Abstract
Induction of neural plasticity through motor learning has been demonstrated in animals and humans. Brain derived neurotrophic factor (BDNF), a member of the neurotrophin family of growth factors, is thought to play an integral role in modulation of central nervous system plasticity during learning and motor skill recovery. Thirty percent of humans possess a single nucleotide polymorphism on the BDNF gene (Val66Met), which has been linked to decreased activity dependent release of BDNF. Presence of the polymorphism has been associated with altered cortical activation, short term plasticity and altered skill acquisition, and learning in healthy humans. The impact of the Val66Met polymorphism on motor learning post-stroke has not been explored. The purpose of this study was to examine the impact of the Val66Met polymorphism in learning of a novel locomotor task in subjects with chronic stroke. It was hypothesized that subjects with the polymorphism would have an altered rate and magnitude of adaptation to a novel locomotor walking paradigm (the split-belt treadmill), compared to those without the polymorphism. The rate of adaptation was evaluated as the reduction in gait asymmetry during the first 30 (early adaptation) and last 100 (late adaptation) strides. Twenty-seven individuals with chronic stroke participated in a single session of split-belt treadmill walking and tested for the polymorphism. Step length and limb phase were measured to assess adaptation of spatial and temporal parameters of walking. The rate of adaptation of step length asymmetry differed significantly between those with and without the polymorphism, while the amount of total adaptation did not. These results suggest that chronic stroke survivors, regardless of presence or absence of the polymorphism, are able to adapt their walking pattern over a period of trial and error practice, however the presence of the polymorphism influences the rate at which this is achieved.
Keywords: Brain-derived neurotrophic factor, Val66Met polymorphism, Locomotor adaptation, Chronic stroke, Split-belt treadmill
Introduction
Stroke is the leading cause of long term disability in the United States(Go et al., 2014). The ability to recover motor skill function post-stroke relies largely upon the adaptive capacity of the brain following neurologic insult(Murphy & Corbett, 2009). The mechanisms which enable neural plasticity post-stroke are similar to those which promote neural reorganization in the healthy brain during learning (Kleim & Jones, 2008; Murphy & Corbett, 2009). As such, parameters of neuro-rehabilitation which optimize motor learning and enhance neural plasticity are of great interest to clinicians and researchers in the field of stroke rehabilitation.
Brain derived neurotrophic factor (BDNF), a member of the neurotrophin family of growth factors, is thought to play an integral role in modulation of central nervous system plasticity during learning and motor skill recovery. Unlike other members of the neutrophin family, BDNF has been found to be released in an activity dependent manner in response to neuronal activity making it a prime target for exploration of experience dependent neural plasticity (Kovalchuk, Holthoff, & Konnerth, 2004; Numakawa et al., 2010; Yoshii & Constantine-Paton, 2010). The role of BDNF in neuronal plasticity is dependent upon stimulation and release of the mature form of BDNF (mBDNF). The mature form of BDNF has become a major target of investigation in learning-related neural plasticity secondary to its role in mediation of induction and maintenance of long term potentiation (LTP) (Lu, Christian, & Lu, 2008). Disruption of BDNF synthesis or BDNF-TrkB receptor binding impairs enhancement of neural plasticity, motor skill acquisition and cognitive learning in the animal model (Intlekofer et al., 2013; Ploughman et al., 2009; Vaynman, Ying, & Gomez-Pinilla, 2004; Ying et al., 2008). Current evidence in the animal model indicates that BDNF is a requisite for induction of neural plasticity with motor learning (Ploughman et al., 2009; Wolf-Rüdiger Schäbitz et al., 2007; W-R Schäbitz et al., 2004). Equivalent data in humans is currently non-existent, however, genetic abnormalities in the BDNF gene have been linked to altered neural plasticity (Cheeran et al., 2008; Kleim et al., 2006) and learning (Joundi et al., 2012; McHughen & Cramer, 2013) in the human population.
A common single nucleotide polymorphism (SNP) exists on the BDNF gene resulting in a substitution of methionine (MET) for valine (Val) at position 66 on the amino acid chain (Val66Met) (Egan et al., 2003). This polymorphism is present in roughly 20–50% of the population, with frequency dependent upon ethnic background. (Shimizu, Hashimoto, & Iyo, 2004). The Val66Met SNP has been linked to decreased secretion of the activity dependent mature form of the BDNF protein within the CNS (Chen et al., 2004; Egan et al., 2003). In humans, the SNP is associated with altered brain structure and function (Bath & Lee, 2006) and with decreased prefrontal and hippocampal volumes (Pezawas et al., 2004). In addition, presence of the polymorphism has been associated with altered cortical activation and short term plasticity (Beste et al., 2010; Cheeran et al., 2008; Kleim et al., 2006; McHughen et al., 2010) and altered skill acquisition and learning (Beste et al., 2010; Joundi et al., 2012; Kleim et al., 2006; McHughen et al., 2010), though results regarding the effects of the polymorphism on human motor learning and cortical plasticity have been somewhat mixed(Gajewski, Hengstler, Golka, Falkenstein, & Beste, 2011; Li Voti et al., 2011; McHughen & Cramer, 2013; McHughen, Pearson-Fuhrhop, Ngo, & Cramer, 2011). In particular, neurologically intact individuals with the polymorphism appear to have a decreased rate of adaptation to a visuomotor perturbation and a decreased rate of readaptation 24 hours later compared to those without the polymorphism (Joundi et al., 2012).
While several studies have examined the general impact of the polymorphism on motor recovery, the specific influence of the polymorphism on motor learning post stroke has yet to be explored. To date, studies examining the influence of the polymorphism on motor learning have been confined to neurologically intact individuals (Barton et al., 2014; Cirillo, Hughes, Ridding, Thomas, & Semmler, 2012; Joundi et al., 2012; Kleim et al., 2006; McHughen & Cramer, 2013; McHughen et al., 2010). Of these studies, all have utilized upper extremity paradigms to examine motor learning, with limited application to complex, real world motor tasks (McHughen et al., 2010). It is not known whether the polymorphism may also influence learning of a more complex lower extremity task such as locomotion in non-neurologically intact participants, such as those with stroke.
