food intake suppression [64]. They develop obesity and associated hyperglycemia, hyperinsulinemia, insulin resistance and liver steatosis [65]. Liver steatosis in these mice does not spontaneously advance to steatohepatitis, which however can be induced by additional stimulus, such as the MCD diet [65].
Phosphatase and tensin homolog (PTEN) null mice
PTEN has been extensively studied as a tumor suppressor gene, which negatively regulates several signaling cascades including phosphatidylinositol 3-kinase/Akt pathway. Albumin-cre driven liver-specific PTEN deletion results in substantial hepatomegaly accompanied by liver steatosis due to increased fatty acid synthesis [66]. Strikingly, PTEN liver-specific deletion causes liver insulin hypersensitivity with decreased fasting glucose levels and improved systemic glucose tolerance. In most of these animals, liver steatosis progresses to steatohepatitis with ballooned hepatocytes, Mallory's hyaline, lobular inflammation and mild pericellular fibrosis. These mice eventually develop benign liver adenomas or hepatocellular carcinomas [67]. Although this model spontaneously develops steatohepatitis with histologic features of human NASH, insulin hypersensitivity and decreased body fat mass are not characteristic of the human correlate.
Peroxisome proliferator-activated receptor α (PPARα) null mice
The nuclear receptor PPARα regulates transcription of genes involved in mitochondrial and peroxisomal fatty acid oxidation. Young PPARα knockout mice do not accumulate excess fat in the liver under normal feeding conditions, however when subjected to fasting (24 h) or high fat diet they develop severe steatosis [68,69]. PPARα deficiency prevents upregulation of fatty acid oxidation in response to the increased influx of fatty acids from the adipose tissues to the liver induced either by food deprivation or high-fat diet feeding. In fasted PPARα null mice, hepatic uptake of fatty acids appears to be decreased and VLDL secretion normal, supporting the role of decreased fatty acid oxidation in fat accumulation in the liver of these mice [68]. As PPARα null mice age, they spontaneously develop a sexually dimorphic pattern of obesity (more pronounced in female mice) and liver steatosis, which, however, does not progress to NASH [70,71].
Acyl-coenzyme A oxidase (AOX) null mice
AOX catalyzes the first and rate-limiting step in peroxisomal β-oxidation of fatty acids. The liver of AOX knockout mice thus displays decreased peroxisomal β-oxidation of fatty acids, which start to accumulate within the hepatocytes leading to microvesicular steatosis, later progressing to transient steatohepatitis without marked fibrosis [72,73]. By 6–8 months of age, a compensatory increase of fatty acid oxidation occurs and hepatic steatosis is then completely reversed by hepatocyte regeneration, which display spontaneous peroxisome proliferation and high expression of PPARα target genes [72]. Later in age, AOX null mice develop hepatocellular adenomas and carcinoma due to sustained activation of PPARα [73].
Methionine adenosyl transferase (MAT1A) null mice
MAT1A is a liver-specific enzyme catalyzing conversion of methionine to S-adenosylmethionine, a major biological methyl donor. MAT1A knockout mice have a decreased hepatic content of S-adenosylmethionine and glutathione, and increased expressions of genes involved in lipid peroxidation [74]. By 8 months of age, these mice spontaneously develop features of NASH represented by macrovesicular steatosis and inflammatory cell infiltration mainly in periportal areas, however without features of the metabolic syndrome [75]. Choline-deficient diet promotes severe macrovesicular steatosis in young MAT1A null mice (3 months). MAT1A deletion also causes an increased hepatocyte proliferation and the majority of MAT1A knockout mice eventually advance to hepatocellular carcinoma [74].
Inflammatory animal models of NASH
Activation of a sterile innate-immune system mediated inflammatory response is a key feature of NASH [76,77]. Obesity, insulin resistance, and hepatic steatosis are inherent to the definition of NASH, therefore examination or development of pure inflammatory models of NASH would be somewhat deficient in key components of the disease; however, an alternative approach, which examines the contribution of known inflammatory mediators in dietary disease models of NASH has considerably advanced the understanding of the role of various inflammatory chemokines, cytokines, transcription factors and immune cell types in NASH.
