Abstract
Background
Type-2 diabetes mellitus (T2DM) is a risk factor for progressive non-alcoholic fatty liver disease (NAFLD). Drugs commonly prescribed in patients with T2DM may affect liver histology by interfering with lipid metabolism and insulin resistance/secretion.
Aim
We studied if statins or antidiabetic agents were associated with non-alcoholic steatohepatitis (NASH) and significant fibrosis (SF).
Methods
We performed a cross-sectional study of 346 diabetics with biopsy-proven NAFLD. T2DM was defined as fasting glucose ≥7 mmol/L or glycated haemoglobin ≥6.5% and/or use of antidiabetics. NASH was defined according to the FLIP algorithm and SF as F2–4 Kleiner's stages.
Results
84% of patients were on antidiabetic therapy and 45% on statins. NASH and SF were present in 57% and 48% of patients. Statin-treated patients were older, more frequently male and with poorer glycaemic control despite more frequent antidiabetic therapy than those without statins; however, the prevalence of NASH (57%vs56%, p=0.868) and SF (48%vs48%, p=0.943) was not different between statin users and non-users. NASH was more common in patients on metformin or insulin than in those not treated with these drugs (60%vs47%, p=0.026; 68%vs53%, p=0.017). SF was more common in those treated with sulfonylureas (57%vs44%, p=0.030). Multivariate analyses confirmed that use of statins was independently and negatively associated with both NASH (OR (95% CI) 0.57 (0.32 to 1.01), p=0.055) and SF (OR (95% CI) 0.47 (0.26 to 0.84), p=0.011). Moreover, we found independent associations between insulin use and NASH (OR (95% CI) 2.24 (1.11 to 4.54), p=0.025) and sulfonylureas use and SF (OR (95% CI) 2.04 (1.11 to 3.74), p=0.022).
Conclusions
Several medications used in patients with diabetes are differently associated with NAFLD histology. Statin use is negatively associated, while insulin and sulfonylureas are positively associated with NASH and SF. A wider use of statins may be warranted in this high-risk population.
Keywords: NONALCOHOLIC STEATOHEPATITIS, FIBROSIS, FATTY LIVER, DIABETES MELLITUS, LIPIDS
Summary box.
What is already known about this subject?
-
▸
Type 2 diabetes mellitus is a risk factor for progressive non-alcoholic fatty liver disease (NAFLD).
-
▸
Drugs commonly prescribed in patients with type 2 diabetes mellitus may affect liver histology by interfering with insulin sensitivity and lipid profile.
-
▸
Despite their good safety profile in patients with chronic liver diseases, and current recommendations for a wider use in patients with diabetes, statins remain underprescribed.
What are the new findings?
-
▸
In patients with diabetes with NAFLD, statins show a significant and independent negative association with NASH and significant fibrosis.
-
▸
Conversely, insulin and sulfonylureas are independently and positively associated with the presence of NASH and significant fibrosis, respectively.
-
▸
Therapies commonly used for cardiovascular prevention or glycaemic control are differently associated with necroinflammation and fibrosis in NAFLD diabetic patients.
How might it impact on clinical practice in the foreseeable future?
-
▸
The potential protective effect of statins may warrant their wider use in high-risk, diabetic, NAFLD patients.
Introduction
Non-alcoholic fatty liver disease (NAFLD) covers a spectrum of liver injury strongly associated with type-2 diabetes mellitus (T2DM) as the prevalence of NAFLD in diabetics ranges from 40% to 70%.1 2 Importantly, NAFLD is also associated with cardiovascular disease, and this association is believed to be independent of traditional cardiovascular risk factors.3 NAFLD patients with T2DM are thus at high risk for progressive liver and cardiovascular disease. In this population, statins should be largely prescribed to achieve low-density lipoprotein cholesterol targets, and antidiabetic agents are required for optimal glycaemic control.4 We postulated that these common medications may also affect liver histology and therefore performed a retrospective analysis aimed to establish the association between the use of these drugs and the severity of liver injury in NAFLD.
Statins are generally safe in patients with liver disease and there is no evidence for drug-induced liver injury in patients with chronic liver disease, including NAFLD.5–9 Some data even suggest a potentially beneficial effect of statins on NAFLD histology.10 11 Metformin, the preferred first-line therapy for TD2M,4 does not appear to improve liver histology in NAFLD,12–14 although some evidence suggests a beneficial effect on hepatocellular carcinoma (HCC) development.15–18 Sulfonylureas and exogenous insulin are used as a second-line therapy in patients with more advanced T2DM, or as an alternative to metformin when poorly tolerated. In contrast to metformin, sulfonylureas and insulin have been associated with an increased risk of HCC,16 18 and their effect on liver histology in NAFLD has been insufficiently investigated so far.19
In this cross-sectional study, we investigated whether statin and antidiabetic therapies are associated with steatohepatitis and significant fibrosis in 346 patients with T2DM and biopsy-proven NAFLD.
Methods
This cross-sectional study included 346 consecutive patients with T2DM with biopsy-proven NAFLD, recruited in two centres, covering the entire spectrum of T2DM and NAFLD severity: a liver disease department and a nutrition and obesity clinic at the Pitié-Salpêtrière Hospital, Paris, France.
Among 420 consecutive patients referred to the liver disease department (Hepato cohort) for suspected NAFLD, and submitted to a first liver biopsy between January 2000 and January 2013, we included 138 patients with T2DM and biopsy-proven NAFLD.
Among 625 consecutive morbidly-obese patients who underwent bariatric surgery at the department of nutrition (Bariatric cohort) between January 2003 and January 2011, we analysed 208 patients with T2DM and biopsy-proven NAFLD.
