Abstract
Objective
In the present study, we aimed to evaluate the effects of high doses of dexamethasone (DEX) in early pregnancy on pregnancy outcomes.
Methods
Pregnant BALB/c mice were treated with high-dose DEX in the experimental group or saline in the control group on gestational days (GDs) 0.5 to 4.5. Pregnant mice were sacrificed on GDs 7.5, 13.5, or 18.5 and their peripheral blood, placentas, fetuses, and uterine tissue were collected. Decidual and placenta cell supernatants were examined to evaluate the effect of DEX on the proliferation of mononuclear cells, the quantity of uterine macrophages and uterine natural killer (uNK) cells, and levels of progesterone and 17β-estradiol, as determined by an 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide assay, immunohistochemistry, and enzyme-linked immunosorbent assay, respectively. We also were measured fetal and placental growth parameters on GD 18.5.
Results
We found that high doses of DEX were associated with an increased abortion rate, enhancement of the immunosuppressive effect of the decidua, alterations in placental growth parameters, decreased progesterone and 17β-estradiol levels, and a reduced frequency of macrophages and uNK cells.
Conclusion
Our data suggest that the high-dose administration of DEX during early pregnancy negatively affected pregnancy outcomes.
Keywords: Abortion, Dexamethasone, Estradiol, Macrophage, Natural killer cell, Pregnancy, Progesterone
Introduction
Pregnant women with obstetric complications may take synthetic glucocorticoids (GCs), such as betamethasone, prednisolone, methylprednisolone, and dexamethasone (DEX) during pregnancy. Common indications for GC administration during pregnancy include prophylaxis against refractory nausea and vomiting associated with pregnancy [1], prevention of preterm labor [2], the treatment of asthma [3] and autoimmune diseases [4], and improvement of pregnancy outcomes in women with a history of recurrent miscarriage [5].
Similarly to all drugs, GCs can have negative side effects, and the degree to which they occur is usually dose-dependent. Thus, GCs are riskier when used at larger total daily doses and over longer treatment periods. Previous studies have shown long-term GC use to be associated with immunosuppression and increased risks of infection, hyperglycemia, hypertension, Cushing syndrome, osteoporosis, and electrolyte disturbances [6]. Furthermore, an increasing body of data indicates that the increased exposure of fetuses to GCs in mid- to late pregnancy may result in adverse outcomes, such as intrauterine growth restriction, postnatal hypertension, increased postnatal activity of the hypothalamo-pituitary-adrenal axis, and an increased risk of pre-term labor [7]. The use of GCs during pregnancy is controversial. Although pharmacological monographs have indicated GCs to be relatively safe in pregnancy (safety category B), animal studies have indicated the possibilities of long-term developmental abnormalities [8,9].
In early pregnancy, various events occur in the uterus, especially the endometrium, leading to a receptive uterus. The receptive uterus provides a hospitable environment for blastocyst implantation and the establishment and maintenance of pregnancy [10]. The main events leading to the formation of a receptive uterus are the elevation of estrogen and progesterone levels, the proliferation and differentiation of endometrial cells, the increase of glands and blood vessels in the endometrium [11], the up-regulation of the expression of cell adhesion molecules on the surface of endometrial epithelial cells as well as on the endometrial leukocytes [12], and the secretion of cytokines, chemokines, and prostaglandins in the endometrium [11].
Despite extensive studies on the developmental consequences of increased synthetic GC exposure in mid to late pregnancy, relatively little is known regarding the significance of synthetic GCs in early pregnancy. The existing literature indicates that the use of synthetic GCs in early pregnancy is controversial. Previous studies have demonstrated that synthetic GCs exert many actions that could both negatively and positively influence the key aspects of early pregnancy [13,14]. Synthetic GCs can exert a range of positive effects that would be expected to promote the establishment of early pregnancy, such as suppressing uterine natural killer (uNK) cells and stimulating human chorionic gonadotropin secretion, as well as promoting trophoblast proliferation and invasion. However, synthetic GCs can also exert a range of adverse effects that would be expected to impede pregnancy, induce placental and/or decidual apoptosis, and impair placental nutrient transport [7,14,15].
DEX is a synthetic GC that is used for the treatment of various complications, such as preterm labor [16], intrahepatic cholestasis [17], and inflammatory conditions during pregnancy [18]. The purpose of this study was to evaluate the effect of high doses of DEX in early pregnancy on pregnancy outcomes, fetal and placental growth parameters, serum steroid hormones, and the immune status of the feto-maternal interface.
Methods
1. Determination of gestational age
In this study, six- to eight-week-old inbred male and female BALB/c mice were purchased from the Pasteur Institute of Iran (Karaj, Iran) and housed in the animal house at Tarbiat Modares University (Tehran, Iran) according to international animal care ethics. The experiments were approved by the Institutional Animal Care and Use Committee of Tarbiat Modares University. The experimental protocols of this study were approved by the Ethics Committee on the Use of Animals of Tarbiat Modares University.
Female BALB/c mice were mated with male BALB/c mice and checked for a vaginal plug every morning. The presence of the vaginal plug was considered to indicate day 0.5 of pregnancy.
2. Animal treatment and sample collection
Pregnant BALB/c mice were treated daily with intraperitoneal injections of DEX (5 mg/kg body weight per injection) in the experimental group [19] or an equivalent volume of phosphate-buffered saline (PBS) in the control group on days 0.5 to 4.5 of gestation (n=21 per group).
From each group, seven pregnant BALB/c mice were sacrificed on gestational day (GD) 7.5. The quantity of uNK cells and uterine macrophages as well as the serum concentrations of progesterone and 17β-estradiol were determined in the tissue sections obtained from uterine tissues and blood samples, respectively. Another seven pregnant BALB/c mice were sacrificed on GD 13.5 and uterine samples were taken for the evaluation of pregnancy outcomes and the immune status of the feto-maternal interface. One third of the mice from each group were sacrificed on GD 18.5 for the morphometric quantification of fetal and placental growth parameters in the uterine samples.
3. Pregnancy outcomes
On GD 13.5, the female BALB/c mice were anesthetized, blood samples were taken, and then the mice were sacrificed. The uteri were removed and the total number of implantations and resorption sites were recorded. The percentage of abortions was calculated as the ratio of resorption sites to the total number of implantation sites (resorption plus normal implantation sites), as described previously by Clark et al. [20].
