Abstract
It was recently discovered that the NRAS isoform 5 (20 amino acids) is expressed in melanoma and results in a more aggressive cell phenotype. This novel isoform is responsible for increased phosphorylation of downstream targets such as AKT, MEK, and ERK as well as increased cellular proliferation. This structure report describes the NMR solution structure of NRAS isoform 5 to be used as a starting point to understand its biophysical interactions. The isoform is highly flexible in aqueous solution, but forms a helix‐turn‐coil structure in the presence of trifluoroethanol as determined by NMR and CD spectroscopy.
Keywords: melanoma, NMR, isoform, NRAS
Short abstract
PDB Code(s): 2n9c
Abbreviations
- CD
circular dichroism
- COSY
correlation spectroscopy
- DIPSI
decoupling in the presence of scalar interactions
- HSQC
heteronuclear single quantum coherence
- NMR
nuclear magnetic resonance
- NOESY
nuclear overhauser effect spectroscopy
- TOCSY
total correlation spectroscopy.
Introduction
The incidence of the aggressive form of skin cancer known as melanoma is quickly rising.1 Recent predictions suggest that over 73,000 new cases of melanoma and 9000 deaths will be attributed to the disease in 2015.2 Early stage melanoma can be treated by surgical resection. However, metastatic disease has a poor overall survival. For therapeutic purposes, metatastic disease can be divided into those tumors that contain a mutation in the BRAF protein and those that do not. There have been recent advances in BRAF specific therapies combined with MEK inhibition.3 However, even in this specific case, the clinical response rate is only around 50% and the responses often last less than one year. When patients fail BRAF therapies there are multiple modes of resistance. One of these modes of resistance is the occurrence of new BRAF isoforms.4, 5 Other modes of therapy, including immune‐based treatment (i.e. CTLA4, PD‐1 inhibitors) that can be utilized in patients with BRAF mutant and wild‐type cancers, have experienced recent success. However, the patient's melanoma often becomes refractory or sometimes does not respond to the initial treatment.6 Therefore, even though the survival of these patients has been lengthened, new therapeutic modalities are desperately needed for those who fail initial treatment or whose tumors become refractory to treatment.
Upstream of BRAF in melanoma cells is the protein NRAS that is part of the NRAS‐BRAF‐MEK‐ERK signaling cascade.7 Recently, five different isoforms of NRAS were discovered, and it was determined that overexpression of isoform 5 results in a more aggressive phenotype.8 Given the importance of this pathway in driving melanoma progression, this structure report details the structural characterization of the novel NRAS isoform 5 by two biophysical techniques, NMR and CD spectroscopy.
Results and Discussion
NRAS isoform 5 does not have GTPase activity
The NRAS isoform 5 is only 20 amino acid residues (MTEYKLVVVGAGGVGKSHVW) long, but the structural report is unavailable and the biophysical interactions by which NRAS isoform 5 increases proliferation is unknown to date. We have previously reported that increased expression of NRAS isoform 5 in cell culture systems results in the increased phosphorylation of downstream targets such as AKT, MEK, and ERK. In addition, overexpression of this isoform results in increased proliferation of cells.8 More recently our group has shown that NRAS isoform 5 is found elevated in three BRAF wild‐type cell lines and six BRAF mutated cell lines compared with normal controls.9 Therefore, NRAS isoform 5 appears to be elevated in melanoma as compared with normal melanocytes independent of the BRAF mutation status. As the full length NRAS protein is known to have GTPase activity, the GTPase activity of NRAS isoform 5 was assessed using in vitro assays. GTPase activity was negligible for NRAS isoform 5 as it was in the negative controls. Therefore, NRAS isoform 5 appears to have important role in regulating the malignant potential of cells, but its biological function is most likely governed by mechanisms other than GTPase activity. In addition, the isoform is too small to have the canonical GTP binding region.10 Residues 1 to 17 of NRAS isoform 5 are also conserved in multiple RAS family members HRAS, KRAS, and p21 (BLASTP search;11 Supporting Information Table I) and are therefore likely important for a distinct biological function. As a first step to understanding the role of NRAS isoform 5, the structure of this novel isoform was investigated using NMR and circular dichroism (CD) spectroscopy techniques.
