Abstract
Bile acid malabsorption is a common cause of chronic diarrhoea in people, however it has never previously been investigated in dogs, despite clinical suspicion of its existence. The goal of this study was to assess the feasibility of measuring serum 7α-hydroxy-4-cholesten-3-one (C4) in dogs, as a potential marker of bile acid malabsorption, and to see whether this is related to clinical disease severity or the presence of hypocobalaminaemia. Serum C4 concentration was measured in 20 clinically healthy control dogs and 17 dogs with chronic diarrhoea. Three of the 17 affected dogs (17.6 per cent) had a C4 concentration significantly above the range of clinically healthy dogs; these dogs were all poorly responsive to conventional therapy. These results suggest that bile acid malabsorption may be a clinically relevant disorder in dogs with chronic diarrhoea and serum C4 may be a useful tool to investigate this further.
Keywords: Gastroenterology, Internal medicine, Dogs, Gastrointestinal
Introduction
Malabsorption of bile acids, as a cause of diarrhoea, has been recognised in human medicine for some time (Fromm and Malavolti 1986). It is known that in patients who have undergone terminal ileal resection or have reduced ileal function due to disease, bile acid absorption is reduced, leading to the presence of bile acids in the colon. Here they cause chloride and water secretion and increase colonic motility, resulting in diarrhoea, so-called secondary or type 1 bile acid malabsorption (Johnston and others 2011). The prevalence of bile acid malabsorption is between 10 and 35.3 per cent in people with chronic diarrhoea, depending on the serum C4 cut-off used (Wedlake and others 2009, Slattery and others 2015).
Various methods have been described to diagnose bile acid malabsorption in humans (Vijayvargiya and others 2013). Until recently, the most commonly utilised was the 75selenium homotaurocholic acid test (SeHCAT); this uses a synthetic radioactive bile acid, the retention time of which can be measured using a gamma camera (Balzer 1995). A downstream product of one of the major enzymes in the bile acid synthesis pathway is 7α-hydroxy-4-cholesten-3-one (C4) and, in people, blood concentrations show good correlation with the rate of bile acid synthesis (Galman and others 2003). Bile acid synthesis is increased in patients with bile acid malabsorption as they have increased faecal bile acid losses and therefore less recycling of bile acids through the process of enterohepatic recirculation (Camilleri and others 2009). Multiple studies have now shown good correlation between serum C4 and SeHCAT in the diagnosis of bile acid malabsorption with a sensitivity of 87–90 per cent and specificity of 79–86 per cent compared with SeHCAT (Eusufzai and others 1993, Brydon and others 1996, Fan and Sellin 2009, Johnston and others 2011, Grutzner and others 2014). C4 has significant advantages over other methods as it only requires one blood sample, avoids radiation risks and does not require immediate access to expensive equipment. To the authors' knowledge, bile acid malabsorption has not been documented in dogs or cats, nor has SeHCAT been described. However, the authors have anecdotal evidence of a clinical response of patients with refractory chronic enteropathies to the bile acid sequestrant cholestyramine, which is a commonly used drug for this condition in people (Pattni and Walters 2009). An improved ability to recognise and diagnose cases of bile acid malabsorption would have significant clinical benefit as these cases may require specific treatments for this condition and may be less likely to respond to conventional therapy. The purpose of this study was to document serum C4 concentration in a group of dogs with chronic diarrhoea and to compare this to a group of clinically healthy control dogs. The authors hypothesised that approximately 10 per cent of dogs with chronic diarrhoea would be affected by bile acid malabsorption, indicated by an elevated serum C4 concentration; this figure was based on the reported prevalence in people with chronic enteropathies (Wedlake and others 2009). They also hypothesised that dogs with hypocobalaminaemia may be more likely to have elevated serum C4 as cobalamin and bile acids are absorbed in a similar region of the small intestine. Lastly, serum C4 concentration was compared with the canine chronic enteropathy activity index in order to determine whether bile acid malabsorption was related to clinical disease severity.