4. Preparation of decidual and placenta cell supernatants and mid-pregnancy serum
The uteri of pregnant BALB/c mice were completely removed under sterile conditions on GD 13.5. Decidual cell supernatants (DS) were prepared according the method used by Bibak et al. [21]. Briefly, after washing with PBS, the uterus was incised along the antimesometrial axis and the embryos with their surrounding membranes were removed. The decidua basalis was then peeled away from the surface of the placental disks and washed three times with PBS. The decidual fragments were finely minced between two scalpels in a small volume of RPMI 1640 medium (Gibco BRL, Paisley, UK) and placed in an enzymatic cocktail composed of a digestive solution (2 mg/mL of type II collagenase and 30 µg/mL of DNase I) at 37℃ for 1 hour. The supernatant was discarded and the cell pellet was suspended in RPMI 1640 containing 20% fetal calf serum and cultured in a 24-well microplate (4×105 cells per well) at 37℃ for 48 hours. Subsequently, the supernatant was collected from all wells and centrifuged at 12,000 g for 15 minutes at 4℃. The supernatant samples were then collected and stored at –70℃ for further use.
For placenta cell supernatant (PS) preparation, a similar method was used to the method of DS preparation described above. Due to the abundance of erythrocytes in the placental tissue, the placental cells were treated with 0.83% ammonium chloride (Merck, Darmstadt, Germany) for erythrocyte lysis before culturing the placenta cells.
5. Effects of decidual and PS on the mitogen-induced proliferation of spleen mononuclear cells
In order to analyze decidua or placenta-associated immunoregulatory activity in the pregnant BALB/c mice treated with DEX or PBS, spleen mononuclear cells from normal BALB/c mice were isolated by the Ficoll-isopaque (Baharafshan, Tehran, Iran). Mononuclear cells were cultured in complete RPMI 1640 medium at the concentration of 2×105 splenocytes/0.2 mL in 96-well plates. The cultures were incubated in the presence and absence of phytohemagglutinin (PHA) (Sigma-Aldrich, St. Louis, MO, USA) or lipopolysaccharide (LPS) (from Escherichia coli O111: B4, Sigma-Aldrich) at 37℃ and 5% CO2 for 72 hours. PHA and LPS were added at final concentrations of 5 µg/mL and 10 µg/mL per well, respectively.
DS or PS from the pregnant BALB/c mice treated with DEX or PBS was added to the culture wells at various concentrations (5%, 10%, and 20% of the total volume) concomitantly with PHA or LPS in triplicate. Positive controls, containing cells and mitogen without any supernatant, were also set up. Cells in the medium alone, without PHA/LPS, PS, or DS served as negative controls. The plates were incubated at 37℃ and 5% CO2 for 72 hours.
Subsequently, cell proliferation was measured using the 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide reduction assay. The test results were expressed as the stimulation index, which was the ratio of the amount of OD540 nm in the simulated cells divided by the OD540 nm for the unstimulated cells on the same day of culture.
6. Determination of progesterone and 17β-estradiol concentrations in serum
The serum collected from pregnant BALB/c mice on GD 7.5 was used to measure the concentration of progesterone and 17β-estradiol. Sandwich enzyme-linked immunosorbent assay kits obtained from R&D Systems (Minneapolis, MN, USA) were used for detecting the levels of these hormones according to the manufacturer's instructions.
7. Immunohistochemical analysis
Pregnant mice were sacrificed on GD 7.5 and the middle third of the left horn of the uterus was removed. Paraffin sections and frozen sections of the uterine horn were prepared for the immunohistochemical staining of uterine macrophages and uNK cells, respectively.
Frozen sections of the tissues were cut to be 7 µm thick, transferred to glass slides, air-dried at room temperature for 4 hours, and fixed in ice-cold acetone for 2 minutes. Acetone-fixed cryostat sections of theuteri were thawed and washed three times with 0.15 M Tris-buffered saline (TBS) at pH 7.4. The samples were incubated in a protein-blocking reagent (Dako, Carpinteria, CA, USA) for 10 minutes and normal goat serum (Dako) for 15 minutes prior to the antibody incubation. In order to localize and characterize the uterine macrophages, the slides were then incubated for 2 hours with rat anti-mouse F4/80 (dilution 1:100, Abcam, Cambridge, MA, USA). After excess antibody was washed out using TBS, the samples were incubated with goat biotinylated anti-rat IgG antibody (dilution 1:50, BD, Franklin Lakes, NJ, USA) for 45 minutes. Excess antibody was removed by washing for three times with TBS followed by the addition of streptavidin-HRP (BD) for 40 minutes. Finally, the sections were stained with a 3,3'-diaminobenzidine (DAB) substrate chromogen system (Dako) for 5 minutes and were counterstained with Harris's hematoxylin (Merck) for 30 seconds.
Paraffin sections 7 µm in thickness were deparaffinized in xylene and rehydrated in a graded ethanol series. After the antigens were treated in a microwave oven at 70℃ for 1 hour, the sections were incubated in protein blocking reagent (Dako, Glostrup, Denmark) for 10 minutes. In order to localize and characterize uNK cells, the slides were then incubated with biotinylated dolichos biflorus agglutinin lectin (dilution 1:200, Sigma-Aldrich) for 1 hour. After washing, they were incubated with streptavidin-HRP (BD) for 30 minutes. The reactions were visualized using a DAB substrate system (Dako) for 5 minutes and were rinsed in tap water. Then, they were serially incubated in 1% periodic acid solution (Sigma-Aldrich) for 10 minutes, Schiff's reagent for 15 minutes, and 0.5% sodium bisulfate solution (Sigma-Aldrich) for 5 minutes. Finally, the slides were counterstained with Harris's hematoxylin for 30 seconds.
For cell counting, at least five sections in each sample were selected randomly, several images were captured at 400× using an Olympus BX41 microscope with a digital camera using Magnafire (Optronics, Goleta, CA, USA), and the total cells (blue-stained), macrophages, and NK cells (brown-stained) were counted separately using Image J software ver. 1.45 (National Institutes of Health, Bethesda, MD, USA).
