Abstract
The risk for respiratory complications and infections is substantially increased in old age, which may be due, in part, to sarcopenia (aging-related weakness and atrophy) of the diaphragm muscle (DIAm), reducing its force generating capacity and impairing the ability to perform expulsive non-ventilatory motor behaviors critical for airway clearance. The aging-related reduction in DIAm force generating capacity is due to selective atrophy of higher force generating type IIx and/or IIb muscle fibers, whereas lower force generating type I and IIa muscle fiber sizes are preserved. Fiber type specific DIAm atrophy is also seen following unilateral phrenic nerve denervation and in other neurodegenerative disorders. Accordingly, the effect of aging on DIAm function resembles that of neurodegeneration and suggests possible common mechanisms, such as the involvement of several neurotrophic factors in mediating DIAm sarcopenia. This review will focus on changes in two neurotrophic signaling pathways that represent potential mechanisms underlying the aging-related fiber type specific DIAm atrophy.
Keywords: Diaphragm muscle, Aging, Atrophy, Brain derived neurotrophic factor, Neuregulin, Denervation
1. Introduction
The population of elderly (>65 years of age) individuals is steadily increasing worldwide, e.g., in the USA, the elderly population will more than double by 2030, exceeding 80 million people (Federal Interagency Forum on Aging-Related Statistics, 2012). This demographic shift will bring a multitude of challenges, including an increased economic burden secondary to a greater incidence of chronic disease. Respiratory diseases associated with old age, such as pneumonia (3-fold higher incidence in those >65 years of age) (Chong and Street, 2008; Janssens and Krause, 2004), already account for ~7% of direct healthcare expenditures in the USA amounting to nearly $100 billion (National Institutes of Health: Fact Book Fiscal Year, 2012). Not only do these respiratory diseases diminish the quality of life in old age (Berry et al., 2012), they are also a leading cause of death in the elderly population (Heron, 2011; Xu et al., 2010). Indeed, the elderly are at greater risk for hospitalization and many are admitted to intensive care units because of respiratory failure frequently requiring prolonged mechanical ventilation (Creditor, 1993; de Jonghe et al., 2002, 2009; Hamel et al., 2005; Ray et al., 2006; Turrentine et al., 2006). For this reason, understanding the etiology of aging-related respiratory disease is vital to the future development of therapeutic strategies designed to lessen this increasing economic burden (Kirkland and Peterson, 2009).
2. Sarcopenia
Irwin Rosenberg proposed the term ‘sarcopenia’ in 1989 to describe the aging-related decrease of skeletal muscle mass, based on the Greek terms ‘sarx’ for flesh and ‘penia’ for poverty (Rosenberg, 1989). Later, Evans revised this definition of sarcopenia to include an aging-related loss in skeletal muscle strength (Evans, 1995; Evans and Campbell, 1993); a description that aligns with current consensus definitions (Cesari et al., 2012; Cruz-Jentoft et al., 2010; Evans, 2010; Fielding et al., 2011). In humans, the progressive aging-related decline in skeletal muscle mass and strength (sarcopenia) begins at ~30 years of age (similar to other physiologic systems; (Sehl and Yates, 2001)), progressing thereafter at a rate of 0.5–1% of muscle mass lost per year with a more rapid decline in humans >65 years of age (Frontera et al., 2000; Lexell et al., 1988; Nair, 2005). Although the specific mechanisms mediating sarcopenia remain poorly understood, its etiology appears to be multifactorial and sarcopenia can often co-exist with other diseases or conditions that are also associated with cachexia (e.g., inactivity and muscle disuse, cancer, poor nutrition, chronic inflammation, insulin resistance, declining levels of anabolic hormones, etc.) (Biolo et al., 2014; Buford et al., 2010; Evans, 2010; Muscaritoli et al., 2010; Thomas, 2007; Thompson, 2007). In our studies, we are exploring whether sarcopenia involves aging-related alterations in neurotrophic influences affecting the entire motor unit (Fig. 1), which comprises a motor neuron and the muscle fibers it innervates through the neuromuscular junction (NMJ).
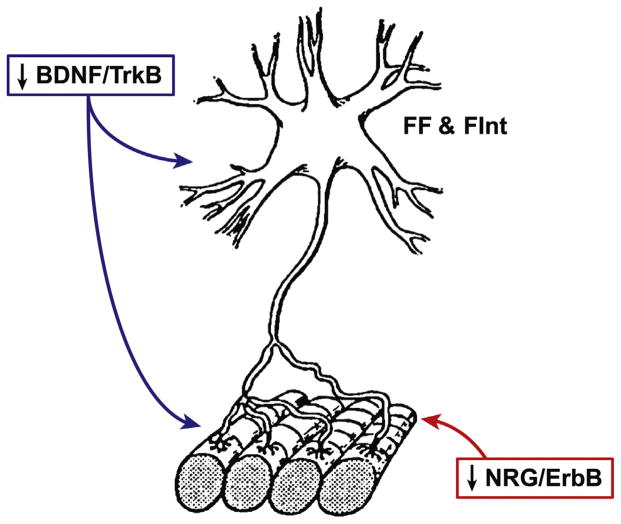
Fig. 1.
Schematic illustrating a fast-twitch fatigable (type FF) and fast-twitch fatigue intermediate (type FInt) motor unit. Muscle fibers corresponding to type FF and FInt motor units are most susceptible to atrophy and weakness secondary to the natural aging process and denervation. Two neurotrophic factors that may be mediating this fiber type specific effect of atrophy and weakness are: (1) brain derived neurotrophic factor (BDNF) acting through the tropomyosin-related kinase receptor B (TrkB) which is involved in motor neuron survival, excitability, and neuromuscular synaptic transmission, and (2) the trophic factor family of neuregulins (NRG) which activate tyrosine kinases of the ErbB receptor family that is released from the motor neuron exerting an anabolic effect on muscle fibers and is implicated in the matching of motor neuron to muscle fiber properties.
3. Muscle fiber type and motor unit classification
The concept of the motor unit as the basic functional element of neuromotor control was first proposed by Sherrington in 1925 (Liddell and Sherrington, 1925). Motor units are classified into specific types based on the mechanical and fatigue properties of the muscle fibers comprising each unit (Burke, 1981; Burke et al., 1971, 1973; Fournier and Sieck, 1988a). Accordingly, fast-twitch (type F) and slow-twitch (type S) motor units are distinguished by the rate of twitch force generation, as well as the presence (type F) or absence (type S) of “sag” in an unfused tetanic force response. Type F motor units are further sub-classified into three distinct types based on their resistance to fatigue during tetanic stimulation: fast-twitch fatigue resistant (type FR); fast-twitch fatigue intermediate (type FInt); and fast-twitch fatigable (type FF). Type S motor units are fatigue resistant. In the diaphragm muscle (DIAm), motor unit types also vary in their contractile strength, with type FInt and FF motor units generating greater peak twitch (Pt) and maximum tetanic (Po) force compared to type FR and S motor units (Fournier and Sieck, 1988a; Sieck, 1988, 1991, 1994; Sieck et al., 1989a).
Each motor unit type is composed of muscle fibers that are homogeneous with respect to their metabolic properties and contractile protein composition, specifically the expression of myosin heavy chain (MyHC) isoforms (Enad et al., 1989; Gransee et al., 2012; Greising et al., 2012; Sieck, 1994; Sieck et al., 1989a, 1996). In fact, this relationship forms the basis of muscle fiber type classification (Brooke and Kaiser, 1970; Edstrom and Kugelberg, 1968; Schiaffino et al., 1989; Sieck et al., 1985). Accordingly, type S motor units comprise type I muscle fibers that have higher oxidative capacity and express a MyHCSlow isoform. Type FR motor units comprise type IIa muscle fibers that also have higher oxidative capacity and express the MyHC2A isoform. More fatigable type FInt motor units comprise type IIx muscle fibers that have lower oxidative capacity and express the MyHC2X isoform. Type FF motor units comprise lower oxidative type IIb muscle fibers primarily expressing MyHC2B although co-expression of the MyHC2X isoform in these fibers commonly occurs (Sieck, 1995; Sieck et al., 1989a). The mechanical properties of single muscle fibers reflect the expression of MyHC isoforms (Geiger et al., 1999, 2000, 2001a,b, 2002), which underlies differences in motor unit force generation. In addition, the number of muscle fibers innervated by each motor neuron (innervation ratio) varies, which also contributes to differences in motor unit force generation. Motor unit fatigue resistance is associated with differences in mitochondrial volume density and the oxidative capacity of muscle fiber types (Sieck et al., 1989b, 1996).