Macrophages
Macrophages are primary mediators of the sterile inflammatory response in NASH. Proinflammatory macrophages are recruited to the liver in NASH from hematopoietic stem cell derived myeloid lineage cells rather than yolk sac-derived resident hepatic macrophages, historically named Kupffer cells [78–80]. Indeed, Kupffer cells may also be proinflammatory in the obese state by secreting monocyte chemotactic protein 1 (MCP-1), a chemokine critical to recruiting myeloid cells to the liver via its receptor, chemokine (C-C motif) receptor 2 (CCR-2), which was highly expressed in recruited hepatic macrophages [78]. Further, several independent animal studies have demonstrated the importance of the CCR-2 and MCP-1 signaling in NASH pathogenesis [78,81]. Mice deficient in CCR-2 (Ccr2−/−) are protected from the development of hepatic steatosis, inflammation, macrophage infiltration and fibrosis compared to wild-type mice [81]. Indeed, mice deleted in upstream receptors which utilize activation of MCP-1 expression as a final common pathway, such as toll-like receptor (TLR) 4, TLR 9 or myeloid cell differentiation (MyD) 88 knockout mice, all demonstrate reduced hepatic macrophage accumulation due to deficient MCP-1 and CCR-2 signaling.
Toll-like receptor signaling
Several toll-like receptors have been implicated in NASH. Using the apolipoprotein E knockout mouse model of NASH, the whole body deletion of TLR4 protects against the development of liver injury and inflammation [82]. Mice deficient in TLR4 or its co-receptor myeloid differentiation protein 2 (MD-2) are protected from MCD diet-induced NASH [83]. TLR9 deletion, interleukin 1 receptor deletion, or MyD88 deletion were all protective against the CDAA diet-induced steatohepatitis. However, in an alternative approach utilizing high fat diet feeding, MyD88−/− mice showed exacerbation of liver injury and insulin resistance in spite of equal body weight gain [84]. Thus, these studies suggest that there is model-specific activation of individual toll-like receptors and results from these studies may not be generalizable. Furthermore, the cellular compartment wherein the activation of TLRs is important varies. The tissue specific deletion approach has recently been utilized to demonstrate that hepatocyte TLR4 and not myeloid cell TLR4 mediates hepatic steatosis. Mice lacking TLR4 in hepatocytes were protected from a nutrient excess diet-induced insulin resistance and fatty liver, though not the development of obesity [85]. Furthermore, myeloid cell TLR4 deletion had no protective effect. Interestingly, markers of liver injury and inflammation were not reported in this study.
C-jun N-terminal kinases (JNK)
Activation of JNK 1 and 2 occurs in both liver and adipose tissue in the obese, insulin resistant state. In the liver these kinases are activated both in parenchymal cells and non-parenchymal cells. The contribution of these kinases in myeloid cells have been examined utilizing chimeric mice generated by bone marrow transplantation or using tissue-specific promoters to delete JNK expression in myeloid cells [86,87]. Mice deleted in both JNK 1 and 2 in myeloid cells, when challenged with a high fat diet for 4 weeks developed similar increases in body mass, however, had a significant reduction in liver inflammation, macrophage accumulation, hepatic steatosis and improved insulin sensitivity [87]. It is not known if longer feeding studies with this strain of mice would demonstrate a sustained reduction in liver injury and fibrosis, though the prediction is that a reduction in pro-inflammatory myeloid cells infiltration would be beneficial over the long term. Mixed lineage kinase (MLK) 3 is an upstream kinase, mediating JNK activation. Indeed, consistent with the JNK knockout data, mice deleted in MLK3 are also protected against the development of dietary NASH [88]. In this context, it remains to be seen whether hepatocyte MLK3 or myeloid cell MLK3 signaling is the principal mediator of NASH. This question can be addressed by pursuing tissue specific deletion models.