Materials and methods
Clinical cases were identified by searching the clinical pathology database at the Queen's Veterinary School Hospital, from January 2012 to March 2015, to identify patients in which serum cobalamin had been measured. The clinical records of these dogs were then reviewed and cases were included if they had undergone investigation of chronic diarrhoea and an adequate volume of stored, frozen serum was available in the sample archive.
Diagnostic investigations were performed as appropriate to each case, but typically included some combination of routine haematology and biochemistry, basal cortisol, faecal analysis, canine trypsin-like immunoreactivity, folate, cobalamin, abdominal ultrasound and gastrointestinal biopsies (most commonly gastric, duodenal, ileal and colonic biopsies). The final diagnosis was recorded for each case. Canine chronic enteropathy activity index scores were estimated and classified according to previously published guidance (Allenspach and others 2007) either at the time the case was investigated or retrospectively based on clinical notes. This involved severity scoring, on a scale of 0–3, 9 parameters: attitude/activity, appetite, frequency of vomiting, stool consistency, stool frequency, severity of weight loss, serum albumin levels, presence of ascites/oedema and severity of pruritus. Cases are then classified into insignificant disease (score 0–3), mild disease (4–5), moderate disease (6–8), severe disease (9–11) or very severe disease (12–27).
Controls were clinically healthy dogs identified by searching the clinical records database, over the same time period as clinical cases, for cases in which blood had been collected as part of routine pre-anaesthetic screening, most commonly prior to orthopaedic surgery. Dogs were only included if they had no history of gastrointestinal signs, an unremarkable haematology and biochemistry, and an adequate volume of stored, frozen serum was available in the sample archive. Dogs were age matched to the clinical cases.
All blood samples were taken following an overnight fast into serum gel tubes; these were separated after clotting and the samples frozen at –80°C for storage, as is standard practice for all residual samples from clinical cases at this hospital. When required, the samples were shipped from the laboratory on dry ice for batch analysis.
Serum C4 was measured using liquid chromatography-mass spectrometry (LC-MS/MS) (see online supplementary appendix 1). Briefly, to 250 μl of the sample was added 250 μl of deuterated internal standard working solution (containing 125 nmol/l of C4-d7 in acetonitrile) followed by a further 500 μl of ice-cold acetonitrile in a tube. Samples were then vortexed for 30 seconds followed by centrifugation for 10 minutes at 16,000g. The supernatant of this is transferred to a glass high-performance liquid chromatography injection phial and 50 μl in injected onto the LC-MS/MS system.
vetreco-2015-000163supp.pdf (56KB, pdf)
For LC-MS/MS, an Aria Transcend TLX-II system and TSQ Vantage were utilised (both ThermoFisher Scientific, San Jose, USA). Isocratic chromatography with a 5:95 (V/V) mixture of (A) 0.1 per cent (V/V) aqueous formic acid and (B) 0.1 per cent (V/V) formic acid in methanol was performed using an Acentis fused-core C18 analytical column (150×4.6 mm, particle size 2.7 µm, Sigma-Aldrich). Flow rate was 0.8 ml/minute with the column being maintained at 40°C using a HotPocket (ThermoFisher Scientific). LC-MS/MS was carried out in selected reaction monitoring mode (two m/z transitions) using positive atmospheric-pressure chemical ionisation.
Statistical analysis was performed using commercially available software (SPSS, IBM). A Mann-Whitney U test was used to compare the age and C4 measurements of the two groups. A Spearman's rank correlation test was used to assess the relationship between C4 concentration, serum cobalamin and clinical activity index. A receiver operating characteristic (ROC) curve was produced in order to define the sensitivity and specificity of serum C4 concentration for presumptive diagnosis of bile acid malabsorption using different cut-off values.