The split-belt treadmill paradigm has previously been well-characterized as a tool to probe short–term locomotor learning in neurologically intact and individuals post-stroke (Reisman, Bastian, & Morton, 2010; Reisman, Block, & Bastian, 2005; Reisman, McLean, Keller, Danks, & Bastian, 2013; Reisman, Wityk, Silver, & Bastian, 2007). The short-term learning process of locomotor adaptation involves relearning an already well-known movement pattern, similar to the re-learning process of those post-stroke early within a therapeutic intervention. Given neural plasticity and motor learning are inherently linked to functional recovery post-stroke, the behavioral effects of altered mBNDF secretion, through presence of the SNP, may be more apparent in this population.
Therefore, in the current study we sought to examine the impact of the Val66Met polymorphism in learning of a novel locomotor task in subjects with chronic stroke (> 6 months post stroke) utilizing the split-belt treadmill paradigm. We hypothesized that subjects with the polymorphism would demonstrate a slowed rate of adaptation to the novel locomotor walking pattern, compared to those without the polymorphism. Additionally, we hypothesized that subjects with the polymorphism would demonstrate a reduced amount of total adaptation relative to those without the polymorphism as well as a limited ability to return to their individual baseline walking asymmetry.
Materials and Methods
Participants
Participants at least 6 months post-stroke were recruited from Delaware and surrounding states with the assistance of local physical therapists, physicians and advertising. All participants provided written informed consent, with the study protocol approved by the University of Delaware Human Subjects Review Board. To be included, subjects must have sustained one single stroke at least 6 months prior to study participation, the ability to ambulate independently with or without bracing, and walk for at least 4 minutes at a self-selected speed without assistance from another person. In addition, to be included participants provided written informed consent to supply a saliva sample for genetic testing for the BDNF Val66Met polymorphism. Exclusion criteria included history of cerebellar stroke, presence of cerebellar signs (ataxic gait or decreased coordination during rapid alternating hand or foot movements), neurologic conditions other than stroke, sensorimotor neglect, intermittent claudication, inability to walk outside the home prior to the stroke, or orthopedic problems of the lower extremities or spine that limited walking. In addition, those with a coronary artery bypass graft or myocardial infarction within 3 months, or unexplained dizziness within 6 months of study participation were excluded.
Instrumentation and Procedures
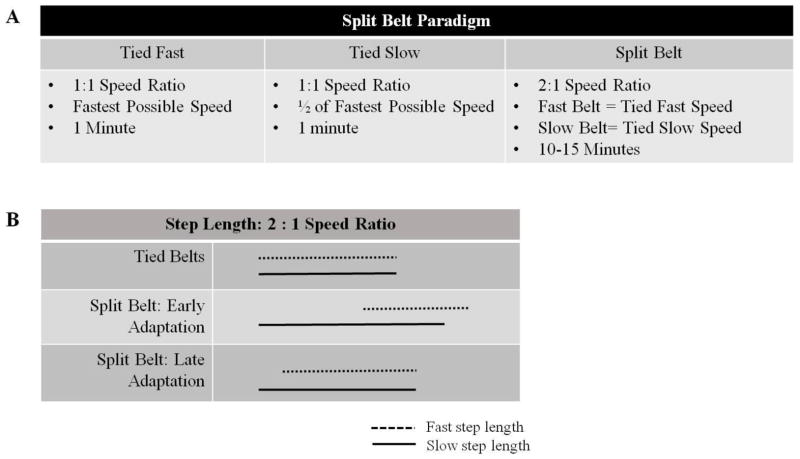
All subjects participated in a single session of split-belt treadmill walking in which the belts were set to a 2:1 speed ratio. Prior to split-belt treadmill walking, subjects were asked to walk on the treadmill with the belts tied at a 1:1 ratio at their fastest speed possible for 1 minute, followed by a speed half of their fastest possible for 2 minutes in order to assess baseline step and limb phase asymmetry (Fig. 1). To achieve the subject’s fastest possible speed the treadmill was increased by 0.1 m/s until the subject reported inability to tolerate a further increase or the researcher felt the subject would be unsafe at a faster speed. All subjects participated in split-belt treadmill walking for 10 to 15 minutes, consisting of walking at a constant 2:1 speed ratio (Fig. 1). Prior to Split Belt treadmill walking subjects were asked to walk 5–10 times on a 13 foot long instrumented walkway (GaitMat II™ E.Q. Inc., Chalfont, PA) that measures basic gait characteristics including step length. Step length of the hemiparetic and contralateral leg was examined for the leg taking the longer step. The leg with the greater step length was placed over the slower belt in order to provide a split-belt configuration that would exaggerate baseline step length asymmetry(Reisman et al., 2013, 2007; Tyrell, Helm, & Reisman, 2014). The fast belt speed was set to the subject’s fastest walking speed achieved on the treadmill and the slow belt was set to half of this speed. Subjects ambulated with this speed ratio throughout the entire session.
Fig. 1.
A Split-belt paradigm. B Schematic of a typical response of a neurologically intact subject to the split-belt paradigm. Dashed lines represent the fast leg step length and solid lines represent the slow leg step length for both the Tied Belt and Split Belt conditions. With the tied belt condition both slow and fast leg step lengths are similar. Asymmetry between the fast and slow leg step length is noted in early adaptation, with reduction in step length asymmetry during late adaptation.
All participants walked on a split-belt treadmill instrumented with two independent six degree of freedom force platforms (AMTI, Watertown, MA) from which ground reaction force data was continuously collected at 1000Hz. Kinematic data was continuously collected using an 8-camera Vicon Motion Capture System (Vicon MX, Los Angeles, CA) at 100Hz. Retro-reflective markers (14-mm diameter) secured to rigid plastic shells were placed on the pelvis, bilateral thighs and bilateral shanks. Single markers were placed on the most prominent superior portion of the bilateral iliac crests, greater trochanters, medial and lateral knee joint lines, medial and lateral malleoli, bilateral heels, and the first and fifth metatarsal heads. During walking all subjects were instructed to gently rest fingertips on the treadmill handrail, and were given verbal cues, as necessary, to avoid excessive use of the handrail while walking. Subjects were instructed to maintain fingertip contact on the handrail throughout all treadmill walking.