Inflammasome
The inflammasome is a cellular signaling platform activated both by microbial insults and sterile inflammatory stimuli such as uric acid [89]. It is composed of several sensor and signaling proteins, which combine to form active caspase 1 from procaspase 1. Caspase 1 is necessary for the secretion of interleukin (IL)-1 beta (IL-1β) and IL-18. NOD-like receptors (NLR) proteins, namely NLRP3 (NOD-, LRR- and pyrin domain-containing 3) has been implicated in fatty liver. Mice deficient in NLRP3 are protected from the development of diet-induced hepatic steatosis [90]. In contrast, in another study deficient NLRP3 signaling worsened fatty liver due to changes in intestinal microbiota, which led to increased hepatic activation of TLR4 and TLR9 from microbial products [91]. Using an inducible overexpression model for hepatic NLRP3, it was demonstrated that activation of this protein markedly exacerbated diet-induced steatohepatitis induced by a CDAA diet [92]. Thus, with respect to NLRP3, intestinal versus hepatic activation have divergent outcomes in NASH pathogenesis.
Though the presence and progression of fibrosis are increasingly recognized as clinically relevant histologic features in NASH, fibrosis does not occur in the absence of inflammation. The progression to fibrosis in cases with known inflammation is not universal either. Furthermore, what separates fibrogenic inflammation from non-fibrogenic inflammation is unknown, and remains a question for future studies.
Conclusion
This review has explored the different characteristics and limitations of the current dietary and genetic murine models used to study human NAFLD (Table 1). We have also presented some of the pathways involved in the development of the sterile inflammatory response associated with NASH and underscored adipose tissue dysregulation as a key feature of human NASH [51]. Future animal studies should also interrogate adipose tissue inflammation in addition to liver steatosis, inflammation and fibrosis. Without the assessment of both adipose tissue and liver in future models, their utility for mimicking the human disease will be imperfect. The optimal model in our opinion will be a diet-induced obesity model with both liver and adipose tissue dysfunction, such as the FFC diet.
Table 1.
Main features of commonly used animal models of NAFLD.
| Model | Obesity | Insulin resistance | Steatosis | Steatohepatits | Fibrosis | Ballooning |
|---|---|---|---|---|---|---|
|
| ||||||
| Dietary | ||||||
| MCD | − | Hepatic insulin resistance only | + | + | + | − |
|
| ||||||
| CDAA | − | − | + | + | + | − |
|
| ||||||
| Atherogenic diet | − | Hepatic insulin resistance only | + | + | + | + |
|
| ||||||
| Lieber-HF-liquid | + | + | + | + | − | − |
|
| ||||||
| Kohli-HFHC | + | + | + | + | + | − |
|
| ||||||
| Charlton-FFC | + | + | + | + | + | + |
|
| ||||||
| Verbeek-HF-HSD | + | + | + | + | + | + |
|
| ||||||
| Genetic | ||||||
| ob/ob | + | + | + | − | − | − |
|
| ||||||
| db/db | + | + | + | − | − | − |
|
| ||||||
| SREBP-1c overexpression (adipocyte specific) | − | + | + | + | + | + |
|
| ||||||
| KK-Ay | + | + | + | − | − | − |
|
| ||||||
| PTEN−/− (liver specific) | − | − | + | + | + | + |
|
| ||||||
| PPAR α −/− | Late onset | − | + | − | − | − |
|
| ||||||
| AOX −/− | − | − | + | Transient | − | − |
|
| ||||||
| MAT1A−/− (liver specific) | − | − | + | + | ? | ? |
|
| ||||||
| Combined | ||||||
| db/db + MCD | + | + | + | + | + | − |
|
| ||||||
| ob/ob + MCD | + | + | + | + | − | − |
Methionine and choline deficient diet (MCD), Choline-deficient L-amino-defined (CDAA), High Fat (HF), High Fat High Carbohydrate (HFHC), high fat, fructose and cholesterol (FFC), high fat high sugar diet (HF-HSD), leptin gene deficiency (ob/ob), leptin receptor deficiency (db/db), sterol regulatory element-binding protein 1c (SREBP-1c), phosphatase and tensin homolog (PTEN), peroxisome proliferator-activated receptor α (PPARα), acyl-coenzyme A oxidase (AOX), methionine adenosyltransferase (MAT1A).
Key Findings / Future Unmet Needs / Implications.