8. Morphometric quantification of embryonic and placental growth parameters
For the morphometric quantification of fetal and placental growth parameters in mice and to determine the weight of the uterus (WU), the pregnant BALB/c mice treated with DEX or PBS were sacrificed on GD 18.5 and their uteri were removed. The uterus was incised along the antimesometrial axis and the embryos and placenta were removed. Measurement instruments with high precision and high accuracy, such as a scientific balance, micrometer (Mahr, Göttingen, Germany), and digital caliper (Mitutoyo, Tokyo, Japan), were used to measure WU and fetal and placental growth parameters including the weight of the placenta (WP), the placenta diameter (PD), the crown-rump length (CRL), the biparietal diameter (BPD), and the body weight of the embryo (BWE).
9. Statistical analyses
All statistical analyses were carried out using GraphPad Prism 5.0 (GraphPad, San Diego, CA, USA). The percentage of abortions was compared using the nonparametric Mann-Whitney U test in two independent experiments. The Student's t-test and one-way analysis of variance (with the Tukey test) were used to determine the significance of differences for all other comparisons. The minimum level of statistical significance was set at p<0.05. Data were presented as mean±standard error of the mean.
Results
1. Pregnancy outcomes
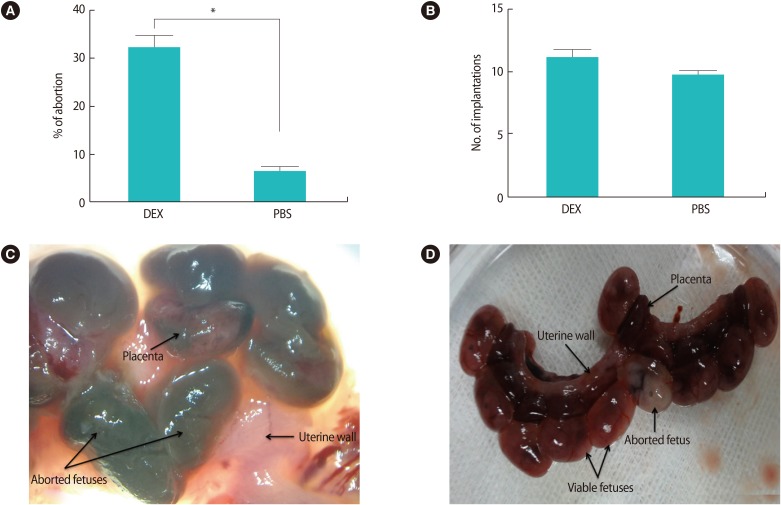
The macroscopic observations of the embryos attached to the uterus on GD 13.5 showed that the treatment of pregnant BALB/c mice with high doses of DEX in early pregnancy significantly increased the abortion rate compared with the control group (32% vs. <6.4%, p<0.01) (Figure 1A). No significant difference was found in the number of implantation sites between the two groups (Figure 1B).
Figure 1. The effects of high doses of DEX in early pregnancy on pregnancy outcomes and appearance of the uterus on gestational day 13.5. (A) Treatment of pregnant BALB/c mice with DEX in early pregnancy significantly the increased abortion rate in comparison with PBS-treated pregnant BALB/c mice (*p<0.01). (B) No significant difference was found in the number of implantation sites between DEX- and PBS-treated pregnant BALB/c mice. (C) Aborted fetuses in the uteri of DEX-treated pregnant BALB/c mice were identified by their small size accompanied by a necrotic appearance in comparison with normal embryos and placentas. (D) The nine viable fetuses and one aborted fetus in the uterine horns were obtained from PBS-treated pregnant BALB/c mice on gestational day 13.5. Results are expressed as mean±standard error of measurement from seven independent experiments and were analyzed using the nonparametric Mann-Whitney U test in two independent experiments. DEX, dexamethasone; PBS, phosphate buffer saline.
2. Effects of decidual and PSs on mitogen-stimulated splenocyte proliferation
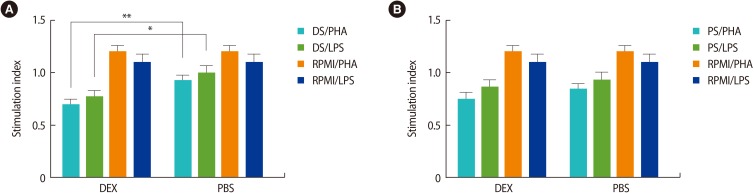
The results of our study showed that DS from pregnant BALB/c mice treated with DEX exhibited significantly decreased PHA- and LPS-stimulated proliferation of mononuclear cells in comparison with the DS from the control group (p<0.05) (Figure 2A). The results also demonstrated that the PS from pregnant BALB/c mice treated with DEX or PBS had the same inhibitory effect on the PHA- and LPS-stimulated proliferation of mononuclear cells (Figure 2B). In all experiments, a 5% concentration of DS or PS had the best effect on the proliferation of mononuclear cells, and the trypan blue exclusion assay indicated a viability rate of higher than 90%.
Figure 2. The effects of DS and PS on the proliferation of mitogen-stimulated mouse spleen mononuclear cells. (A) DS from DEX-treated pregnant BALB/c mice significantly suppressed the proliferation of PHA- and LPS-stimulated mononuclear cells in comparison with DS from PBS-treated pregnant BALB/c mice. (B) PS from DEX-treated pregnant BALB/c mice and PS from PBS-treated pregnant BALB/c had a similar inhibitory effect on PHA- and LPS-stimulated mononuclear cell proliferation. All data presented in these charts were obtained by the treatment of mouse spleen mononuclear cells with a 5% concentration of DS or PS. The stimulation index was calculated by dividing the optical density test of each group by the optical density control for each group. The results are expressed as mean±standard error of measurement from seven independent experiments. DS, decidual cell supernatant; PS, placenta cell supernatant; DEX, dexamethasone; PHA, phytohemagglutinin; LPS, lipopolysaccharides; PBS, phosphate-buffered saline. *p<0.05, **p<0.01 was used for the statistical analysis.