All subjects wore a safety harness around their chest for fall prevention; however the harness did not provide body weight support. Blood pressure, heart rate and rating of perceived exertion (RPE) (Borg, 1982) were monitored throughout the treadmill walking session and subjects were provided with optional standing or sitting rest breaks. During optional rest breaks, subjects were not permitted to dismount from the treadmill.
Genotyping
Each subject provided a 2 mL saliva sample in a DNA Self-Collection Kit (DNA Genotek, Kanata, Canada) containing a DNA stabilizing buffer. The samples were sent to DNA Genotek (GenoFIND Services, Salt Lake City, UT) for processing. Genotek created a set of primers to amplify the region surrounding the SNP (Val66Met: rs6265) of the BDNF gene and then examined the sample for the presence or absence of the Val66Met polymorphism. Extracted DNA results of genotyping were sent to the primary investigator with remaining saliva samples destroyed following analysis.
Data Analysis
All kinematic and kinetic data were exported from Vicon-Nexus software, and further processed using Visual 3D (C-Motion, Inc, Germantown) and Matlab (MathWorks, Natick, MA). Gait events of foot strike and lift off were determined for each limb individually using an automatic algorithm in Visual 3D. Foot strike was identified when the vertical ground reaction force exceeded 20 Newtons for at least 8 frames, and lift-off identified when the vertical ground reaction force dropped below 20 Newtons for at least 8 frames. All gait events were visually checked for accuracy.
Dependent variables
Spatial and temporal parameters of gait have been found to respond differently during split-belt walking (Malone & Bastian, 2010; Malone, Vasudevan, & Bastian, 2011; Tyrell et al., 2014). Therefore, both spatial (step length) and temporal (limb phasing) variables were evaluated. Both variables were calculated for each leg continuously throughout treadmill walking. The spatiotemporal measure of step length was calculated as the sagittal distance between the right and left heel markers at foot strike. Step length was labeled as Left or Right based on leading leg. Stride by stride symmetry data for step length was calculated as:
Where symmetrical step length = (paretic step length + non-paretic step length)/2 (Tyrell et al., 2014; Tyrell, Helm, & Reisman, 2015).
Based on the above calculations, a value of 0 would indicate that the subject has achieved perfect symmetry based on their individual stride length. A negative value denotes the leg on the slow belt has a decreased step length relative to perfect symmetry. This method is preferred over the calculation of a ratio (paretic/non-paretic) because it prevents extremely large values when the denominator of the ratio is small due to a “step to” gait pattern in which one leg does not pass the other leg (Patterson, Gage, Brooks, Black, & McIlroy, 2010).
The temporal measure of limb phasing was calculated as previously reported (Tyrell et al., 2014, 2015). Briefly, a calculation of limb phase for each leg provides a measure of the difference in time between the contralateral limb’s peak flexion and the ipsilateral limb’s peak extension, normalized by the ipsilateral limb’s stride duration. Stride-by-stride limb phase symmetry was calculated by dividing the limb phase value for the leg on the slow belt by the contralateral limb phase value.
Locomotor adaptation to the split-belt treadmill paradigm, through trial and error practice, has previously been well characterized (Reisman et al., 2010, 2005, 2007). By splitting the treadmill belts in a 2:1 ratio, the split-belt paradigm requires both neurologically intact, and subjects post-stroke to alter their coordination while walking (Reisman et al., 2005, 2007). Initially characteristics of gait symmetry, including limb phasing and step length, are altered, however over a period of ten to fifteen minutes this asymmetry is reduced through use of trial and error practice (Reisman et al., 2005, 2007), Fig 1B.
To evaluate differences in locomotor adaptation in those with (MET) and without (VAL) the Val66Met polymorphism we examined: the rate of adaptation, the magnitude of total adaptation, and return to baseline. Calculations were performed for both step and limb phase symmetry.
Rate of adaptation
For each variable the rate of adaptation was calculated by first removing baseline asymmetry from each raw symmetry value to provide a value that reflects the deviation from the individual’s baseline asymmetry pattern (Tyrell et al., 2014, 2015; Vasudevan, Torres-Oviedo, Morton, Yang, & Bastian, 2011). Subtraction of the baseline asymmetry pattern allows for comparison of data across subjects who may demonstrate different levels of baseline asymmetry. A value of 0 reflects a pattern identical to baseline asymmetry.
In order to account for individual differences in the initial asymmetry at the start of the split-belt paradigm (initial perturbation) individual stride data was normalized by initial perturbation (Vasudevan et al., 2011). Normalization was achieved by dividing each symmetry value by the initial perturbation value, where initial perturbation was defined as the average of the first 3 strides during adaptation (Vasudevan et al., 2011). This normalization allows individual subject data to be scaled to a proportion of the initial perturbation (Vasudevan et al., 2011). Individual stride data, was separated into “Early” and “Late” adaptation. A value of 30 strides was selected to represent Early adaptation for step length asymmetry. Previous literature indicates that adaptation to limb phase asymmetry occurs on a much shorter timescale than step length adaptation (Malone & Bastian, 2010; Tyrell et al., 2014). In order to accurately capture rapid adjustments in limb phase asymmetry we utilized the first 10 strides to assess Early adaptation for limb phase. Late adaptation for both step and limb phase asymmetry were represented by the last 100 strides of adaptation for each individual subject.
Group (presence vs. absence of Val66Met polymorphism) averages of stride by stride data for Early and Late adaptation were then compared through a linear regression.
Magnitude of total adaptation
To evaluate the total amount of adaptation for both step length and limb phase symmetry during split-belt treadmill walking, the magnitude of total adaptation was calculated as follows:
This calculation represents the difference between the asymmetry pattern utilized at the start of adaptation and the asymmetry pattern utilized at the end of adaptation. A larger positive number would indicate a larger amount of adaptation.