NASH is currently the most common liver disease and will become the most common indication for liver transplantation in the near future.
The majority of current NASH mouse models do not fully recapitulate the human condition.
The most “accurate” nutritional dietary model incorporate all dietary component of the western diet, including fat, fructose, and cholesterol. Such a diet needs to be maintained over a prolonged feeding period of 20 weeks or more to reproduce the histopathological features NASH.
NASH induced by genetic manipulations has a distinct etiology from human NASH associated with the metabolic syndrome, which contributes to the major limitation of these models.
Although activation of a sterile innate-immune system mediated inflammatory response is a key feature of non-alcoholic steatohepatitis, examination of a pure inflammatory models of NASH would be deficient in the metabolic key components of the disease.
Acknowledgements
The authors thank Courtney Hoover for her excellent secretarial assistance, Dr. Thomas Smyrk, and Dr. Kyoko Tomita for their help in acquiring the histological pictures.
Financial Support: This work was supported by the Center for Clinical and Translational Science (CCaTS), KL2 program KL2TR000136-09 (SHI), a Pilot and Feasibility Award (SHI), and the optical microscopy core of the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567), NIH grant DK97178 (HM), NIH grant DK41876 (GJG), and the Mayo Clinic, Rochester, MN.
Abreviations in order of appearance in the manuscript
- (NAFLD)
nonalcoholic fatty liver disease
- (NASH)
nonalcoholic steatohepatitis
- (MCD)
methionine and choline deficient
- (CDAA)
choline-deficient L-amino-defined
- (VLDL)
very low density lipoprotein
- (HFD)
high fat diet
- (FF)
fast food
- (HF)
high fat
- (FFC)
high fat, fructose and cholesterol
- (HF-HSD)
high fat high sugar diet
- (SREBP-1)
sterol regulatory element-binding protein 1
- (PTEN)
phosphatase and tensin homolog
- (PPARα)
peroxisome proliferator-activated receptor α
- (AOX)
acyl-coenzyme A oxidase
- (MAT1A)
methionine adenosyl transferase
- (MCP-1)
monocyte chemotactic protein 1
- (CCR-2)
chemokine (C-C motif) receptor 2
- (TLR)
toll-like receptor
- (My88)
myeloid cell differentiation 88
- (MD-2)
myeloid differentiation protein 2
- (JNK)
C-jun N-terminal kinase
- (MLK) 3
mixed lineage kinase
- (IL)
interleukin
- (NLR)
NOD-like receptors
- (NLRP3)
NOD-LRR- and pyrin domain-containing 3
Footnotes
Conflict of Interest: The authors report no conflict of interest.
References
- 1.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;(17 Suppl):S186–190. doi: 10.1046/j.1440-1746.17.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 4.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 5.Vanni E, Marengo A, Mezzabotta L, Bugianesi E. Systemic complications of nonalcoholic fatty liver disease: When the liver is not an innocent bystander. Semin Liver Dis. 2015;35:236–249. doi: 10.1055/s-0035-1562944. [DOI] [PubMed] [Google Scholar]
- 6.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: A follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 8.Propst A, Propst T, Judmaier G, Vogel W. Prognosis in nonalcoholic steatohepatitis. Gastroenterology. 1995;108:1607. doi: 10.1016/0016-5085(95)90724-6. [DOI] [PubMed] [Google Scholar]
- 9.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Lackner C. Hepatocellular ballooning in nonalcoholic steatohepatitis: The pathologist's perspective. Expert Rev Gastroent. 2011;5:223–231. doi: 10.1586/egh.11.8. [DOI] [PubMed] [Google Scholar]
- 11.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 12.Carter-Kent C, Brunt EM, Yerian LM, Alkhouri N, Angulo P, Kohli R, Ling SC, Xanthakos SA, Whitington PF, Charatcharoenwitthaya P, Yap J, Lopez R, McCullough AJ, Feldstein AE. Relations of steatosis type, grade, and zonality to histological features in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2011;52:190–197. doi: 10.1097/MPG.0b013e3181fb47d3. [DOI] [PubMed] [Google Scholar]