Often in pathological conditions, e.g., hypothyroidism (Geiger et al., 2002), spinal muscular atrophy (Ben Hamida et al., 1994) and amyotrophic lateral sclerosis (Baloh et al., 2007), muscle fibers co-express MyHC isoforms obviating unambiguous fiber type classification. In vastus lateralis muscle biopsies from subjects ranging in age from 21 to 66 years of age, we found a significant number of fibers from older subjects co-expressed MyHCSlow and MyHC2A isoforms, as well as MyHC2X and MyHC2A isoforms (Han et al., 2001). These results were consistent with a prior study by Klitgaard et al. (1990) in which they found an increased incidence of co-expression of both MyHCSlow and MyHC2A and MyHC2A and MyHC2B isoforms in medial vastus lateralis and medial biceps brachii muscle fibers from older (68–70 years of age) compared to younger (21–31 years of age) men. In addition to pathological conditions and aging, this type of co-expression of MyHC isoforms also occurs following denervation and removal of neurotrophic influence on muscle fibers (Geiger et al., 2001a); thus, there may be a common underlying mechanism responsible for the loss of matching between motor neurons and muscle fiber type. We believe this common mechanism involves the influence of neurotrophic signaling (see below).
3.1. Motor unit recruitment and frequency coding
Charles Sherrington first proposed that motor units are recruited in an all-or-none fashion, such that force generated by the whole muscle represents the sum of forces produced by the recruitment (recruitment coding) and frequency of activation (frequency coding) of individual motor units (Liddell and Sherrington, 1925; Sherrington, 1925, 1929). Later, Elwood Henneman proposed a mechanism to explain the recruitment of motor units that relates to the intrinsic size-related properties of motor neurons—Henneman’s “Size Principle” (Henneman, 1957; Henneman and Olson, 1965; Henneman et al., 1965). According to the Size Principle, both intrinsic membrane resistance and membrane capacitance (Cm) of a motor neuron is size dependent. For a given level of synaptic input (i.e., input current; Ic), the rate of change of membrane potential (dVm/dt—reflecting motor neuron excitability), is inversely proportional to Cm; such that, dVm/dt = Ic/Cm. Thus, for a given level of synaptic input, smaller motor neurons, with a lower Cm are more excitable (increased dVm/dt) compared to larger motor neurons. Motor neurons innervating type S motor units are the smallest and therefore more excitable and recruited first during motor behaviors followed in rank order by motor neurons innervating type FR, FInt, and FF motor units (Burke, 1975; Henneman and Mendell, 1981). Frequency coding of motor units also depends on intrinsic size-related membrane properties of motor neurons (Kernell, 1983, 2003), such that, type S motor units have the lowest onset and peak discharge rates, whereas type FR, FInt and FF motor units display increasingly higher onset and peak discharge rates (Sieck et al., 1984, 2013). The range of discharge rates (onset to peak) of motor neurons generally matches the force-frequency characteristics of motor units, thereby optimizing frequency coding of motor unit force production (Fournier and Sieck, 1988a; Kernell, 2003; Seven et al., 2014; Sieck et al., 1984).
3.2. Diaphragm motor unit recruitment
In 1988, we introduced a model of DIAm neuromotor control across a range of motor behaviors, in which we assumed that DIAm motor unit recruitment order was consistent with Henneman’s Size Principle (Sieck, 1988; Sieck and Fournier, 1989). Indeed, it is now clear that DIAm motor units obeyed the Size Principle (Dick et al., 1987; Jodkowski et al., 1987, 1988; Seven et al., 2014). This model of DIAm motor unit recruitment also depends on estimates of the proportion of motor unit types and the forces they contribute. The proportion of different motor unit types can be estimated based on the proportion of different muscle fiber types and measurements of the innervation ratio of each motor unit type (based on glycogen depletion) (Enad et al., 1989; Fournier and Sieck, 1988b). The force generated by each motor unit type depends on the total cross-sectional area (CSA) of the muscle fibers comprising each unit, which can be estimated from fiber type proportion, average fiber CSA and the innervation ratio. In some studies, we directly measured the force generated by different motor unit types by isolating single motor units following dissection of cervical ventral root filaments and graded electrical stimulation (verified by waveform analysis of evoked motor unit action potentials) (Fournier and Sieck, 1988a). In other more recent studies, we measured the specific force of single permeabilized DIAm fibers (type identified by MyHC isoform expression) activated by extracellular Ca2+ (Geiger et al., 2000, 2001a, 2002). Using in vivo measurements of transdiaphragmatic pressure (Pdi—a surrogate of DIAm force), we determined DIAm force generation across a range of ventilatory and higher force non-ventilatory motor behaviors (Fig. 2). Based on our model of DIAm motor unit recruitment, both eupneic ventilation and ventilation stimulated by a hypoxic-hypercapnic gas mixture (10% O2, 5% CO2, balance N2) require the recruitment of only type S and FR motor units, whereas higher force non-ventilatory motor behaviors require the additional recruitment of more fatigable type FInt and FF motor units (Fournier and Sieck, 1988a; Gransee et al., 2012; Greising et al., 2012; Mantilla et al., 2010; Mantilla and Sieck, 2011; Sieck, 1988, 1989, 1990, 1991, 1994, 1995; Sieck et al., 1989a, 2013; Sieck and Fournier, 1989; Sieck and Gransee, 2012).
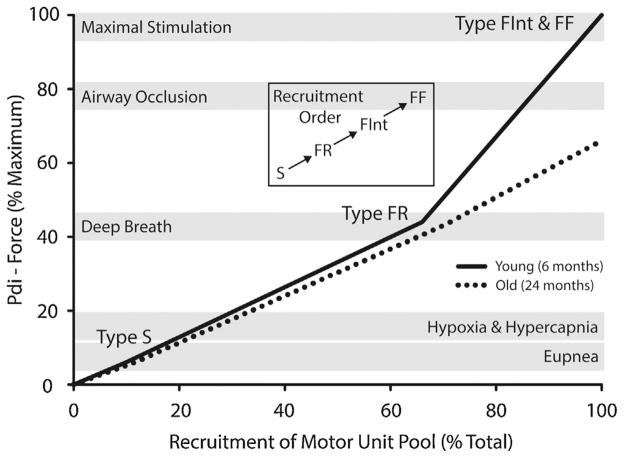
Fig. 2.
Model of diaphragm muscle motor unit recruitment during ventilatory and non-ventilatory behaviors in mice at 6 and 24 months of age. Motor units are assumed to be recruited in an orderly fashion in rank order beginning with slow-twitch (Type S), followed by fast-twitch fatigue resistant (Type FR), fast-twitch fatigue intermediate (Type FInt), and finally fast-twitch fatigable (Type FF) motor units. Ventilatory behaviors can be accomplished without the recruitment of more fatigable motor units (i.e., FInt and FF). Recruitment of these motor units is only required during more forceful non-ventilatory behaviors that are associated with clearing the airways. Data adapted from (Greising et al., 2015b).
3.3. Diaphragm muscle sarcopenia and motor unit recruitment
The presence of sarcopenia is well characterized in limb muscles in both humans (Doherty, 2003; Proctor et al., 1995) and rodent models of aging (Brooks and Faulkner, 1988). Sarcopenia also exists in the DIAm. For example, work from our lab in Fischer 344 rats showed an ~20% reduction in DIAm specific force in old (24 months of age; 50% survival) compared to young (6 months of age; 100% survival) animals (Gosselin et al., 1994). Subsequent work in Fischer 344 (Criswell et al., 1997; Powers et al., 1996; Smith et al., 2002) Fischer 344 × Brown Norway (Criswell et al., 2003) and Wistar (Imagita et al., 2009) rats confirmed an aging-related reduction in DIAm specific force. An aging-related reduction in DIAm specific force has also been reported in golden hamsters (Zhang and Kelsen, 1990). More recently, work by our group used a mouse model (C57BL/6 × 129) of natural aging (6 and 24 months of age, corresponding to 100% and 75% survival, respectively) to investigate DIAm sarcopenia. In these studies, we showed an ~25% reduction in DIAm specific force (Fig. 3A) (Greising et al., 2013, 2015b) together with a selective atrophy of type IIx and/or IIb DIAm fibers (Fig. 3B). Importantly, the CSA of type I and IIa DIAm fibers was not reduced in older mice. In our earlier study (Greising et al., 2013), we also found that in the transgenic BubR1H/H mouse model of early onset aging, there was fiber type specific DIAm fiber atrophy and a reduction in specific force characteristic of sarcopenia in the naturally aging mice.