NRAS isoform 5 is flexible
To test if NRAS isoform 5 had any secondary or tertiary structure, CD and NMR spectroscopy techniques were utilized. CD data were collected from 300 nm to 190 nm on NRAS isoform 5 (100–200 μM) in 4 mM sodium phosphate buffer, pH 6.5, 50 μM EDTA, 10 μM NaN3. It was evident from the CD data that NRAS isoform 5 is most likely a random coil when it is not bound to its target [Fig. 1(a), Supporting Information Fig. 1].
Figure 1.

NRAS isoform 5 is a flexible peptide that is induced to form an α helix in the presence of trifluoroethanol. A) The CD dichroism spectrum of NRAS isoform 5 in the absence of TFE is presented and represents a spectrum that would be expected for a random coil. B) The CD dichroism spectrum of NRAS isoform 5 in the presence of 56% TFE is presented and represents a spectrum with α helical components. In the Supporting Information data, the complete TFE titration is presented.
NMR spectroscopy was utilized to verify the CD results. Two‐dimensional (2D) homonuclear and heteronuclear experiments were acquired for the chemical shift assignments of the amino acids and determination of the structure of the peptide. The NMR chemical shifts for the 1H atoms (e.g., Hα, Hβ, and HN) were assigned using 2D TOCSY, COSY, and NOESY experiments. A 2D 1H‐13C‐HSQC was used to assist in the assignment of the alpha and side chain aliphatic protons. In the absence of trifluoroethanol (TFE), there were no observable NOEs in aqueous buffer that were characteristic of helical or ordered secondary structures. Other than intraresidue and short range sequential NOEs (i–j ≤2), only a weak medium range NOE was observed from the aromatic protons of Tyr 4 to Ηγ of Val 7/8. In addition, there was no measurable difference in the chemical shifts between the aromatic protons (δ1/δ2 or ɛ2/ɛ2) of Tyr 4 indicating no stable secondary structure in the region of the peptide around Tyr 4. Taken together, both CD and NMR data suggested that the NRAS isoform 5 is flexible and lacks any stable secondary or tertiary structure in aqueous solution. In addition, CD and NMR data were acquired in presence of magnesium to test GTPase activity. There were no changes observed to the CD or NMR spectra when magnesium was added (data not shown). This is significant as magnesium acts as a cofactor for GTPase proteins.10 This provides further evidence that the biophysical interactions associated with this isoform likely have other functions than GTPase activity. Given that the target peptide for NRAS isoform 5 is not known, TFE was utilized to induce secondary structure elements that would likely be present when it is bound to its target protein.12, 13
TFE induces secondary structure as determined by CD spectroscopy
The CD spectra of NRAS isoform 5 was measured using various amounts (11%, 14%, 21%, 45%, 56%, and 85%) of TFE. While the secondary structural elements were induced starting at ∼14 to 15% TFE, it was not until 50% TFE concentration was achieved that there was a constant amount of the predicted alpha helical components present [Fig. 1(b), Supporting Information Fig. 1]. Therefore 56% deuterated TFE was chosen for the NMR experiments (4 mM sodium phosphate, pH 6.5, 10 μM NaN3, 50 μM EDTA).
NMR spectroscopy of NRAS isoform 5 confirmed alpha helical content
To confirm secondary or tertiary helical structural elements in the presence of TFE, NMR spectroscopy was utilized. Two‐dimensional NMR experiments were acquired on Bruker AVANCE III HD 800 MHz instruments equipped with triple resonance TXI cryoprobe to determine the structure of NRAS isoform 5 in the presence of 56% TFE. The NMR chemical shifts for the atoms (Cα, Hα, Hβ, and HN) were assigned using 2D TOCSY, COSY, NOESY, and 13C‐HSQC experiments. Given some overlap in the homonuclear 2D dataset, sequential and proton side chain assignments were confirmed with 2D 1H‐13C HSQC‐TOCSY and 1H‐13C HSQC‐NOESY experiments. 100% peptide amino acid 1H was assigned. To demonstrate an example of adequate dispersion of the data, the spectrum corresponding to Hβ and Hγ for the five valines in the Cα region is presented in the Supporting Information Figure 2. Once the chemical shift assignments were done, secondary structural elements were analyzed using chemical shift indices (CSI).14, 15 The Cα chemical shifts of amino acids in random coils were subtracted from the Cα chemical shifts obtained from the 2D 1H‐13C HSQC spectrum and confirmed with 2D 1H‐13C HSQC‐TOCSY/HSQC‐NOESY spectra to calculate CSI [Fig. 2(a)]. A preponderance of positive values (≥2) for four or more consecutive residues (residues 4–9) strongly suggested the presence of α‐helical components in NRAS 5 peptide in presence of TFE.
