Abstract
We conducted a prospective, randomized controlled trial of an internet-based safer-sex intervention to reduce HIV transmission risk behaviors. HIV-infected men who have sex with men (n = 179) were randomized to receive a monthly internet survey alone or a monthly survey plus tailored risk reduction messages over 12 months. The primary outcome was the cumulative sexually transmitted infection (STI) incidence over 12 months. Secondary outcomes included self-reported unprotected sex with an at risk partner and disclosure of HIV status to partners. In a modified intent to treat analysis, there was no difference in 12-month STI incidence between the intervention and control arms (30 vs. 25 %, respectively; p = 0.5). Unprotected sex decreased and disclosure increased over time in both study arms. These improvements suggest that addition of the risk-reduction messages provided little benefit beyond the self-monitoring of risky behavior via regular self-report risk behavior assessments (as was done in both study arms).
Keywords: Internet, Men who have sex with men, HIV, Risk behaviors, Intervention, Randomized controlled trial, Sexually transmitted infection
Introduction
The annual incidence of new HIV infections in the United States remains persistently high in men who have sex with men (MSM), the risk group which accounts for the majority of new HIV infections [1]. Use of new technologies (e.g., internet, mobile phone applications) among MSM to seek sex partners has been linked with sexually transmitted infections (STIs), more sexual partners and unprotected anal intercourse [2, 3]. However, the Internet can also be utilized by public health providers for health behavior interventions [4]. Therefore, using the Internet to deliver HIV prevention is an attractive avenue for prevention program implementation as the highest risk population already uses this medium [5, 6.] However, few HIV prevention interventions, delivered through these technologies, have been adequately tested for efficacy [7, 8].
Internet-based interventions tend to have small, but significant, effects on health behaviors [9] [5, 6]. Despite the small effects (perhaps due to the less personable and passive nature of the medium), the accessibility and ease of diffusion of this approach applied over a large audience can still lead to behavioral change that has a population level impact. Larger effects are found among interventions with a theoretical basis, such as social cognitive theory (SCT) [10], the transtheoretical model (TTM) [11], and the theory of reasoned action/planned behavior (TPB) [12]. A previous meta-analysis of theory-based HIV prevention interventions found the most common program elements to include educational information, behavioral skills/attitudinal persuasions, and behavioral skills training [13].
There are a growing number of effective HIV prevention interventions that are delivered in clinical settings. For example, clinic-based interventions have reduced unsafe sex among persons living with HIV [7, 14–16]. However, it is unclear whether the safer sex messages communicated via health care providers/educators in these studies would have similar effects when communicated through a technology-based medium. Emerging prevention efforts targeting new technologies, such as internet-based safer sex interventions and outreach efforts to promote HIV/STI testing have shown promise [2, 17–21]. In this randomized controlled trial among HIV-infected MSM, we evaluated the efficacy of a brief internet-based intervention, provided monthly for 1 year, to reduce STIs and HIV transmission behaviors. Messages in this internet-based intervention were adapted from the clinic/provider-based Partnership for Health intervention [14], which has shown efficacy in reducing unsafe sex.
Methods
Study Setting
The study was conducted from November 2010 to July 2012 at three Southern California sites (University of California San Diego, University of Southern California, and Harbor-University of California Los Angeles) of the California Collaborative Treatment Group (CCTG), a multi-institutional, HIV clinical research network.
Eligibility Criteria
Eligible participants were HIV-infected MSM (age <18 years) in care at any of the CCTG clinics with risk of HIV transmission as determined by having one or more of the following criteria: (1) self-reported unprotected anal sex (either receptive or insertive) with any partner in the past 3 months; (2) more than two partners in the past year; (3) having an HIV-uninfected or unknown status partner in the past 3 months; and/or (4) any STI in the past year. Other eligibility criteria included English speaking, adequate computer skills for the study and no uncontrolled psychiatric condition. All study participants gave informed consent, and study procedures were approved by the institutional review boards at all sites. Participants were recruited from patients engaged in ongoing clinical care in primary care HIV services.
Study and Intervention Design
The study was a randomized, controlled study comparing the efficacy of an internet-based intervention to reduce the incidence of STIs and high-risk sexual behavior by HIV-infected MSM. Participants were randomized 1:1 to receive either a monthly brief, computer accessed, sexual behavior survey alone for 12 months (control), or the same monthly survey plus Internet-delivered tailored messages concerning safer-sex, disclosure of HIV status to sex partners, and the initiation of antiretroviral therapy (ART) (intervention). Randomization was stratified based on site, having a computer at home (yes/no), and ART use (yes/no) to ensure balance between the groups across each of these.