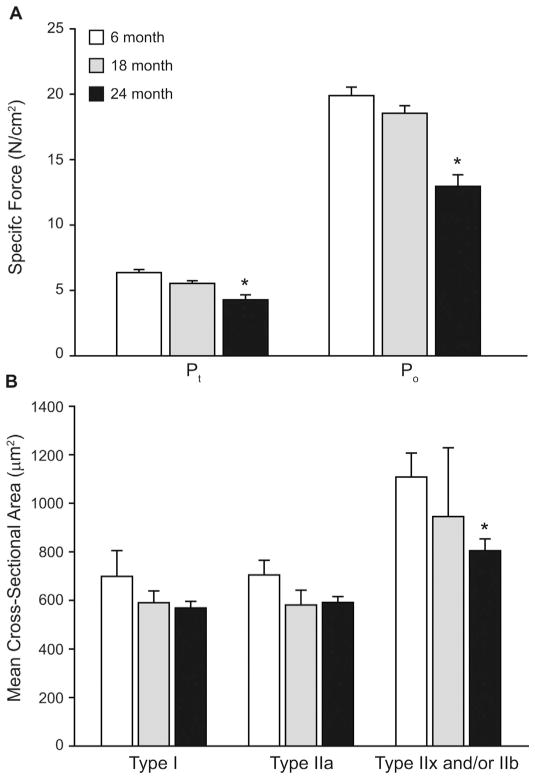
Fig. 3.
Diaphragm muscle sarcopenia is event by ~75% survival in mice. (A) Maximal isometric twitch force (Pt ) and maximal tetanic force (Po ), normalized to physiological cross-sectional area, of midcostal diaphragm muscle (DIAm) of mice across the lifespan (aged 6, 18 and 24 months). There is a significant (*) loss of force by 24 months of age but not between 6 and 18 months. Reprinted with permission from (Greising et al., 2015a). (B) Fiber cross-sectional area (CSA) of the DIAm of across the lifespan. There is a significant (*) loss of type IIx and/or IIb DIAm fiber CSA by 24 months of age. Data at 6 and 24 months of age adapted from (Greising et al., 2013), data at 18 months of age is pilot (n = 4). Mean ± SE.
Overall, it appears that DIAm sarcopenia exists and that muscle fiber atrophy and weakness is fiber type specific such that the higher force-generating type IIx and/or IIb fibers are most affected whereas lower force-generating type I and IIa fibers are spared. These results are consistent with work in limb muscles in which it has been reported that aging-related atrophy predominantly affects type IIx and/or IIb fibers, whereas CSA and force-generating capacity of type I and IIa fibers is relatively preserved (Aniansson et al., 1986; Grimby et al., 1982; Larsson et al., 1978; Lexell et al., 1988; Nilwik et al., 2013; Oertel, 1986; Proctor et al., 1995; Tomonaga, 1977). Importantly, an effect of sarcopenia on type IIx and/or IIb fibers would impact the relative contributions of more fatigable type FF and FInt motor units. Thus, lower force motor behaviors would be minimally affected, whereas higher force motor behaviors would be primarily impacted (Fig. 2). For the DIAm, this would involve higher force non-ventilatory behaviors primarily involved in airway clearance.
The functional consequence of DIAm sarcopenia in elderly humans was explored by Tolep et al., where the maximum Pdi generated through a combined expulsive-Mueller maneuver was found to be ~25% lower in older (65–75 years of age) individuals (Tolep et al., 1995). Subsequently, Polkey et al. confirmed aging-related DIAm weakness in subjects 67–83 years old as reflected by a ~14% reduction in Pdi generated by a maximal sniff test (Polkey et al., 1997). Similar to the performance of expulsive, airway clearance maneuvers (e.g., coughing and sneezing), the performance of these maximal inspiratory efforts requires the recruitment of all available motor units, particularly the higher force generating type FInt and FF motor units (Fig. 2) (Fournier and Sieck, 1988a; Greising et al., 2012; Mantilla et al., 2010; Sieck, 1988, 1991, 1994; Sieck et al., 1989a). Accordingly, the aging-related reduction in maximum DIAm strength, does not appear to impact the ability to sustain normal ventilatory behaviors (i.e., eupneic ventilation in normoxia) but does impair the ability to perform higher force, expulsive airway clearance maneuvers (Greising et al., 2015b). In this way, DIAm sarcopenia may put elderly individuals at an increased risk for developing pneumonia or other respiratory system infections.
4. Aging-related motor neuron loss and sarcopenia
Although the underlying etiology and pathogenesis for the aging-related selective atrophy of type IIx and/or IIb fibers in the DIAm and other muscles remains undetermined, evidence suggests that the entire motor unit (motor neuron, NMJ, and muscle fibers) is involved, which may reflect alterations in neurotrophic influence (Fig. 1). There is an aging-related progressive loss of motor neurons innervating limb muscles (Brown, 1972; Kwan, 2013; Lexell et al., 1988; McNeil et al., 2005; Rosenheimer, 1990; Tomlinson and Irving, 1977) that is correlated with the presence of sarcopenia (Drey et al., 2014). This frank loss of motor neurons results in denervation of muscle fibers, with possible reinnervation by remaining motor neurons (via axonal sprouting), and consequent motor unit expansion (Gordon and Stein, 1982). With axonal sprouting and reinnervation, there is fiber type transition leading to a clustering of fiber types. In a recent study, we found an aging-related increase in fiber type clustering in the mouse DIAm providing indirect evidence of motor neuron loss (Greising et al., 2015c). Importantly, in a number of studies, we have shown that denervation of DIAm fibers induces a decrease in maximum specific force and a selective atrophy of type IIx and/or IIb fibers (Geiger et al., 2001a; Miyata et al., 1995; Zhan et al., 1997), a pattern very similar to aging-related sarcopenia. Thus, motor neuron loss and denervation may at least partially underlie sarcopenia.
In a study, examining the effect of unilateral phrenic nerve denervation on the morphological and mechanical properties of single rat DIAm fibers (Geiger et al., 2001a), we reported that at 14 days after denervation, there was atrophy of type IIx and/or IIb fibers and a decrease in maximum Ca2+-activated force (similar to sarcopenia). Furthermore, there was a decrease in MyHC content per half-sarcomere in type IIx and/or IIb DIAm fibers. Importantly, maximum force was reduced even when normalized for CSA (specific force) as well as MyHC content per half-sarcomere (indicating reduced force per cross bridge). In contrast, unilateral denervation slightly increased the CSA of type I and IIa fibers with no effect on MyHC content per half-sarcomere. However, denervation did induce a decrease in specific force in type I and IIa DIAm fibers, but much less in contrast to that observed in type IIx and/or IIb fibers. It is unlikely that the selective atrophy of type IIx and/or IIb DIAm fibers following unilateral phrenic nerve denervation is due to muscle paralysis and inactivity per se, since there is no change in the CSA of type IIx and/or IIb fibers following DIAm paralysis induced by unilateral spinal hemisection at C2 (Miyata et al., 1995; Prakash et al., 1999; Zhan et al., 1997). Accordingly, we concluded that the selective atrophy and reduction of specific force of type IIx and/or IIb DIAm fibers was due to the removal of a neurotrophic influence (Fig. 1). Thus, it is likely that an aging-related disruption in neurotrophic influence (either via motor neuron loss or decreased expression) underlies DIAm sarcopenia (Fig. 4).
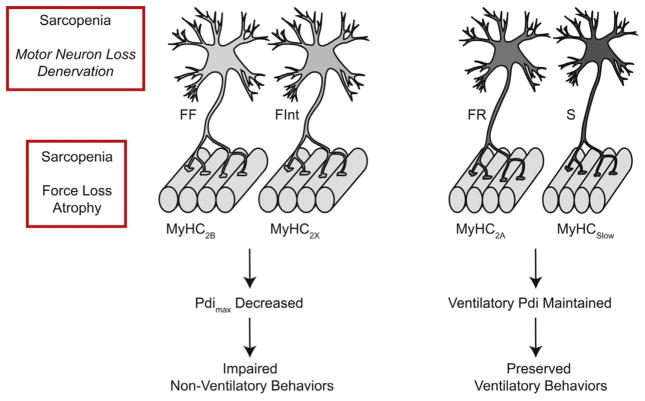
Fig. 4.
Schematic illustrating the aging-related functional consequences of diaphragm muscle (DIAm) fiber type specific sarcopenia (atrophy and weakness). In this model, DIAm sarcopenia is predominantly present in the fast-twitch fatigable (type FF) and fast-twitch fatigue intermediate (type FInt) motor units, which in turn are associated with type IIb and IIx DIAm fibers that express the MyHC2B and MyHC2X isoforms, respectively. In contrast, DIAm sarcopenia is less evident in the fast-twitch fatigue resistant (type FR) and slow (type S) motor units, which in turn are associated with type IIa and I DIAm fibers that express the MyHC2A and MyHCSlow isoforms, respectively. The functional consequences of this fiber type specific effect of DIAm sarcopenia is a decrease in the maximum transdiaphragmatic pressure (Pdimax ) the DIAm is capable of generating, and therefore an impaired ability to perform expulsive, high force non-ventilatory behaviors (i.e., coughing and sneezing). However, the Pdi required to perform ventilatory behaviors remains preserved.