Exclusion Criteria were: chronic liver diseases secondary to other aetiologies (notably viral, autoimmune and inherited chronic liver diseases), alcohol consumption (>30 g/day for men and >20 g/day for women), drug-induced NASH. All patients were negative for antihepatitis C virus antibodies and hepatitis B surface antigen.
The following data were recorded at the time of liver biopsy: age, medical history, body mass index (BMI) and blood pressure. Self-reported statin and antidiabetic therapies at the time of liver biopsy were recorded. The intensity of statin therapy was graded as low-to-moderate (simvastatin, pravastatin, fluvastatin or lovastatin) or moderate-to-high (atorvastatin, rosuvastatin or any statin in combination with ezetimibe). Laboratory tests included: aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ glutamyl transpeptidase (GGT), fasting glucose, glycated haemoglobin (HbA1c) and lipid profile.
T2DM was defined as fasting glucose ≥7 mmol/L or HbA1c ≥6.5% and/or the use of any antidiabetic treatment, according to the ADA statement.20 metabolic syndrome (MS) was defined according to the IDF criteria.21
All liver biopsies were adequate in terms of length, absence of fragmentation and quality of staining with H&E and Picrosirius Hemalun. All slides were read by expert liver pathologists (FC and PB). NAFLD was defined as the presence of steatosis in ≥5% of hepatocytes. NASH was diagnosed when steatosis, lobular inflammation and hepatocellular ballooning were present, according to the FLIP algorithm.22 Fibrosis was staged according to Kleiner's criteria and significant fibrosis was defined as ≥F2.23 All patients provided informed consent for performing liver biopsy.
Statistical analysis
Results were expressed as median (IQR) for continuous variables and frequencies (percentages) for categorical variables. Medians were compared by the Mann-Whitney test and nominal variables by Fisher's exact test. One-way ANalysis Of VAriance (ANOVA) and χ2 were used to test for linear trends. Several multivariate binary logistic regression models were carried out to identify if statin and antidiabetic therapies were independently associated with NASH and significant fibrosis. Variables were chosen on the basis of clinical judgement and on the results of univariate analyses: cohort origin, age, sex, BMI, high blood pressure, lipid profile, HbA1c control and liver enzymes (ALT, AST and GGT) were used in all the models. Model 1 included a single drug among statins, metformin, sulfonylureas or insulin; model 2 included a number of antidiabetic drugs and a single drug among statins, metformin, sulfonylureas or insulin; model 3 included statins and antidiabetic (metformin, sulfonylureas and insulin) medications at the same time. Model 3 was also used in additional multivariate binary logistic regression analyses to assess the independent association between the intensity of statin therapy and liver damage, and in sensitivity analyses. To exclude selection bias in the decision of treating with statins based on suspicion of advanced liver disease or high aminotransferase levels, we carried out two sensitivity analyses. The first sensitivity analysis was carried in non-cirrhotic patients, and the second one after excluding patients with ALT ≥40 U/L and/or AST ≥40 U/L. ORs and 95% CIs were reported with p values. A two-sided p value <0.05 was considered significant. Statistical analyses were performed using the statistical software package SPSS, V.17.0 for Windows (SPSS Inc, Illinois, USA).
Results
Study population
Three hundred forty six patients with T2DM and biopsy-proven NAFLD, 40% from the Hepato cohort and 60% from the Bariatric cohort, were analysed (table 1). There was a predominance of females and median age was 53 (46–60) years. Median BMI was 42 (32–49) kg/m2 and 93% of patients had MS. One hundred fifty four patients (45%) were statin-treated, 36 on low-to-moderate intensity (23 on simvastatin, 10 on pravastatin and 3 on fluvastatin) and 116 on moderate-to-high intensity (82 on atorvastatin, 24 on rosuvastatin and 10 on statin combined with ezetimibe). In two patients (excluded from the subanalysis on statin treatment intensity), the type of statin was not recorded. Eighty four per cent of patients were on antidiabetic therapy. Metformin (74%), sulfonylureas (33%) and insulin (24%) were most commonly used, and were considered for further analyses. Dipeptidyl peptidase-4 inhibitors (8%), thiazolidinediones (7%), glucagon-like peptide 1 analogues (5%), acarbose (4%) and glinides (1%) were used only in a minority of patients and their association with liver injury could not be specifically investigated. Thirty two per cent of patients received a single antidiabetic agent, 35% a combination of two and 17% a combination of three or more. Median HbA1c levels were 7.1 (6.5–8.1)%. NASH was diagnosed in 196 patients (57%), and significant fibrosis in 167 (48%).
Table 1.