An electronic data management system was created using a fully validated, secure, web-enabled software (that conformed to 21 CFR Part 11 requirements), that enable data collection and intervention delivery. The implementation of the computer intervention enabled study participants to input and receive their personal information in a confidential and user-friendly manner. As participants completed the web-based intervention, the data gathered was automatically integrated with their clinical and research data. This design facilitated real-time quality and compliance monitoring (e.g., to actively monitor study retention) by study staff. Clinicians did not have access to this information and were blind to group assignment.
Messages for those randomized to the intervention arm were tailored based on the participants’ prior month reported risk of transmission, which was classified as: (1) ‘Very Low’—0 %; (2) ‘Low’—<0.1 %; (3) ‘High’—0.1–1.0 %; and (4) ‘Very High’—>1.0 %. Risk of transmission was calculated using the number (N) of unprotected receptive anal (Nra), insertive anal (Nai), insertive vaginal sex acts (Niv), oral sex acts (No), and needle sharing (Nn) multiplied by a probability (P) estimates of per contact transmission rate for each act (Pra = 0.65*0.0011, Pai = 5*0.0011, Piv = 0.0011, Po = 0.1*0.0011, Pnd = 3*0.0011), multiplied for adjustment of recent STI (if Yes sti = 3.7 times increased risk, if No sti = 1) and ART use (if Yes art = 0.1, if No art = 1) [22–24]. Thus estimated HIV transmission risk is the combined risk of not transmitting HIV subtracted from one or:
(Risk probability scores were implemented using R, an open source scientific computing package.). Each group received a unique web page that would give a risk appropriate message. For example, if they were ‘Very Low’ they would be told they had a low chance of transmitting HIV in past month and were supported in continued low risk behavior, whereas those with ‘High’ were told they had significant risk of transmitting in the past month. Based on this stratification, there were different intensities of other static internet pages that had specific themes: (1) condom use; (2) disclosure to sex partners; (3) reduced use of drugs and alcohol; (4) initiation of ART (for those not reporting being on ART). The theoretical framework for the intervention approach and risk behavior messages was based on SCT [10] and the TTM of Change [11]. Messages used social influences and promoted positive movements in behavior based on the participant's current behavior/intent (e.g. those not on ART were encouraged to consider ART or take steps to start ART depending on whether they had no intention to start ART or intended to start ART, respectively). Messages were partially adapted from the clinic-based Partnership for Health intervention [14] and pre-tested through focus groups with HIV-infected MSM who informed development and changes to the intervention content and approach. Intervention text and flow is provided in a supplementary document (actual webpages were in color with pictures).
Study Procedures and Measures
Data was collected by both confidential in-person interview and computer assisted survey self-report for all enrolled participants. In-person interviews were used to determine basic demographics, medical history, history of STIs, ART use, concomitant medications, medication adherence, psychiatric history, and depressive symptoms using Center for Epidemiological Studies Depression Scale (CES-D). Plasma HIV RNA and CD4 counts were abstracted from clinical records. STI screening assessments at baseline and every 3 months over 12 months included syphilis (serum RPR and if positive confirmatory treponemal test), as well as nucleic acid amplification testing (NAAT) of urine and swabs of pharynx and rectum for chlamydia and gonorrhea using Hologic Aptima. (Study clinic visits occurred every 3 months and included both STI screening and the web-based assessments and did not need to coincide with clinic treatment visits.) Newly diagnosed STIs were communicated to participants and referral was made to their provider or a local sexually transmitted disease clinic. Treatment was confirmed by completion of a medication record review. All STIs were verified by an independent and blinded adjudication committee.
The primary outcome was the composite incidence variable of any new STI at any anatomic site (syphilis, gonorrhea, chlamydia) during the 12 months study period. Secondary outcomes were derived from the computer assisted self-report surveys for: (1) any unprotected anal/vaginal sex with an HIV negative/unknown status partner during the past month, and (2) disclosure of status to HIV negative/unknown status partners (defined at each visit as disclosure to all partners). There were up to six disclosure questions (one per partner type: regular male partners, casual male partners, regular female partners, casual female partners, regular transgender partners, casual transgender partners) per assessment. Each question had four options (non-disclosure, ≤50 % of the time, >50 % of the time, all disclosure), although we a priori planned to analyze this variable as all versus not all disclosed. Descriptive summaries at each visit by study arm revealed only a few subjects (<10 %) selected the middle two options at each visit. Thus we retained this disclosure coding plan. Those not sexually active in subsequent months we treated as all safe and all disclosed.
Because so few participants were not on ART at baseline (n = 22), we were unable to meaningfully compare ART initiation between study arms during the study period.
Statistical Analysis
The study was powered to compare the incidence rates of the primary endpoint between the two study arms using a two-sample binomial test for proportions. Initially, to achieve 80 % power to detect a reduction in STI incidence rates from 25 to 10 %, with a two-sided alpha of 0.05, we estimated a need for 200 participants. Enrollment was halted at 181 participants because of slow recruitment. However, the statistical power of the study was not impacted because the STI incidence rate was higher than originally anticipated.