4.1. Aging reduces neurotrophic influence on muscle fibers
The importance of neurotrophic interactions in the establishment and maintenance of muscle fiber properties was first demonstrated in 1960 by Buller et al. (1960) in a study in which the nerves innervating the flexor digitorum longus (predominantly fast) and soleus (predominantly slow) muscles were cut and then switched resulting in reinnervation and a reversal of the mechanical properties of these muscles. It is possible that the pattern of neural activation (e.g., frequency of activation) caused the switch in mechanical properties; however, it is now apparent that motor unit-specific neurotrophic factors are primarily responsible. The problem is which ones?
One potential candidate that we are exploring is neuregulin (NRG), which is a member of the epidermal growth factor family of trophic factors that acts through receptor tyrosine kinases of the epidermal growth factor (ErbB) family. To date, six NRG genes (NRG1-6) have been identified, each with multiple splice isoforms (e.g., >30 for NRG-1) that interact with four different ErbB receptors (ErbB1-4) (Buonanno and Fischbach, 2001; Burden and Yarden, 1997; Falls, 2003; Mei and Nave, 2014; Mei and Xiong, 2008; Yarden and Sliwkowski, 2001; Zhu et al., 1995). The number of potential permutations between NRG and ErbB is further increased by ErbB receptor dimerization, which allows for 6 different ErbB receptor complexes (Jones et al., 1999). We have primarily focused on NRG-1 and NRG-2, due to their relevant expression in muscle fibers and motor neurons (Falls et al., 1993; Moscoso et al., 1995; Rimer et al., 2004; Trinidad et al., 2000; Zhu et al., 1995). Neither NRG-1 nor NRG-2 appreciably binds ErbB1, and both are unable to bind ErbB2. Although NRG-1 and NRG-2 can bind ErbB3 and ErbB4, ErbB3 lacks a functional tyrosine kinase domain and requires dimerization with other ErbB receptor complexes for activation. Compared to ErbB3 and ErbB4 homodimers, the ErbB2/3 and ErbB2/4 heterodimers display an ~100 fold greater binding affinity for NRG-1 and NRG-2 (Jones et al., 1999).
We found that activation of NRG/ErbB signaling has an anabolic effect in DIAm and therefore, may have an integral role in the maintenance of muscle mass (Hellyer et al., 2006). The signal transduction initiated from NRG/ErbB binding in muscle is mediated by phosphorylation of the ErbB receptor and subsequent activation of the phosphoinositide 3-kinase (PI3K) and protein kinase B (Akt) intracellular pathways (Citri et al., 2003). Indeed, activation of PI3K/Akt and subsequent initiation of mammalian target of rapamycin (mTOR) signaling is a critical regulator of muscle protein synthesis (Argadine et al., 2009; Argadine et al., 2011; Bodine et al., 2001). Although all four ErbB receptors can activate PI3K/Akt/mTOR signaling, the ErbB2/3 receptor heterodimer appears uniquely suited to activating the PI3K/Akt/mTOR pathway in DIAm (Hellyer et al., 2001).
The anabolic effect of NRG/ErbB signaling makes the removal of this neurotrophic factor particularly well suited to mediate the aging-related atrophy of muscle fibers due to a negative protein balance, with a reduction in MyHC content per half-sarcomere and resulting decrease in force generation (Fig. 1). At the present time it is unknown whether there are fiber type specific changes in NRG/ErbB signaling that may contribute to muscle fiber atrophy and weakness characteristic of sarcopenia.
4.2. Neurotrophic signaling at phrenic motor neurons
It is clear that neural-derived trophic influence has a pro-found effect on determining muscle fiber metabolic and mechanical properties within motor units, but neurotrophic factors can also substantially influence properties of the motor neuron as well. Our own studies have focused on brain-derived neurotrophic factor (BDNF), which is a member of the neurotrophin family that includes nerve growth factor (NGF), neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4), all of which act through sub-classes of the high affinity tropomyosin-related kinase (Trk) receptors (Koliatsos et al., 1993; Reichardt, 2006). Acting through its high affinity Trk subtype B receptor (TrkB), BDNF is broadly involved in several aspects of motor unit function, including the regulation of motor neuron survival during embryonic development (pruning process) and possibly during aging (Fig. 1), enhancement of presynaptic release of neurotransmitters and postsynaptic regulation of receptor expression (c.f. Kalinkovich and Livshits, 2015; Raschke and Eckel, 2013; Sakuma et al., 2015).
Recently, we demonstrated a role for BDNF/TrkB signaling in enhancing functional recovery of rhythmic phrenic motor neuron activity following upper cervical spinal cord injury (SCI) (Gransee et al., 2013, 2015; Mantilla et al., 2013, 2014a). Intrathecal infusion of BDNF in the cervical spinal cord promoted recovery after SCI, whereas competitive inhibition of BDNF by intrathecal infusion of TrkB-Fc blunted recovery. Knocking down TrkB receptor expression in phrenic motor neurons by intrapleural injection of siRNA also blunted recovery after SCI, while increasing TrkB receptor expression using an AAV7 vector enhanced recovery. Inhibition of TrkB kinase activity in a knockin TrkBF616A mouse model (reversible inhibition that is sensitive to the phosphoprotein phosphatase-1 derivative, 1NMPP1) also blunted functional recovery after SCI. The postsynaptic effect of BDNF/TrkB signaling at phrenic motor neuron is to enhance excitability through an increased expression of serotonergic (5HT2A) and glutamatergic (NMDA and GluR) receptors (Mantilla et al., 2012).
4.3. Aging effect on neuromuscular transmission
There is substantial evidence of an aging-related remodeling of the NMJ (Deschenes et al., 2010; Fahim and Robbins, 1982; Gutmann and Hanzlikova, 1965; Kung et al., 2014; Miyata et al., 2008; Prakash and Sieck, 1998; Suzuki et al., 2009; Valdez et al., 2010). Previous work from our laboratory showed aging-related remodeling of NMJs in DIAm from 24-month old Fischer 344 rats (75% survival) with motor end-plate fragmentation found predominantly at type IIx and/or IIb fibers (Prakash and Sieck, 1998). Importantly, a similar fiber type specific remodeling of DIAm NMJs was also reported following DIAm paralysis induced by unilateral spinal hemisection at C2 (Prakash et al., 1999). Cardasis and LaFontaine also reported aging-related NMJ remodeling in the rat DIAm (~24 month) with an increased incidence of frank denervation (Cardasis and LaFontaine, 1987). Deschenes et al. (2010) reported that aging-related NMJ remodeling occurred at muscle fibers in the plantaris and soleus muscles in Fischer 344 rats during early stages of aging (21 month; 80% survival). It should be noted that in these muscles, aging-related NMJ remodeling occurred before muscle fiber atrophy. Accordingly, an aging-related disruption of normal neurotrophic signaling, by way of denervation, may induce morphological and mechanical adaptations of DIAm fibers (predominantly type IIx and/or IIb) that are characteristic of sarcopenia (Fig. 1).
In previous studies, we assessed neuromuscular transmission failure in the DIAm by repetitive stimulation of the phrenic nerve with periodic superimposition of direct muscle stimulation (Kuei et al., 1990; Sieck and Prakash, 1995). In this technique, if neuromuscular transmission failure occurs, the DIAm does not fatigue and there is a greater difference in the forces evoked by nerve vs. direct muscle stimulation (Fig. 5). Using this technique, we demonstrated that significant neuromuscular transmission failure occurs in the DIAm especially at higher rates of stimulation (e.g., >40 Hz). For example, neuromuscular transmission failure accounts for ~43% of rat DIAm force loss at 75 Hz stimulation (Kuei et al., 1990). To assess whether neuromuscular transmission failure was fiber type specific, we used a glycogen depletion technique in which muscle fiber glycogen stores are not depleted in fibers susceptible to neuromuscular transmission failure during repetitive stimulation. Using this technique we demonstrated that type IIx and/or IIb fibers in the rat DIAm are most susceptible to neuromuscular transmission failure (Johnson and Sieck, 1993). Later, it was shown that during repetitive phrenic nerve stimulation, terminals innervating type IIx and/or IIb DIAm fibers demonstrated significantly less synaptic vesicle recycling compared to terminals innervating type I and IIa DIAm fibers (Mantilla et al., 2004a). Furthermore, we showed that during repetitive phrenic nerve stimulation quantal release of acetylcholine (ACh) is depressed to a greater extent at type IIx and/or IIb DIAm fibers, due to a decreased probability of release at nerve terminals (Rowley et al., 2007). With a decrease in quantal ACh release at type IIx and/or IIb DIAm fibers, end-plate potential (EPP) amplitude falls below the safety factor (i.e., ratio of EPP amplitude to action potential activation threshold) and neuromuscular transmission fails (Ermilov et al., 2007).