3. Serum levels of progesterone and 17β-estradiol
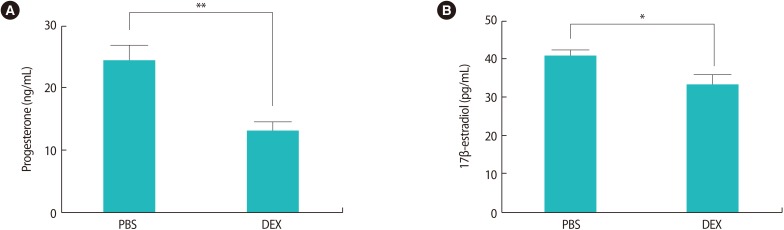
Analysis of the serum samples showed that treatment with DEX markedly decreased progesterone concentration in comparison to treatment with PBS (13±1.38 ng/mL vs. 24.29±2.50 ng/mL, p<0.01) (Figure 3A). This analysis also demonstrated that DEX treatment in early pregnancy significantly decreased serum 17β-estradiol concentrations in the pregnant BALB/c mice in comparison with the PBS group (33.14±2.52 pg/mL vs. 40.29±1.7 pg/mL, p<0.05) (Figure 3B).
Figure 3. The effects of DEX treatment in early pregnancy on serum levels of progesterone and 17β-estradiol. Pregnant BALB/c mice were injected with DEX or PBS on gestational days 0.5 to 4.5 and sacrificed on gestational day 7.5. Levels of progesterone (ng/mL) and 17β-estradiol (pg/mL) were measured by enzyme-linked immunosorbent assay in serum samples from pregnant BALB/c mice. The serum concentration of 17β-estradiol (B), especially progesterone (A), was decreased in DEX-treated pregnant BALB/c mice compared with the PBS-treated pregnant BALB/c mice. Data are reported as mean±standard error of measurement from seven independent experiments and the unpaired Student's t-test with *p<0.05, **p<0.01 was used for the statistical analysis. DEX, dexamethasone; PBS, phosphate-buffered saline.
4. Frequency and distribution of macrophages and NK cells within the uterine tissue
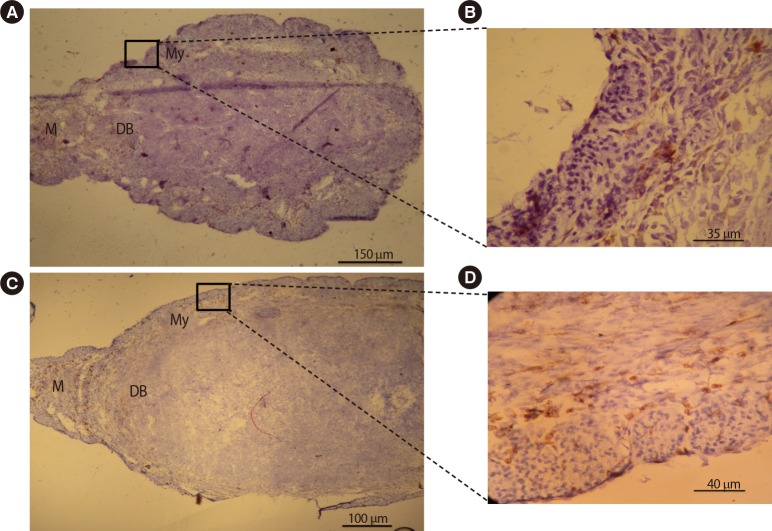
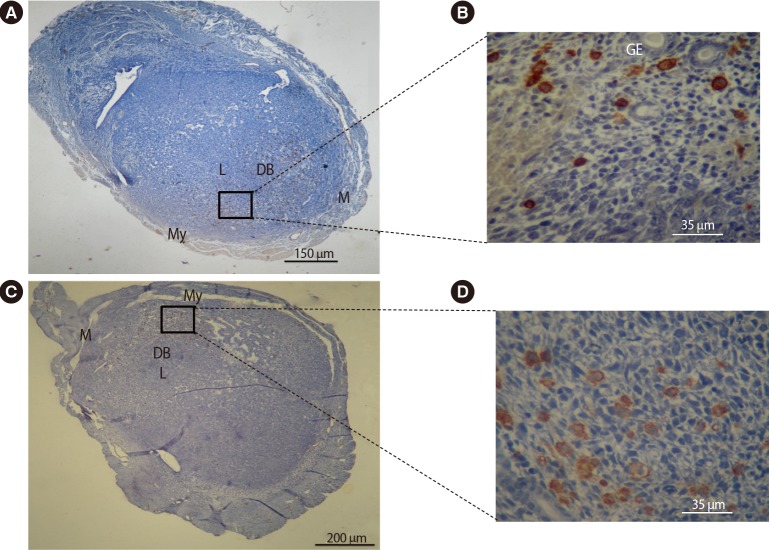
In order to assess the effects of DEX on the distribution and localization of NK cells and macrophages within the uterus, an immunohistochemical analysis was conducted on the uterine tissue from pregnant BALB/c mice treated with PBS or DEX. The results of this evaluation showed similar distribution and localization patterns of uterine macrophages and uNK cells in the experimental and control groups. Uterine macrophages were present in the myometrium, mesometrium, and decidua basalis (Figure 4), while uNK cells accumulated in the decidua basalis and around the glandular and luminal epithelial layers (Figure 5). The frequency of uterine macrophages significantly decreased in pregnant BALB/c mice treated with DEX compared with the control group (13.14%±1.33% vs. 17.29%±1.30%, p<0.05). Similarly, pregnant BALB/c mice treated with DEX had significantly lower uNK cell percentages than the control group (36.14%±2.64% vs. 47.57%±1.83%, p<0.01).
Figure 4. Immunohistochemical comparative analysis of macrophages in the uteri of pregnant BALB/c mice on gestational day 7.5. Cryosections were prepared from the uteri of pregnant BALB/c mice and stained with F4/80 antibody. Tissues were also counterstained with hematoxylin. Positive cells were counted and expressed as a percentage of the total number of nucleated cells. Macrophages were present in the myometrium, mesometrium, and decidua basalis. The frequency of uterine macrophages significantly decreased in pregnant BALB/c mice treated with DEX (A, B) when compared with pregnant BALB/c mice treated with PBS (C, D). DEX, dexamethasone; PBS, phosphate-buffered saline; M, mesometrial side; DB, decidual basalis; My, myometrium.