General features of the study population
| Whole study population (n=346) | Hepato cohort (n=138) | Bariatric cohort (n=208) | |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 53 (46–60) | 57 (51–63) | 50 (43–56) |
| Male sex (n (%)) | 138 (40) | 72 (52) | 66 (32) |
| Cohort origin (hepato/bariatric) (n (%)) | 138 (40)/208 (60) | − | − |
| Metabolic data | |||
| BMI (kg/m2) | 42 (32–49) | 31 (27–34) | 47 (42–54) |
| High blood pressure (n (%)) | 261 (76) | 109 (79) | 152 (73) |
| On statins (n (%)) | 154 (45) | 60 (44) | 94 (45) |
| On antidiabetic drugs (n (%)) | 292 (84) | 121 (88) | 171 (82) |
| Number of antidiabetic drugs | 2 (1–2) | 2 (1–2) | 2 (1–2) |
| Metformin (n (%)) | 256 (74) | 101 (73) | 155 (75) |
| Sulfonylureas (n (%)) | 113 (33) | 54 (39) | 59 (28) |
| Insulin (n (%)) | 84 (24) | 31 (23) | 53 (26) |
| Metabolic syndrome (IDF) (n (%)) | 312 (93) | 117 (89) | 195 (96) |
| Biochemical data | |||
| HbA1c (%) | 7.1 (6.5–8.1) | 7.1 (6.5–7.9) | 7.1 (6.6–8.2) |
| Triglyceridaemia (mmol/L) | 1.7 (1.2–2.5) | 1.7 (1.2–2.7) | 1.7 (1.1–2.4) |
| Total cholesterol (mmol/L) | 4.7 (4.1–5.4) | 4.8 (4.0–5.5) | 4.6 (4.1–5.4) |
| HDL cholesterol (mmol/L) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.1 (1.0–1.4) |
| AST (U/L) | 31 (24–46) | 40 (29–58) | 27 (22–38) |
| ALT (U/L) | 41 (27–62) | 55 (38–80) | 33 (23–49) |
| GGT (U/L) | 51 (32–90) | 65 (44–120) | 44 (29–70) |
| Histological data | |||
| NASH (n (%)) | 196 (57) | 84 (61) | 112 (54) |
| Significant fibrosis (F≥2) (n (%)) | 167 (48) | 79 (57) | 88 (42) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, γ glutamyl transpeptidase; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein cholesterol; IDF, International Diabetes Federation.
Statin users versus non-users
Statin-treated patients were significantly older, more frequently male, hypertensive and with MS. As expected, total cholesterol was significantly lower in statin-treated patients than in patients without statins. Of note, patients on statins had poorer glycaemic control than those without statins despite more frequent antidiabetic therapy, probably reflecting more severe T2DM. However, the prevalence of NASH (57% vs 56%, p=0.868) and significant fibrosis (48% vs 48%, p=0.943) was not different between statin users and non-users (table 2).
Table 2.
Comparisons according to statin use
| No statins (n=192) | On statins (n=154) | p Value | |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 52 (42–58) | 55 (48–61) | <0.001 |
| Male sex (n (%)) | 65 (34) | 73 (47) | 0.011 |
| Bariatric cohort (n (%)) | 114 (59) | 94 (61) | 0.753 |
| Metabolic data | |||
| BMI (kg/m2) | 42 (32–50) | 42 (33–49) | 0.873 |
| High blood pressure (n (%)) | 123 (64) | 138 (90) | <0.001 |
| On antidiabetic drugs (n (%)) | 150 (78) | 142 (92) | <0.001 |
| Number of antidiabetic drugs | 1 (1–2) | 2 (1–2.25) | <0.001 |
| Metformin (n (%)) | 125 (65) | 131 (85) | <0.001 |
| Sulfonylureas (n (%)) | 48 (25) | 65 (42) | 0.001 |
| Insulin (n (%)) | 33 (17) | 51 (33) | 0.001 |
| Metabolic syndrome (n (%)) | 160 (88) | 152 (100) | <0.001 |
| Biochemical data | |||
| HbA1c (%) | 7.0 (6.3–7.7) | 7.5 (6.7–8.5) | <0.001 |
| Triglyceridaemia (mmol/L) | 1.7 (1.2–2.7) | 1.7 (1.1–2.4) | 0.567 |
| Total cholesterol (mmol/L) | 5.0 (4.3–5.7) | 4.3 (3.8–5.1) | <0.001 |
| HDL cholesterol (mmol/L) | 1.1 (0.9–1.4) | 1.1 (1.0–1.3) | 0.518 |
| AST (U/L) | 32 (25–46) | 31 (23–43) | 0.178 |
| ALT (U/L) | 42 (29–64) | 39 (25–61) | 0.218 |
| GGT (U/L) | 50 (31–86) | 54 (33–92) | 0.286 |
| Histological data | |||
| NASH (n (%)) | 108 (56) | 88 (57) | 0.868 |
| Significant fibrosis (F≥2) (n (%)) | 93 (48) | 74 (48) | 0.943 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, γ glutamyl transpeptidase; HbA1c, glycated haemoglobin.
Clinical profile according to antidiabetic therapy
We found significantly positive linear trends between the number of antidiabetic agents and age, prevalence of arterial hypertension and MS, and HbA1c levels (p for linear trend, all <0.001), reflecting more advanced T2DM in patients treated with multiple antidiabetic drugs. Moreover, patients treated with multiple antidiabetic agents were more frequently treated with statins (no antidiabetic drugs 22% vs 1 drug 30% vs 2 drugs 58% vs 3 or more drugs 63%, p<0.001); accordingly, they showed lower levels of total cholesterol (p<0.001). Of note, the higher the number of antidiabetic drugs, the higher the prevalence of NASH (no drugs 43% vs 1 drug 53% vs 2 drugs 63% vs 3 or more drugs 65%, p for linear trend=0.005) and significant fibrosis (no drugs 37% vs 1 drug 39% vs 2 drugs 55% vs 3 or more drugs 62%, p for linear trend=0.001).
Patients on metformin were significantly older, had significantly higher HbA1c levels and arterial hypertension and MS more frequently, and were more frequently treated with statins than patients not on metformin. NASH was significantly more prevalent in patients treated with metformin than in those not on this treatment (60% vs 47%, p=0.026), whereas the prevalence of significant fibrosis did not significantly differ according to metformin treatment (51% vs 41%, p=0.114) (table 3).
Table 3.