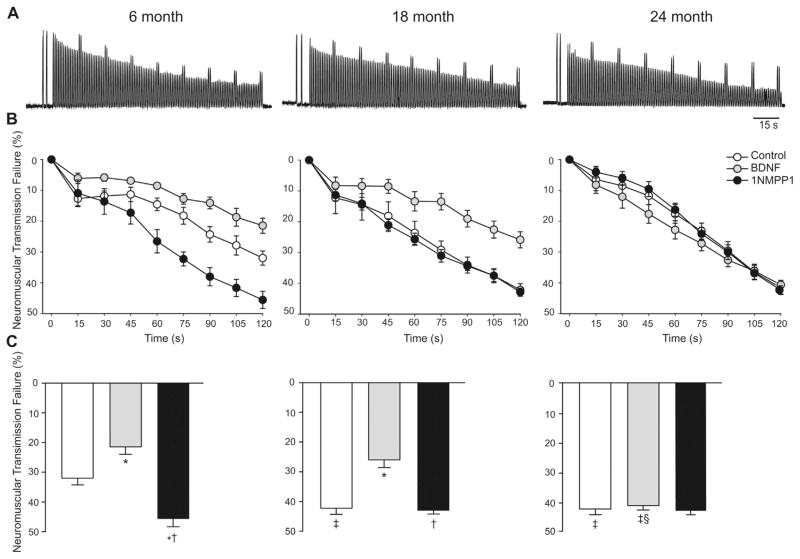
Fig. 5.
Across the lifespan in mice (aged 6, 18 and 24 months) the contribution of neuromuscular transmission failure to diaphragm muscle fatigue over a 2-min period of repetitive nerve stimulation and superimposed intermittent muscle stimulation. (A) Representative tracings for control (vehicle-treated) diaphragm muscle-phrenic nerve preparations of each age group. (B) Time course of neuromuscular transmission failure during repetitive stimulation in control, brain derived neurotrophic factor (BDNF), or 1NMPP1-treated (inhibition of TrkB kinase activity) preparations at each age group, all for 30 min in vitro. In all age and treatment groups, there is progressively greater neuromuscular transmission failure over time. (C) Neuromuscular transmission failure following 2 min of repetitive stimulation in control, BDNF- and 1NMPP1-treated groups. *Significantly different from control at the same age; †significantly different from BDNF at the same age; ‡significantly different from 6 months within the same treatment; §significantly different from 18 months within the same treatment. Mean ± SE. Reprinted with permission from (Greising et al., 2015a).
In hippocampal neurons, exogenous BDNF treatment increases quantal content, and the frequency of miniature excitatory postsynaptic potentials (mEPSPs), indicating an effect on the probability of presynaptic neurotransmitter release (Tyler and Pozzo-Miller, 2001; Tyler et al., 2006). Through this mechanism, BDNF/TrkB signaling improves synaptic transmission (Boulanger and Poo, 1999; Lohof et al., 1993). We also found that at the rat DIAm NMJ, BDNF increases quantal release of ACh (unpublished observations), and thereby improves synaptic transmission (Greising et al., 2015a; Mantilla and Ermilov, 2012; Mantilla et al., 2004b; Zhan et al., 2003). This effect of BDNF/TrkB signaling on synaptic transmission is particularly evident at the DIAm NMJ (Greising et al., 2015a; Mantilla and Ermilov, 2012; Mantilla et al., 2004b, 2014b; Zhan et al., 2003).
In a recent study, we showed an aging-related increase in neuromuscular transmission failure in the mouse DIAm (Fig. 5) (Greising et al., 2015a). Furthermore, we found that aging diminishes the beneficial impact of BDNF/TrkB signaling on neuromuscular transmission. In younger mice and rats (3–6 months of age), BDNF/TrkB signaling significantly improves neuromuscular transmission (Fig. 5) (Greising et al., 2015a; Mantilla et al., 2004b). A similar effect is seen using the BDNF agonist, 7,8-dihydroxyflavone (Mantilla and Ermilov, 2012). In contrast, inhibition of TrkB using the non-specific tyrosine kinase inhibitor, K252a (Mantilla et al., 2004b) or more specifically by inhibiting TrkB tyrosine kinase activity in a knockin TrkBF616A mouse model sensitive to 1NMPP1 (Greising et al., 2015a; Mantilla et al., 2014b), markedly impairs neuromuscular transmission. However, in older mice (24 months of age), the beneficial effects of BDNF/TrkB signaling on DIAm neuromuscular transmission are largely absent (Fig. 5) (Greising et al., 2015a). Furthermore, in TrkBF616A mice at 24 months of age, inhibition of TrkB kinase activity (by 1NMPP1 treatment) has no effect on neuromuscular transmission (Fig. 5). Interestingly, in TrkBF616A mice at 18 months of age, BDNF improved neuromuscular transmission, while 1NMPP1-induced inhibition of TrkB kinase activity had no effect on neuromuscular transmission (Fig. 5). Together, these results suggest that with aging there is a reduction in endogenous BDNF production in phrenic motor neurons that precedes alterations in TrkB expression and/or activity (Fig. 1).
5. Conclusion
As the elderly population increases across the world, so will the economic burden associated with a greater incidence of aging-related chronic diseases, particularly the ubiquitous patho-physiological effects of sarcopenia. Unlike sarcopenia of the limb muscles that manifests as systemic weakness, an increased risk for falling and limitations in activities of daily living, sarcopenia of the respiratory muscles rarely contributes to ventilatory failure. Instead, respiratory muscle sarcopenia, particularly of the DIAm, manifests as an impaired ability to generate higher levels of Pdi necessary for expulsive airway clearance behaviors (e.g., coughing and sneezing). Accordingly, DIAm sarcopenia may directly contribute to the significantly increased risk for pneumonia and other respiratory infections common in elderly populations. Unfortunately, the mechanisms underlying sarcopenia remain unknown. However, the similarity in the physiological consequences associated with sarcopenia and unilateral phrenic nerve denervation suggest a common mechanism. With unilateral phrenic nerve denervation, neurotrophic influence on DIAm fibers is removed resulting in atrophy and decreased specific force that is most pronounced in type IIx and/or IIb fibers. Importantly, with aging, there are similar morphological and functional changes in type IIx and/or IIb DIAm fibers that may reflect a progressive removal of neurotrophic influence. In this regard, there is evidence for a loss of phrenic motor neurons with aging. Two potential neurotrophic signaling pathways may be affected by aging. At DIAm fibers, nerve-derived NRG/ErbB signaling exerts an anabolic effect that maintains contractile protein content in type IIx and/or IIb DIAm fibers. The influence of NRG/ErbB signaling may be lost during aging by the loss of phrenic motor neurons. At phrenic motor neurons, BDNF/TrkB signaling may be essential to promote motor neuron survival, possibly by enhancing synaptic transmission. An aging-related decrease in BDNF expression and/or a decrease in TrkB signaling may lead to phrenic motor neuron loss. Together, NRG/ErbB and BDNF/TrkB provide important regulatory signals serving to maintain synaptic transmission, integrity of the neuromuscular junction and muscle fiber mass. Further work aimed at investigating the aging-related impairments in the signaling of these neurotrophic factors may provide important mechanistic insight into the etiology of not only sarcopenia, but potentially other neurodegenerative diseases as well.
Acknowledgments
Supported by National Institutes of Health grants R01-AG044615 (C.B.M. & G.C.S.) and T32-HL105355 (J.E.E. & S.M.G.).