Results
Serum cobalamin measurements were available for 74 dogs over the study period. A total of 57 cases were excluded due to insufficient stored serum (49 cases) or inappropriate clinical history (8 cases), leaving 17 cases for analysis. Serum C4 measurement was consequently available for 17 dogs with chronic diarrhoea and 20 control dogs.
Clinical cases had an age range from 1.5 to 12 years (median 8 years), control dogs had an age range from 1.1 to 12 years (median 5.5 years) and there was no significant difference between the ages of these groups (P=0.170). The breeds of the dogs in both groups are listed in Table 1.
TABLE 1:
Breed, C4, serum cobalamin concentration and clinical features of the control and clinical cases
| Breed | Age (years) | Cobalamin (pg/ml) Normal >200 |
C4 (nmol/l) | Canine chronic enteropathy activity index | Diagnosis |
|---|---|---|---|---|---|
| Clinically healthy dogs | |||||
| Border terrier | 2.7 | N/A | 15.1 | N/A | N/A |
| Hungarian vizsla | 1.7 | N/A | 22.7 | N/A | N/A |
| Labrador retriever | 5.0 | N/A | 29.9 | N/A | N/A |
| Lurcher | 3.0 | N/A | 35.9 | N/A | N/A |
| Golden retriever | 2.0 | N/A | 40.0 | N/A | N/A |
| Cross breed | 7.0 | N/A | 41.1 | N/A | N/A |
| Cocker spaniel | 2.5 | N/A | 8.9 | N/A | N/A |
| Labrador cross | 8.2 | N/A | 58.8 | N/A | N/A |
| Yorkshire terrier | 1.3 | N/A | 74.8 | N/A | N/A |
| Labrador retriever | 6.0 | N/A | 77.2 | N/A | N/A |
| German shepherd dog | 9.0 | N/A | 84.6 | N/A | N/A |
| Rottweiler | 8.0 | N/A | 100.4 | N/A | N/A |
| Schnauzer | 8.0 | N/A | 105.0 | N/A | N/A |
| Japanese spitz | 4.5 | N/A | 105.3 | N/A | N/A |
| Labrador retriever | 3.0 | N/A | 108.2 | N/A | N/A |
| Labrador retriever | 10.0 | N/A | 143.0 | N/A | N/A |
| Labrador retriever | 12.0 | N/A | 146.5 | N/A | N/A |
| Shiba inu | 9.0 | N/A | 164.0 | N/A | N/A |
| Mastiff | 1.1 | N/A | 176.5 | N/A | N/A |
| Boxer | 9.5 | N/A | 180.2 | N/A | N/A |
| Group median | 5.5 | N/A | 80.9 | ||
| Clinical cases | |||||
| Greyhound | 8.0 | 523 | 21.3 | 6 | IBD |
| Tibetan terrier | 10.0 | 338 | 25.1 | 13 | IBD |
| Miniature poodle | 4.0 | 556 | 25.8 | 8 | IBD |
| Wheaten terrier | 7.0 | 199 | 26.2 | 15 | IBD |
| Greyhound | 10.0 | 121 | 34.2 | 7 | IBD |
| Cocker spaniel | 8.0 | 834 | 37.1 | 5 | ARE |
| German shepherd dog | 4.25 | 217 | 40.1 | 6 | ARE |
| Airedale terrier | 5.0 | 338 | 43.9 | 5 | DRE |
| German shepherd dog | 2.9 | 443 | 59.9 | 5 | EPI |
| Staffordshire bull ter. | 9.5 | 162 | 119.8 | 12 | IBD |
| Miniature schnauzer | 9.0 | 272 | 120.0 | 11 | IBD |
| Rottweiler | 1.5 | 558 | 129.3 | 5 | IBD |
| Rottweiler | 8.8 | 190 | 178.9 | 4 | DRE |
| Shiba inu | 12.0 | 353 | 194.3 | 7 | Coronavirus |
| Jack Russell ter. cross | 5.0 | 310 | 362.7 | 10 | IBD |
| Border collie | 6.0 | 100 | 438.0 | 9 | IBD |
| Border terrier | 11.0 | 550 | 518.6 | 6 | IBD |
| Group median | 8.0 | 338 | 59.9 | 7 | |
ARE, antibiotic-responsive enteropathy; DRE, diet-responsive enteropathy; EPI, exocrine pancreatic insufficiency; IBD, inflammatory bowel disease; N/A, not applicable
The final diagnosis in the cases with chronic diarrhoea included idiopathic inflammatory bowel disease (11 cases), diet-responsive enteropathy (2 cases), antibiotic-responsive enteropathy (2 cases), exocrine pancreatic insufficiency (1 case) and a suspected corona virus infection (1 case). The diagnosis of inflammatory bowel disease was based on histopathological examination of gastrointestinal biopsies, most commonly gastric, duodenal and ileal biopsies. The diagnosis of corona virus was based on faecal PCR testing and eventual resolution of clinical signs with symptomatic treatment.