Comparison according to antidiabetic therapy
| On metformin |
On sulfonylureas |
On insulin |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No (n=90) | Yes (n=256) | p Value | No (n=233) | Yes (n=113) | p Value | No (n=262) | Yes (n=84) | p Value | |
| Demographic data | |||||||||
| Age (years) | 50 (42–57) | 54 (46–60) | 0.010 | 51 (43–58) | 56 (49–61) | <0.001 | 53 (46–60) | 53 (46–59) | 0.985 |
| Male sex (n (%)) | 35 (39) | 103 (40) | 0.823 | 90 (39) | 48 (43) | 0.493 | 102 (39) | 36 (43) | 0.523 |
| Bariatric cohort (n (%)) | 53 (59) | 155 (61) | 0.782 | 149 (64) | 59 (52) | 0.037 | 155 (59) | 53 (63) | 0.522 |
| Metabolic data | |||||||||
| BMI (kg/m2) | 42 (29–52) | 41 (33–49) | 0.879 | 42 (33–51) | 40 (31–48) | 0.063 | 42 (32–50) | 42 (33–49) | 0.890 |
| High blood pressure (n (%)) | 56 (63) | 205 (80) | 0.001 | 167 (72) | 94 (83) | 0.023 | 190 (73) | 71 (85) | 0.029 |
| On statins (n (%)) | 23 (26) | 131 (51) | <0.001 | 89 (38) | 65 (58) | 0.001 | 103 (39) | 51 (61) | 0.001 |
| Number of Antidiabetic drugs | 0 (0–1) | 2 (1–2) | <0.001 | 1 (1–2) | 2 (2–3) | <0.001 | 1 (1–2) | 2 (2–3) | <0.001 |
| Metformin (n (%)) | − | − | − | 156 (67) | 100 (89) | <0.001 | 190 (73) | 66 (79) | 0.271 |
| Sulfonylureas (n (%)) | 13 (14) | 100 (39) | <0.001 | − | − | − | 91 (35) | 22 (26) | 0.146 |
| Insulin (n (%)) | 18 (20) | 66 (26) | 0.271 | 62 (27) | 22 (20) | 0.146 | − | − | − |
| Metabolic syndrome (n (%)) | 71 (84) | 241 (97) | <0.001 | 206 (92) | 106 (97) | 0.049 | 232 (92) | 80 (99) | 0.026 |
| Biochemical data | |||||||||
| HbA1c (%) | 6.9 (6.5–7.6) | 7.2 (6.6–8.3) | 0.014 | 7.0 (6.4–7.9) | 7.5 (6.8–8.4) | <0.001 | 6.9 (6.3–7.5) | 8.2 (7.5–9.0) | <0.001 |
| Triglyceridaemia (mmol/L) | 1.8 (1.2–2.8) | 1.7 (1.2–2.3) | 0.379 | 1.7 (1.2–2.5) | 1.7 (1.1–2.3) | 0.435 | 1.7 (1.2–2.3) | 1.8 (1.2–2.8) | 0.064 |
| Total cholesterol (mmol/L) | 5.1 (4.3–5.9) | 4.5 (4.0–5.2) | <0.001 | 4.7 (4.0–5.5) | 4.6 (4.1–5.4) | 0.767 | 4.7 (4.1–5.5) | 4.6 (3.8–5.2) | 0.170 |
| HDL cholesterol (mmol/L) | 1.1 (1.0–1.4) | 1.1 (0.9–1.3) | 0.265 | 1.1 (0.9–1.3) | 1.2 (1.0–1.4) | 0.014 | 1.1 (0.9–1.4) | 1.1 (0.9–1.3) | 0.179 |
| AST (U/L) | 31 (25–46) | 31 (23–46) | 0.388 | 32 (24–46) | 28 (24–43) | 0.198 | 31 (23–46) | 30 (25–45) | 0.842 |
| ALT (U/L) | 41 (28–62) | 40 (26–62) | 0.561 | 41 (28–64) | 40 (26–61) | 0.585 | 40 (26–64) | 42 (27–57) | 0.570 |
| GGT (U/L) | 49 (35–75) | 52 (31–93) | 0.940 | 49 (30–90) | 55 (36–90) | 0.156 | 49 (31–82) | 65 (34–116) | 0.016 |
| Histological data | |||||||||
| NASH (n (%)) | 42 (47) | 154 (60) | 0.026 | 132 (57) | 64 (57) | 0.998 | 139 (53) | 57 (68) | 0.017 |
| Significant fibrosis (F≥2) (n (%)) | 37 (41) | 130 (51) | 0.114 | 103 (44) | 64 (57) | 0.030 | 120 (46) | 47 (56) | 0.105 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, γ glutamyl transpeptidase; HbA1c, glycated haemoglobin.
Similarly, patients treated with sulfonylureas were significantly older, had significantly higher HbA1c levels and arterial hypertension and MS more frequently as well as being on concurrent treatment with statins than patients without sulfonylureas. There were no significant differences in NASH prevalence according to treatment with sulfonylureas (57% vs 57%, p=0.998); however, significant fibrosis was more common in patients with sulfonylureas than in patients not taking these drugs (57% vs 44%, p=0.030) (table 3).
Insulin-treated patients had still higher HbA1c levels, presented more frequently with arterial hypertension and MS and were more frequently treated with statins than patients not on insulin. NASH was significantly more common in patients with than in those without insulin (68% vs 53%, p=0.017), whereas the prevalence of significant fibrosis did not differ significantly according to insulin treatment (56% vs 46%, p=0.105) (table 3).