References
- Federal Interagency Forum on Aging-Related Statistics. Older Americans 2012: Key Indicators of Well-Being. U.S. Government Printing Office; Washington, DC: 2012. [Google Scholar]
- National Institutes of Health; National Heart Lung and Blood Institute, editor. Fact Book Fiscal Year, 2012. Bethesda, MD: [Google Scholar]
- Aniansson A, Hedberg M, Henning GB, Grimby G. Muscle morphology, enzymatic activity, and muscle strength in elderly men: a follow-up study. Muscle Nerve. 1986;9:585–591. doi: 10.1002/mus.880090702. [DOI] [PubMed] [Google Scholar]
- Argadine HM, Hellyer NJ, Mantilla CB, Zhan WZ, Sieck GC. The effect of denervation on protein synthesis and degradation in adult rat diaphragm muscle. J Appl Physiol. 2009;107:438–444. doi: 10.1152/japplphysiol.91247.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argadine HM, Mantilla CB, Zhan WZ, Sieck GC. Intracellular signaling pathways regulating net protein balance following diaphragm muscle denervation. Am J Physiol Cell Physiol. 2011;300:C318–C327. doi: 10.1152/ajpcell.00172.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloh RH, Rakowicz W, Gardner R, Pestronk A. Frequent atrophic groups with mixed-type myofibers is distinctive to motor neuron syndromes. Muscle Nerve. 2007;36:107–110. doi: 10.1002/mus.20755. [DOI] [PubMed] [Google Scholar]
- Ben Hamida C, Soussi-Yanicostas N, Butler-Browne GS, Bejaoui K, Hentati F, Ben Hamida M. Biochemical and immunocytochemical analysis in chronic proximal spinal muscular atrophy. Muscle Nerve. 1994;17:400–410. doi: 10.1002/mus.880170407. [DOI] [PubMed] [Google Scholar]
- Berry CE, Drummond MB, Han MK, Li D, Fuller C, Limper AH, Martinez FJ, Schwarz MI, Sciurba FC, Wise RA. Relationship between lung function impairment and health-related quality of life in COPD and interstitial lung disease. Chest. 2012;142:704–711. doi: 10.1378/chest.11-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr. 2014;33:737–748. doi: 10.1016/j.clnu.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Poo MM. Presynaptic depolarization facilitates neurotrophin-induced synaptic potentiation. Nat Neurosci. 1999;2:346–351. doi: 10.1038/7258. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WF. A method for estimating the number of motor units in thenar muscles and the changes in motor unit count with ageing. J Neurol Neurosurg Psychiatry. 1972;35:845–852. doi: 10.1136/jnnp.35.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, Leeuwenburgh C, Pahor M, Manini TM. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010;9:369–383. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of responses. J Physiol. 1960;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Burden S, Yarden Y. Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron. 1997;18:847–855. doi: 10.1016/s0896-6273(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Burke RE. Motor units: anatomy, physiology, and functional organization. In: York N, editor. Handbook of Physiology. Raven Press; 1975. pp. 345–420. [Google Scholar]
- Burke RE. Motor units: anatomy, physiology and functional organization. In: Peachey LD, editor. Handbook of Physiology, The Nervous System, Motor Control. American Physiological Society; Bethesda, MD: 1981. pp. 345–422. [Google Scholar]
- Burke RE, Levine DN, Zajac FE., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971;174:709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardasis CA, LaFontaine DM. Aging rat neuromuscular junctions: a morphometric study of cholinesterase stained whole mounts and ultrastructure. Muscle Nerve. 1987;10:200–213. doi: 10.1002/mus.880100303. [DOI] [PubMed] [Google Scholar]
- Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M, Van Kan GA, Anker SD, Rutkove S, Vrijbloed JW, Isaac M, Rolland Y, M’Rini C, Aubertin-Leheudre M, Cedarbaum JM, Zamboni M, Sieber CC, Laurent D, Evans WJ, Roubenoff R, Morley JE, Vellas B. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3:181–190. doi: 10.1007/s13539-012-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong CP, Street PR. Pneumonia in the elderly: a review of the epidemiology, pathogenesis, microbiology, and clinical features. South Med J. 2008;101:1141–1145. doi: 10.1097/SMJ.0b013e318181d5b5. [DOI] [PubMed] [Google Scholar]
- Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- Criswell DS, Powers SK, Herb RA, Dodd SL. Mechanism of specific force deficit in the senescent rat diaphragm. Respir Physiol Neurobiol. 1997;107:149–155. doi: 10.1016/s0034-5687(96)02509-1. [DOI] [PubMed] [Google Scholar]
- Criswell DS, Shanely RA, Betters JJ, McKenzie MJ, Sellman JE, Van Gammeren DL, Powers SK. Cumulative effects of aging and mechanical ventilation on in vitro diaphragm function. Chest. 2003;124:2302–2308. doi: 10.1378/chest.124.6.2302. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, Raphael JC, Outin H, Bastuji-Garin S. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- de Jonghe B, Lacherade JC, Sharshar T, Outin H. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med. 2009;37:S309–S315. doi: 10.1097/CCM.0b013e3181b6e64c. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45:389–393. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Kong FJ, Berger AJ. Recruitment order of diaphragmatic motor units obeys Henneman’s size principle. In: Sieck GC, Gandevia SC, Cameron WE, editors. Respiratory Muscles and Their Neuromotor Control. Alan R Liss, Inc; New York: 1987. pp. 239–247. [Google Scholar]
- Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Drey M, Krieger B, Sieber CC, Bauer JM, Hettwer S, Bertsch T. Motoneuron loss is associated with sarcopenia. J Am Med Dir Assoc. 2014;15:435–439. doi: 10.1016/j.jamda.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Edstrom L, Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968;31:424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enad JG, Fournier M, Sieck GC. Oxidative capacity and capillary density of diaphragm motor units. J Appl Physiol. 1989;67:620–627. doi: 10.1152/jappl.1989.67.2.620. [DOI] [PubMed] [Google Scholar]
- Ermilov LG, Mantilla CB, Rowley KL, Sieck GC. Safety factor for neuromuscular transmission at type-identified diaphragm fibers. Muscle Nerve. 2007;35:800–803. doi: 10.1002/mus.20751. [DOI] [PubMed] [Google Scholar]
- Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med. 1995;50(Spec):5–8. doi: 10.1093/gerona/50a.special_issue.5. [DOI] [PubMed] [Google Scholar]
- Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S–1127S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993;123:465–468. doi: 10.1093/jn/123.suppl_2.465. [DOI] [PubMed] [Google Scholar]
- Fahim MA, Robbins N. Ultrastructural studies of young and old mouse neuromuscular junctions. J Neurocytol. 1982;11:641–656. doi: 10.1007/BF01262429. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993;72:801–813. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol. 1988a;59:1055–1066. doi: 10.1152/jn.1988.59.3.1055. [DOI] [PubMed] [Google Scholar]
- Fournier M, Sieck GC. Somatotopy in the segmental innervation of the cat diaphragm. J Appl Physiol. 1988b;64:291–298. doi: 10.1152/jappl.1988.64.1.291. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol. 2000;279:C611–618. doi: 10.1152/ajpcell.2000.279.3.C611. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Sieck GC. Force-calcium relationship depends on myosin heavy chain and troponin isoforms in rat diaphragm muscle fibers. J Appl Physiol. 1999;87:1894–1900. doi: 10.1152/jappl.1999.87.5.1894. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol. 2000;89:695–703. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Macken RL, Bayrd ME, Sieck GC. Effect of unilateral denervation on maximum specific force in rat diaphragm muscle fibers. J Appl Physiol. 2001a;90:1196–1204. doi: 10.1152/jappl.2001.90.4.1196. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Macken RL, Bayrd ME, Sieck GC. Mechanisms underlying increased force generation by rat diaphragm muscle fibers during development. J Appl Physiol. 2001b;90:380–388. doi: 10.1152/jappl.2001.90.1.380. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Han YS, Hunter LW, Zhan WZ, Sieck GC. Effects of hypothyroidism on maximum specific force in rat diaphragm muscle fibers. J Appl Physiol. 2002;92:1506–1514. doi: 10.1152/japplphysiol.00095.2001. [DOI] [PubMed] [Google Scholar]
- Gordon T, Stein RB. Reorganization of motor-unit properties in reinnervated muscles of the cat. J Neurophysiol. 1982;48:1175–1190. doi: 10.1152/jn.1982.48.5.1175. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Johnson BD, Sieck GC. Age-related changes in diaphragm muscle contractile properties and myosin heavy chain isoforms. Am J Respir Crit Care Med. 1994;150:174–178. doi: 10.1164/ajrccm.150.1.8025746. [DOI] [PubMed] [Google Scholar]