- 13.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 14.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 15.Day CP, James OF. Steatohepatitis: A tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 16.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating tnf-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 17.Tilg H, Diehl AM. Mechanisms of disease: Cytokines in alcoholic and nonalcoholic steatohepatitis. New Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 18.Duval C, Thissen U, Keshtkar S, Accart B, Stienstra R, Boekschoten MV, Roskams T, Kersten S, Muller M. Adipose tissue dysfunction signals progression of hepatic steatosis towards nonalcoholic steatohepatitis in c57bl/6 mice. Diabetes. 2010;59:3181–3191. doi: 10.2337/db10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, Masuoko H, Gores G. Fast food diet mouse: Novel small animal model of nash with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011;301:G825–834. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in pnpla3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S, Tsukamoto H. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42:905–914. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 22.Ito M, Suzuki J, Tsujioka S, Sasaki M, Gomori A, Shirakura T, Hirose H, Ishihara A, Iwaasa H, Kanatani A. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol Res. 2007;37:50–57. doi: 10.1111/j.1872-034X.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- 23.Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol. 2004;40:47–51. doi: 10.1016/j.jhep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Svegliati-Baroni G, Candelaresi C, Saccomanno S, Ferretti G, Bachetti T, Marzioni M, De Minicis S, Nobili L, Salzano R, Omenetti A, Pacetti D, Sigmund S, Benedetti A, Casini A. A model of insulin resistance and nonalcoholic steatohepatitis in rats: Role of peroxisome proliferator-activated receptor-alpha and n-3 polyunsaturated fatty acid treatment on liver injury. The American journal of pathology. 2006;169:846–860. doi: 10.2353/ajpath.2006.050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte cyp2e1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 26.Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe nafld with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008;295:G987–995. doi: 10.1152/ajpgi.90272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiri-Sverdlov R, Wouters K, van Gorp PJ, Gijbels MJ, Noel B, Buffat L, Staels B, Maeda N, van Bilsen M, Hofker MH. Early diet-induced non-alcoholic steatohepatitis in apoe2 knock-in mice and its prevention by fibrates. J Hepatol. 2006;44:732–741. doi: 10.1016/j.jhep.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 28.Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, Tang PH, Miles L, Miles MV, Balistreri WF, Woods SC, Seeley RJ. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52:934–944. doi: 10.1002/hep.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbeek J, Lannoo M, Pirinen E, Ryu D, Spincemaille P, Vander Elst I, Windmolders P, Thevissen K, Cammue BPA, van Pelt J, Fransis S, Van Eyken P, Ceuterick-De Groote C, Van Veldhoven PP, Bedossa P, Nevens F, Auwerx J, Cassiman D. Roux-en-y gastric bypass attenuates hepatic mitochondrial dysfunction in mice with non-alcoholic steatohepatitis. Gut. 2015;64:673–683. doi: 10.1136/gutjnl-2014-306748. [DOI] [PubMed] [Google Scholar]
- 30.Lieber CS, Leo MA, Mak KM, Xu YQ, Cao Q, Ren CL, Ponomarenko A, DeCarli LM. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- 31.Hewitt KN, Pratis K, Jones MEE, Simpson ER. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology. 2004;145:1842–1848. doi: 10.1210/en.2003-1369. [DOI] [PubMed] [Google Scholar]
- 32.Newberry EP, Kennedy S, Xie Y, Luo J, Jiang H, Ory DS, Davidson NO. Phenotypic divergence in two lines of l-fabp −/− mice reflects substrain differences and environmental modifiers. Am J Physiol Gastrointest Liver Physiol. 2015 doi: 10.1152/ajpgi.00170.2015. ajpgi 00170 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao ZM, Vance DE. Reduction in vldl, but not hdl, in plasma of rats deficient in choline. Biochem Cell Biol. 1990;68:552–558. doi: 10.1139/o90-079. [DOI] [PubMed] [Google Scholar]