Return to baseline
To assess whether subjects were able to fully adapt back to their baseline asymmetry, the amount of adaptation relative to their individual baseline was calculated as follows:
This calculation represents the difference between the asymmetry pattern achieved at the end of adaptation and the subject’s baseline asymmetry pattern with the belts tied at a 1:1 speed ratio. A value of 0 would indicate the subject has completely adapted to the split-belt treadmill and has returned back to their baseline asymmetry pattern, despite the continued split-belts.
Statistical Analysis
All statistical analyses were completed with SPSS v22. In order to test our hypothesis that subjects with the polymorphism (Met) would demonstrate a slowed rate of adaptation compared to those without the polymorphism (Val), a linear regression was performed for Early and Late adaptation separately. In the regression analyses, the n would then be the number of steps. To ensure that averaging across individuals for adaptation within a group was appropriate, each individual’s stride data over time was examined for the nature of the relationship using a modified Box-Cox test for linearity (Draper, 1998). To ascertain the appropriate use of a linear model (Osborne, 2010) an apriori decision was made to utilize group data when greater than 70% of subjects within each group (Val vs. Met) met the criteria for a linear relationship. A linear relationship was defined as 95% confidence interval around the Box-Cox lambda containing one. Moderated regression was then used to test if the relationship between the change in asymmetry and stride number during both Early and Late adaptation differed with presence or absence of the polymorphism. Normality of the data was assessed using Kolmogorov-Smirnov test.
To test our hypothesis that subjects with the polymorphism would demonstrate a reduced Magnitude of Total Adaptation and Return to baseline, group differences (presence vs. absence of Val66Met polymorphism) were assessed utilizing an ANCOVA, with the initial perturbation value as the covariate to adjust for individual differences in the initial perturbation (Vasudevan et al., 2011).
Results
A total of twenty seven participants, 11 with the polymorphism (67.75+/− 9.5 yr) and 16 without the polymorphism (67.0+/− 6.7 yr), participated in this study (Table 1). There were no significant differences in baseline demographics or clinical scores between subjects with and without the polymorphism (all p > 0.05, Table 2). Subjects with and without the polymorphism also did not differ in amount of initial perturbation to the split belt treadmill for step length and limb phase asymmetry (p=.312 and p=.187 respectively).
Table 1.
Subject Characteristics
| Subject ID | Location of Stroke | Type of Stroke | Gender | Polymorphism |
|---|---|---|---|---|
| S1 | R pons | Ischemic | M | MET |
| S110 | R ICA | Ischemic | F | MET |
| S115 | L pons and medulla | Ischemic | M | MET |
| S136 | L frontal and temporal lobes | Hemorrhagic | M | MET |
| S155 | R MCA | Ischemic | M | MET |
| S61 | R MCA | Ischemic | M | MET |
| S138 | R MCA | Ischemic | M | MET |
| S192 | L MCA ACA | Ischemic | F | MET |
| S196 | L fronto-parietal region | Ischemic | F | MET |
| S294 | Medial upper pons, L lower midbrain | Ischemic | F | MET |
| S425 | R MCA | Ischemic | M | MET |
| S14 | L posterior temporal, parietal lobe | Ischemic | M | VAL |
| S53 | R ICA and MCA | Ischemic | M | VAL |
| S67 | R MCA | Ischemic | M | VAL |
| S142 | R MCA | Ischemic | F | VAL |
| S171 | R frontal lobe | Ischemic | M | VAL |
| S128 | L MCA | Ischemic | F | VAL |
| S177 | R cerebral intraparenchymal | Hemorrhagic | M | VAL |
| S186 | R pontine basis | Hemorrhagic | F | VAL |
| S187 | R MCA | Ischemic | F | VAL |
| S194 | R frontal lobe | Ischemic | F | VAL |
| S200 | R MCA | Hemorrhagic | M | VAL |
| S241 | R frontal, temporal, parietal lobes | Ischemic | F | VAL |
| S265 | R Pons | Ischemic | M | VAL |
| S383 | L MCA | Ischemic | F | VAL |
| S575 | R parietal, corona radiata, and putamen | Ischemic | M | VAL |
| S447 | R temporal and parietal lobes | Hemorrhagic | F | VAL |
Table 2.
Group Demographics
| VAL66VAL (n=16) | VAL66Met (n=11) | |
|---|---|---|
| Age (years) (mean±std) | 65.10 ± 9.26 | 61.45 ± 6.19 |
| Time since Stroke (months) (mean±std) | ||
| Time since Stroke (months) (mean±std) | 43.8 ± 42.45 | 32.18 ± 21.9 |
| Total Fugl Meyer (mean±std) | 22.50 ± 4.62 | 23.30 ± 5.30 |
| Fast speed on treadmill (m/s) (mean±std) | 0.64± 0.24 | 0.78 ± 0.08 |
| Baseline Step Length (a)symmetry (m) (mean±std) | 0.14 ± 0.15 | 0.07 ± 0.08 |
| Baseline Limb Phase (a)symmetry (m) (mean±std) | 0.90 ± 0.24 | 0.90 ± 0.20 |
Rate of Adaptation
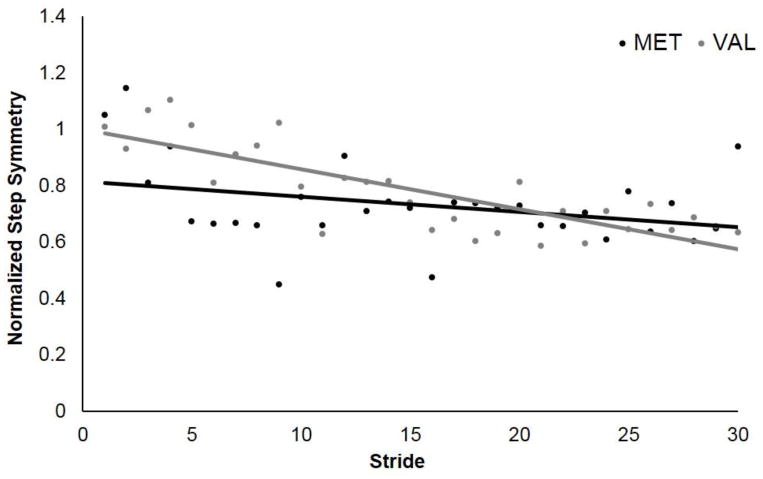