- Gransee HM, Mantilla CB, Sieck GC. Respiratory Muscle Plasticity. Compr Physiol. 2012;2:1441–1462. doi: 10.1002/cphy.c110050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PloS One. 2013;8:e64755. doi: 10.1371/journal.pone.0064755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Localized delivery of brain-derived neurotrophic factor-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury. J Neurotrauma. 2015;32:185–193. doi: 10.1089/neu.2014.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Gransee HM, Mantilla CB, Sieck GC. Systems biology of skeletal muscle: fiber type as an organizing principle. Wiley Interdiscip Rev Syst Biol Med. 2012;4:457–473. doi: 10.1002/wsbm.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol. 2013;48:881–887. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Ermilov LG, Sieck GC, Mantilla CB. Ageing and neurotrophic signalling effects on diaphragm neuromuscular function. J Physiol. 2015a;593:431–440. doi: 10.1113/jphysiol.2014.282244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Mantilla CB, Medina-Martinez JS, Stowe JM, Sieck GC. Functional impact of diaphragm muscle sarcopenia in both male and female mice. Am J Physiol Lung Cell Mol Physiol. 2015b;309:L46–L52. doi: 10.1152/ajplung.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Medina-Martínez JS, Vasdev AK, Sieck GC, Mantilla CB. Analysis of muscle fiber clustering in the diaphragm muscle of sarcopenic mice. Muscle Nerve. 2015c;52:76–82. doi: 10.1002/mus.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimby G, Danneskiold-samsoe B, Hvid K, Saltin B. Morphology and enzymatic capacity in arm and leg muscles in 78–81 year old men and women. Acta Physiol Scand. 1982;115:125–134. doi: 10.1111/j.1748-1716.1982.tb07054.x. [DOI] [PubMed] [Google Scholar]
- Gutmann E, Hanzlikova V. Age changes of motor endplates in muscle fibres of the rat. Gerontologia. 1965;11:12–24. [Google Scholar]
- Hamel MB, Henderson WG, Khuri SF, Daley J. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc. 2005;53:424–429. doi: 10.1111/j.1532-5415.2005.53159.x. [DOI] [PubMed] [Google Scholar]
- Han YS, Proctor DN, Geiger PC, Sieck GC. Reserve capacity of ATP consumption during isometric contraction in human skeletal muscle fibers. J Appl Physiol. 2001;90:657–664. doi: 10.1152/jappl.2001.90.2.657. [DOI] [PubMed] [Google Scholar]
- Hellyer NJ, Kim MS, Koland JG. Heregulin-dependent activation of phosphoinositide 3-kinase and Akt via the ErbB2/ErbB3 co-receptor. J Biol Chem. 2001;276:42153–42161. doi: 10.1074/jbc.M102079200. [DOI] [PubMed] [Google Scholar]
- Hellyer NJ, Mantilla CB, Park EW, Zhan WZ, Sieck GC. Neuregulin-dependent protein synthesis in C2C12 myotubes and rat diaphragm muscle. Am J Physiol Cell Physiol. 2006;291:C1056–C1061. doi: 10.1152/ajpcell.00625.2005. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell LM. Functional organization of motoneuron pool and its inputs. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology. American Physiological Society; Bethesda, MD: 1981. pp. 423–507. [Google Scholar]
- Henneman E, Olson CB. Relations between structure and function in the design of skeletal muscles. J Neurophysiol. 1965;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Heron M. Deaths: leading causes for 2007. Natl Vital Stat Rep. 2011;59:1–95. [PubMed] [Google Scholar]
- Imagita H, Yamano S, Tobimatsu Y, Miyata H. Age-related changes in contraction and relaxation of rat diaphragm. Biomed Res. 2009;30:337–342. doi: 10.2220/biomedres.30.337. [DOI] [PubMed] [Google Scholar]
- Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect Dis. 2004;4:112–124. doi: 10.1016/S1473-3099(04)00931-4. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Viana F, Dick TE, Berger AJ. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol. 1987;58:105–124. doi: 10.1152/jn.1987.58.1.105. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Viana F, Dick TE, Berger AJ. Repetitive firing properties of phrenic motoneurons in the cat. J Neurophysiol. 1988;60:687–702. doi: 10.1152/jn.1988.60.2.687. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Sieck GC. Differential susceptibility of diaphragm muscle fibers to neuromuscular transmission failure. J Appl Physiol. 1993;75:341–348. doi: 10.1152/jappl.1993.75.1.341. [DOI] [PubMed] [Google Scholar]
- Jones JT, Akita RW, Sliwkowski MX. Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett. 1999;447:227–231. doi: 10.1016/s0014-5793(99)00283-5. [DOI] [PubMed] [Google Scholar]
- Kalinkovich A, Livshits G. Sarcopenia—the search for emerging biomarkers. Ageing Res Rev. 2015;22:58–71. doi: 10.1016/j.arr.2015.05.001. http://dx.doi.org/10.1016/j.arr.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Kernell D. Functional properties of spinal motoneurons and gradation of muscle force. Adv Neurol. 1983;39:213–226. [PubMed] [Google Scholar]
- Kernell D. Principles of force gradation in skeletal muscles. Neural Plast. 2003;10:69–76. doi: 10.1155/NP.2003.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Peterson C. Healthspan, translation, and new outcomes for animal studies of aging. J Gerontol A Biol Sci Med Sci. 2009;64:209–212. doi: 10.1093/gerona/gln063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand. 1990;140:55–62. doi: 10.1111/j.1748-1716.1990.tb08975.x. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Clatterbuck RE, Winslow JW, Cayouette MH, Price DL. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- Kuei JH, Shadmehr R, Sieck GC. Relative contribution of neurotransmission failure to diaphragm fatigue. J Appl Physiol. 1990;68:174–180. doi: 10.1152/jappl.1990.68.1.174. [DOI] [PubMed] [Google Scholar]
- Kung TA, Cederna PS, van der Meulen JH, Urbanchek MG, Kuzon WM, Jr, Faulkner JA. Motor unit changes seen with skeletal muscle sarcopenia in oldest old rats. J Gerontol A Biol Sci Med Sci. 2014;69:657–665. doi: 10.1093/gerona/glt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P. Sarcopenia, a neurogenic syndrome? J Aging Res. 2013;2013:791679. doi: 10.1155/2013/791679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103:31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Liddell EGT, Sherrington CS. Recruitment and some other factors of reflex inhibition. Proc R Soc Lond (Biol) 1925;97:488–518. [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Ermilov LG. The novel TrkB receptor agonist 7,8-dihydroxyflavone enhances neuromuscular transmission. Muscle Nerve. 2012;45:274–276. doi: 10.1002/mus.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol. 2011;179:57–63. doi: 10.1016/j.resp.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Rowley KL, Fahim MA, Zhan WZ, Sieck GC. Synaptic vesicle cycling at type-identified diaphragm neuromuscular junctions. Muscle Nerve. 2004a;30:774–783. doi: 10.1002/mus.20173. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC. Neurotrophins improve neuromuscular transmission in the adult rat diaphragm. Muscle Nerve. 2004b;29:381–386. doi: 10.1002/mus.10558. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol. 2010;173:101–106. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Bailey JP, Zhan WZ, Sieck GC. Phrenic motoneuron expression of serotonergic and glutamatergic receptors following upper cervical spinal cord injury. Exp Neurol. 2012;234:191–199. doi: 10.1016/j.expneurol.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Gransee HM, Zhan WZ, Sieck GC. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp Neurol. 2013;247C:101–109. doi: 10.1016/j.expneurol.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Greising SM, Stowe JM, Zhan WZ, Sieck GC. TrkB kinase activity is critical for recovery of respiratory function after cervical spinal cord hemisection. Exp Neurol. 2014a;261:190–195. doi: 10.1016/j.expneurol.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Stowe JM, Sieck DC, Ermilov LG, Greising SM, Zhang C, Shokat KM, Sieck GC. TrkB Kinase activity maintains synaptic function and structural integrity at adult neuromuscular junctions. J Appl Physiol. 2014b;117:910–920. doi: 10.1152/japplphysiol.01386.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31:461–467. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2014;83:27–49. doi: 10.1016/j.neuron.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H, Suzuki T, Maruyama A, Wada N. Age-related three-dimensional morphological changes in rat motoneurons innervating diaphragm and longissimus muscles. Anat Histol Embryol. 2008;37:394–399. doi: 10.1111/j.1439-0264.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol. 1995;79:1640–1649. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- Moscoso LM, Chu GC, Gautam M, Noakes PG, Merlie JP, Sanes JR. Synapse-associated expression of an acetylcholine receptor-inducing protein, ARIA/heregulin, and its putative receptors, ErbB2 and ErbB3, in developing mammalian muscle. Dev Biol. 1995;172:158–169. doi: 10.1006/dbio.1995.0012. [DOI] [PubMed] [Google Scholar]
- Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by special interest groups (SIG) cachexia-anorexia in chronic wasting diseases and nutrition in geriatrics. Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Nair KS. Aging muscle. Am J Clin Nutr. 2005;81:953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB, van Loon LJ. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol. 2013;48:492–498. doi: 10.1016/j.exger.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Oertel G. Changes in human skeletal muscles due to ageing. Histological and histochemical observations on autopsy material. Acta Neuropathol. 1986;69:309–313. doi: 10.1007/BF00688309. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Harris ML, Hughes PD, Hamnegard CH, Lyons D, Green M, Moxham J. The contractile properties of the elderly human diaphragm. Am J Respir Crit Care Med. 1997;155:1560–1564. doi: 10.1164/ajrccm.155.5.9154857. [DOI] [PubMed] [Google Scholar]