Control dogs had a serum C4 range from 15.1 to 180.1 nmol/l (median 80.9 nmol/l), dogs with chronic diarrhoea had a serum C4 range from 21.3 to 518.6 nmol/l (median 59.9 nmol/l) and there was no significant difference in the C4 concentration between these two groups (P=0.8). Figure 1 shows a boxplot of the serum C4 concentration in the two groups. Serum cobalamin and canine chronic enteropathy activity index were available for all clinical cases. Cobalamin concentration ranged from 100 to 834 pg/ml (median 338 pg/ml), and canine chronic enteropathy activity index scores ranged from 4 to 15 (median 7). When assessing the relationship between serum C4 concentration and serum cobalamin, no statistically significant relationship was identified (P=0.6, correlation coefficient=−0.11). There was also no statistically significant relationship between canine chronic enteropathy activity index and serum C4 (P=0.6, correlation coefficient=−0.04).
FIG 1:
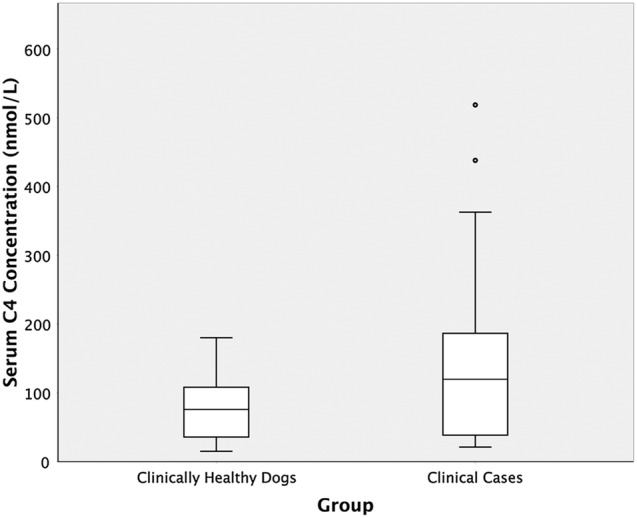
A box plot demonstrating serum C4 concentrations in clinically healthy dogs and dogs with chronic diarrhoea
A cut-off value for serum C4 concentration of 187.2 nmol/l on the ROC curve resulted in a specificity of 100 per cent (95 per cent CI 81.5 to 100 per cent) and a sensitivity of 25 per cent (95 per cent CI 5.3 to 48.6 per cent) for presumptive diagnosis of bile acid malabsorption in this group of dogs (Fig 2), with a positive predictive value (PPV) of 100 per cent (95 per cent CI 31 to 100 per cent) and a negative predictive value (NPV) of 59 per cent (95 per cent CI 41 to 75 per cent). If 187.2 nmol/l is set as the upper limit of normal, then three (17.6 per cent; 95 per cent CI 6.2 to 41.0 per cent) of the clinical cases with chronic diarrhoea had a serum C4 concentration significantly above this (at least twice this upper limit). The greatest serum C4 concentration (518.6 nmol/l) was observed in an 11-year-old, neutered male border terrier. This dog was diagnosed with suspected inflammatory bowel disease, which was partially, although never fully, responsive to dietary modification and immunosuppressive medication (prednisolone and azathioprine). This dog had a serum cobalamin concentration within the reference range (550 pg/ml). The second case with a marked serum C4 elevation (438.0 nmol/l) was a 6-year-old, neutered male border collie. This dog had a complex medical history of previous primary hypoparathyroidism and more recent inflammatory bowel disease. Concerns regarding