Figure 5. Immunohistochemical comparative analysis of NK cells in the uteri of pregnant BALB/c mice on day 7.5 of gestation. Cryosections were prepared from the uterus of pregnant BALB/c mice and stained with biotinylated dolichos biflorus agglutinin lectin. Tissues were also counterstained with hematoxylin. Positive cells were counted and expressed as a percentage of the total number of nucleated cells. The mesometrial regions are at the top of each image. The NK cells accumulated in the decidua basalis and around the glandular and luminal epithelial layers. The frequency of uterine NK cells significantly decreased in pregnant BALB/c mice treated with DEX (A, B) when compared with pregnant BALB/c mice treated with PBS (C, D). NK, natural killer; DEX, dexamethasone; PBS, phosphate-buffered saline; M, mesometrial side; My, myometrium; L, luminal epithelium; DB, decidual basalis; GE, glandular epithelium.
5. Morphometric quantification of fetal and placental growth parameters
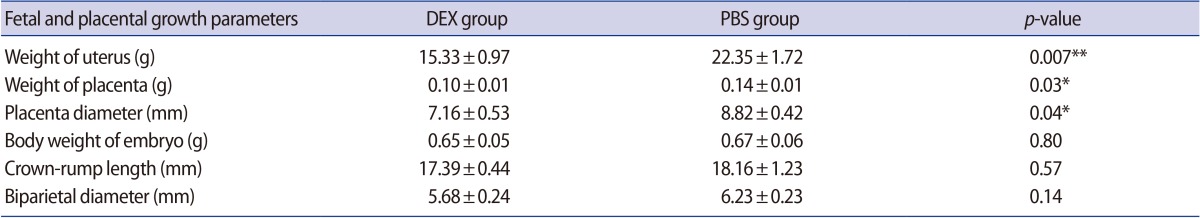
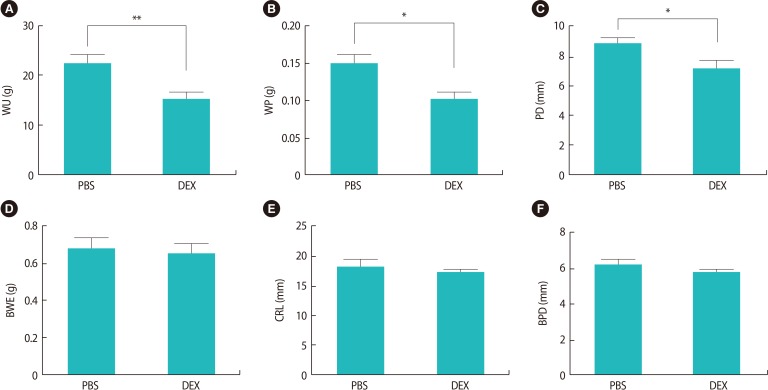
Evaluation of the pregnant BALB/c mice treated with DEX or PBS on GD 18.5 showed that, although the number of live fetuses in the mice treated with DEX was lower than in the control group, no intrauterine growth retardation or abnormal embryo development was observed in the fetuses of either group. The fetal and placental growth parameters and the weight of the uteri from the pregnant BALB/c mice treated with DEX or PBS on GD 18.5 are presented in Table 1. We found that fetal growth parameters such as CRL, BPD, and BWE were the same between the experimental and control groups, whereas WU, WP, and PD were significantly lower in the pregnant BALB/c mice treated with DEX than in the control group (Figure 6).
Table 1. Quantitative morphometric analysis of fetal and placental growth parameters in DEX- and PBS-treated pregnant BALB/c mice on day 18.5 of gestation.
Values are presented as mean±standard deviation.
DEX, dexamethasone; PBS, phosphate-buffered saline.
*p<0.05; **p<0.01.
Figure 6. The in vitro quantification of fetal and placental growth parameters in pregnant BALB/c mice treated with DEX or PBS on gestational day 18.5 (*p<0.05; **p<0.01). The results are expressed as mean concentrations±standard error of the mean. DEX, dexamethasone; PBS, phosphate-buffered saline; WU, weight of the uterus; WP, weight of placenta; PD, placenta diameter; BWE, body weight of embryo; CRL, crown-rump length; BPD, biparietal diameter.
Discussion
In this study, the effects of high doses of DEX in early pregnancy were evaluated in pregnant BALB/c mice. An increased abortion rate, enhancement of the immunosuppressive effect of the decidua, alterations in placental growth factor levels, decreased serum progesterone and 17β-estradiol levels, and reductions in the frequency of uterine macrophages and uNK cells were the main findings of this study.
In the past few decades, several studies have investigated the effect of DEX on pregnancy outcomes in different animals and human groups; Wichtel et al. [22] showed that a single intra-allantoic infusion of DEX induced elective termination of midterm pregnancy in mares. Additionally, Zone et al. [23] reported that oral administration of DEX appeared to be a potentially useful pharmacologic treatment for the termination of unwanted pregnancy in female dogs. Furthermore, in a prospective case-control study, the antenatal administration of GCs to women in the first trimester significantly increased the incidence of miscarriages compared to the rate observed in control patients [15]. The results of the present study are in agreement with those of previous studies, demonstrating a significant increase in the abortion rate among pregnant BALB/c mice treated with high doses of DEX in early pregnancy (Figure 1).
The immune status of the feto-maternal interface during pregnancy has been shown to be important for the successful maintenance of pregnancy. The local environment of the feto-maternal interface is characterized not only by the cell types evaluated in this study, but also by the soluble factors produced therein. The decidua as a fetal-independent tissue and the placenta as a fetal-dependent tissue may be ideal sources for the production and secretion of immunoregulatory factors [24].
In the present study, the immunomodulatory changes occurring at the feto-maternal interface in response to high doses of DEX among pregnant BALB/c mice were investigated. The results demonstrated that the DS from the pregnant BALB/c mice treated with DEX exhibited significantly decreased PHA-stimulated (due to polyclonal T cell activation) and LPS-stimulated (due to polyclonal B cell activation) proliferation of mononuclear cells compared to the DS of the control group (p<0.05) (Figure 2A). However, the PS collected from the pregnant BALB/c mice treated with DEX or PBS had the same inhibitory effect on the PHA- and LPS-stimulated proliferation of mononuclear cells (Figure 2B).
It has been well established that GCs such as DEX have inhibitory effects on a broad range of specific immune responses mediated by T and B cells [25,26]. Previous studies showed that immune responses at the feto-maternal interface regulate a range of reproductive functions, such as embryo implantation, endometrial angiogenesis, decidual formation, and maternal tolerance to the fetus [27,28]. Many disorders of pregnancy can be caused by alterations in the various beneficial immunological responses of normal pregnancy [29]. It is possible, therefore, that high doses of DEX in early pregnancy, with the corresponding suppression of immune responses at the feto-maternal interface (especially the decidua), impact pregnancy outcomes.