- Powers SK, Criswell D, Herb RA, Demirel H, Dodd S. Age-related increases in diaphragmatic maximal shortening velocity. J Appl Physiol. 1996;80:445–451. doi: 10.1152/jappl.1996.80.2.445. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Miyata H, Zhan WZ, Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 1999;22:307–319. doi: 10.1002/(sici)1097-4598(199903)22:3<307::aid-mus3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve. 1998;21:887–895. doi: 10.1002/(sici)1097-4598(199807)21:7<887::aid-mus6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PWR. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol. 1995;78:2033–2038. doi: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- Raschke S, Eckel J. Adipo-myokines: two sides of the same coin-mediators of inflammation and mediators of exercise. Mediators Inflamm. 2013;2013:320724. doi: 10.1155/2013/320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P, Birolleau S, Lefort Y, Becquemin MH, Beigelman C, Isnard R, Teixeira A, Arthaud M, Riou B, Boddaert J. Acute respiratory failure in the elderly: etiology, emergency diagnosis and prognosis. Crit Care. 2006;10:R82. doi: 10.1186/cc4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimer M, Prieto AL, Weber JL, Colasante C, Ponomareva O, Fromm L, Schwab MH, Lai C, Burden SJ. Neuregulin-2 is synthesized by motor neurons and terminal Schwann cells and activates acetylcholine receptor transcription in muscle cells expressing ErbB4. Mol Cell Neurosci. 2004;26:271–281. doi: 10.1016/j.mcn.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Rosenberg IH. Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr. 1989;50:1231–1233. [Google Scholar]
- Rosenheimer JL. Factors affecting denervation-like changes at the neuromuscular junction during aging. Int J Dev Neurosci. 1990;8:643–654. doi: 10.1016/0736-5748(90)90059-b. [DOI] [PubMed] [Google Scholar]
- Rowley KL, Mantilla CB, Ermilov LG, Sieck GC. Synaptic vesicle distribution and release at rat diaphragm neuromuscular junctions. J Neurophysiol. 2007;98:478–487. doi: 10.1152/jn.00251.2006. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Aoi W, Yamaguchi A. Current understanding of sarcopenia: possible candidates modulating muscle mass. Pflugers Arch Eur J Physiol. 2015;467:213–229. doi: 10.1007/s00424-014-1527-x. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lomo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Sehl ME, Yates FE. Kinetics of human aging: I. Rates of senescence between ages 30 and 70 years in healthy people. J Gerontol A Biol Sci Med Sci. 2001;56:B198–B208. doi: 10.1093/gerona/56.5.b198. [DOI] [PubMed] [Google Scholar]
- Seven YB, Mantilla CB, Sieck GC. Recruitment of rat diaphragm motor units across motor behaviors with different levels of diaphragm activation. J Appl Physiol. 2014;117:1308–1316. doi: 10.1152/japplphysiol.01395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. Remarks on some aspects of reflex inhibition. Proc R Soc Lond (Biol) 1925;97:519–545. [Google Scholar]
- Sherrington CS. Some functional problems attaching to convergence. Proc R Soc Lond (Biol) 1929;105:332–362. [Google Scholar]
- Sieck GC. Diaphragm muscle: structural and functional organization. In: Belman MJ, editor. Clinics in Chest Medicine, Respiratory Muscles: Function in Health and Disease. W.B. Saunders Company; Philadelphia, PA: 1988. pp. 195–210. [PubMed] [Google Scholar]
- Sieck GC. Recruitment and frequency coding of diaphragm motor units during ventilatory and non-ventilatory behaviors. In: Swanson GD, Grodins FS, Hughson RL, editors. Respiratory Control. Plenum Press; New York: 1989. pp. 441–450. [Google Scholar]
- Sieck GC. Conceptual model of ventilatory muscle recruitment and diaphragmatic fatigue. In: Khoo MCK, editor. Modeling and Parameter Estimation in Respiratory Control. Plenum Press; New York: 1990. pp. 113–123. [Google Scholar]
- Sieck GC. Neural control of the inspiratory pump. NIPS. 1991;6:260–264. [Google Scholar]
- Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med. 1994;15:641–659. [PubMed] [Google Scholar]
- Sieck GC. Organization and recruitment of diaphragm motor units. In: Roussos C, editor. The Thorax. 2. Marcel Dekker; New York, NY: 1995. pp. 783–820. [Google Scholar]
- Sieck GC, Ferreira LF, Reid MB, Mantilla CB. Mechanical properties of respiratory muscles. Compr Physiol. 2013;3:1553–1567. doi: 10.1002/cphy.c130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M, Belman MJ. Physiological properties of motor units in the diaphragm. In: Bianchi AL, Denavit-Saubie M, editors. Neurogenesis of Central Respiratory Rhythm. MTP; Hingham MA: 1985. pp. 227–229. [Google Scholar]
- Sieck GC, Fournier M, Enad JG. Fiber type composition of muscle units in the cat diaphragm. Neurosci Lett. 1989a;97:29–34. doi: 10.1016/0304-3940(89)90134-1. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Lewis MI, Blanco CE. Effects of undernutrition on diaphragm fiber size, SDH activity, and fatigue resistance. J Appl Physiol. 1989b;66:2196–2205. doi: 10.1152/jappl.1989.66.5.2196. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M, Prakash YS, Blanco CE. Myosin phenotype and SDH enzyme variability among motor unit fibers. J Appl Physiol. 1996;80:2179–2189. doi: 10.1152/jappl.1996.80.6.2179. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Gransee HM. Respiratory muscles: structure, function & regulation. Morgan Claypool Life Sci. 2012:1–87. http://dx.doi.org/10.4199/C00057ED1V01Y2012ISP034.
- Sieck GC, Prakash YS. Fatigue at the neuromuscular junction. Branch point vs presynaptic vs postsynaptic mechanisms. Adv Exp Med Biol. 1995;384:83–100. [PubMed] [Google Scholar]
- Sieck GC, Trelease RB, Harper RM. Sleep influences on diaphragmatic motor unit discharge. Exp Neurol. 1984;85:316–335. doi: 10.1016/0014-4886(84)90143-2. [DOI] [PubMed] [Google Scholar]
- Smith WN, Dirks A, Sugiura T, Muller S, Scarpace P, Powers SK. Alteration of contractile force and mass in the senescent diaphragm with beta(2)-agonist treatment. J Appl Physiol. 2002;92:941–948. doi: 10.1152/japplphysiol.00576.2001. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Maruyama A, Sugiura T, Machida S, Miyata H. Age-related changes in two- and three-dimensional morphology of type-identified endplates in the rat diaphragm. J Physiol Sci. 2009;59:57–62. doi: 10.1007/s12576-008-0005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Thompson DD. Aging and sarcopenia. J Musculoskelet Neuronal Interact. 2007;7:344–345. [PubMed] [Google Scholar]
- Tolep K, Higgins N, Muza S, Criner G, Kelsen SG. Comparison of diaphragm strength between healthy adult elderly and young men. Am J Respir Crit Care Med. 1995;152:677–682. doi: 10.1164/ajrccm.152.2.7633725. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977;34:213–219. doi: 10.1016/0022-510x(77)90069-7. [DOI] [PubMed] [Google Scholar]
- Tomonaga M. Histochemical and ultrastructural changes in senile human skeletal muscle. J Am Geriatr Soc. 1977;25:125–131. doi: 10.1111/j.1532-5415.1977.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Trinidad JC, Fischbach GD, Cohen JB. The Agrin/MuSK signaling pathway is spatially segregated from the neuregulin/ErbB receptor signaling pathway at the neuromuscular junction. J Neurosci. 2000;20:8762–8770. doi: 10.1523/JNEUROSCI.20-23-08762.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203:865–877. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Zhang XL, Hartman K, Winterer J, Muller W, Stanton PK, Pozzo-Miller L. BDNF increases release probability and the size of a rapidly recycling vesicle pool within rat hippocampal excitatory synapses. J Physiol. 2006;574:787–803. doi: 10.1113/jphysiol.2006.111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Kang H, Clemenson GD, Jr, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: final data for 2007. Natl Vital Stat Rep. 2010;58(19):1–136. [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Mantilla CB, Sieck GC. Regulation of neuromuscular transmission by neurotrophins. Sheng Li Xue Bao. 2003;55:617–624. [PubMed] [Google Scholar]
- Zhan WZ, Miyata H, Prakash YS, Sieck GC. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol. 1997;82:1145–1153. doi: 10.1152/jappl.1997.82.4.1145. [DOI] [PubMed] [Google Scholar]
- Zhang YL, Kelsen SG. Effects of aging on diaphragm contractile function in golden hamsters. Am Rev Respir Dis. 1990;142:1396–1401. doi: 10.1164/ajrccm/142.6_Pt_1.1396. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lai C, Thomas S, Burden SJ. Neuregulin receptors, erbB3 and erbB4, are localized at neuromuscular synapses. EMBO J. 1995;14:5842–5848. doi: 10.1002/j.1460-2075.1995.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]