- 34.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. Cyp2e1 and cyp4a as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larter CZ, Yeh MM, Williams J, Bell-Anderson KS, Farrell GC. Mcd-induced steatohepatitis is associated with hepatic adiponectin resistance and adipogenic transformation of hepatocytes. J Hepatol. 2008;49:407–416. doi: 10.1016/j.jhep.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 37.Dela Pena A, Leclercq I, Field J, George J, Jones B, Farrell G. Nf-kappab activation, rather than tnf, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005;129:1663–1674. doi: 10.1053/j.gastro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent pparalpha agonist, wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- 39.Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastro Hepat. 2011;8:34–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 40.Nakae D, Mizumoto Y, Andoh N, Tamura K, Horiguchi K, Endoh T, Kobayashi E, Tsujiuchi T, Denda A, Lombardi B, Konishi Y. Comparative changes in the liver of female fischer-344 rats after short-term feeding of a semipurified or a semisynthetic l-amino acid-defined choline-deficient diet. Toxicol Pathol. 1995;23:583–590. doi: 10.1177/019262339502300504. [DOI] [PubMed] [Google Scholar]
- 41.Kodama Y, Kisseleva T, Iwaisako K, Miura K, Taura K, De Minicis S, Osterreicher CH, Schnabl B, Seki E, Brenner DA. C-jun n-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology. 2009;137:1467–1477. doi: 10.1053/j.gastro.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou YH, Li J, Lu C, Wang JQ, Ge JF, Huang Y, Zhang L, Wang YY. High-fat emulsion-induced rat model of nonalcoholic steatohepatitis. Life Sci. 2006;79:1100–1107. doi: 10.1016/j.lfs.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ, Duimel H, Lutjohann D, Kerksiek A, van Kruchten R, Maeda N, Staels B, van Bilsen M, Shiri-Sverdlov R, Hofker MH. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 2008;48:474–486. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- 44.Vergnes L, Phan J, Strauss M, Tafuri S, Reue K. Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J Biol Chem. 2003;278:42774–42784. doi: 10.1074/jbc.M306022200. [DOI] [PubMed] [Google Scholar]
- 45.Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, Yokoyama M, Honda M, Zen Y, Nakanuma Y, Miyamoto K, Kaneko S. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–1403. doi: 10.1002/hep.21874. [DOI] [PubMed] [Google Scholar]
- 46.Ichimura M, Kawase M, Masuzumi M, Sakaki M, Nagata Y, Tanaka K, Suruga K, Tamaru S, Kato S, Tsuneyama K, Omagari K. High-fat and high-cholesterol diet rapidly induces non-alcoholic steatohepatitis with advanced fibrosis in sprague-dawley rats. Hepatology Research. 2015;45:458–469. doi: 10.1111/hepr.12358. [DOI] [PubMed] [Google Scholar]
- 47.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM, Clini NS. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee L, Alloosh M, Saxena R, Van Alstine W, Watkins BA, Klaunig JE, Sturek M, Chalasani N. Nutritional model of steatohepatitis and metabolic syndrome in the ossabaw miniature swine. Hepatology. 2009;50:56–67. doi: 10.1002/hep.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alisi A, Manco M, Pezzullo M, Nobili V. Fructose at the center of necroinflammation and fibrosis in nonalcoholic steatohepatitis. Hepatology. 2011;53:372–373. doi: 10.1002/hep.23873. [DOI] [PubMed] [Google Scholar]
- 51.du Plessis J, van Pelt J, Korf H, Mathieu C, van der Schueren B, Lannoo M, Oyen T, Topal B, Fetter G, Nayler S, van der Merwe T, Windmolders P, Van Gaal L, Verrijken A, Hubens G, Gericke M, Cassiman D, Francque S, Nevens F, van der Merwe S. Association of adipose tissue inflammation with histologic severity of nonalcoholic fatty liver disease. Gastroenterology. 2015;149:635–648. e614. doi: 10.1053/j.gastro.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 52.Idrissova L, Malhi H, Werneburg NW, LeBrasseur NK, Bronk SF, Fingas C, Tchkonia T, Pirtskhalava T, White TA, Stout MB, Hirsova P, Krishnan A, Liedtke C, Trautwein C, Finnberg N, El-Deiry WS, Kirkland JL, Gores GJ. Trail receptor deletion in mice suppresses the inflammation of nutrient excess. J Hepatol. 2015;62:1156–1163. doi: 10.1016/j.jhep.2014.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mayer J, Bates MW, Dickie MM. Hereditary diabetes in genetically obese mice. Science. 1951;113:746–747. doi: 10.1126/science.113.2948.746. [DOI] [PubMed] [Google Scholar]
