Abstract
Toxoplasmosis is a serious and life-threatening disease in humans with a high prevalence in immunocompromised persons. The disease has a wide spectrum, depending on the immune status of the person. A CNS manifestation of toxoplasmosis in an immunocompetent person is very rare and often undetected. Our case of CNS toxoplasmosis in an immunocompetent person emphasizes the radiological diagnosis, which was further confirmed by advanced microbiology technique.
Introduction
Toxoplasmosis, an infection with worldwide distribution, is the most common cause of cerebral abscess in immunocompromised patients when the absolute CD4 count is less than 100 u/L, with an incidence of 10–34% (1). The causative organism is Toxoplasma gondii (T. gondii), an obligate intracellular protozoan parasite. One-fourth of patients with HIV infection and latent T. gondii will eventually develop toxoplasmosis during the natural course of the disease (2). The incidence of CNS toxoplasmosis among HIV-infected patients in India is 1.33–3.33% (3), and if it is diagnosed late, the results are considerable morbidity and mortality. The incidence of primary toxoplasmosis in immunocompetent individuals in French Guiana, according to a study done by Carme et al, is very minimal, about 0.018% (4). However, at present, no data is available for the Indian population.
Case report
A 42-year-old male presented to our hospital with complaints of headache and neck pain for one week. He gave a history of loss of weight and appetite for the past month. There was no history of fever, altered sensorium, diplopia, or vomiting. Nor was there past history of surgeries or any other comorbid illness. On examination, his vitals were stable, and systemic examination was normal. Biochemical laboratory investigations showed elevated ESR. All other blood investigations were within normal limits. HIV and VDRL samples showed negative results. In view of the patient's headache, he was sent for MRI imaging of the brain; it showed multiple lesions that appeared hypointense on T1 sequence (Figs. 1A and B) and hyperintense on T2/FLAIR sequences (Figs. 2A, B, and C). The lesions that involved both the cerebral hemispheres showed intense homogeneous enhancement on contrast administration (Figs. 3A and B).
Figure 1.
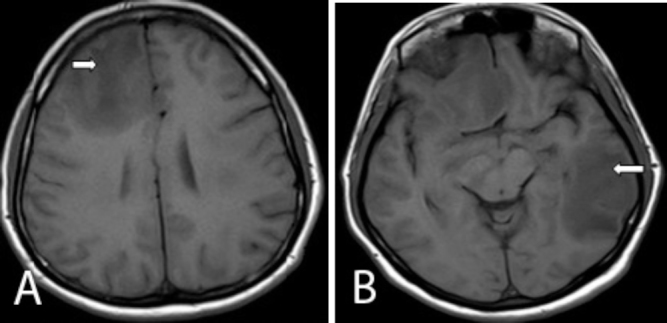
Axial T1 images of the brain show multiple hypointense lesions involving the right frontal (right arrow) and left temperoparietal lobes (left arrow).
Figure 2.
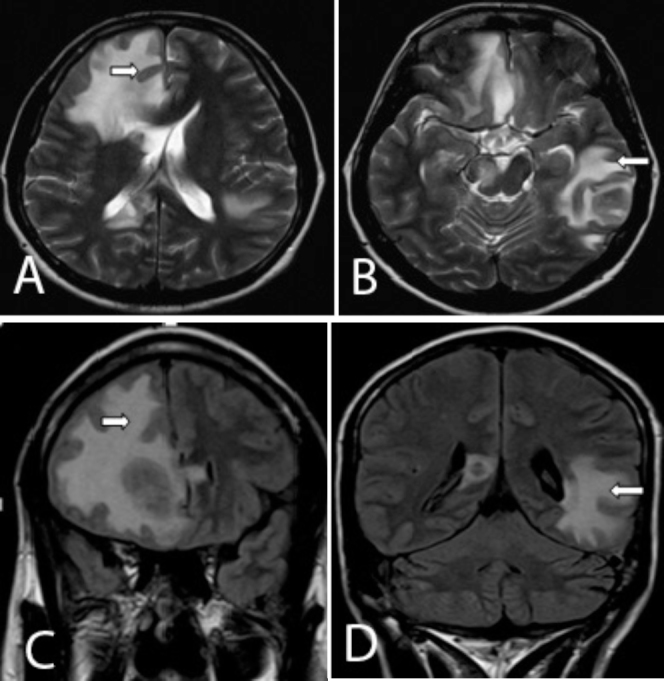
Axial T2 and coronal FLAIR images of the brain show multiple hyperintense lesions involving the right frontal (right arrow) and the left temperoparietal lobes (left arrow).