Independent predictors of NASH
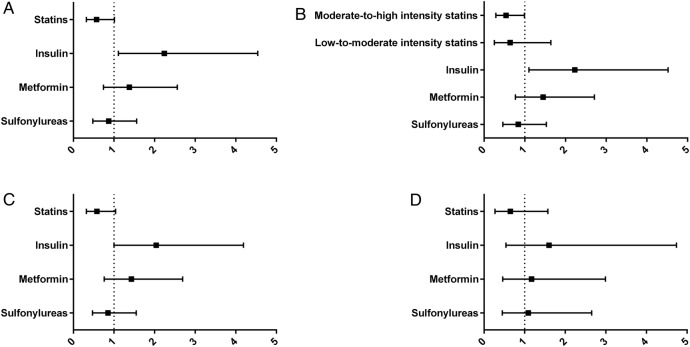
By multivariate binary logistic regression analyses, treatment with statins was negatively associated with NASH irrespective of the model used (adjusted OR (95% CI) model 1 0.61 (0.35 to 1.07), p=0.086; model 2 0.57 (0.32 to 1.01), p=0.055; model 3 0.57 (0.32 to 1.01), p=0.055), although the association did not reach statistical significance (table 4 and figure 1A).
Table 4.
Independent predictors of NASH and significant fibrosis
| NASH |
Significant fibrosis |
|||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Model 1 (cohort origin, age, sex, BMI, high blood pressure, triglycerides, total cholesterol and HDL cholesterol levels, HbA1c, ALT, AST and GGT+a single drug among statins, metformin, sulfonylureas or insulin) | ||||
| Statin use | 0.61 (0.35 to 1.07) | 0.086 | 0.52 (0.29 to 0.93) | 0.026 |
| Metformin use | 1.24 (0.68 to 2.27) | 0.476 | 1.06 (0.58 to 1.96) | 0.841 |
| Sulfonylureas use | 0.78 (0.44 to 1.36) | 0.377 | 1.73 (0.98 to 3.05) | 0.061 |
| Insulin use | 2.15 (1.08 to 4.28) | 0.029 | 1.26 (0.65 to 2.42) | 0.493 |
| Model 2 (cohort origin, age, sex, BMI, high blood pressure, triglycerides, total cholesterol and HDL cholesterol levels, HbA1c, ALT, AST and GGT+number of antidiabetic drugs and a single drug among statins, metformin, sulfonylureas or insulin) | ||||
| Statin use | 0.57 (0.32 to 1.01) | 0.055 | 0.46 (0.26 to 0.83) | 0.010 |
| Metformin use | 1.00 (0.47 to 2.12) | 0.997 | 0.58 (0.27 to 1.26) | 0.170 |
| Sulfonylureas use | 0.55 (0.28 to 1.07) | 0.076 | 1.34 (0.69 to 2.62) | 0.392 |
| Insulin use | 2.03 (0.98 to 4.17) | 0.055 | 1.01 (0.51 to 2.01) | 0.978 |
| Model 3 (cohort origin, age, sex, BMI, high blood pressure, triglycerides, total cholesterol and HDL cholesterol levels, HbA1c, ALT, AST and GGT+statins, metformin, sulfonylureas and insulin at the same time) | ||||
| Statin use | 0.57 (0.32 to 1.01) | 0.055 | 0.47 (0.26 to 0.84) | 0.011 |
| Metformin use | 1.38 (0.74 to 2.56) | 0.315 | 1.00 (0.53 to 1.89) | 0.992 |
| Sulfonylureas use | 0.87 (0.48 to 1.56) | 0.632 | 2.04 (1.11 to 3.74) | 0.022 |
| Insulin use | 2.24 (1.11 to 4.54) | 0.025 | 1.58 (0.79 to 3.14) | 0.196 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, γ glutamyl transpeptidase; HbA1c, glycated haemoglobin.
Figure 1.
Adjusted ORs of statin and antidiabetic therapies for NASH. Association between the use of statins and antidiabetic agents and the risk of NASH (adjusted ORs and 95% CI). (A) Whole study population. (B) Study population with statin therapy graded as low-to-moderate (simvastatin, pravastatin, fluvastatin or lovastatin) or moderate-to-high (atorvastatin, rosuvastatin or any statin in combination with ezetimibe) intensity. (C) In non-cirrhotic patients only (exclusion of 18 individuals with histological cirrhosis). D) In 158 individuals with normal aminotransferases (ALT<40 U/L and AST<40 U/L). ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Metformin and sulfonylureas use was not significantly associated with NASH in any models. However, insulin use showed a positive independent significant association with NASH (adjusted OR (95% CI) model 1, 2.15 (1.08 to 4.28), p=0.029; model 2, 2.03 (0.98 to 4.17), p=0.055; model 3, 2.24 (1.11 to 4.54), p=0.025) (table 4 and figure 1A).
Independent predictors of significant fibrosis
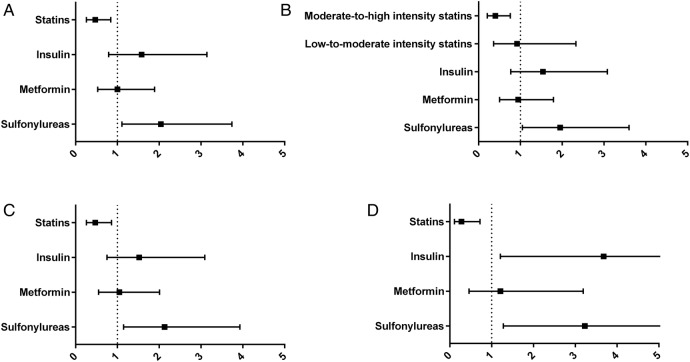
At multivariate binary logistic regression analyses, treatment with statins was significantly and negatively associated with significant fibrosis irrespective of the model used (adjusted OR (95% CI) model 1 0.52 (0.29 to 0.93), p=0.026; model 2 0.46 (0.26 to 0.83), p=0.010; model 3 0.47 (0.26 to 0.84), p=0.011) (table 4, figure 2A).