Baseline characteristics were summarized and compared between study arms using Fisher's exact test for categorical variables and Wilcoxon Rank-Sum test for continuous variables. Primary analyses were performed on a modified intent-to-treat (mITT) population, defined as randomized participants who completed the baseline visit (n = 179) [25]. (A total of 181 participants were randomized but two did not complete their baseline visit.) A logistic regression model was used to compare the difference in any new STI during the study between the study arms, adjusting for the baseline STI status, ART use and methamphetamine use at baseline. Secondary outcomes (self-reported unprotected anal/vaginal sex and disclosure) were assessed using a generalized estimating equation (GEE) model with study arm, visit (treated as a categorical variable), study arm-by-visit interaction as the dependent variables. Additional prespecified analyses (as determined by the investigators when the protocol was developed) were performed on a subset that completed 75 % or more of monthly internet visits (as-treated- 9/12 months; n = 107) and for those that continued on study through month 12 (regardless of many internet visits they completed; study completers, n = 140). In subsequent sensitivity analyses, we examined changes over time in the secondary outcomes using mixed models and pairwise testing. Because the pattern of results were similar, we present only the GEE model results below. A p value of <0.05 was considered statistically significant. Statistical analyses were performed in R (http://cran.r-pro ject.org), version 3.0.2.
Results
Baseline Analysis
From 188 screened, a total of 181 MSM met the eligibility criteria for inclusion in the study and were randomized to intervention or control and 179 individuals completed the baseline visit (included in mITT; Table 1). All 181 participants were MSM with approximately one-third White Non-Hispanic, one-third Black Non-Hispanic and one-third Hispanic. The majority (78 %) of participants were daily internet users. Most were on ART (84 %), and 65 % had plasma HIV RNA levels below detection. Baseline data and factors associated with participants having detectable HIV RNA values have been previously published [26].
Table 1.
Baseline characteristics for the modified intention to treat population (randomized and completed baseline visit)
| Intervention (n = 90) N (%) | Control (n = 89) N (%) | p | |
|---|---|---|---|
| Mean Age | 44.6 | 42.7 | 0.15 |
| Race/ethnicity | |||
| White | 32 (36) | 27 (30) | 0.36 |
| Black | 28 (31) | 27 (30) | |
| Hispanic | 28 (31) | 29 (32) | |
| Other | 2 (3) | 7 (8) | |
| English as primary language | 77 (86) | 77 (86) | >0.99 |
| Internet use | |||
| More than once a day | 43 (48) | 45 (51) | 0.84 |
| Daily | 29 (32) | 23 (26) | |
| Weekly or less | 18 (20) | 21 (23) | |
| Education more than high school | 69 (77) | 67 (75) | 0.33 |
| Income ≥$2000/montha | 14 (22) | 16 (24) | 0.74 |
| Mean CD4, cells/mm3 | 559 | 531 | 0.36 |
| On ART | 76 (84) | 74 (83) | 0.84 |
| Mean months on ARTb | 46.3 | 34.8 | 0.03 |
| HIV RNA undetectable | 60 (67) | 56 (63) | 0.64 |
| AIDS diagnosis | 24 (27) | 21 (24) | 0.73 |
| STI | 25 (28) | 27 (30) | 0.74 |
| Unprotected anal/vaginal sex past month | 51 (57) | 44 (50) | 0.37 |
| Incomplete disclosure of status | 60 (67) | 44 (50) | 0.02 |
| Any illicit drug use | 32 (36) | 43 (48) | 0.10 |
ART antiretroviral therapy, STI sexually transmitted infection
Of those who responded to this question
Of those on ART
Most baseline factors were balanced between arms. Participants in the intervention arm were more likely to have had incomplete disclosure of their HIV status to their partners at baseline (67 % compared to 50 %, p = 0.02). Among those on ART, participants in the intervention arm had been on therapy for longer (p = 0.03). Both arms had similar high levels of baseline STIs: 28 and 30 % in the intervention and control arms, respectively (p = 0.74). Methamphetamine use in the past month was similar in the intervention (18 %) and control (19 %) arms (p = 0.85). Since the intervention focused on reducing HIV transmission and changing behaviors in the context of making participants aware of potential risk, we evaluated the participants’ baseline perception of their risk for transmitting HIV. Self-perception of risk was balanced between arms, based on the response to the question “could you have infected someone with HIV in the past month”. Overall 11 (12 %) in the intervention arm and 9 (10 %) in the control arm responded that they believed it was “somewhat likely” or “almost certain”.