Figure 2.
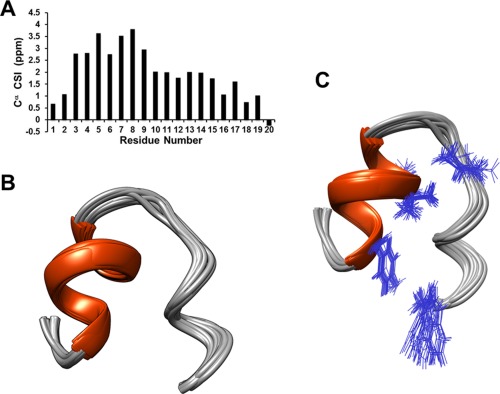
NRAS isoform 5 main structural features include a helix, turn, and coil. A) Cα chemical shift Index (CSI) is presented. This CSI is higher between residues 4 and 9 compared with other regions. Together with NOE data it was determined that the alpha helix extends from Tyr 4 to Gly 10. B) Twenty‐five independently derived NMR structures from the program CYANA are superimposed. C) The structural ensemble is shown with the side chains of residues (Tyr 4, Val 8, Val 9, Val 14, and Trp 20) important for the tertiary fold of NRAS isoform 5.
In order to determine the tertiary structure, 2D NOESY data were acquired at two different mixing times (200 and 400 ms). The NOESY data with 400 ms NOE mixing time was used for distance restraints to determine the tertiary structure. We assigned ∼21.0% of the total NOEs and then this NOE list was entered into the program CYANA to automatically assign the remaining assignable NOEs.16 The NOE derived distance restraint list was improved by iteratively comparing the CYANA output to the NOE peaks present in the NOESY spectrum. The secondary structure was defined by 69 sequential NOEs and 39 medium range NOEs. More importantly, many NOEs characteristic of helical structure were observed in the NOESY data. For example NOEs were measured from Hα (i) → HN (i + 3, i + 4) for residues Tyr 4 and Leu 6. In addition, NOEs were measured from Hα (i) → Hβ (i + 3) for residues Tyr 4, Lys 5, and Leu 6. The helix breaks with the sequence GAGG as is predicted from previous studies.17, 18 The tertiary fold was determined by 10 key long‐range NOEs. The flexible and unstructured C‐terminal (in aqueous solution) is brought in close proximity to the helix by four NOEs between Val 8 and/or 9 (V8(Hγ), V9(Ηα), and V9(Ηγ)) to Val 14 (Hβ, HN), and five NOEs between Tyr 4 (Hɛ(1)) and Try 20 (Hβ(2), Hβ(3), Hδ(1), Hɛ(3)) to form a helix‐turn‐coil structure in presence of TFE. In addition, these interactions were key to fix the tyrosine aromatic ring leading to distinct proton chemical shifts for the aromatic Hδ(1) (7.16), Hδ(2) (7.08),Hɛ(1) (6.81), and Hɛ(2) (6.77). The structural characteristics presented in Table 1 suggest that this peptide is highly flexible in aqueous solution but will most likely adopt a tertiary structure (helix‐turn‐coil) in the presence of its natural binding partner [Table 1, Fig. 2(b)].
Table 1.
