Abstract
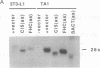
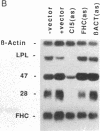
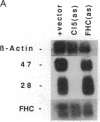
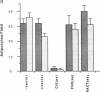
We have recently characterized an adipocyte cDNA (clone 5) that is enhanced in expression by environmental and hormonal conditions favoring adipogenic differentiation. Moreover, certain agents including fibroblast growth factor and phorbol 12-myristate 13-acetate (but not epidermal growth factor) markedly inhibit clone 5 gene expression and prevent TA1 cell differentiation. These results led us to propose that a threshold level of the clone 5 gene product (AP27 protein) is required for triggering adipocyte differentiation. We have constructed vectors that direct the synthesis of clone 5 antisense RNA to reduce the levels of AP27 in adipogenic cell lines TA1 and 3T3-L1. We show here that when these cells express clone 5 antisense RNA, they fail to undergo morphological differentiation, whereas adipogenesis is unaffected in cells expressing antisense beta-actin or ferritin heavy-chain RNA. We further show that cells expressing clone 5 antisense RNA (but not the other antisense RNAs) are unable to induce the expression of characteristic "adipocyte-specific" mRNAs. The level of inhibition of differentiation by clone 5 antisense RNA correlates with decreased levels of AP27 protein. These results provide strong evidence that expression of AP27 is linked to adipogenic differentiation and that AP27 may be a component of an as-yet-uncharacterized signal-transduction pathway required for the triggering of adipocyte differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal A. K., Monder C., Eckstein B., White P. C. Cloning and expression of rat cDNA encoding corticosteroid 11 beta-dehydrogenase. J Biol Chem. 1989 Nov 15;264(32):18939–18943. [PubMed] [Google Scholar]
- Amini S., DeSeau V., Reddy S., Shalloway D., Bolen J. B. Regulation of pp60c-src synthesis by inducible RNA complementary to c-src mRNA in polyomavirus-transformed rat cells. Mol Cell Biol. 1986 Jul;6(7):2305–2316. doi: 10.1128/mcb.6.7.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. E. A common ancestor for human placental 17 beta-hydroxysteroid dehydrogenase, Streptomyces coelicolor actIII protein, and Drosophila melanogaster alcohol dehydrogenase. FASEB J. 1990 Feb 1;4(2):222–226. doi: 10.1096/fasebj.4.2.2153594. [DOI] [PubMed] [Google Scholar]
- Braun T., Buschhausen-Denker G., Bober E., Tannich E., Arnold H. H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989 Mar;8(3):701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
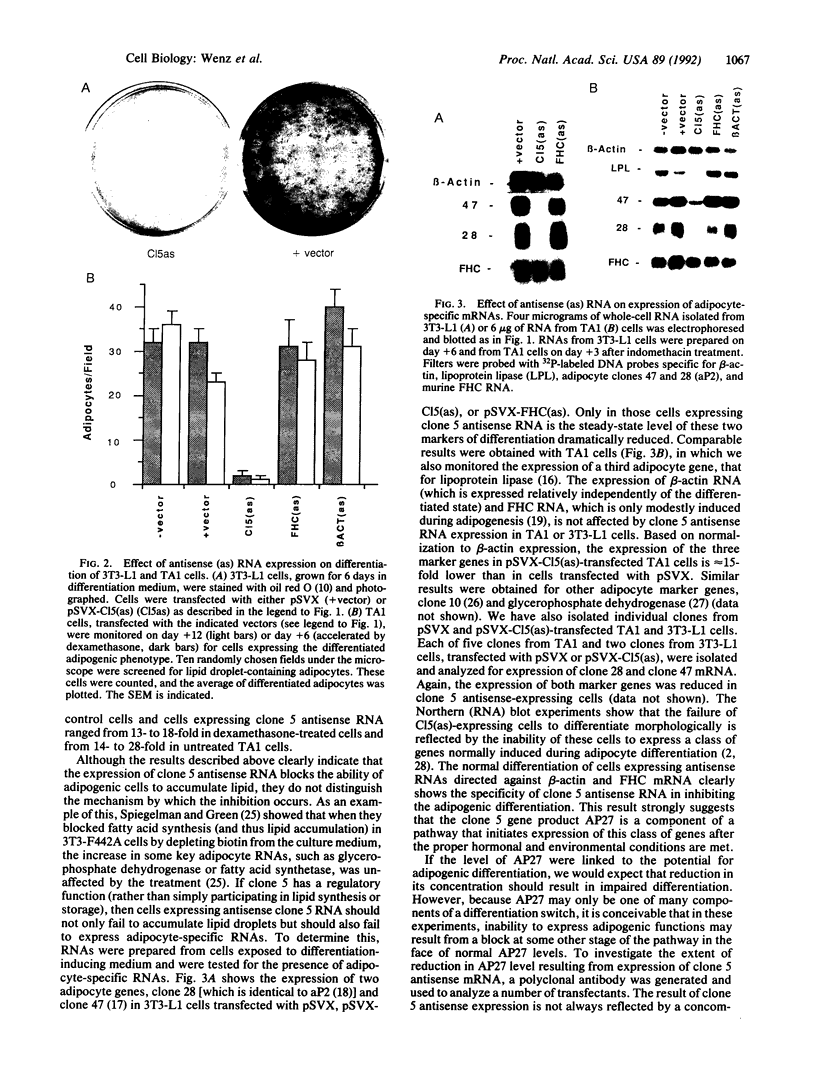
- Chapman A. B., Knight D. M., Dieckmann B. S., Ringold G. M. Analysis of gene expression during differentiation of adipogenic cells in culture and hormonal control of the developmental program. J Biol Chem. 1984 Dec 25;259(24):15548–15555. [PubMed] [Google Scholar]
- Chapman A. B., Knight D. M., Ringold G. M. Glucocorticoid regulation of adipocyte differentiation: hormonal triggering of the developmental program and induction of a differentiation-dependent gene. J Cell Biol. 1985 Oct;101(4):1227–1235. doi: 10.1083/jcb.101.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Teicher L. C., Kazim D., Pollack R. E., Wise L. S. Commitment of mouse fibroblasts to adipocyte differentiation by DNA transfection. Science. 1989 May 5;244(4904):582–585. doi: 10.1126/science.2470149. [DOI] [PubMed] [Google Scholar]