Study Follow-Up
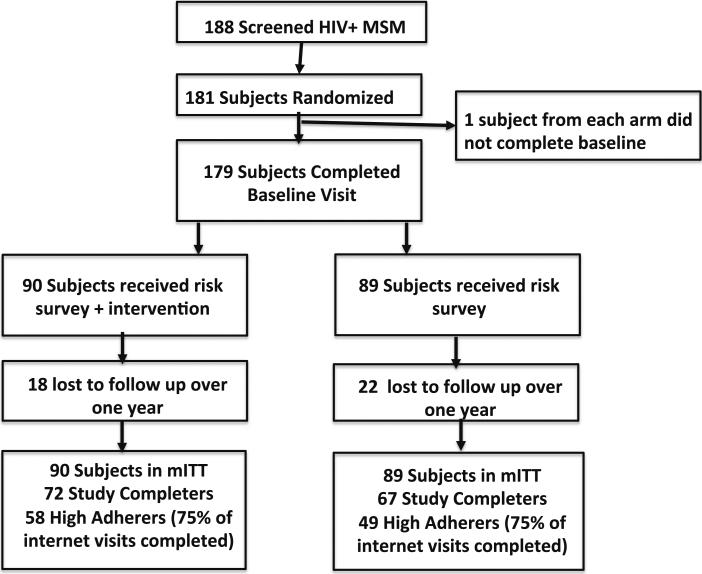
There were seven individuals that chose not to participate or did not return to the randomization visit. From the 181 MSM that were randomized, 179 completed baseline assessments (Fig. 1). There were 39 loss to follow ups during study and 140 study completers who attended the 12 month study visit. There was no difference in the number of study completers by arm of study, 67 in the control and 73 in the intervention arm (p = 0.37), nor was there difference in time to premature discontinuation. The majority of loss to follow ups were due to participants not returning for study visits. There were 107 participants that completed 75 % or more (as-treated) of the internet-based component of the study (i.e. ≥9/12 months consisting of any combination of the four visits in clinic and eight visits out of clinic). Of the as-treated, 58 were in control and 49 were in the intervention arm.
Fig. 1.
consort diagram of study participants. Study completers are those who continued on study through month 12
At the close of the internet-based intervention visit, each subject was asked to choose one risk behavior that they would like to focus on in the next month and asked if they had achieved their goals from the previous month. Affirmative responses to these target/goal questions ranged from 71 to 86 % across the study visits.
Primary Endpoint
In the mITT analysis, the occurrence of any incident STI during the study (Table 2) was 27 % overall, with no difference between the intervention and control arm (30 vs. 25 %, respectively; p = 0.50). Of the 27 participants with any STI in the intervention arm, six subjects had two STIs and two had three STIs while seven participants in the control arm had two STIs. The number of visits with incident STIs showed no difference between the arms (p = 0.57). The multivariable logistic regression also showed no difference comparing intervention to control (OR = 1.35, 95 % CI 0.68–2.70, p = 0.38), adjusted for STI status, ART and meth use at baseline. Subset analyses did not show lower rates of STIs in the intervention arm. The occurrence of any incident STI was 33 versus 28 % (p = 0.59) among study completers and 24 versus 24 % among the as-treated groups.
Table 2.
New sexual transmitted infections (STI) on study
| Intervention (n = 90) | Control (n = 89) | p | |
|---|---|---|---|
| Any incident STI after baseline (%) | 27 (30) | 22 (25) | 0.50 |
| Visits with new STI per subject | |||
| 1 | 19 (21) | 15 (17) | 0.57 |
| 2 | 6 (7) | 7 (8) | |
| 3 | 2 (2) | 0 (0) |
To evaluate if the lack of difference between arms in STIs could potentially be explained by serosorting, we evaluated the number of HIV-infected partners over the course of the study; there was no between arm difference.
Secondary Endpoints
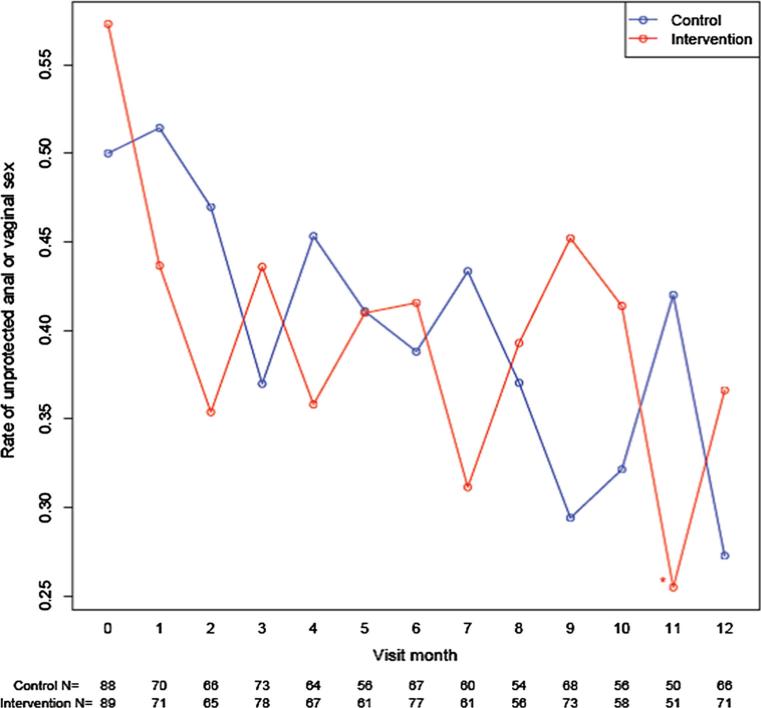
In the mITT, self-reported unprotected anal/vaginal sex declined over time in both arms (Fig. 2). In the intervention arm, the decline in unprotected anal/vaginal sex from baseline was significant in 10/12 study months. In the control arm, the decline in unprotected anal/vaginal sex was significant in only three of the follow up months (months 9, 10, 12). The intervention arm had greater relative decline in unprotected anal/vaginal sex only at month 11 compared to the control arm. In sub-analyses of the study completers, the trend was similar. The intervention arm had greater relative decline in unprotected anal/vaginal sex than the control arm in months 4, 7 and 11. Among the as-treated, the intervention arm had greater relative decline in unprotected anal/vaginal sex than the control in months 1, 2, 4, 7 and 11.