Figure 3.
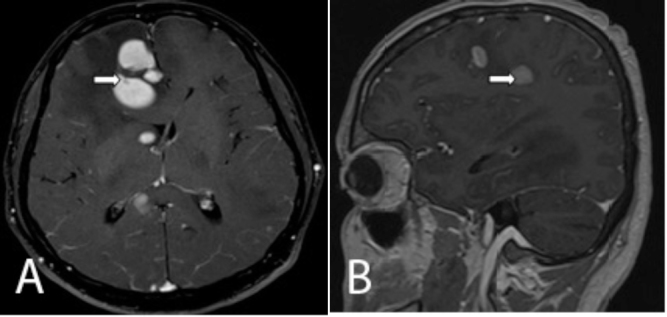
Axial and sagittal postcontrast T1W images show intensely enhancing lesions involving the bilateral cerebral hemispheres (right arrow),
Initially, in view of the patient's age, history, and imaging features, a diagnosis of metastasis from an unknown primary was made. The patient was further evaluated for localization of the primary lesion. USG of the abdomen, bone scintigraphy, and CT of the thorax were performed and found to be normal. The patient's symptoms worsened in the next three days, and a repeat CECT of the brain was done. This showed multiple homogeneously enhancing, hyperdense lesions with surrounding perilesional edema involving both the cerebral hemispheres (Figs. 4A and B). Adding this information to that on the MRI and CT brain images, the possibility of primary CNS lymphoma was raised.
Figure 4.
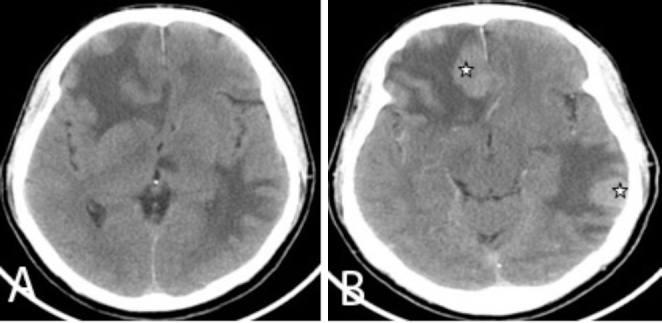
Axial pre- and postcontrast CT brain images show multiple intensely enhancing lesions (star) involving the bilateral cerebral hemispheres.
The patient underwent MRI of the brain on the same day. This showed new lesions involving the left thalamus and globus pallidus (Fig. 5A). The lesion involving the left thalamus showed restricted diffusion on DWI (Fig. 5B) and low signal on ADC (Fig. 5C). MR spectroscopy showed an elevated lipid lactate peak (Figs. 6A and B). In view of the multiplicity of lesions and the onset of newer lesions within a span of one week (showing mixed, nodular enhancement pattern and a raised lipid lactate peak on MR spectroscopy), the possibility of CNS toxoplasmosis was raised. A sample from lumbar puncture was sent for PCR analysis. CSF analysis showed elevated Toxoplasma IgG of more than 200 (Ref range > 10 in an immunocompetent person) and an IgM greater than 3.1 (Ref range > 1.2 in an immunocompetent person). Toxoserology confirmed the radiological diagnosis of CNS toxoplasmosis. The patient was discharged against medical advice. Followup revealed that the patient had died due to respiratory failure. However, no autopsy was performed.