Figure 2.
Adjusted ORs of statin and antidiabetic therapies for Significant Fibrosis. Association between the use of statins and antidiabetic agents and the risk of significant fibrosis (adjusted ORs and 95% CI). (A) Whole study population. (B) Study population with statin therapy graded as low-to-moderate (simvastatin, pravastatin, fluvastatin or lovastatin) or moderate-to-high (atorvastatin, rosuvastatin or any statin in combination with ezetimibe) intensity. (C) In non-cirrhotic patients only (exclusion of 18 individuals with histological cirrhosis). (D) In 158 individuals with normal aminotransferases (ALT<40 U/L and AST<40 U/L). ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Metformin and insulin use was not associated with significant fibrosis in any models. However, sulfonylureas use showed a positive independent association with significant fibrosis in all the models, with the exception of model 2 (adjusted OR (95% CI) for model 1 1.73 (0.98 to 3.05), p=0.061; model 2 1.34 (0.69 to 2.62), p=0.392; model 3 2.04 (1.11 to 3.74), p=0.022) (table 4, figure 2A).
Associations between intensity of statin therapy and liver damage
When assessing the independent associations between the intensity of statin therapy and liver damage, moderate-to-high intensity treatment remained the only significant factor for NASH and significant fibrosis. Indeed, adjusted ORs of low-to-moderate intensity statin therapy for NASH and significant fibrosis were 0.64 (0.25 to 1.64), p=0.354 and 0.92 (0.36 to 2.33), p=0.852, respectively; in contrast, adjusted ORs of moderate-to-high intensity regimens for NASH and significant fibrosis were 0.54 (0.29 to 0.99), p=0.047 and 0.40 (0.21 to 0.76), p=0.005, respectively (figure 1B, 2B).
Sensitivity analyses
After excluding the 18 individuals with histological liver cirrhosis, the associations between statin use and liver damage did not show substantial modifications; in particular, adjusted ORs of NASH and significant fibrosis for statin use were 0.58 (0.32 to 1.04), p=0.067 and 0.47 (0.26 to 0.86), p=0.014, respectively. Similarly, insulin use remained associated with NASH (adjusted OR (95% CI) 2.04 (1.00 to 4.19), p=0.051) and sulfonylureas use with significant fibrosis (adjusted OR (95% CI) 2.13 (1.15 to 3.93), p=0.016) (figure 1C, 2C).
When analysing the 158 individuals with normal aminotransferases, the association between statin use and NASH was lost (adjusted OR (95% CI) 0.65 (0.27 to 1.57), p=0.340). However, statin use remained significantly and negatively associated with significant fibrosis (adjusted OR (95% CI) 0.28 (0.11 to 0.72), p=0.008). Interestingly, no antidiabetic agents were significantly associated with NASH in patients with normal aminotransferases. However, in this particular population, sulfonylureas and insulin were significantly and positively associated with significant fibrosis (adjusted OR (95% CI) of sulfonylureas: 3.23 (1.28 to 8.12), p=0.013; adjusted OR (95% CI) of insulin: 3.68 (1.21 to 11.18), p=0.022) (figure 1D, 2D).
Discussion
The main finding of this study is that common medications used for cardiovascular prevention or glycaemic control in diabetics are differently associated with NAFLD histological severity. Statins demonstrated a significant and independent negative association with NASH and significant fibrosis. This was confirmed even after excluding patients in whom the clinical suspicion of advanced liver disease may have biased the decision to treat with statins. Moreover, this beneficial association may be largely due to moderate-to-high intensity statin regimens, including atorvastatin, rosuvastatin and ezetimibe-containing combinations. In contrast, insulin and sulfonylureas were independently and positively associated with the presence of NASH and of significant fibrosis, respectively.
In addition to their lipid-lowering activity, statins also have systemic anti-inflammatory, antioxidant and antithrombotic properties.6 This could confer a certain level of protection against steatohepatitis and fibrosis. In this series, the negative association between statin use and histology was independent of demographic, anthropometric and metabolic factors and concurrent antidiabetic treatments. Statins with the highest efficacy for the reduction of cholesterol levels were also those associated with a reduced risk of histological lesions. This finding corroborates the possible link between the intensity of statin effects and hepatic protection. The few available trials of statins in NAFLD have shown improvement of steatosis and biomarkers of liver injury, but not on histological necroinflammation or fibrosis.10 14 24–26 These underpowered trials contrast with several cross-sectional studies, confirming our current findings that statin-treated patients are less likely to have the full spectrum of liver damage related to NAFLD.6 11 19 A multicentre European study showed that statins were independently associated with protection from steatosis, NASH and significant fibrosis in 1201 individuals at high risk for NASH. In line with our findings, the authors found a dose–dependent relationship between the intensity of statin treatment and the reduced risk of liver damage.11 A cross-sectional study of US NAFLD patients with T2DM confirmed that statin therapy is negatively associated with advanced fibrosis.19 Further support for a beneficial effect of statins comes from longitudinal studies showing an independent reduction in overall and liver-related morbidity and mortality in NAFLD patients.27 Nonetheless, these data need to be interpreted with caution. Associations from transversal studies do not necessarily support a causative process. In addition and short of randomised interventions, they do not provide specific evidence that exposure to statins will result in histological improvement. However, the data available so far collectively favour a wider use of statins in NAFLD, given their cardiovascular benefits and the demonstration that their use is not associated with more advanced liver injury. Statins are underprescribed28 despite their good safety profile in patients with chronic liver diseases,7–9 and current recommendations for use in all patients with T2DM older than 40.29 In our high-risk cohort of diabetic, mainly obese, NAFLD individuals, less than half of the patients were on statins.