Fig. 2.
Self report of any unprotected anal/vaginal sex in past month
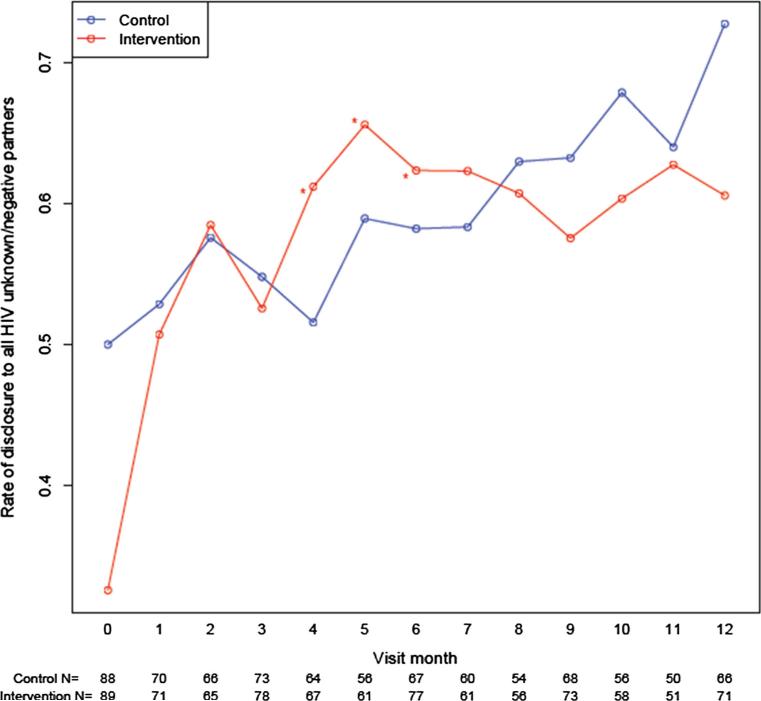
Self-reported disclosure to all HIV unknown/negative partners increased over time in both arms (Fig. 3). In the intervention arm, disclosure increased in all study months compared to baseline (all p's < 0.05). The increase in disclosure from baseline was greater in the intervention arm (vs. control) at months 4, 5, and 6. Results were similar among study completers and the as-treated. In sub-analyses of the study completers, disclosure increased in all follow up months compared to baseline and was higher than the control arm in months 4, 6 and 7. Among the as-treated, the intervention arm had higher rates of disclosure in eleven of the follow up months compared to baseline and was higher than the control in months 6 and 7.
Fig. 3.
Self report of disclosure of status to sex partners in past month
Discussion
This randomized, controlled trial enrolled a cohort of sexually active MSM living with HIV to receive a brief, monthly risk assessment survey alone or in combination with a web-based risk reduction intervention aimed to reduce risky behavior. While there was no difference between study arms in the primary endpoint of STI rates over the course of the study, participants in the study in either arm reduced self-reported unprotected anal/vaginal sex behavior and increased disclosure of HIV status to at risk (unknown/negative) partners.