the effect of prednisolone on serum calcium concentrations prevented use of this drug, and so the dog was primarily managed with chlorambucil and dietary modification; however, his diarrhoea was also poorly responsive to this treatment. Interestingly, at a later time this case was trialled on cholestyramine and showed an improvement in faecal consistency; however, a further dietary modification was made at the same time, making the response difficult to interpret. This dog also had significant hypocobalaminaemia (100 pg/ml), suggesting marked distal small intestinal disease. The third case (serum C4 concentration 362.7 nmol/l) was a 5-year-old, neutered female Jack Russell terrier cross. This dog presented for investigation of chronic diarrhoea and panhypoproteinaemia and was diagnosed with idiopathic inflammatory bowel disease. She was treated with prednisolone, chlorambucil and metronidazole, along with dietary modification. This resulted in an improvement of clinical signs and normalisation of serum proteins; however, the diarrhoea persisted intermittently. Malabsorption of bile acids may offer a potential explanation for the limited response of the diarrhoea to therapy; however, no therapeutic trials were ever performed to confirm this. This dog had a serum cobalamin concentration at the lower end of the reference range (310 pg/ml).
FIG 2:
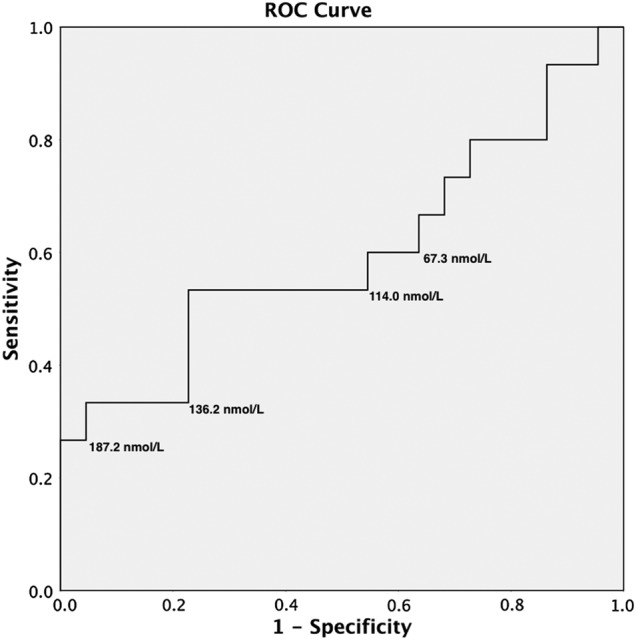
A receiver operating characteristic (ROC) curve for various cut-off levels of serum C4 concentration demonstrating the sensitivity and specificity for the presumed diagnosis of bile acid malabsorption
The remaining 14 clinical cases were treated with a variety of medications, depending on diagnosis and had a range of outcomes. The two cases of antibiotic-responsive enteropathy, two cases of diet-responsive enteropathy, two case of suspected enteric coronavirus and two case of exocrine pancreatic insufficiency were all treated appropriately for those conditions with an excellent response. The eight cases with idiopathic inflammatory bowel disease were treated with a number of medications, including prednisolone alone (five cases), prednisolone with azathioprine (one case), prednisolone with chlorambucil (one case) and prednisolone with ciclosporin (one case). Seven of these eight cases responded well to treatment with good long-term control of clinical signs; one case deteriorated despite therapy resulting in eventual euthanasia. This dog had a low serum C4 level (25.12 nmol/l).
