Abstract
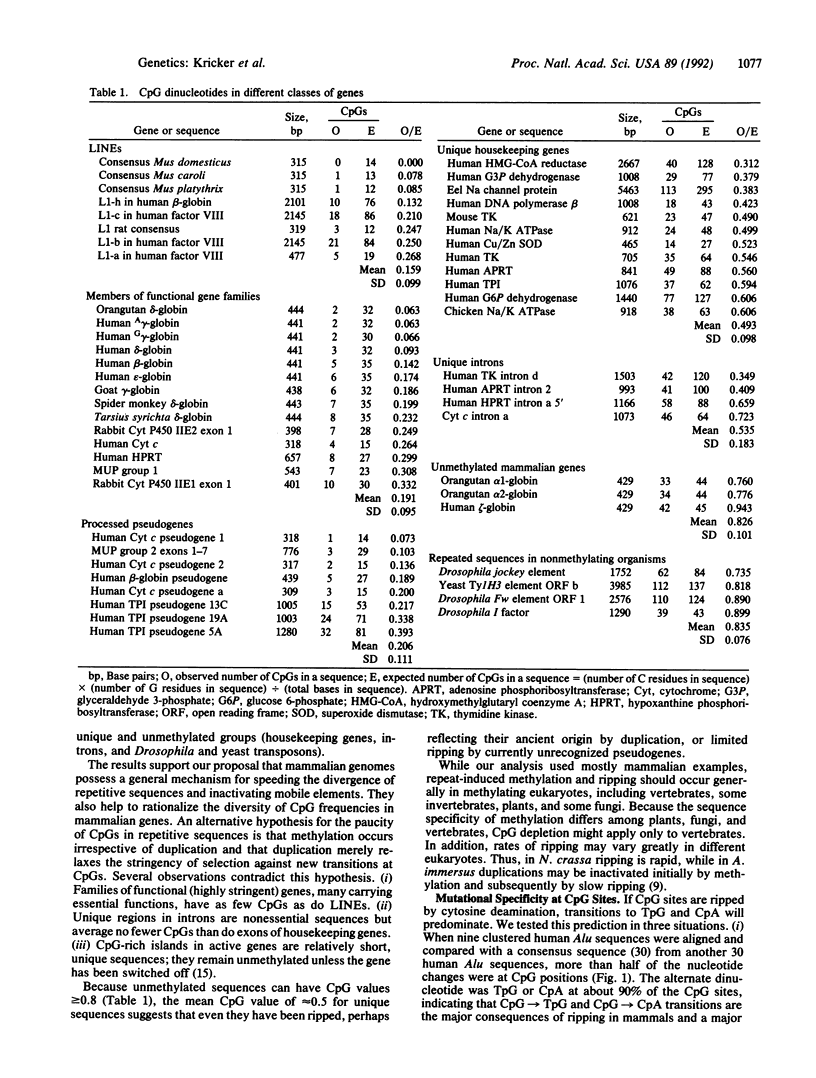
Mammalian genomes are threatened with gene inactivation and chromosomal scrambling by recombination between repeated sequences such as mobile genetic elements and pseudogenes. We present and test a model for a defensive strategy based on the methylation and subsequent mutation of CpG dinucleotides in those DNA duplications that create uninterrupted homologous sequences longer than about 0.3 kilobases. The model helps to explain both the diversity of CpG frequencies in different genes and the persistence of gene fragmentation into exons and introns.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antequera F., Boyes J., Bird A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell. 1990 Aug 10;62(3):503–514. doi: 10.1016/0092-8674(90)90015-7. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Bird A., Taggart M., Frommer M., Miller O. J., Macleod D. A fraction of the mouse genome that is derived from islands of nonmethylated, CpG-rich DNA. Cell. 1985 Jan;40(1):91–99. doi: 10.1016/0092-8674(85)90312-5. [DOI] [PubMed] [Google Scholar]
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Baron W. F., Stout D. B., Davidson E. H. Sources and evolution of human Alu repeated sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4770–4774. doi: 10.1073/pnas.85.13.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. R., Daar I. O., Krug J. R., Maquat L. E. Characterization of the functional gene and several processed pseudogenes in the human triosephosphate isomerase gene family. Mol Cell Biol. 1985 Jul;5(7):1694–1706. doi: 10.1128/mcb.5.7.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorit R. L., Schoenbach L., Gilbert W. How big is the universe of exons? Science. 1990 Dec 7;250(4986):1377–1382. doi: 10.1126/science.2255907. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Scarpulla R. C. The human somatic cytochrome c gene: two classes of processed pseudogenes demarcate a period of rapid molecular evolution. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9625–9629. doi: 10.1073/pnas.85.24.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W. Evolution of the P450 gene superfamily: animal-plant 'warfare', molecular drive and human genetic differences in drug oxidation. Trends Genet. 1990 Jun;6(6):182–186. doi: 10.1016/0168-9525(90)90174-5. [DOI] [PubMed] [Google Scholar]
- Hardies S. C., Edgell M. H., Hutchison C. A., 3rd Evolution of the mammalian beta-globin gene cluster. J Biol Chem. 1984 Mar 25;259(6):3748–3756. [PubMed] [Google Scholar]
- Mehtali M., LeMeur M., Lathe R. The methylation-free status of a housekeeping transgene is lost at high copy number. Gene. 1990 Jul 16;91(2):179–184. doi: 10.1016/0378-1119(90)90086-7. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Frequency of abnormal human haemoglobins caused by C----T transitions in CpG dinucleotides. J Mol Biol. 1990 May 20;213(2):203–206. doi: 10.1016/S0022-2836(05)80178-0. [DOI] [PubMed] [Google Scholar]
- Petes T. D., Hill C. W. Recombination between repeated genes in microorganisms. Annu Rev Genet. 1988;22:147–168. doi: 10.1146/annurev.ge.22.120188.001051. [DOI] [PubMed] [Google Scholar]