The primary endpoint of incident STIs was selected as an objective, biological marker of high-risk sexual behavior. Prevalent STIs were common at baseline (29 %), reflecting the high risk characteristics of our study population and confirming the need for risk reduction interventions. Over the course the study, in contrast with the improvement in the self-reported secondary endpoints, incident STIs were frequent with a cumulative incidence of 27 %. Precedent for discordance in STI rates, such as syphilis, without concomitant rise in HIV rates has been seen in epidemiological data [27]. In this study, the discordance between changes in unprotected sex and lack of reduction in STIs did not appear to be explained by increased serosorting, the number of partners, or strategic positioning (i.e., receptive vs. insertive sex). However, the absolute number of sexual acts (i.e. protected and unprotected anal/vaginal sex acts) with HIV-infected partners was not assessed. Thus, changes in serosorting risk behaviors could not be entirely excluded. Likewise, because the majority of participants were on ART, their perceived risk of HIV transmission may have been discordant with STI acquisition, the primary endpoint of this study. That is, a lower perceived risk of HIV transmission (attributed to ART use) could drive an increased/continued risk of STI transmission. Although this is a potential limitation of using STIs as primary endpoint in current HIV intervention efforts, it remains relevant as an indicator of condomless sex and greater potential for HIV exposure, including the potential for higher levels of the virus in the fluids/tissues of STI infected persons [28, 29].
In HIV-infected individuals, having unprotected anal sex with HIV unknown or uninfected partners is the highest risk behavior for possible onward transmission events [22, 30]. Reduction in these events through fewer sexual encounters or consistent use of condoms represents a cornerstone in the combination prevention strategy in addition to ARV therapy [31]. Although our intervention delivered messages to minimize unprotected sexual encounters with serodiscordant partners, we observed reductions in unprotected sex in both study arms at follow up visits compared to baseline assessments. Unprotected sex in the intervention arm was lower than in the control arm at several follow up months during the study. Further, participants with high adherence to the study protocol had even fewer unprotected anal/vaginal sex acts and the rates were significantly lower than in the control group at five time points. It may be possible that these differences were random fluctuations that are not meaningful. However, this reduction in unprotected acts supports a potential dose effect of study participation, either receiving the intervention or simply having repeated assessment of risk behavior.
Consistent disclosure of HIV status can also reduce HIV transmissions from HIV-infected MSM [32]. Disclosure facilitates serosorting behaviors that are commonly practiced, but seroadpative practices are associated with increased risk of transmission compared to avoiding all unprotected sex [33]. Our intervention provided some possible strategies to enable disclosure and the risk survey questions (given to all participants) queried about disclosure to regular and casual sexual partners. Compared to baseline, disclosure of status was higher within participants in the intervention arm and, in some months, the intervention arm was significantly higher than the control arm for both unprotected anal/vaginal sex and disclosure outcomes.
Limitations of the present study were identified that could be helpful in development of future on-line or mobile interventions. The study intervention was built on html platform that would not adjust to fit on a tablet or smart-phone screen, both of which became more common in use during the course of this study. Because digital approaches have shown promise in pilot studies to reduce HIV transmission risk behaviors, [34–36] future work should include more dynamic content. The survey and intervention were static and largely non personal (with few decision trees). Future interventions can be enhanced by (i) rotating content; (ii) use of an avatar to deliver messages; (iii) development of a more user-friendly experiences; and (iv) adaption to a mobile device platform. Further, although many study visits were integrated with clinic visits, clinicians did not have access to the reporting of their patients who were study participants. More fully integrated visits, with consented transparent risk monitoring by the patient and clinician may increase the efficacy of this intervention approach. For example, although the intervention group was able to select behavioral goals for their next visit, clinicians were unaware of these goals. For outcomes, the self-reported measures for sexual risk behavior are subject to bias that even when done on a computer could suffer over the course of study from a tendency to report a more desirable result. Because the secondary outcomes improved in both groups, there may have been reporting bias or alternatively some effect from participation and/or self-reflection on reported risk behaviors. To address these issues, more sensitive assessment tools need to be used. One way to do this may be more frequent data capture such as daily electronic diaries or text reporting where there may be less bias.
Since participants in both study arms reduced their risk behaviors, technology-based, brief, self-administered risk assessments in general may influence transmission risk behaviors in HIV-infected MSM. These findings are consistent with prior research documenting positive behavior change and risk sensitization following behavioral assessment [37–39]. It may be that behavioral assessments alone (versus none) could be sufficient or superior in promoting behavior change compared to health education and messages alone [20.] Behavioral self-assessment is well suited for large-scale delivery through new technologies; such low intensity interventions could contribute significantly to combination prevention strategies. Thus, future risk reduction interventions should focus on the broader implementation of regular risk-behavior monitoring and utilization of newer mobile technologies that can be more dynamic and pervasive. This may require a change in approach to how clinical trials are done with new technologies that allow for larger scale of implementation that can measure smaller effect sizes with sufficient power.
Acknowledgments
The authors would like to thank the California Collaborative Treatment Group study nurses Edward Seefried, Connie Funk, and Angela Grbic, the administrative help of Eric Ellorin, Fang Wang, and Luis Mendez, the University of California, San Diego (UCSD) Antiviral Research Center staff for their support in data collection, and the study participants for volunteering for this study.