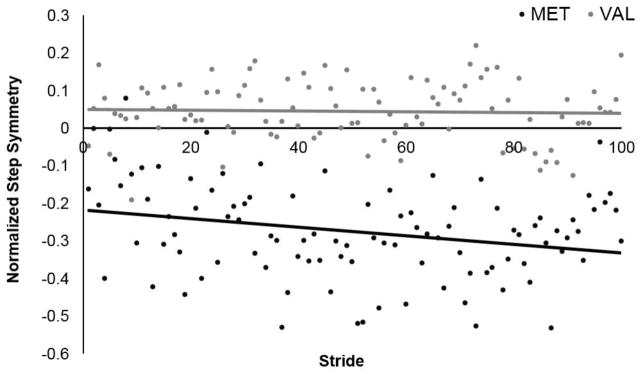
Figures 2 and 3 illustrate the pattern of changes in step length asymmetry with exposure to the split-belt treadmill in the group of participants with (MET) and without (VAL) the Val66Met polymorphism. When the treadmill belt speeds are set to a 2:1 speed ratio with the paretic leg walking on the slow belt and non-paretic leg walking on the fast belt, both groups of subjects (Val and Met) demonstrate an increase in step length asymmetry relative to their baseline. With a period of trial and error practice (“Adaptation”), both groups demonstrate the ability to reduce this asymmetry despite the belts still moving at a 2:1 speed ratio. The two groups, however, demonstrate two divergent patterns of adaptation. Those with the polymorphism (MET) demonstrate a slowed rate of initial adaptation relative to those without the polymorphism (VAL) (Fig. 2). In addition, those with the polymorphism (MET) continue to adapt their step length asymmetry throughout adaptation, while those without the polymorphism (VAL) appear to plateau near their baseline symmetry (Fig. 3).
Fig. 2.
Early Adaptation. Group averaged stride by stride data for normalized step asymmetry over the first 30 strides of adaptation for VAL (gray) and MET (black) subjects. Each data point represents the average of the individual step length asymmetry value per group for each stride.
Fig. 3.
Late Adaptation. Group averaged stride by stride data for normalized step asymmetry over the last 100 strides of adaptation for VAL (gray) and MET (black) subjects. Each data point represents the average of the individual step length asymmetry value per group for each stride.
These qualitative results are supported by the quantitative data. The results of the linear regression show that those with the polymorphism (MET) demonstrate a slowed rate of step length adaptation relative to those without the polymorphism (VAL) within Early adaptation (Fig. 2; p=0.000). In the first block, Group (presence vs. absence of the polymorphism) and Stride (group step symmetry values for each of the first 30 strides) were able to predict change in asymmetry within Early adaptation (R2=0.331; p= 0.000; Table 2). Addition of the interaction term (Group x Stride) significantly improved the model (ΔR2=0.108; p=0.002; Table 2), indicating that the groups differed in how step length asymmetry was reduced over time. For Late adaptation, the first block of Group and Stride, predicted change in asymmetry (R2 =0.711; p= 0.000; Table 3). Addition of the interaction term (Group x Stride) improved the model (ΔR2 =.006; p=0.041; Table 3), indicating a small difference in the relationship between stride and asymmetry for VAL and MET subjects. In contrast to rate of step length adaptation, there were no differences in the rate of limb phase adaptation for those with and without the polymorphism.
Table 3.
Sequential linear regression model predicting change in asymmetry over the first 30 strides (Early adaptation) for those with (MET) and without (VAL) the polymorphism.
| Model # | Predictors | Model p | ΔR2 | ΔR2 p |
|---|---|---|---|---|
| 1 | Group Stride |
.000 | .331 | .000 |
| 2 | Group Stride Group x Stride |
.000 | .108 | .002 |
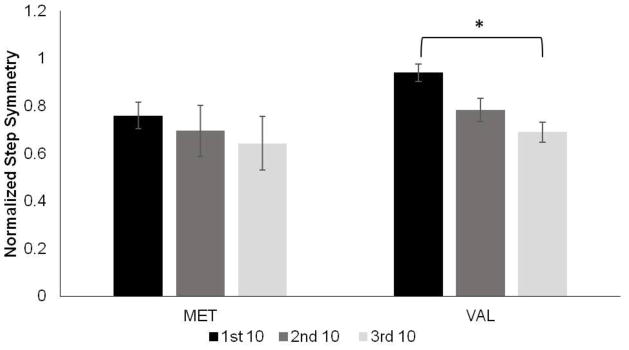
Given the significant group differences in the rate of Early step length adaptation, it was important to examine how the groups may differ in the pattern of this adaptation. To assess differences in the pattern of Early step length adaptation between those with and without the polymorphism, stride by stride symmetry data were group averaged as the first, second, and third 10 symmetry values within the first 30 strides (Fig. 4). Each group of ten symmetry values within the first 30 strides were then compared using a repeated measures ANOVA for each group individually. Those without the polymorphism (VAL) demonstrated a significant decrease in step length asymmetry within the first 30 strides, while those with the polymorphism (MET) did not (Fig. 4; p= 0.015 and p=0.522, respectively).
Fig. 4.
Early Adaptation. Group step symmetry data for the first, second and third ten step symmetry values within the first 30 strides. *p=0.015
Magnitude of Adaptation
Despite a slowed rate of step-length adaptation, those with the polymorphism did not demonstrate a reduced magnitude of total adaptation relative to those without the polymorphism. The groups did not differ significantly in the total amount of adaptation for step length or limb phase (Table 4). In addition, subjects with the polymorphism were able to achieve a magnitude of step and limb phase asymmetry relative to baseline (return to baseline) that did not differ significantly from that achieved by those without the polymorphism (Table 4).
Table 4.