Structural Statistics for the Final Structures
| Completeness of resonance assignments | |
| Cα | 20/20 |
| 1H | 100/100 |
| Distance restraints | |
| Total NOE‐derived distances | 202 |
| Intraresidue | 84 |
| Inter‐residue | |
| Sequential (|i – j| = 1) | 69 |
| Medium‐range (1 < |i – j | < 5) | 39 |
| Long‐range (|i – j| ≥ 5) | 10 |
| Distance restraints per residue | 10.1 |
| Restraint violations (per structure) | |
| NOE violations > 0.4 Å 0 | |
| Ramachandran analysis (PROCHECK) | |
| Most favored region | 77.80% |
| Allowed | 17.10% |
| Generously allowed | 5.10% |
| Disallowed | 0.00% |
| RMSD to mean structures | |
| Backbone | 0.29 ± 0.15 Å |
| Heavy atoms | 0.49 ± 0.21 Å |
The helix‐turn‐coil structure of NRAS isoform 5 is supported by the literature. While the majority of structures solved in the presence of TFE are alpha helical in nature, many other structures may be found in the presence of TFE.19 It is known that TFE induces a compact state for protein structures.19, 20 In addition, it has been observed that clusters of TFE may mimic the interior of a protein, induce turns, and fix aromatic rings.19, 21, 22, 23, 24 Therefore, our calculated structure of NRAS isoform 5 is consistent with the TFE literature.
NRAS Isoform 5 is constructed by the fusion of the first 17 codons of exon 2 and 3 codons toward the end of exon 5.8 There is one deposited structure in the PDB database for the native NRAS isoform (3CON, 2009), but to date this is unpublished in a journal citation. In this structure, there is a beta strand from amino acids 2 to 9 and an alpha helix beginning at residue 16. However, in NRAS isoform 5 there is no additional beta strand to form a sheet. In addition, amino acids 18 to 20 in NRAS isoform 5 are derived from a different portion of the NRAS native protein. Therefore, it was not unexpected that NRAS isoform 5 has a different structure than the one suggested in the pdb database that was not published. In addition, the PDBeFold server was utilized using the default settings of 70% lowest acceptable match of the query (NRAS isoform 5) and the target (entire pdb database).25 When this is done 856 matches are obtained. Upon manually inspecting the results several large proteins binding to small peptides were found in the search. These include a peptidyl bivalent inhibitor bound to α‐thrombin and the RB tumor suppressor bound to the transactivation domain of E2F‐2. Therefore, it is likely that NRAS isoform 5 binds to another protein to increase the aggressiveness of melanoma cells.8, 9
Conclusion
In this report, we have determined a three‐dimensional solution structural model of NRAS isoform 5 in the presence of TFE. Given the increased growth shown by cells overexpressing this isoform, the structural characteristics in the presence of TFE can be used to find the putative target. The isoform forms a helix‐turn‐coil type structure. The availability of this structure will likely have significance for understanding the biology of melanoma.
Data deposition
The PDB coordinates of the NRAS isoform 5 solution structure have been deposited in the PDB as entry 2N9C. The BMRB entry assigned accession number is 25900.
Methods
NMR spectroscopy
NMR spectroscopy
The NMR sample consisted of ∼1 mM NRAS isoform 5 in 4 mM phosphate buffer, pH 6.5 with 50 to 200 µM deuterated EDTA and 10 µM NaN3, ± deuterated TFE. Deuterated chemicals were purchased from Cambridge Isotopes (Tewksbury, MA) or Sigma (St. Louis, MO), and NMR tubes were from Wilmad Glass (Vineland, NJ). NRAS Isoform 5 was synthesized by Genscript (Piscataway Township, NJ). The peptide was C‐acetylated and N‐amidated. NMR data were acquired at 25°C with a Bruker Avance III HD 600 and 800 MHz NMR spectrometers (Campus Chemical Instrument Center NMR facility), each equipped with a triple resonance z ‐axis gradient TXI (1H, 13C, 15N) cryoprobe. The experiments included 2D 1H‐homonulcear DQF‐COSY, TOCSY (60 ms DIPSI2 mixing time), NOESY (200 ms and 400 ms mixing times), 2D heteronuclear 1H‐13C HSQC, 2D 1H‐13C HSQC‐NOESY (400 ms mixing time), and 2D 1H‐13C HSQC‐TOCSY (60 ms DIPSI2), all using standard Bruker pulse sequences.