Figure 5.
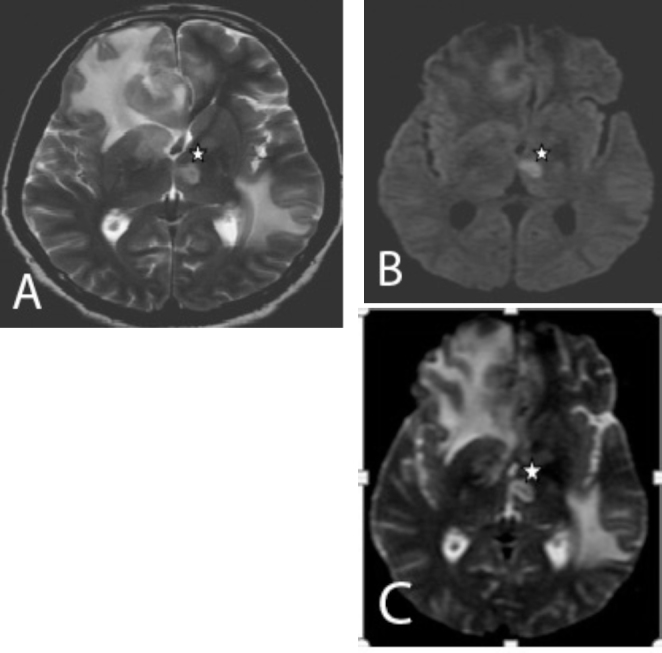
A and B. Axial T2W and DWI images show new hyperintense lesions with restricted diffusion in the left thalamus and the globus pallidus (star). C. Axial ADC image shows low signal in the left thalamus, suggestive of true restriction.
Figure 6.
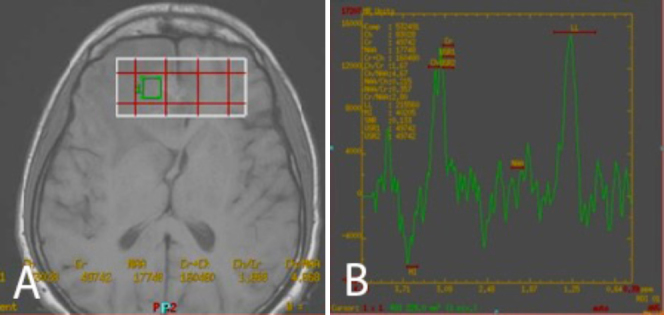
MR spectroscopy of the lesion involving the right frontal lobe shows elevated lipid lactate peak.
Discussion
Toxoplasmosis is a disease caused by Toxoplasmosis gondii, an intracellular obligate protozoal parasite. T. gondii has three forms: the tachyzoite (the rapidly reproducing form), the bradyzoite (a slower reproducing form contained in tissue cysts), and the sporozoite (contained in oocysts) (5, 6).
Immunocompetent persons with an acute primary infection are usually asymptomatic. However, during the dormant phase of the disease, the organisms may be seen within the gray and white matter of the brain, skeletal muscle, heart, and alveolar lining of the lungs, mimicking Pneumocystis jirovecii infection (6, 7).
A study done by Jeffery et al in the United States listed the possible risk factors for toxoplasmosis, which include consumption of uncooked or raw foods and exposure to kittens (8). However, in our case, the cause of CNS toxoplasmosis was idiopathic.
The common presenting symptom of cerebral toxoplasmosis is headache, often accompanied by fever and altered mental status (9). Individuals may also present with visual disturbances, seizures, cranial nerve abnormalities, and sensory disturbances. The common neurological signs include motor weakness and speech disturbances (7).
The most common affected areas in CNS include the basal ganglia, corticomedullary junction, white matter, and periventricular regions.