Sequential linear regression model predicting change in asymmetry over the last 100 strides (Late adaptation) for those with (MET) and without (VAL) the polymorphism.
| Model # | Predictors | Model p | ΔR2 | ΔR2 p |
|---|---|---|---|---|
| 1 | Group Stride |
.000 | .711 | .000 |
| 2 | Group Stride Group x Stride |
.000 | .006 | .041 |
Discussion
The results of this study demonstrate that chronic stroke survivors, regardless of presence or absence of the BDNF Val66Met polymorphism, are able to adapt their walking pattern over a period of trial and error practice, however the presence of the polymorphism influences the rate at which this is achieved. Specifically, our results suggest that the process of modifying a spatial parameter of gait to a novel locomotor task is slowed in those with the polymorphism. The current study provides a crucial first step in identifying mechanisms of neural plasticity and motor learning that may influence the response to rehabilitation interventions. In particular the current results identify a population that may benefit from increased practice or differing practice parameters to facilitate optimal motor learning and identifies a potential biomarker for individualization of rehabilitation post-stroke.
Within the current study chronic stroke survivors with the BDNF Val66Met polymorphism demonstrated a slowed rate of step length adaptation to a novel locomotor task, however were able to achieve a similar amount of total adaptation relative to those without the polymorphism. Similar findings have been reported within a visuomotor adaptation task in neurologically intact individuals (Joundi et al., 2012). Specifically, Joundi et al. found that neurologically intact participants with the polymorphism demonstrated a decreased rate of adaptation to a 60 degree visuomotor deviation during skill acquisition as well as during a 24 hour retention test. The subjects, however, did not differ significantly in mean error at the end of adaptation, indicating subjects with and without the polymorphism had similar levels of total adaptation (Joundi et al., 2012). Together these findings indicate that subjects with and without stroke with the BDNF Val66Met polymorphism are able overcome slowed adaptation with repetition. This is in line with previous evidence showing that intense training on a marble navigation task can overcome deficits in motor map plasticity in those with the polymorphism (McHughen et al., 2011).
How are subjects with the polymorphism able to achieve similar amounts of total step length adaptation despite differing rates of step length adaptation? As shown in Figure 1, subjects with the polymorphism show a subtle, continued reduction in step length asymmetry over the last 100 strides, while subjects without the polymorphism show no change. While the results of the regression confirm these differences (Table 3), the change in R2 is very small and would generally not be considered meaningful. Nevertheless, it appears that these small changes in late adaptation in those with the polymorphism allowed them to achieve similar amounts of total step length adaptation as those without the polymorphism. The contrasting behavioral patterns during both Early and Late adaptation, may be due in part to a deficit in error processing in those with the Val66Met polymorphism (Beste et al., 2010). When performing a stimulus-response flanker task, neurologically intact subjects with the polymorphism demonstrated a decreased neural response to error and thereby a lessened behavioral response (Beste et al., 2010). It is plausible that decreased error recognition in those with the polymorphism limited the drive to detect and reduce the “error” signal induced through the exaggeration of step length asymmetry in the current study. This lack of drive may have been demonstrated behaviorally through a reduced rate of adaptation. If present, this reduced error recognition could also limit the ability to plateau at one’s previous baseline. Although not significant in the current study, qualitatively, subjects with the polymorphism appear to continue past their baseline asymmetry (Fig. 3). This is an important concept for those with chronic stroke whose nervous system may no longer perceive gait deviations as errors, and require an exaggeration of this error in order to make a correction. As such, the polymorphism may present an additional obstacle for motor learning in those post-stroke.
Early adaptation represents a period of rapid adjustment to the split-belt perturbation for both step length and limb phase asymmetry while late adaptation is typically characterized by a plateau in adaptation near the individual’s baseline gait pattern. In the current study, individuals with the polymorphism (MET) showed a significantly slowed rate of adaptation within the early phase of adaptation, while in the late phase continued to demonstrate adaptation to the walking task. It is plausible the differences in adaptation to the split belt paradigm may be due to limitations in the ability to exploit mechanisms of long term potentiation through BDNF. Evidence within an animal model suggests that the polymorphism leads to an altered trafficking of intracellular BDNF within the CNS, resulting in reduced activity dependent secretion of the mature protein(Chen et al., 2004; Egan et al., 2003). Reduction in the amount of BDNF secreted may dampen the potential for excitability of the post-synaptic cell and thus reduce early long term potentiation. This may be demonstrated behaviorally through an inability to quickly reduce the gait asymmetry induced through the split belt treadmill. The polymorphism, however, does not attenuate the regulated release of mature BDNF, therefore the nervous system retains the ability to engage in long term potentiation, however may require increased stimulation over a longer duration to facilitate early and late LTP(Carvalho, Caldeira, Santos, & Duarte, 2008). This may be characterized behaviorally through an ability to achieve a magnitude of adaptation similar to participants without the polymorphism (VAL) through continued trial and error practice.
The current results demonstrate deficits in the rate of adaptation to spatial (step length) but not temporal (limb phase) parameters of gait, in those with the polymorphism. This discrepancy between the spatial and temporal variables is not entirely surprising. Differences in adaptation rates of temporal versus spatial characteristics of gait have been previously demonstrated in neurologically intact subjects (Malone & Bastian, 2010) and in those with stroke (Tyrell et al, 2014). Temporal characteristics of gait appear to be much more resistant to manipulations of practice structure(Malone & Bastian, 2010; Malone et al., 2011) as well as influenced by developmental stage (Vasudevan et al., 2011). In addition, a previous study of subjects with chronic stroke adapting to the split-belt treadmill showed a slowed rate of adaptation compared to neurologically intact subjects for step length, but not for limb phase (Tyrell et al, 2014). These differences in the adaptation of temporal versus spatial gait characteristics have been postulated to occur because different neural structures are involved in these two control processes, (Malone et al., 2011; Torres-Oviedo, Vasudevan, Malone, & Bastian, 2011). Specifically it has be suggested that spatial control may be more readily accessed using cerebral resources whereas temporal control may occur at a lower level in the nervous system, such as the brainstem or spinal cord. For further review see Torres-Oviedo, Vasudevan, Malone, & Bastian, 2011. As such, temporal characteristics may be more resistant to manipulation with the split-belt paradigm, regardless of presence or absence of the polymorphism.