Antidiabetic therapies include drugs that improve insulin sensitivity such as metformin, drugs that increase circulating insulin levels such as sulfonylureas, or exogenous insulin in advanced patients with relative insulinopaenia. Our study suggests that antidiabetic medications that increase insulin levels or deliver exogenous insulin are independently associated with an increased risk of progressive NAFLD. Patients treated with sulfonylureas had a twofold increase in the risk of significant fibrosis, while those treated with insulin had a twofold increase in the risk of NASH. Moreover, in the subset of individuals with normal aminotrasferases, sulfonylureas and insulin were significantly associated with significant fibrosis. These findings could be simply due to the fact that the severity of T2DM negatively impacts on liver histology. In fact, patients treated with sulfonylureas and/or insulin often have a longer duration of T2DM or a poorer glycaemic control. However, we performed several multivariate analyses adjusting for covariates that are associated with steatohepatitis or significant fibrosis in NAFLD, and all confirmed an independent association of these drugs with liver injury. Of note, other studies suggested that sulfonylureas and insulin are associated with an increased risk of HCC.16 18 Insulin and the insulin-like growth factor (IGF) axis play a pivotal role in hepatocarcinogenesis by stimulating proliferation of liver cells, inhibiting apoptosis and promoting cell migration.30 The effect of insulin signalling on pathways of liver injury, inflammation and fibrogenesis is less well established. However, it has been suggested that hyperinsulinaemia and disruption of the IGF axis, further to their direct hepatocarcinogenic potential, may also be associated with progression of chronic liver disease, including NAFLD.31 32 Consistent with our findings, a recent study showed that use of insulin and sulfonylureas were both positively and independently associated with advanced fibrosis in diabetic patients with NAFLD.19
In contrast with insulin and sulfonylureas, metformin was not associated with advanced fibrosis in one report.19 Our study also shows that metformin is not associated with steatohepatitis or significant fibrosis. Therapeutic trials assessing the impact of metformin on NAFLD histology did not show beneficial effects.12 13 More recently, however, a few preclinical and retrospective human studies have suggested that metformin, by inhibiting hepatocyte proliferation, is associated with a significantly decreased risk of HCC in patients with diabetes.15–18 While our data favour a safe use of metformin in NAFLD diabetics, they also alert to the possibility of more advanced liver damage associated with the use of sulfonylureas or exogenous insulin. Our study has several limitations. Data on duration of diabetes, and dose and duration for these drugs were not available, so it is not possible to draw conclusions about the minimal drug exposure required to detect an effect on liver histology. Importantly, owing to its cross-sectional design, we have to emphasise that only statistical associations are described and that causative relationships between drug intake and effects on liver histology cannot be established on the basis of this study. Despite these limitations, a strength of this study is the large number of patients with thorough clinical and histological characterisation, allowing adjustment for multiple confounders.
In conclusion, our data support the hypothesis that statin and antidiabetic therapies commonly prescribed in patients with diabetes are differently associated with necroinflammation and fibrosis in NAFLD. Diabetic patients with NAFLD who are treated with statins, expecially with moderate-to-high intensity regimens, have a lower risk of being diagnosed with advanced forms of NAFLD. Conversely, sulfonylureas and exogenous insulin are associated with an increased risk of NASH and significant fibrosis. No associations were found for metformin. These findings suggest a potential protective effect of statins which may warrant their wider use in high-risk, diabetic, NAFLD patients. Conversely, since sulfonylureas and insulin are indicated for glycaemic control despite a possible worsening of liver injury, specific pharmacological therapy for NASH is acutely needed.
Acknowledgments
The authors thank members of the LIDO (Liver Injury in Diabetes and Obesity) Study Group: André Grimaldi, Philippe Giral, Eric Bruckert, Arnaud Basdevant, Karine Clement, Jean-Michel Oppert, Agnès Hartemann-Heurtier, Fabrizio Andreelli, Sophie Gombert, Sophie Jacqueminet, Arnaud Cocaul, Fabienne Fouffelle, Joseph Moussalli, Dominique Thabut, Pascal Lebray, Philippe Podevin, Dominique Bonnefont-Rousselot, Randa Bittar, Yves Benhamou, Carole Bernhardt, Hôpital Pitié-Salpêtrière; Christian Boitard, Etienne Larger, Agnès Sola, Martine El-Etr, Hôpital Hotel-Dieu; Jean-François Gautier, Hôpital Saint-Louis; Lawrence Serfaty, Chantal Housset, Jacqueline Capeau, Hôpital Saint-Antoine, all in Paris, France.