Funding This work was supported by the California HIV Research Program (CHRP- MC08-SD-700 and EI-11-SD-005). Additional funding- NIAID Grants: AI 064086 (K24 to RH); AI 36214 (CFAR Clinical Investigation and Biostatistics Core). Portions of this work were previously presented at the 21st Conference on Retroviruses and Opportunistic Infections, Boston, MA.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors do not have any conflicts of interest to report.
References
- 1.Prejean J, Song RG, Hernandez A, Ziebell R, Green T, Walker F, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS ONE. 2011;6(8):13. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benotsch EG, Kalichman S, Cage M. Men who have met sex partners via the Internet: prevalence, predictors, and implications for HIV prevention. Arch Sex Behav. 2002;31(2):177–83. doi: 10.1023/a:1014739203657. [DOI] [PubMed] [Google Scholar]
- 3.Ross MW, Tikkanen R, Mansson SA. Differences between Internet samples and conventional samples of men who have sex with men: implications for research and HIV interventions. Soc Sci Med. 2000;51(5):749–58. doi: 10.1016/s0277-9536(99)00493-1. [DOI] [PubMed] [Google Scholar]
- 4.Hen L, Boniel-Nissim M, Shapira N. A comprehensive review and a meta-analysis of the effectiveness of internet-based psychotherapeutic interventions. J Technol Hum Serv. 2008;26(2/4):109–60. [Google Scholar]
- 5.Pellowski JA, Kalichman SC. Recent advances (2011–2012) in technology-delivered interventions for people living with HIV. Curr HIV/AIDS Rep. 2012;9(4):326–34. doi: 10.1007/s11904-012-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tufts KA, Johnson KF, Shepherd JG, Lee J-Y, Ajzoon MSB, Mahan LB, et al. Novel interventions for HIV self-management in African American women: a systematic review of mHealth interventions. J Assoc Nurses AIDS Care. 2014;26(2):139–50. doi: 10.1016/j.jana.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Crepaz N, Tungol-Ashmon MV, Higa DH, Vosburgh W, Mullins MM, Barham T, et al. A systematic review of interventions for reducing HIV risk behaviors among people living with HIV in the United States, 1988–2012. Aids. 2014;28(5):633–56. doi: 10.1097/QAD.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noar SM, Black HG, Pierce LB. Efficacy of computer technology-based HIV prevention interventions: a meta-analysis. Aids. 2009;23(1):107–15. doi: 10.1097/QAD.0b013e32831c5500. [DOI] [PubMed] [Google Scholar]
- 9.Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):6. doi: 10.2196/jmir.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44(9):1175–84. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- 11.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–5. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 12.Schifter DE, Ajzen I. Intention, perceived control, and weight loss: an application of the theory of planned behavior. J Pers Soc Psychol. 1985;49(3):843–51. doi: 10.1037//0022-3514.49.3.843. [DOI] [PubMed] [Google Scholar]
- 13.Albarracin D, Gillette JC, Earl AN, Glasman LR, Durantini MR, Ho MH. A test of major assumptions about behavior change: a comprehensive look at the effects of passive and active HIV-prevention interventions since the beginning of the epidemic. Psychol Bull. 2005;131(6):856–97. doi: 10.1037/0033-2909.131.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson JL, Milam J, McCutchan A, Stoyanoff S, Bolan R, Weiss J, et al. Effect of brief safer-sex counseling by medical providers to HIV-1 seropositive patients: a multi-clinic assessment. Aids. 2004;18(8):1179–86. doi: 10.1097/00002030-200405210-00011. [DOI] [PubMed] [Google Scholar]
- 15.DiClemente RJ, Wingood GM, Rose E, Sales JM, Crosby RA. Evaluation of an HIV/STD sexual risk-reduction intervention for pregnant African American adolescents attending a prenatal clinic in an urban public hospital: preliminary evidence of efficacy. J Pediatr Adolesc Gynecol. 2010;23(1):32–8. doi: 10.1016/j.jpag.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers J, Shade S, Rose C, Koester K, Maiorana A, Malitz F, et al. Interventions delivered in clinical settings are effective in reducing risk of HIV transmission among people living with HIV: results from the Health Resources and Services Administration (HRSA)'s Special Projects of National Significance Initiative. AIDS Behav. 2010;14(3):483–92. doi: 10.1007/s10461-010-9679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bull SS, Levine DK, Black SR, Schmiege SJ, Santelli J. Social media-delivered sexual health intervention a cluster randomized controlled trial. Am J Prev Med. 2012;43(5):467–74. doi: 10.1016/j.amepre.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holloway IW, Cederbaum JA, Ajayi A, Shoptaw S. Where are the young men in HIV prevention efforts? Comments on HIV prevention programs and research from young men who sex with men in Los Angeles County. J Prim Prev. 2012;33(5–6):271–8. doi: 10.1007/s10935-012-0282-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter KM, Stoner SA, Mikko AN, Dhanak LP, Parsons JT. Efficacy of a web-based intervention to reduce sexual risk in men who have sex with men. AIDS Behav. 