A normal pattern of progesterone and estradiol secretion is necessary for the establishment and maintenance of pregnancy. The impairment of progesterone and estradiol production is a risk factor for pregnancy loss. In the present study, the effect of high doses of DEX in early pregnancy on serum progesterone and 17β-estradiol levels was evaluated and it was found that treatment with DEX significantly decreased the serum concentration of these hormones (Figure 3).
Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) regulate levels of 17β-estradiol and progesterone during pregnancy. In an in vivo study, Sowers et al. [30] showed that the short-term administration of DEX suppressed the secretion of LH and FSH via a direct effect on the anterior pituitary. It has been well demonstrated that endogenous or exogenous GCs, such as DEX in excess, lead to the development of acquired hypogonadotropic hypogonadism. This complication is characterized by the decreased production of estrogen and progesterone by female ovarian follicular cells [31,32]. Therefore, the administration of high doses of DEX to pregnant BALB/c mice during early pregnancy may inhibit progesterone and estradiol secretion via the down-regulation of FSH and LH released from gonadotrophic cells in the anterior pituitary gland.
In recent years, a growing body of evidence has indicated that immune–immune interactions, as well as immune–endocrine interactions, build up a complex network of immune regulation that ensures fetal survival within the maternal uterus [33]. Hormones such as estrogens and progesterone modulate immunological responses to induce maternal-fetal tolerance [34]. The hormonal changes that occur in pregnant BALB/c mice treated with high dose DEX may underlie some of the distinct immunological changes associated with unfavorable pregnancy outcomes.
Macrophages and NK cells are the dominant uterine immune cells in pregnancy, especially in the decidua during early pregnancy [35,36,37]. The regulation of trophoblast invasion, the induction of feto-maternal tolerance, and the successful implantation and decidualization are the main roles of uterine macrophages and uNK cells [36,38]. With the critical role of uterine macrophages and uNK cells in successful pregnancy in mind, we evaluated their uterine frequency in pregnant BALB/c mice treated with DEX or PBS. The results of this study showed that the presence of uterine macrophages and uNK cells was reduced in the DEX-treated group compared to the control group (Figures 4, 5).
These results seem to be consistent with those of other studies that have found anti-inflammatory drugs such as DEX to suppress leukocyte migration [39]. These drugs bind to intracellular receptors and inhibit gene expression by binding to negative regulatory promoter regions or through protein/protein interactions [39]. Similarly, in another study, Sugimoto et al. [40] found that the anti-inflammatory effects of GCs such as DEX were mediated by the inhibition of leukocyte migration. In contrast, DeLoia et al. [41] demonstrated that the frequency of uterine macrophages and uNK cells had a direct relationship with serum estrogen levels. It can thus be suggested that the administration of high-dose DEX in pregnant BALB/c mice during early pregnancy can, directly or indirectly, alter the frequency of uterine macrophages and uNK cells. Since uterine macrophages and uNK cells play important roles in the secretion of angiogenic and growth factors [42,43], decreased frequencies of these cells can change spiral artery remodeling and trophoblast invasion, as well as lead to adverse pregnancy outcomes.
In several studies, the side effects of antenatal GC therapy with drugs such as DEX and betamethasone on the fetus have been evaluated. Reductions in fetal heart rate [44], growth discordance, low birth weight [45], reduction in fetal body movements and activity periods [46], and congenital adrenal hyperplasia [47] are some of the side effects of antenatal GC therapy. In this study, we evaluated the effect of high doses of DEX in early pregnancy on fetal growth parameters. In contrast to earlier findings, the present results indicated that the treatment of pregnant BALB/c mice with high doses of DEX during early pregnancy did not affect fetal growth parameters (BWE, CRL, and BPD), although a significant difference was found in the WU between the experimental and control groups (Figure 4).
Several possible explanations may be suggested for this result. It is clear that the side effects of GCs such as DEX are correlated with the dose and time of exposure. In this experiment, we used a high dose of DEX before the implantation and development of the placenta. Additionally, we utilized measurement instruments such as a scientific balance, micrometer, and digital calipers to determine fetal growth parameters. For these reasons, our findings were different from those of the studies mentioned above. It may thus be suggested that the effects of different doses and time intervals of DEX on fetal growth parameters should be investigated using highly accurate methods, such as ultrasonography and skeletal staining.
Since fetal growth and survival are critically dependent on successful placental development, we aimed to investigate the effects of high doses of DEX in early pregnancy on placental growth parameters. The results of this study showed that placental growth parameters such as WP and PD were significantly decreased in the pregnant BALB/c mice treated with DEX compared with the control group (Figure 4). These alterations were more severe in resorbed sites than in non-resorbed sites, which was in agreement with Lee et al. [48], who showed that the prenatal administration of DEX on GDs 7.5, 8.5, and 9.5 in pregnant mice negatively affected placental development and efficiency. Newnham et al. [49] also showed that the infusion of corticosteroids into ewes in late pregnancy resulted in decreased placental size. In another study, Ain et al. [50] demonstrated that pregnant rats treated with DEX in the second half of gestation exhibited decreased placental weight. These data suggest that the administration of GCs (especially DEX) during pregnancy can interfere with placental development. This leads to the question of what mechanism is involved in the reduction of WP, PD, and WU.
To answer this question, several reports have shown that ovarian steroid hormones and immune responses at the feto-maternal interface play key roles in both uterine remodeling and placental development [33,51]. The current study found that treatment with high doses of DEX in early pregnancy induced placental and uterine shrinkage. We suggest that these alterations could be related to changes in the frequency of uterine macrophages and uNK cells, immune responses at the feto-maternal interface, and serum levels of progesterone and 17β-estradiol.
Overall, we found that the administration of high doses of DEX during early pregnancy negatively affected pregnancy outcomes. Enhancements of the immunosuppressive effect of the decidua, alterations in placental growth factor levels, decreased serum progesterone and 17β-estradiol levels, and the reduced frequency of uterine macrophages and uNK cells could be the possible reasons for the unfavorable pregnancy outcomes that we observed. Nonetheless, further experiments are still needed to clarify the relevant mechanisms.
Acknowledgments
The authors thank the members of the Department of Pathobiology and Medical Laboratory Science of the North Khorasan University of Medical Sciences for technical assistance.