Post hoc power analysis was performed and showed that two groups of 76 cases would be required to detect a statistically significant difference (α=0.05 and power, 1−β=0.80) if similar results were obtained in the two groups.
Discussion
In the small group of dogs examined in this study, there was no overall difference in the serum C4 concentration between dogs with and without chronic diarrhoea. In people, the reference range for serum C4 is 14.3–60.7 nmol/l. However, 12 (60 per cent) of the control dogs in this study had a concentration outside that reference range, suggesting that the reference range in dogs may be higher. The ROC analysis suggests that a cut-off value of 187.2 nmol/l would give a high specificity for the suspected diagnosis of bile acid malabsorption. The sensitivity in this study was low; largely due to the small sample size. This is likely to be improved if only dogs with a poor response to conventional therapy were tested, in which the suspicion of bile acid malabsorption might be higher. The high specificity and high PPV suggest that this test would be most useful as a screening test to increase clinical suspicion of bile acid malabsorption in dogs with chronic diarrhoea; however, false negative results are likely and a normal serum C4 concentration does not exclude this disease. Larger studies are needed to refine the reference range for dogs, with the aim of improving test sensitivity and NPV. This test is relatively inexpensive to perform (approximately £30 per sample), but is currently only available through a small number of specialist human hospital labs. However, it is being run more commonly in people and so it is likely to become more widely available in the future. The reliability of the test is also excellent, with a low coefficient of variation both intra-assay and inter-assay (see online supplementary appendix 1).
While the small numbers in this study make definite conclusions difficult, elevated serum C4 was identified in three dogs with chronic diarrhoea and, interestingly, all of these cases had clinical disease with a limited response to traditional therapy. Ideally these cases would have undergone SeHCAT in order to increase the clinical suspicion, but the authors’ results suggest that bile acid malabsorption may well be a factor in some cases of chronic enteropathy. Measuring serum C4 could therefore be useful in cases of chronic enteropathy that fail to respond to standard treatments.
We hypothesised that there may be a relationship between an elevated serum C4 concentration and hypocobalaminaemia, based on the knowledge that both abnormalities may be associated with distal small intestinal disease (Berghoff and others 2013); however, there was no evidence of such a relationship. One of the cases with particularly high serum C4 concentration did have a very low serum cobalamin, suggesting that significant distal small intestinal pathology may lead to both these changes in some cases. There was also no evidence of an association between the clinical severity of the disease, as measured by the clinical activity index, and serum C4 concentration. However, the numbers were very small in this pilot study, which was significantly underpowered to find any associations, as demonstrated by the post hoc power estimations.
It is currently unclear, in both dogs and people, why bile acid malabsorption occurs. Proposed mechanisms include a defective ileal bile acid transport system, a reduced contact time of bile acids in the ileum due to increased motility and an increased bile acid pool size due to disordered negative feedback mechanisms on bile acid synthesis (Pattni and Walters 2009).
A number of potential treatments exist for bile acid malabsorption if it is identified or suspected. Glucocorticoids have been shown to upregulate the bile acid transporter apical sodium linked bile acid transporter (ASBT) in people (Johnston and others 2011), which may explain the positive response to these medications in many cases. Glucocorticoids form the mainstay of immunosuppressive treatment in inflammatory intestinal diseases and may be treating bile acid malabsorption as a useful side effect of therapy. The bile acid sequestrant cholestyramine is the first-line treatment in most people with bile acid malabsorption and is effective in between 63 and 100 per cent of people in different studies (Wilcox and others 2014). This has been used successfully in both dogs and cats; however, it is not licensed in either of these species. An alternative sequestrant is colesevelam, which has shown benefit in patients who were intolerant or unresponsive to cholestyramine (Beigel and others 2014). However, to the authors' knowledge, colesevelam has not been used on canine patients. It is likely that dietary management is also important in managing these cases. Increasing dietary fat has been shown to increase faecal bile acid secretion in people (Koga and others 1984), and therefore, a low-fat diet is probably preferred (Westergaard 2007).