Footnotes
Contributors: FN, the guarantor of the article, analysed the data and wrote the paper; JA-W, RP, CP, FC and PB performed the research, collected the data and revised the paper; JT, TP and KC contributed to the design of the study; VR designed the research study and revised the paper. All authors approved the final version of the article, including the authorship list.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Leite NC, Salles GF, Araujo AL, et al. . Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int 2009;29:113–19. doi:10.1111/j.1478-3231.2008.01718.x [DOI] [PubMed] [Google Scholar]
- 2.Williamson RM, Price JF, Glancy S, et al. . Prevalence of and risk factors for hepatic steatosis and nonalcoholic fatty liver disease in people with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care 2011;34:1139–44. doi:10.2337/dc10-2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341–50. doi:10.1056/NEJMra0912063 [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014;37(Suppl 1):S14–80. doi:10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- 5.Nascimbeni F, Pais R, Bellentani S, et al. . From NAFLD in clinical practice to answers from guidelines. J Hepatol 2013;59:859–71. doi:10.1016/j.jhep.2013.05.044 [DOI] [PubMed] [Google Scholar]
- 6.Pastori D, Polimeni L, Baratta F, et al. . The efficacy and safety of statins for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis 2015;47:4–11. doi:10.1016/j.dld.2014.07.170 [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N, Aljadhey H, Kesterson J, et al. . Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology 2004;126:1287–92. doi:10.1053/j.gastro.2004.02.015 [DOI] [PubMed] [Google Scholar]
- 8.Browning JD. Statins and hepatic steatosis: perspectives from the Dallas heart study. Hepatology 2006;44:466–71. doi:10.1002/hep.21248 [DOI] [PubMed] [Google Scholar]
- 9.Lewis JH, Mortensen ME, Zweig S, et al. . Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 2007;46:1453–63. doi:10.1002/hep.21848 [DOI] [PubMed] [Google Scholar]
- 10.Ekstedt M, Franzén LE, Mathiesen UL, et al. . Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol 2007;47:135–41. doi:10.1016/j.jhep.2007.02.013 [DOI] [PubMed] [Google Scholar]
- 11.Dongiovanni P, Petta S, Mannisto V, et al. . Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol 2015;63:705–12. doi:10.1016/j.jhep.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 12.Uygun A, Kadayifci A, Isik AT, et al. . Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2004;19:537–44. doi:10.1111/j.1365-2036.2004.01888.x [DOI] [PubMed] [Google Scholar]
- 13.Bugianesi E, Gentilcore E, Manini R, et al. . A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 2005;100:1082–90. doi:10.1111/j.1572-0241.2005.41583.x [DOI] [PubMed] [Google Scholar]
- 14.Musso G, Cassader M, Rosina F, et al. . Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia 2012;55:885–904. doi:10.1007/s00125-011-2446-4 [DOI] [PubMed] [Google Scholar]
- 15.Donadon V, Balbi M, Mas MD, et al. . Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int 2010;30:750–8. doi:10.1111/j.1478-3231.2010.02223.x [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Singh PP, Singh AG, et al. . Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol 2013;108:881–91; quiz 92 doi:10.1038/ajg.2013.5 [DOI] [PubMed] [Google Scholar]
- 17.Chen HP, Shieh JJ, Chang CC, et al. . Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut 2013;62:606–15. doi:10.1136/gutjnl-2011-301708 [DOI] [PubMed] [Google Scholar]
- 18.Hassan MM, Curley SA, Li D, et al. . Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer 2010;116:1938–46. doi:10.1002/cncr.24982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goh GBB, Pagadala MR, Dasarathy J, et al. . Diabetes mellitus, insulin, sulfonylurea and advanced fibrosis in non-alcoholic fatty liver disease. J Diabetes Metab 2014;5:410. [Google Scholar]
- 20.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl 1):S62–9. doi:10.2337/dc10-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberti KG, Eckel RH, Grundy SM, et al. . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the study of obesity. Circulation 2009;120:1640–5. doi:10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 22.Bedossa P, Consortium FP. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014;60:565–75. doi:10.1002/hep.27173 [DOI] [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM, Van Natta M, et al. . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. doi:10.1002/hep.20701 [DOI] [PubMed] [Google Scholar]
- 24.Athyros VG, Tziomalos K, Gossios TD, et al. . Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet 2010;376:1916–22. doi:10.1016/S0140-6736(10)61272-X [DOI] [PubMed] [Google Scholar]
- 25.Foster T, Budoff MJ, Saab S, et al. . Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol 2011;106:71–7. doi:10.1038/ajg.2010.299 [DOI] [PubMed] [Google Scholar]
- 26.Nelson A, Torres DM, Morgan AE, et al. . A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo-controlled trial. J Clin Gastroenterol 2009;43:990–4. doi:10.1097/MCG.0b013e31819c392e [DOI] [PubMed] [Google Scholar]
- 27.Angulo P, Kleiner DE, Dam-Larsen S, et al. . Liver fibrosis, but no other histologic features, Is associates with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–97.e10. doi:10.1053/j.gastro.2015.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blais P, Lin M, Kramer JR, et al. . Statins are underutilized in patients with nonalcoholic fatty liver disease and dyslipidemia. Dig Dis Sci 2015. doi:10.1007/s10620-015-4000-6 [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association. (8) Cardiovascular disease and risk management. Diabetes Care 2015;38(Suppl):S49–57. doi:10.2337/dc15-S011 [DOI] [PubMed] [Google Scholar]
- 30.Enguita-Germán M, Fortes P. Targeting the insulin-like growth factor pathway in hepatocellular carcinoma. World J Hepatol 2014;6:716–37. doi:10.4254/wjh.v6.i10.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svegliati-Baroni G, Ridolfi F, Di Sario A, et al. . Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology 1999;29:1743–51. doi:10.1002/hep.510290632 [DOI] [PubMed] [Google Scholar]
- 32.Paradis V, Perlemuter G, Bonvoust F, et al. . High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology 2001;34Pt 1):738–44. doi:10.1053/jhep.2001.28055 [DOI] [PubMed] [Google Scholar]