2010;14(3):549–57. doi: 10.1007/s10461-009-9578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lightfoot M, Rotheram-Borus MJ, Comulada WS, Reddy VS, Duan N. Efficacy of brief interventions in clinical care settings for persons living with HIV. J Acquir Immune Defic Syndr. 2010;53(3):348–56. doi: 10.1097/QAI.0b013e3181c429b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey M, Senn T, Vanable P, Coury-Doniger P, Urban M. Brief and intensive behavioral interventions to promote sexual risk reduction among STD clinic patients: results from a randomized controlled trial. AIDS Behav. 2010;14(3):504–17. doi: 10.1007/s10461-009-9587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis. 2002;29(1):38–43. doi: 10.1097/00007435-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Røttingen J-A, Cameron DW, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex Transm Dis. 2001;28(10):579–97. doi: 10.1097/00007435-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 25.Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109. doi: 10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumenthal J, Haubrich R, Jain S, Sun X, Dube M, Daar E, et al. Factors associated with high transmission risk and detectable plasma HIV RNA in HIV-infected MSM on ART. Int J STD AIDS. 2014;25(10):734–41. doi: 10.1177/0956462413518500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Truong HM, Kellogg T, Klausner JD, Katz MH, Dilley J, Knapper K, et al. Increases in sexually transmitted infections and sexual risk behaviour without a concurrent increase in HIV incidence among men who have sex with men in San Francisco: a suggestion of HIV serosorting? Sex Transm Infect. 2006;82(6):461–6. doi: 10.1136/sti.2006.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pao D, Fisher M, Hué S, Dean G, Murphy G, Cane PA, et al. Transmission of HIV-1 during primary infection: relationship to sexual risk and sexually transmitted infections. Aids. 2005;19(1):85–90. doi: 10.1097/00002030-200501030-00010. [DOI] [PubMed] [Google Scholar]
- 29.Mayer K, O'Cleirigh C, Skeer M, Covahey C, Leidolf E, Vanderwarker R, et al. Which HIV-infected men who have sex with men in care are engaging in risky sex and acquiring sexually transmitted infections: findings from a Boston community health centre. Sex Transm Infect. 2010;86(1):66–70. doi: 10.1136/sti.2009.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9(2):118–29. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardner EM, Young B. The HIV care cascade through time. Lancet Infect Dis. 2014;14(1):5. doi: 10.1016/S1473-3099(13)70272-X. [DOI] [PubMed] [Google Scholar]
- 32.Pinkerton SD, Galletly CL. Reducing HIV transmission risk by increasing serostatus disclosure: a mathematical modeling analysis. AIDS Behav. 2007;11(5):698–705. doi: 10.1007/s10461-006-9187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallabhaneni S, Li X, Vittinghoff E, Donnell D, Pilcher CD, Buchbinder SP. Seroadaptive practices: association with HIV acquisition among HIV-negative men who have sex with men. PLoS ONE. 2012;7(10):e45718. doi: 10.1371/journal.pone.0045718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirshfield S, Chiasson MA, Joseph H, Scheinmann R, Johnson WD, Remien RH, et al. An online randomized controlled trial evaluating HIV prevention digital media interventions for men who have sex with men. PLoS ONE. 2012;7(10):e46252. doi: 10.1371/journal.pone.0046252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnall R, Travers J, Rojas M, Carballo-Diéguez A. eHealth interventions for HIV prevention in high-risk men who have sex with men: a systematic review. J Med Internet Res. 2014;16(5):e134. doi: 10.2196/jmir.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiasson MA, Hirshfield S, Rietmeijer C. HIV prevention and care in the digital age. J Acquir Immune Defic Syndr. 2010;55:S94–7. doi: 10.1097/QAI.0b013e3181fcb878. [DOI] [PubMed] [Google Scholar]
- 37.Weinhardt LS, Carey KB, Carey MP. HIV risk sensitization following a detailed sexual behavior interview: a preliminary investigation. J Behav Med. 2000;23(4):393–8. doi: 10.1023/a:1005505018784. [DOI] [PubMed] [Google Scholar]
- 38.Lightfoot M, Rotheram-Borus M, Comulada S, Gundersen G, Reddy V. Self-monitoring of behaviour as a risk reduction strategy for persons living with HIV. AIDS care. 2007;19(6):757–63. doi: 10.1080/09540120600971117. [DOI] [PubMed] [Google Scholar]
- 39.Safren SA, O'Cleirigh CM, Skeer M, Elsesser SA, Mayer KH. Project enhance: a randomized controlled trial of an individualized HIV prevention intervention for HIV-infected men who have sex with men conducted in a primary care setting. Health Psychol. 2013;32(2):171. doi: 10.1037/a0028581. [DOI] [PMC free article] [PubMed] [Google Scholar]