In addition to being underpowered, the retrospective nature of this study did not allow a comparison of serum C4 measurement to the gold standard of SeHCAT. It was therefore not possible to confirm that serum C4 elevations indicate malabsorption of bile acids. It is possible that serum C4 was elevated by an alternative mechanism in these patients. However, this pilot study suggests that this is a worthwhile area to investigate further. Second, accurate clinical follow-up on cases was restricted and cases with abnormal C4 concentrations were not able to be assessed for response to specific therapy. Lastly, it would have been interesting to measure serum cobalamin for the control cases as well as the clinical cases; however, as the samples came from residual archived blood, insufficient volumes were available to measure both parameters.
In conclusion, this study suggests that C4 measurement is feasible in dogs and may indicate the presence of bile acid malabsorption in some cases. Assuming that bile acid malabsorption occurs in cases with elevated serum C4, 17.6 per cent (3/17; 95 per cent CI 6.2 to 41.0 per cent) of the dogs with chronic diarrhoea in this study may have had bile acid malabsorption, similar to what has been found in people. However, larger studies are needed to define the true prevalence in dogs as the prevalence in this study is speculative. While further studies are needed to explore the clinical significance, clinicians should be aware of the potential for bile acid malabsorption in cases that are poorly responsive to conventional therapies.
Acknowledgments
ACCK is very grateful to the Alice Noakes Trust for sponsorship of his Senior Clinical Training Scholarship.
Footnotes
Contributors: All the authors made a full and individual contribution to this study.
Competing interests: None declared.
Patient consent: All owners had given prior consent for the storage and use of blood for research purposes as the time of clinical investigation.
Ethics approval: The Ethics and Welfare Committee of the Department of Veterinary Medicine, University of Cambridge.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data sharing statement: No additional data are available.
References
- Allenspach K., Wieland B., Grone A., Gaschen F. (2007) Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. Journal of Veterinary Internal Medicine 21, 700–708 doi:10.1111/j.1939-1676.2007.tb03011.x [DOI] [PubMed] [Google Scholar]
- Balzer K. (1995) The 75SeHCAT retention test—methodology and clinical applications. Medizinische Klinik (Munich, Germany: 1983) 90, 35–39 [PubMed] [Google Scholar]
- Beigel F., Teich N., Howaldt S., Lammert F., Maul J., Breiteneicher S., Rust C., Goke B., Brand S., Ochsenkuhn T. (2014) Colesevelam for the treatment of bile acid malabsorption-associated diarrhea in patients with Crohn's disease: a randomized, double-blind, placebo-controlled study. Journal of Crohn's and Colitis 8, 1471–1479 doi:10.1016/j.crohns.2014.05.009 [DOI] [PubMed] [Google Scholar]
- Berghoff N., Parnell N. K., Hill S. L., Suchodolski J. S., Steiner J. M. (2013) Serum cobalamin and methylmalonic acid concentrations in dogs with chronic gastrointestinal disease. American Journal of Veterinary Research 74, 84–89 doi:10.2460/ajvr.74.1.84 [DOI] [PubMed] [Google Scholar]
- Brydon W. G., Nyhlin H., Eastwood M. A., Merrick M. V. (1996) Serum 7α-hydroxy-4-cholesten-3-one and selenohomocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea. European Journal of Gastroenterology & Hepatology 8, 117–124 doi:10.1097/00042737-199602000-00005 [DOI] [PubMed] [Google Scholar]