On plain CT images, cerebral toxoplasmosis usually appears as multiple hypoattenuating or isoattenuating lesions representing abscesses with surrounding vasogenic edema and mass effect. Solitary lesions are very rare but have been reported (10). Calcifications are usually rare but can be seen after therapy with antitoxoplasmic agents. However, calcifications are very commonly seen in congenital toxoplasmosis (11). On contrast-enhanced CT, the lesions show either a thin, smooth, or ill-defined rim of enhancement or a solid, eccentric nodular enhancement. At times, no obvious enhancement has been observed (12).
On MRI, cerebral toxoplasmosis appears as hypointense lesions on T1-weighted images and may show peripheral hyperintensity (which helps to differentiate it from CNS lymphoma). The lesions on T2 and FLAIR images have high or mixed signal intensity. On contrast-enhanced T1-weighted images, the lesions show rimlike enhancement with surrounding hypointense areas (representing edema) (12).
A study done by Vastava et al observed imaging characteristics of cerebral toxoplasmosis in immunocompetent persons. This showed radiating enhancement in cortical/subcortical regions having very few nodular or ring-enhancing lesions—quite different from those in the immunocompromised patients (10). A previous report by Brightbill et al on 27 patients with toxoplasma encephalitis demonstrated three different MR imaging patterns: 37% had predominantly T2-weighted hyperintense lesions, 37% had T2-weighted isointense lesions, and 26% had lesions with mixed signal on T2-weighted images (13).
On DWI, the central portion of toxoplasmic lesions does not show restricted diffusion, which is a characteristic finding observed in pyogenic abscesses. However, the presence of hemorrhage within the walls of toxoplasmic lesions may demonstrate peripheral hyperintensity. In addition, the measured ADC values are greater than that of unaffected white matter (14).
Like toxoplasmosis, CNS lymphoma also has a predilection for the basal ganglia. Unifocal and multifocal involvements are observed in both conditions. Both have varied patterns of enhancement, edema, and mass effect on CT images, and increased signal intensity on T2-weighted MR images. Lesions in lymphoma are usually more locally infiltrative; hence, a butterfly-like pattern of spread and enhancement favors lymphoma more than toxoplasmosis. In addition to this, lymphomatous lesions are usually larger than those of toxoplasmosis (15) and tend to have a periventricular distribution (7).
SPECT and PET have been known to play a role in distinguishing toxoplasmosis and other infections from CNS lymphoma. In comparison with cerebral toxoplasmosis or other infections, lymphoma has a greater thallium uptake on SPECT images (16).
The other differential diagnoses for multiple intraparenchymal brain lesions include tuberculoma, aspergillosis, progressive multifocal leukoencephalopathy, bacterial abscess, and cryptococcosis (17, 18).
In our case, with the multiplicity of lesions and onset of newer lesions within a span of one week, showing a mixed, nodular enhancement pattern and raised lipid lactate peak on MR spectroscopy, a diagnosis of CNS toxoplasmosis was made.
CNS toxoplasmosis should be considered as an important differential diagnosis in immunocompetent patients with neurological findings and unknown etiology. CT and MRI play an important role in early diagnosis and assist the clinician to provide effective management and prevent further complications.