Data were processed on Linux workstations using the processing program nmrPipe and Pine‐SPARKY and Topspin®.26, 27 All chemical shifts are calibrated against an external reference, 2,2‐dimethy‐2‐silapentane‐5‐sulfonate (DSS) (0.0 ppm) at 25°C. Sequential and NOE assignments were performed using visual aid of the programs SPARKY and Topspin®.27 NOE assignments were converted to CYANA format. CYANA was utilized to assign ambiguous assignments in an iterative fashion as previously described and calculate the peptide structures using 10,000 calculation energy steps for each peptide.16 The resulting pdb files containing the peptide structures were analyzed with pdbSTAT and PROCHECK to categorize the structural characteristics of the peptide.28, 29 The Ramachandran plot was produced using the CYANA software using the PROCHECK criteria.
Circular dichroism
The Jasco J‐815 circular dichroism spectrometer was utilized to measure CD spectra with or without TFE.30 Typical CD sample conditions include 100 to 200 μM NRAS isoform 5, 4 mM sodium phosphate buffer, pH 6.5, 50 μM EDTA, 10 μM NaN3 ± TFE. CD data was collected from 300 nm to 190 nm at a rate of 100 nm/min, bandwidth of 1.0 nm, step resolution of 1.0 nm, path length of 0.1 cm, and three scans for each concentration of TFE at a temperature of 20°C.
Measurement of GTPase activity
NRAS isoform 5 was placed into pT7CFE1‐Chis vector for in vitro translation of the peptide per product recommendation (Themo Scientific, #88882). A Bradford assay was utilized to normalize the protein concentrations and the GTPase activity was measured using a colorimetric malachite green assay to detect the free inorganic phosphate using the GTPase assay kit (Innova Biosicences, #602‐0120).
Supporting information
Supporting Information
Supporting Information Table.
Acknowledgments
Authors acknowledge Marina Bakhtina, Ph.D. for her technical assistance on the Jasco CD spectrometer in the OSU Biophysical Interaction and Characterization Facility and Isaac P. Foote for his assistance in producing the circular dichroism figures for this paper. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the Pelotonia Fellowship Program. J. Markowitz is currently supported by the Donald A. Adam Comprehensive Melanoma Research Center at Moffitt Cancer Center.
References
- 1. Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA (2009) Increasing burden of melanoma in the United States. J Invest Dermatol 129:666–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A (2015) Cancer Stat 65:5–29. [DOI] [PubMed] [Google Scholar]
- 3. Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, Kudchadkar R, III Burris HA, , Falchook G, Algazi A, Lewis K, Long GV, Puzanov I, Lebowitz P, Singh A, Little S, Sun P, Allred A, Ouellet D, Kim KB, Patel K Weber J (2012) Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. New Engl J Med 367:1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hartsough EJ, Basile KJ, Aplin AE (2014) Beneficial effects of RAF inhibitor in mutant BRAF splice variant‐expressing melanoma. Mol Cancer Res 12:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basile KJ, Le K, Hartsough EJ, Aplin AE (2014) Inhibition of mutant BRAF splice variant signaling by next‐generation, selective RAF inhibitors. Pigment Cell Melanoma Res 27:479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sondak VK, Gibney GT (2014) Indications and options for systemic therapy in melanoma. Surgical Clin North Am 94:1049. [DOI] [PubMed] [Google Scholar]
- 7. Cantwell‐Dorris ER, O'Leary JJ, Sheils OM (2011) BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Therapeut 10:385–394. [DOI] [PubMed] [Google Scholar]
- 8. Eisfeld AK, Schwind S, Hoag KW, Walker CJ, Liyanarachchi S, Patel R, Huang X, Markowitz J, Duan W, Otterson GA, III Carson WE, , Marcucci G, Bloomfield CD de la Chapelle A (2014) NRAS isoforms differentially affect downstream pathways, cell growth, and cell transformation. Proc Natl Acad Sci USA 111:4179–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duggan M, Stiff A, Latchana N, Markowitz J, Eisfeld AK, de la Chapelle A, Carson WE (2015) NRAS mRNA is differentially spliced to give five distinct isoforms: implications for melanoma therapy. Late Breaking Abstract. Society for Melanoma Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kjeldgaard M, Nyborg J, Clark BF (1996) The GTP binding motif: variations on a theme. FASEB J 10:347–1368. [PubMed] [Google Scholar]