- 54.Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: Implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brix AE, Elgavish A, Nagy TR, Gower BA, Rhead WJ, Wood PA. Evaluation of liver fatty acid oxidation in the leptin-deficient obese mouse. Mol Genet Metab. 2002;75:219–226. doi: 10.1006/mgme.2002.3298. [DOI] [PubMed] [Google Scholar]
- 56.Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206–213. doi: 10.1016/s0168-8278(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 57.Chitturi S, Farrell G, Frost L, Kriketos A, Lin R, Fung C, Liddle C, Samarasinghe D, George J. Serum leptin in nash correlates with hepatic steatosis but not fibrosis: A manifestation of lipotoxicity? Hepatology. 2002;36:403–409. doi: 10.1053/jhep.2002.34738. [DOI] [PubMed] [Google Scholar]
- 58.Uygun A, Kadayifci A, Yesilova Z, Erdil A, Yaman H, Saka M, Deveci MS, Bagci S, Gulsen M, Karaeren N, Dagalp K. Serum leptin levels in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2000;95:3584–3589. doi: 10.1111/j.1572-0241.2000.03297.x. [DOI] [PubMed] [Google Scholar]
- 59.Chalasani N, Crabb DW, Cummings OW, Kwo PY, Asghar A, Pandya PK, Considine RV. Does leptin play a role in the pathogenesis of human nonalcoholic steatohepatitis? Am J Gastroenterol. 2003;98:2771–2776. doi: 10.1111/j.1572-0241.2003.08767.x. [DOI] [PubMed] [Google Scholar]
- 60.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 61.Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whitington PF. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: Role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1035–1043. doi: 10.1152/ajpgi.00199.2004. [DOI] [PubMed] [Google Scholar]
- 62.Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear srebp-1c in adipose tissue: Model for congenital generalized lipodystrophy. Genes Dev. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakayama H, Otabe S, Ueno T, Hirota N, Yuan X, Fukutani T, Hashinaga T, Wada N, Yamada K. Transgenic mice expressing nuclear sterol regulatory element-binding protein 1c in adipose tissue exhibit liver histology similar to nonalcoholic steatohepatitis. Metabolism. 2007;56:470–475. doi: 10.1016/j.metabol.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 64.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 65.Okumura K, Ikejima K, Kon K, Abe W, Yamashina S, Enomoto N, Takei Y, Sato N. Exacerbation of dietary steatohepatitis and fibrosis in obese, diabetic kk-a(y) mice. Hepatol Res. 2006;36:217–228. doi: 10.1016/j.hepres.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 66.Stiles B, Wang Y, Stahl A, Bassilian S, Lee WP, Kim YJ, Sherwin R, Devaskar S, Lesche R, Magnuson MA, Wu H. Liver-specific deletion of negative regulator pten results in fatty liver and insulin hypersensitivity [corrected] Proc Natl Acad Sci U S A. 2004;101:2082–2087. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, Naito M, Enomoto K, Watanabe S, Mak TW, Nakano T. Hepatocyte-specific pten deficiency results in steatohepatitis and hepatocellular carcinomas. The Journal of clinical investigation. 2004;113:1774–1783. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. The Journal of clinical investigation. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (pparalpha) in the cellular fasting response: The pparalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kroetz DL, Yook P, Costet P, Bianchi P, Pineau T. Peroxisome proliferator-activated receptor alpha controls the hepatic cyp4a induction adaptive response to starvation and diabetes. J Biol Chem. 1998;273:31581–31589. doi: 10.1074/jbc.273.47.31581. [DOI] [PubMed] [Google Scholar]
- 71.Costet P, Legendre C, More J, Edgar A, Galtier P, Pineau T. Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J Biol Chem. 1998;273:29577–29585. doi: 10.1074/jbc.273.45.29577. [DOI] [PubMed] [Google Scholar]