Footnotes
Published: March 4, 2014
References
- 1.Kornienko VN, Pronin IN. Diagnostic neuroradiology. Springer Verlag; 2009. [Google Scholar]
- 2.Grant I, Gold J, Rosenblum M, Niedzwiecki D, Armstrong D. Toxoplasma gondii serology in HIV-infected patients: the development of central nervous system toxoplasmosis. AIDS. 1990;4:519–521. [PubMed] [PubMed] [Google Scholar]
- 3.Venkatasatya SA, Kumar GA, Pratap VS, Vivekananda DV, Madhukar R, Shyam S. Neurological manifestations in HIV infected patients around Varnasi, India. African Journal of Neuroscience. 2006;25:33–40. [Google Scholar]
- 4.Carme B., Bissuel F., Ajzenberg D., Bouyne R., Aznar C., Demar M., Bichat S., Louvel D., Bourbigot A.M., Peneau C., Neron P., Dardé M.L. Severe acquired toxoplasmosis in immunocompetent adult patients in French Guiana. Journal of clinical microbiology. Nov. 2002;40:4037–4044. doi: 10.1128/JCM.40.11.4037-4044.2002. [PubMed] No. 11 0095-1137/02/$04.000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alice B, Smith, Smirniotopoulos James G., MD, Rushing Elisabeth J., COL, MC, USA Central nervous system infections associated with human immunodeficiency virus infection: Radiologic-pathologic correlation. Radiographics. November-December 2008 doi: 10.1148/rg.287085135. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Khan Ali Nawaz, MBBS, FRCS, FRCP, FRCR, Smirniotopoulos James G. Imaging in CNS toxoplasmosis. Medscape. June 11 2013 [Google Scholar]
- 7.Lee Gregory Tse, MD, Antelo Fernando, MD, Mlikotic Anton A., MD Cerebral toxoplasmosis. Radiographics. July-August 2009 doi: 10.1148/rg.294085205. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Jeffrey L, Jones, Dargelas Valerie, Roberts Jacquelin, Cindy, Press, Remington Jack S, Montoya Jose G. Risk factors for toxoplasma gondii infection in the United States. Journal of Clinical Infections Disease. September 2009 doi: 10.1086/605433. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327(23):1643–1648. doi: 10.1056/NEJM199212033272306. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Vastava P.B., Pradhan S., Jha S., Prasad K.N., Kumar S., Gupta R.K., MRI Features of toxoplasma encephalitis in the immunocompetent host. Neuroradiology. 2002 Oct;44(10):834–838. doi: 10.1007/s00234-002-0852-5. [PubMed] Epub 2002 Aug 24. [DOI] [PubMed] [Google Scholar]
- 11.Grossman RI, Yousem DM. Neuroradiology: the requisites. 2nd ed. Mosby, Elsevier Inc.; Philadelphia, Pa: 2003. p. 224. 223. [Google Scholar]
- 12.Osborn AG, Blaser SI, Salzman KL. Diagnostic imaging: brain. Amirsys; Salt Lake City, Utah: 2004. pp. 70–73. [Google Scholar]
- 13.Brightbill TC, Post MJ, Hensley GT, Ruiz A. MR of toxoplasma encephalitis: signal characteristics on T2-weighted images and pathologic correlation. J Comput Assist Tomogr. 1996;20:417–422. doi: 10.1097/00004728-199605000-00019. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Chong-Han CH, Cortez C, Tung GA. Diffusion-weighted MRI of cerebral toxoplasma abscess. Am J Roentgenol. 2003;181:1711–1714. doi: 10.2214/ajr.181.6.1811711. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Balakrishnan J, Becker PS, Kumar AJ, Zinreich SJ, McArthur JC, Bryan RN. Acquired immunodeficiency syndrome: correlation of radiologic and pathologic findings in brain. Radiographics. 1990;10(2):201–215. doi: 10.1148/radiographics.10.2.2326512. [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.Lorberboym M, Wallach F, Estok L. Thallium-201 retention in focal intracranial lesions for differential diagnosis of primary lymphoma and non-malignant lesions in AIDS patients. J Nucl Med. 1998;39(8):1366–1369. [PubMed] [PubMed] [Google Scholar]
- 17.Ciricillo SF, Rosenblum ML. Use of CT and MR imaging to distinguish intracranial lesions and to define the need for biopsy in AIDS patients. J Neurosurg. 1990;73(5):720–724. doi: 10.3171/jns.1990.73.5.0720. [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Chang L, Cornford ME, Chiang FL, Ernst TM, Sun NC, Miller BL. Radiologic-pathologic correlation: cerebral toxoplasmosis and lymphoma in AIDS. AJNR Am J Neuroradiol. 1995;16(8):1653–1663. [PubMed] [PMC free article] [PubMed] [Google Scholar]