Within the current paradigm chronic stroke subjects demonstrated a slowed rate of step length adaptation with continued use of trial and error practice throughout treadmill walking. It is currently unknown if providing additional practice would result in a plateau in adaptation in the subjects with the polymorphism. In a recent study of longer-term learning of the split-belt walking pattern, it was shown that although those with chronic stroke took an additional day of practice to reach a stable plateau in learning, compared to neurologically intact controls, they were able to learn the pattern with this additional practice (Tyrell et al., 2014). It may be that chronic stroke survivors with the BDNF Val66Met polymorphism require even more practice, or different practice parameters, to achieve longer term learning of a novel walking pattern. Additional studies in the post-stroke population are needed to better understand the impact of the BDNF polymorphism on post-stroke motor learning and the interaction of the polymorphism on various rehabilitation interventions and outcomes.
Limitations
The current paradigm demonstrates a slowed rate of adaptation to a novel locomotor task in individuals with chronic stroke who have the Val66Met polymorphism. It is currently unknown however if the polymorphism would confer the same results in the acute or subacute stroke population. In addition, the current study, with a small sample size, lacks gender and age matching. Although not significant, the difference in the time since stroke for those with (MET) and without the polymorphism (VAL) cannot be discounted as a possible source of bias given previous literature suggesting null or beneficial effects of the Val66Met polymorphism with increasing age (McHughen et al, 2013, Gajewski et al., 2011). Additional studies at various time points of post-stroke recovery are needed to better understand the impact of the BDNF polymorphism on post-stroke motor learning.
Conclusions
The goal of this study was to examine the role of a single nucleotide polymorphism (SNP) in the BDNF gene in moderating motor learning post-stroke. To our knowledge this is first study to address the role of BDNF in motor learning post-stroke. The results suggest that chronic stroke survivors, regardless of presence or absence of the polymorphism, are able to adapt their walking pattern over a period of trial and error practice. The process of locomotor adaptation, however, is slowed in those with the Val66Met polymorphism. These results have important implications for motor learning and rehabilitation post-stroke because they identify a population (those with the polymorphism) that may benefit from increased practice or differing practice parameters to facilitate optimal motor learning. In addition, the current results identify a potential biomarker that may be utilized to further individualize treatment approaches within rehabilitation post-stroke.
Table 5.
Non significant step and limb phase symmetry variables. Average and standard deviation for Total Adaptation (average of first 10 symmetry values - last 10 symmetry values) and Return to Baseline (average of last 10 symmetry values - baseline symmetry values).
| Step Symmetry (m) | ||
|---|---|---|
| Total Adaptation | Return to Baseline | |
| VAL | 0.19± 0.11 | 0.02 ± 0.10 |
| MET | 0.15 ± 0.08 | −0.02 (+/− 0.08) |
| Limb Phase Symmetry | ||
| Total Adaptation | Return to Baseline | |
| VAL | 0.27 ± 0.20 | 0.20 ± 0.25 |
| MET | 0.31 ± 0.24 | 0.15 ± 0.13 |
Acknowledgments
Grants: National Institutes of Health: Research Project Grant (R01) R01 HD078330-01A1 and 2T32HD007490-16
Footnotes
Disclosures: No conflicts of interest, financial or otherwise, are declared by the author(s)
References
- Barton B, Treister A, Humphrey M, Abedi G, Cramer SC, Brewer AA. Paradoxical visuomotor adaptation to reversed visual input is predicted by BDNF Val66Met polymorphism. Journal of Vision. 2014;14(9) doi: 10.1167/14.9.4. http://doi.org/10.1167/14.9.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Lee FS. Variant BDNF (Val66Met) impact on brain structure and function. Cognitive, Affective, & Behavioral Neuroscience. 2006;6(1):79–85. doi: 10.3758/cabn.6.1.79. http://doi.org/10.3758/CABN.6.1.79. [DOI] [PubMed] [Google Scholar]
- Beste C, Kolev V, Yordanova J, Domschke K, Falkenstein M, Baune BT, Konrad C. The role of the BDNF Val66Met polymorphism for the synchronization of error-specific neural networks. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2010;30(32):10727–33. doi: 10.1523/JNEUROSCI.2493-10.2010. http://doi.org/10.1523/JNEUROSCI.2493-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. International Journal of Sports Medicine. 1982;3(3):153–8. doi: 10.1055/s-2008-1026080. http://doi.org/10.1055/s-2008-1026080. [DOI] [PubMed] [Google Scholar]
- Carvalho aL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. British Journal of Pharmacology. 2008;153(Suppl December 2007):S310–24. doi: 10.1038/sj.bjp.0707509. http://doi.org/10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Rothwell JC. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. The Journal of Physiology. 2008;586(Pt 23):5717–25. doi: 10.1113/jphysiol.2008.159905. http://doi.org/10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2004;24(18):4401–11. doi: 10.1523/JNEUROSCI.0348-04.2004. http://doi.org/10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo J, Hughes J, Ridding M, Thomas PQ, Semmler JG. Differential modulation of motor cortex excitability in BDNF Met allele carriers following experimentally induced and use-dependent plasticity. The European Journal of Neuroscience. 2012;36(5):2640–9. doi: 10.1111/j.1460-9568.2012.08177.x. http://doi.org/10.1111/j.1460-9568.2012.08177.x. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Weinberger DR. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. http://doi.org/10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Hengstler JG, Golka K, Falkenstein M, Beste C. The Met-allele of the BDNF Val66Met polymorphism enhances task switching in elderly. Neurobiology of Aging. 2011;32(12):2327 e7–19. doi: 10.1016/j.neurobiolaging.2011.06.010. http://doi.org/10.1016/j.neurobiolaging.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Turner MB. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. http://doi.org/10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer KA, Berchtold NC, Malvaez M, Carlos AJ, McQuown SC, Cunningham MJ, Cotman CW. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2013;38(10):2027–34. doi: 10.1038/npp.2013.104. http://doi.org/10.1038/npp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joundi RA, Lopez-Alonso V, Lago A, Brittain JS, Fernandez-Del-Olmo M, Gomez-Garre P, Brown P. The effect of BDNF val66met polymorphism on visuomotor adaptation. Experimental Brain Research. 