- Cook K. S., Hunt C. R., Spiegelman B. M. Developmentally regulated mRNAs in 3T3-adipocytes: analysis of transcriptional control. J Cell Biol. 1985 Feb;100(2):514–520. doi: 10.1083/jcb.100.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florini J. R., Roberts A. B., Ewton D. Z., Falen S. L., Flanders K. C., Sporn M. B. Transforming growth factor-beta. A very potent inhibitor of myoblast differentiation, identical to the differentiation inhibitor secreted by Buffalo rat liver cells. J Biol Chem. 1986 Dec 15;261(35):16509–16513. [PubMed] [Google Scholar]
- Graves R. A., Tontonoz P., Ross S. R., Spiegelman B. M. Identification of a potent adipocyte-specific enhancer: involvement of an NF-1-like factor. Genes Dev. 1991 Mar;5(3):428–437. doi: 10.1101/gad.5.3.428. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975 May;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C. R., Ro J. H., Dobson D. E., Min H. Y., Spiegelman B. M. Adipocyte P2 gene: developmental expression and homology of 5'-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3786–3790. doi: 10.1073/pnas.83.11.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Constitutive and conditional suppression of exogenous and endogenous genes by anti-sense RNA. Science. 1985 Jul 26;229(4711):345–352. doi: 10.1126/science.2990048. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984 Apr;36(4):1007–1015. doi: 10.1016/0092-8674(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., von Bahr-Lindström H., Jany K. D., Ulmer W., Fröschle M. Extended superfamily of short alcohol-polyol-sugar dehydrogenases: structural similarities between glucose and ribitol dehydrogenases. FEBS Lett. 1984 Jan 9;165(2):190–196. doi: 10.1016/0014-5793(84)80167-2. [DOI] [PubMed] [Google Scholar]
- Kirchgessner T. G., LeBoeuf R. C., Langner C. A., Zollman S., Chang C. H., Taylor B. A., Schotz M. C., Gordon J. I., Lusis A. J. Genetic and developmental regulation of the lipoprotein lipase gene: loci both distal and proximal to the lipoprotein lipase structural gene control enzyme expression. J Biol Chem. 1989 Jan 25;264(3):1473–1482. [PubMed] [Google Scholar]
- Korman A. J., Frantz J. D., Strominger J. L., Mulligan R. C. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. doi: 10.1073/pnas.84.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughton C. Measurement of the specific lipid content of attached cells in microtiter cultures. Anal Biochem. 1986 Aug 1;156(2):307–314. doi: 10.1016/0003-2697(86)90258-7. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Lane M. D., Gluecksohn-Waelsch S. Is CCAAT/enhancer-binding protein a central regulator of energy metabolism? Genes Dev. 1989 Dec;3(12B):2021–2024. doi: 10.1101/gad.3.12b.2021. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navre M., Ringold G. M. A growth factor-repressible gene associated with protein kinase C-mediated inhibition of adipocyte differentiation. J Cell Biol. 1988 Jul;107(1):279–286. doi: 10.1083/jcb.107.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes S. J., Konieczny S. F. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989 Dec;3(12B):2050–2061. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Chapman A. B., Knight D. M. Glucocorticoid control of developmentally regulated adipose genes. J Steroid Biochem. 1986 Jan;24(1):69–75. doi: 10.1016/0022-4731(86)90034-8. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980 Sep 25;255(18):8811–8818. [PubMed] [Google Scholar]
- Spizz G., Roman D., Strauss A., Olson E. N. Serum and fibroblast growth factor inhibit myogenic differentiation through a mechanism dependent on protein synthesis and independent of cell proliferation. J Biol Chem. 1986 Jul 15;261(20):9483–9488. [PubMed] [Google Scholar]
- Torti S. V., Kwak E. L., Miller S. C., Miller L. L., Ringold G. M., Myambo K. B., Young A. P., Torti F. M. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988 Sep 5;263(25):12638–12644. [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]