- Camilleri M., Nadeau A., Tremaine W.J., Lamsam J., Burton D., Odunsi S., Sweetser S., Singh R. (2009) Measurement of serum 7α-hydroxy-4-cholesten-3-one (or 7αC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterology & Motility 21, 734–e43 doi:10.1111/j.1365-2982.2009.01288.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusufzai S., Axelson M., Angelin B., Einarsson K. (1993) Serum 7 alpha-hydroxy-4-cholesten-3-one concentrations in the evaluation of bile acid malabsorption in patients with diarrhoea: correlation to SeHCAT test. Gut 34, 698–701 doi:10.1136/gut.34.5.698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Sellin J. H. (2009) Review article: Small intestinal bacterial overgrowth, bile acid malabsorption and gluten intolerance as possible causes of chronic watery diarrhoea. Alimentary Pharmacology & Therapeutics 29, 1069–1077 doi:10.1111/j.1365-2036.2009.03970.x [DOI] [PubMed] [Google Scholar]
- Fromm H., Malavolti M. (1986) Bile acid-induced diarrhoea. Clinics in Gastroenterology 15, 567–582 [PubMed] [Google Scholar]
- Galman C., Arvidsson I., Angelin B., Rudling M. (2003) Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. The Journal of Lipid Research 44, 859–866 doi:10.1194/jlr.D200043-JLR200 [DOI] [PubMed] [Google Scholar]
- Grutzner N., Suchodolski J. S., Steiner J. M. (2014) Relationship between cobalamin-dependent metabolites and both serum albumin and alpha 1 -proteinase inhibitor concentrations in hypocobalaminemic dogs of 7 different breeds. Veterinary Clinical Pathology 43, 561–566 doi:10.1111/vcp.12204 [DOI] [PubMed] [Google Scholar]
- Johnston I., Nolan J., Pattni S. S., Walters J. R. (2011) New insights into bile acid malabsorption. Current Gastroenterology Reports 13, 418–425 doi:10.1007/s11894-011-0219-3 [DOI] [PubMed] [Google Scholar]
- Koga T., Nishida T., Miwa H., Yamamoto M., Kaku K., Yao T., Okumura M. (1984) Effects of dietary butter fat on fecal bile acid excretion in patients with Crohn's disease on elemental diet. Digestive Diseases and Sciences 29, 994–999 doi:10.1007/BF01311249 [DOI] [PubMed] [Google Scholar]
- Pattni S., Walters J. R. (2009) Recent advances in the understanding of bile acid malabsorption. British Medical Bulletin 92, 79–93 doi:10.1093/bmb/ldp032 [DOI] [PubMed] [Google Scholar]
- Slattery S. A., Niaz O., Aziz Q., Ford A. C., Farmer A. D. (2015) Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Alimentary Pharmacology & Therapeutics 42, 3–11 doi:10.1111/apt.13227 [DOI] [PubMed] [Google Scholar]
- Vijayvargiya P., Camilleri M., Shin A., Saenger A. (2013) Methods for diagnosis of bile acid malabsorption in clinical practice. Clinical Gastroenterology and Hepatology 11, 1232–1239 doi:10.1016/j.cgh.2013.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlake L., A'hern R., Russell D., Thomas K., Walters J. R., Andreyev H. J. (2009) Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Alimentary Pharmacology & Therapeutics 30, 707–717 doi:10.1111/j.1365-2036.2009.04081.x [DOI] [PubMed] [Google Scholar]
- Westergaard H. (2007) Bile Acid malabsorption. Current Treatment Options in Gastroenterology 10, 28–33 doi:10.1007/s11938-007-0054-7 [DOI] [PubMed] [Google Scholar]
- Wilcox C., Turner J., Green J. (2014) Systematic review: the management of chronic diarrhoea due to bile acid malabsorption. Alimentary Pharmacology & Therapeutics 39, 923–939 doi:10.1111/apt.12684 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
vetreco-2015-000163supp.pdf (56KB, pdf)