Footnotes
This study was financially supported by grants from the North Khorasan University of Medical Sciences (1390/P/561), Bojnord, Iran and Tarbiat Modares University (TMU 92-6-65), Tehran, Iran.
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Allen TK, Jones CA, Habib AS. Dexamethasone for the prophylaxis of postoperative nausea and vomiting associated with neuraxial morphine administration: a systematic review and meta-analysis. Anesth Analg. 2012;114:813–822. doi: 10.1213/ANE.0b013e318247f628. [DOI] [PubMed] [Google Scholar]
- 2.Crowley P. Withdrawn: prophylactic corticosteroids for preterm birth. Cochrane Database Syst Rev. 2007;(3):CD000065. doi: 10.1002/14651858.CD000065.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breton MC, Beauchesne MF, Lemiere C, Rey E, Forget A, Blais L. Risk of perinatal mortality associated with inhaled corticosteroid use for the treatment of asthma during pregnancy. J Allergy Clin Immunol. 2010;126:772–777.e2. doi: 10.1016/j.jaci.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Adams Waldorf KM, Nelson JL. Autoimmune disease during pregnancy and the microchimerism legacy of pregnancy. Immunol Invest. 2008;37:631–644. doi: 10.1080/08820130802205886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lash GE, Bulmer JN, Innes BA, Drury JA, Robson SC, Quenby S. Prednisolone treatment reduces endometrial spiral artery development in women with recurrent miscarriage. Angiogenesis. 2011;14:523–532. doi: 10.1007/s10456-011-9237-x. [DOI] [PubMed] [Google Scholar]
- 6.Shaikh S, Verma H, Yadav N, Jauhari M, Bullangowda J. Applications of steroid in clinical practice: a review. Int Sch Res Notices. 2012;2012:1–11. [Google Scholar]
- 7.Michael AE, Papageorghiou AT. Potential significance of physiological and pharmacological glucocorticoids in early pregnancy. Hum Reprod Update. 2008;14:497–517. doi: 10.1093/humupd/dmn021. [DOI] [PubMed] [Google Scholar]
- 8.Edwards HE, Burnham WM. The impact of corticosteroids on the developing animal. Pediatr Res. 2001;50:433–440. doi: 10.1203/00006450-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Morrison JL, Botting KJ, Soo PS, McGillick EV, Hiscock J, Zhang S, et al. Antenatal steroids and the IUGR fetus: are exposure and physiological effects on the lung and cardiovascular system the same as in normally grown fetuses? J Pregnancy. 2012;2012:839656. doi: 10.1155/2012/839656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu H, Hou CC, Luo LF, Hu YJ, Yang WX. Endometrial stromal cells and decidualized stromal cells: origins, transformation and functions. Gene. 2014;551:1–14. doi: 10.1016/j.gene.2014.08.047. [DOI] [PubMed] [Google Scholar]
- 11.Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731–746. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- 12.Singh H, Aplin JD. Endometrial apical glycoproteomic analysis reveals roles for cadherin 6, desmoglein-2 and plexin b2 in epithelial integrity. Mol Hum Reprod. 2015;21:81–94. doi: 10.1093/molehr/gau087. [DOI] [PubMed] [Google Scholar]
- 13.De Blasio MJ, Dodic M, Jefferies AJ, Moritz KM, Wintour EM, Owens JA. Maternal exposure to dexamethasone or cortisol in early pregnancy differentially alters insulin secretion and glucose homeostasis in adult male sheep offspring. Am J Physiol Endocrinol Metab. 2007;293:E75–E82. doi: 10.1152/ajpendo.00689.2006. [DOI] [PubMed] [Google Scholar]
- 14.Boomsma CM, Keay SD, Macklon NS. Peri-implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst Rev. 2012;6:CD005996. doi: 10.1002/14651858.CD005996.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Gur C, Diav-Citrin O, Shechtman S, Arnon J, Ornoy A. Pregnancy outcome after first trimester exposure to corticosteroids: a prospective controlled study. Reprod Toxicol. 2004;18:93–101. doi: 10.1016/j.reprotox.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Mwansa-Kambafwile J, Cousens S, Hansen T, Lawn JE. Antenatal steroids in preterm labour for the prevention of neonatal deaths due to complications of preterm birth. Int J Epidemiol. 2010;39(Suppl 1):i122–i133. doi: 10.1093/ije/dyq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glantz A, Marschall HU, Lammert F, Mattsson LA. Intrahepatic cholestasis of pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42:1399–1405. doi: 10.1002/hep.20952. [DOI] [PubMed] [Google Scholar]
- 18.Ostensen M, Khamashta M, Lockshin M, Parke A, Brucato A, Carp H, et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther. 2006;8:209. doi: 10.1186/ar1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu T, Qiao J, Zhao L, He G, Li K, Wang J, et al. Effect of dexamethasone on acute respiratory distress syndrome induced by the H5N1 virus in mice. Eur Respir J. 2009;33:852–860. doi: 10.1183/09031936.00130507. [DOI] [PubMed] [Google Scholar]
- 20.Clark DA, Petitbarat M, Chaouat G. How should data on murine spontaneous abortion rates be expressed and analyzed? Am J Reprod Immunol. 2008;60:192–196. doi: 10.1111/j.1600-0897.2008.00612.x. [DOI] [PubMed] [Google Scholar]
- 21.Bibak B, Gharib FG, Daneshmandi S, Abbaspour AR, Firizi MN, Ahmadabad HN. The immunomodulatory effects of abortionprone mice decidual and serum soluble factors on macrophages and splenocytes. Eur J Obstet Gynecol Reprod Biol. 2012;165:331–336. doi: 10.1016/j.ejogrb.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Wichtel JJ, Evans LE, Clark TL. Termination of midterm pregnancy in mares using intra-allantoic dexamethasone. Theriogenology. 1988;29:1261–1267. [Google Scholar]
- 23.Zone M, Wanke M, Rebuelto M, Loza M, Mestre J, Duchene A, et al. Termination of pregnancy in dogs by oral administration of dexamethasone. Theriogenology. 1995;43:487–494. doi: 10.1016/0093-691x(94)00041-r. [DOI] [PubMed] [Google Scholar]