- 11. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI‐BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roccatano D, Colombo G, Fioroni M, Mark AE (2002) Mechanism by which 2,2,2‐trifluoroethanol/water mixtures stabilize secondary‐structure formation in peptides: a molecular dynamics study. Proc Natl Acad Sci USA 99:12179–12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Myers JK, Pace CN, Scholtz JM (1998) Trifluoroethanol effects on helix propensity and electrostatic interactions in the helical peptide from ribonuclease T1. Protein Sci 7:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wishart DS, Sykes BD (1994) Chemical shifts as a tool for structure determination. Methods Enzymol 239:363–392. [DOI] [PubMed] [Google Scholar]
- 15. Wishart DS, Sykes BD (1994) The 13C chemical‐shift index: a simple method for the identification of protein secondary structure using 13C chemical‐shift data. J Biomol NMR 4:171–180. [DOI] [PubMed] [Google Scholar]
- 16. Guntert P (2004) Automated NMR structure calculation with CYANA. Methods Mol Biol 278:353–378. [DOI] [PubMed] [Google Scholar]
- 17. Bhattacharjee N, Biswas P (2013) Helical ambivalency induced by point mutations. BMC Struct Biol 13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scholtz JM, Baldwin RL (1992) The mechanism of alpha‐helix formation by peptides. Ann Rev Biophys Biomol Struct 21:95–118. [DOI] [PubMed] [Google Scholar]
- 19. Buck M (1998) Trifluoroethanol and colleagues: cosolvents come of age. Recent studies with peptides and proteins. Quart Rev Biophys 31:297–355. [DOI] [PubMed] [Google Scholar]
- 20. Walgers R, Lee TC, Cammers‐Goodwin A (1998) An indirect chaotropic mechanism of the stabilization of helix conformation of peptides in aqueous trifluoroethanol and hexafluoro‐2‐propanol. J Am Chem Soc 81:3255–3260. [Google Scholar]
- 21. Ramirez‐Alvarado M, Blanco FJ, Niemann H, Serrano L (1997) Role of beta‐turn residues in beta‐hairpin formation and stability in designed peptides. J Mol Biol 273:898–912. [DOI] [PubMed] [Google Scholar]
- 22. Rizzo V, Jackle H (1983) Sidechain vs mainchain conformational flexibility in aromatic dipeptides. J Am Chem Soc 105:4195–4205. [Google Scholar]
- 23. Soler‐Gonzalez AS, Fersht AR (1997) Helix stability in barstar peptides. Eur J Biochem 249:724–732. [DOI] [PubMed] [Google Scholar]
- 24. Zhou NE, Kay CM, Sykes BD, Hodges RS (1993) A single‐stranded amphipathic alpha‐helix in aqueous solution: design, structural characterization, and its application for determining alpha‐helical propensities of amino acids. Biochemistry 32:6190–6197. [DOI] [PubMed] [Google Scholar]
- 25. Krissinel E, Henrick K (2004) Secondary‐structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr 60:2256–2268. D [DOI] [PubMed] [Google Scholar]
- 26. Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293. [DOI] [PubMed] [Google Scholar]
- 27. Lee W, Westler WM, Bahrami A, Eghbalnia HR, Markley JL (2009) PINE‐SPARKY: graphical interface for evaluating automated probabilistic peak assignments in protein NMR spectroscopy. Bioinformatics 25:2085–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM (1996) AQUA and PROCHECK‐NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR 8:477–486. [DOI] [PubMed] [Google Scholar]
- 29. Tejero R, Snyder D, Mao B, Aramini JM, Montelione GT (2013) PDBStat: a universal restraint converter and restraint analysis software package for protein NMR. J Biomol NMR 56:337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greenfield NJ (2006) Using circular dichroism spectra to estimate protein secondary structure. Nature Protoc 1:2876–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information Table.