- 72.Fan CY, Pan J, Chu R, Lee D, Kluckman KD, Usuda N, Singh I, Yeldandi AV, Rao MS, Maeda N, Reddy JK. Hepatocellular and hepatic peroxisomal alterations in mice with a disrupted peroxisomal fatty acyl-coenzyme a oxidase gene. J Biol Chem. 1996;271:24698–24710. doi: 10.1074/jbc.271.40.24698. [DOI] [PubMed] [Google Scholar]
- 73.Fan CY, Pan J, Usuda N, Yeldandi AV, Rao MS, Reddy JK. Steatohepatitis, spontaneous peroxisome proliferation and liver tumors in mice lacking peroxisomal fatty acyl-coa oxidase. Implications for peroxisome proliferator-activated receptor alpha natural ligand metabolism. J Biol Chem. 1998;273:15639–15645. doi: 10.1074/jbc.273.25.15639. [DOI] [PubMed] [Google Scholar]
- 74.Martinez-Chantar ML, Corrales FJ, Martinez-Cruz LA, Garcia-Trevijano ER, Huang ZZ, Chen L, Kanel G, Avila MA, Mato JM, Lu SC. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1a. Faseb J. 2002;16:1292–1294. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- 75.Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, Avila MA, Kanel G, Mato JM. Methionine adenosyltransferase 1a knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci U S A. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–379. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 77.Brunt EM, Kleiner DE, Wilson LA, Unalp A, Behling CE, Lavine JE, Neuschwander-Tetri BA. Portal chronic inflammation in nonalcoholic fatty liver disease (nafld): A histologic marker of advanced nafld-clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:809–820. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morinaga H, Mayoral R, Heinrichsdorff J, Osborn O, Franck N, Hah N, Walenta E, Bandyopadhyay G, Pessentheiner AR, Chi TJ, Chung H, Bogner-Strauss JG, Evans RM, Olefsky JM, Oh da Y. Characterization of distinct subpopulations of hepatic macrophages in hfd/obese mice. Diabetes. 2015;64:1120–1130. doi: 10.2337/db14-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 80.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through ccr2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ye D, Li FY, Lam KS, Li H, Jia W, Wang Y, Man K, Lo CM, Li X, Xu A. Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of x-box binding protein-1 in mice. Gut. 2012;61:1058–1067. doi: 10.1136/gutjnl-2011-300269. [DOI] [PubMed] [Google Scholar]
- 83.Csak T, Velayudham A, Hritz I, Petrasek J, Levin I, Lippai D, Catalano D, Mandrekar P, Dolganiuc A, Kurt-Jones E, Szabo G. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G433–441. doi: 10.1152/ajpgi.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hosoi T, Yokoyama S, Matsuo S, Akira S, Ozawa K. Myeloid differentiation factor 88 (myd88)-deficiency increases risk of diabetes in mice. PloS one. 2010;5 doi: 10.1371/journal.pone.0012537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jia L, Vianna CR, Fukuda M, Berglund ED, Liu C, Tao C, Sun K, Liu T, Harper MJ, Lee CE, Lee S, Scherer PE, Elmquist JK. Hepatocyte toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nature communications. 2014;5:3878. doi: 10.1038/ncomms4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. Jnk1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell metabolism. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 87.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. Jnk expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ibrahim SH, Gores GJ, Hirsova P, Kirby M, Miles L, Jaeschke A, Kohli R. Mixed lineage kinase 3 deficient mice are protected against the high fat high carbohydrate diet-induced steatohepatitis. Liver international : official journal of the International Association for the Study of the Liver. 2014;34:427–437. doi: 10.1111/liv.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nature reviews Immunology. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The nlrp3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature medicine. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of nafld and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wree A, McGeough MD, Pena CA, Schlattjan M, Li H, Inzaugarat ME, Messer K, Canbay A, Hoffman HM, Feldstein AE. Nlrp3 inflammasome activation is required for fibrosis development in nafld. Journal of molecular medicine. 2014;92:1069–1082. doi: 10.1007/s00109-014-1170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]