- 24.van Mourik MS, Macklon NS, Heijnen CJ. Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J Leukoc Biol. 2009;85:4–19. doi: 10.1189/jlb.0708395. [DOI] [PubMed] [Google Scholar]
- 25.Piccinni MP. T cells in normal pregnancy and recurrent pregnancy loss. Reprod Biomed Online. 2007;14(Spec No 1):95–99. doi: 10.1016/S1472-6483(10)61463-0. [DOI] [PubMed] [Google Scholar]
- 26.Fahey AJ, Robins RA, Kindle KB, Heery DM, Constantinescu CS. Effects of glucocorticoids on STAT4 activation in human T cells are stimulus-dependent. J Leukoc Biol. 2006;80:133–144. doi: 10.1189/jlb.0605296. [DOI] [PubMed] [Google Scholar]
- 27.Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 28.Ott TL, Kamat MM, Vasudevan S, Townson DH, Pate JL. Maternal immune responses to conceptus signals during early pregnancy in ruminants. Anim Reprod. 2014;11:237–245. [Google Scholar]
- 29.Arulkumaran S, Sivanesaratnam V, Chatterjee A, Kumar P. Essentials of obstetrics. London: Jaypee Brothers; 2004. [Google Scholar]
- 30.Sowers JR, Rice BF, Blanchard S. Effect of dexamethsone on luteinizing hormone and follicle stimulating hormone responses to LHRH and to clomiphene in the follicular phase of women with normal menstrual cycles. Horm Metab Res. 1979;11:478–480. doi: 10.1055/s-0028-1092765. [DOI] [PubMed] [Google Scholar]
- 31.Rosen H, Jameel ML, Barkan AL. Dexamethasone suppresses gonadotropin-releasing hormone (GnRH) secretion and has direct pituitary effects in male rats: differential regulation of GnRH receptor and gonadotropin responses to GnRH. Endocrinology. 1988;122:2873–2880. doi: 10.1210/endo-122-6-2873. [DOI] [PubMed] [Google Scholar]
- 32.Skalba P, Guz M. Hypogonadotropic hypogonadism in women. Endokrynol Pol. 2011;62:560–567. [PubMed] [Google Scholar]
- 33.Schumacher A, Costa SD, Zenclussen AC. Endocrine factors modulating immune responses in pregnancy. Front Immunol. 2014;5:196. doi: 10.3389/fimmu.2014.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62:263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 36.Lombardelli L, Aguerre-Girr M, Logiodice F, Kullolli O, Casart Y, Polgar B, et al. HLA-G5 induces IL-4 secretion critical for successful pregnancy through differential expression of ILT2 receptor on decidual CD4+ T cells and macrophages. J Immunol. 2013;191:3651–3662. doi: 10.4049/jimmunol.1300567. [DOI] [PubMed] [Google Scholar]
- 37.Gao R, Chen L, Tan W, Tan H, Ou X, Li H, et al. Role of macrophages in mouse uterine during the peri-implantation period. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35:365–369. [PubMed] [Google Scholar]
- 38.Hammer A. Immunological regulation of trophoblast invasion. J Reprod Immunol. 2011;90:21–28. doi: 10.1016/j.jri.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function*. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 40.Sugimoto Y, Ogawa M, Tai N, Kamei C. Inhibitory effects of glucocorticoids on rat eosinophil superoxide generation and chemotaxis. Int Immunopharmacol. 2003;3:845–852. doi: 10.1016/S1567-5769(03)00055-9. [DOI] [PubMed] [Google Scholar]
- 41.DeLoia JA, Stewart-Akers AM, Brekosky J, Kubik CJ. Effects of exogenous estrogen on uterine leukocyte recruitment. Fertil Steril. 2002;77:548–554. doi: 10.1016/s0015-0282(01)03062-x. [DOI] [PubMed] [Google Scholar]
- 42.Warning JC, McCracken SA, Morris JM. A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction. 2011;141:715–724. doi: 10.1530/REP-10-0360. [DOI] [PubMed] [Google Scholar]
- 43.Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol. 2009;174:1959–1971. doi: 10.2353/ajpath.2009.080995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dawes GS, Serra-Serra V, Moulden M, Redman CW. Dexamethasone and fetal heart rate variation. Br J Obstet Gynaecol. 1994;101:675–679. doi: 10.1111/j.1471-0528.1994.tb13183.x. [DOI] [PubMed] [Google Scholar]
- 45.Brownfoot FC, Gagliardi DI, Bain E, Middleton P, Crowther CA. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2013;8:CD006764. doi: 10.1002/14651858.CD006764.pub3. [DOI] [PubMed] [Google Scholar]
- 46.Derks JB, Mulder EJ, Visser GH. The effects of maternal beta-methasone administration on the fetus. Br J Obstet Gynaecol. 1995;102:40–46. doi: 10.1111/j.1471-0528.1995.tb09024.x. [DOI] [PubMed] [Google Scholar]
- 47.Dreger A, Feder EK, Tamar-Mattis A. Prenatal dexamethasone for congenital adrenal hyperplasia: an ethics canary in the modern medical mine. J Bioeth Inq. 2012;9:277–294. doi: 10.1007/s11673-012-9384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JY, Park SJ, Kim SH, Kim MH. Prenatal administration of dexamethasone during early pregnancy negatively affects placental development and function in mice. J Anim Sci. 2012;90:4846–4856. doi: 10.2527/jas.2012-5090. [DOI] [PubMed] [Google Scholar]
- 49.Newnham JP, Evans SF, Godfrey M, Huang W, Ikegami M, Jobe A. Maternal, but not fetal, administration of corticosteroids restricts fetal growth. J Matern Fetal Med. 1999;8:81–87. doi: 10.1002/(SICI)1520-6661(199905/06)8:3<81::AID-MFM3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 50.Ain R, Canham LN, Soares MJ. Dexamethasone-induced intrauterine growth restriction impacts the placental prolactin family, insulin-like growth factor-II and the Akt signaling pathway. J Endocrinol. 2005;185:253–263. doi: 10.1677/joe.1.06039. [DOI] [PubMed] [Google Scholar]
- 51.Care AS, Ingman WV, Moldenhauer LM, Jasper MJ, Robertson SA. Ovarian steroid hormone-regulated uterine remodeling occurs independently of macrophages in mice. Biol Reprod. 2014;91:60. doi: 10.1095/biolreprod.113.116509. [DOI] [PubMed] [Google Scholar]