2012;223(1):43–50. doi: 10.1007/s00221-012-3239-9. http://doi.org/10.1007/s00221-012-3239-9. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nature Neuroscience. 2006;9(6):735–7. doi: 10.1038/nn1699. http://doi.org/10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. Journal of Speech, Language, and Hearing Research : JSLHR. 2008;51(1):S225–39. doi: 10.1044/1092-4388(2008/018). http://doi.org/10.1044/1092-4388(2008/018) [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Holthoff K, Konnerth A. Neurotrophin action on a rapid timescale. Current Opinion in Neurobiology. 2004;14(5):558–63. doi: 10.1016/j.conb.2004.08.014. http://doi.org/10.1016/j.conb.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Li Voti P, Conte A, Suppa A, Iezzi E, Bologna M, Aniello MS, Berardelli A. Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Experimental Brain Research. 2011;212(1):91–9. doi: 10.1007/s00221-011-2700-5. http://doi.org/10.1007/s00221-011-2700-5. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiology of Learning and Memory. 2008;89(3):312–23. doi: 10.1016/j.nlm.2007.08.018. http://doi.org/10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. Journal of Neurophysiology. 2010;103(4):1954–62. doi: 10.1152/jn.00832.2009. http://doi.org/10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Vasudevan EVL, Bastian AJ. Motor adaptation training for faster relearning. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2011;31(42):15136–43. doi: 10.1523/JNEUROSCI.1367-11.2011. http://doi.org/10.1523/JNEUROSCI.1367-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHughen SA, Cramer SC. The BDNF val(66)met polymorphism is not related to motor function or short-term cortical plasticity in elderly subjects. Brain Research. 2013;1495:1–10. doi: 10.1016/j.brainres.2012.12.004. http://doi.org/10.1016/j.brainres.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHughen SA, Pearson-Fuhrhop K, Ngo VK, Cramer SC. Intense training overcomes effects of the Val66Met BDNF polymorphism on short-term plasticity. Experimental Brain Research. 2011;213(4):415–22. doi: 10.1007/s00221-011-2791-z. http://doi.org/10.1007/s00221-011-2791-z. [DOI] [PubMed] [Google Scholar]
- McHughen SA, Rodriguez PF, Kleim JA, Kleim ED, Marchal Crespo L, Procaccio V, Cramer SC. BDNF val66met polymorphism influences motor system function in the human brain. Cerebral Cortex (New York, NY: 1991) 2010;20(5):1254–62. doi: 10.1093/cercor/bhp189. http://doi.org/10.1093/cercor/bhp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nature Reviews. Neuroscience. 2009;10(12):861–72. doi: 10.1038/nrn2735. http://doi.org/10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histology and Histopathology. 2010;25(2):237–58. doi: 10.14670/HH-25.237. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20017110. [DOI] [PubMed] [Google Scholar]
- Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait & Posture. 2010;31(2):241–6. doi: 10.1016/j.gaitpost.2009.10.014. http://doi.org/10.1016/j.gaitpost.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2004;24(45):10099–102. doi: 10.1523/JNEUROSCI.2680-04.2004. http://doi.org/10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploughman M, Windle V, MacLellan CL, White N, Doré JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke; a Journal of Cerebral Circulation. 2009;40(4):1490–5. doi: 10.1161/STROKEAHA.108.531806. http://doi.org/10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Bastian AJ, Morton SM. Neurophysiologic and rehabilitation insights from the split-belt and other locomotor adaptation paradigms. Physical Therapy. 2010;90(2):187–95. doi: 10.2522/ptj.20090073. http://doi.org/10.2522/ptj.20090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? Journal of Neurophysiology. 2005;94(4):2403–15. doi: 10.1152/jn.00089.2005. http://doi.org/10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabilitation and Neural Repair. 2013;27(5):460–8. doi: 10.1177/1545968312474118. http://doi.org/10.1177/1545968312474118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain : A Journal of Neurology. 2007;130(Pt 7):1861–72. doi: 10.1093/brain/awm035. http://doi.org/10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäbitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, Sommer C. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke; a Journal of Cerebral Circulation. 2004;35(4):992–7. doi: 10.1161/01.STR.0000119754.85848.0D. http://doi.org/10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- Schäbitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, Kuhn HG. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke; a Journal of Cerebral Circulation. 2007;38(7):2165–72. doi: 10.1161/STROKEAHA.106.477331. http://doi.org/10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics : The Official Publication of the International Society of Psychiatric Genetics. 2004;126B(1):122–3. doi: 10.1002/ajmg.b.20118. http://doi.org/10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Vasudevan E, Malone L, Bastian AJ. Locomotor adaptation. Progress in Brain Research. 2011;191:65–74. doi: 10.1016/B978-0-444-53752-2.00013-8. http://doi.org/10.1016/B978-0-444-53752-2.00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrell CM, Helm E, Reisman DS. Learning the spatial features of a locomotor task is slowed after stroke. Journal of Neurophysiology. 2014;112(2):480–9. doi: 10.1152/jn.00486.2013. http://doi.org/10.1152/jn.00486.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrell CM, Helm E, Reisman DS. Locomotor adaptation Is influenced by the interaction between perturbation and baseline asymmetry after stroke. Journal of Biomechanics. 2015 doi: 10.1016/j.jbiomech.2015.04.027. http://doi.org/10.1016/j.jbiomech.2015.04.027. [DOI] [PMC free article] [PubMed]
- Vasudevan EVL, Torres-Oviedo G, Morton SM, Yang JF, Bastian AJ. Younger is not always better: development of locomotor adaptation from childhood to adulthood. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2011;31(8):3055–65. doi: 10.1523/JNEUROSCI.5781-10.2011. http://doi.org/10.1523/JNEUROSCI.5781-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. The European Journal of Neuroscience. 2004;20(10):2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. http://doi.org/10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155(4):1070–8. doi: 10.1016/j.neuroscience.2008.06.057. http://doi.org/10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Developmental Neurobiology. 2010;70(5):304–22. doi: 10.1002/dneu.20765. http://doi.org/10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]