- Petit M. A., Dimpfl J., Radman M., Echols H. Control of large chromosomal duplications in Escherichia coli by the mismatch repair system. Genetics. 1991 Oct;129(2):327–332. doi: 10.1093/genetics/129.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proffitt J. H., Davie J. R., Swinton D., Hattman S. 5-Methylcytosine is not detectable in Saccharomyces cerevisiae DNA. Mol Cell Biol. 1984 May;4(5):985–988. doi: 10.1128/mcb.4.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M., Wagner R. Mismatch repair in Escherichia coli. Annu Rev Genet. 1986;20:523–538. doi: 10.1146/annurev.ge.20.120186.002515. [DOI] [PubMed] [Google Scholar]
- Rayssiguier C., Thaler D. S., Radman M. The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature. 1989 Nov 23;342(6248):396–401. doi: 10.1038/342396a0. [DOI] [PubMed] [Google Scholar]
- Rideout W. M., 3rd, Coetzee G. A., Olumi A. F., Jones P. A. 5-Methylcytosine as an endogenous mutagen in the human LDL receptor and p53 genes. Science. 1990 Sep 14;249(4974):1288–1290. doi: 10.1126/science.1697983. [DOI] [PubMed] [Google Scholar]
- Roeder G. S. Chromosome synapsis and genetic recombination: their roles in meiotic chromosome segregation. Trends Genet. 1990 Dec;6(12):385–389. doi: 10.1016/0168-9525(90)90297-j. [DOI] [PubMed] [Google Scholar]
- Seiser C., Knöfler M., Rudelstorfer I., Haas R., Wintersberger E. Mouse thymidine kinase: the promoter sequence and the gene and pseudogene structures in normal cells and in thymidine kinase deficient mutants. Nucleic Acids Res. 1989 Jan 11;17(1):185–195. doi: 10.1093/nar/17.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker E. U. Premeiotic instability of repeated sequences in Neurospora crassa. Annu Rev Genet. 1990;24:579–613. doi: 10.1146/annurev.ge.24.120190.003051. [DOI] [PubMed] [Google Scholar]
- Shen P., Huang H. V. Effect of base pair mismatches on recombination via the RecBCD pathway. Mol Gen Genet. 1989 Aug;218(2):358–360. doi: 10.1007/BF00331291. [DOI] [PubMed] [Google Scholar]
- Shen P., Huang H. V. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics. 1986 Mar;112(3):441–457. doi: 10.1093/genetics/112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Son H. J., Shahan K., Rodriguez M., Costantini F., Derman E. Silent genes in the mouse major urinary protein gene family. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4584–4588. doi: 10.1073/pnas.86.12.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppa-Lyonnet D., Carter P. E., Meo T., Tosi M. Clusters of intragenic Alu repeats predispose the human C1 inhibitor locus to deleterious rearrangements. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1551–1555. doi: 10.1073/pnas.87.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarković D., Plohl M., Gamulin V. Sequence variability of satellite DNA from the mealworm Tenebrio molitor. Gene. 1989 Nov 15;83(1):181–183. doi: 10.1016/0378-1119(89)90417-4. [DOI] [PubMed] [Google Scholar]
- Vnencak-Jones C. L., Phillips J. A., 3rd Hot spots for growth hormone gene deletions in homologous regions outside of Alu repeats. Science. 1990 Dec 21;250(4988):1745–1748. doi: 10.1126/science.1980158. [DOI] [PubMed] [Google Scholar]
- Waldman A. S., Liskay R. M. Differential effects of base-pair mismatch on intrachromosomal versus extrachromosomal recombination in mouse cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5340–5344. doi: 10.1073/pnas.84.15.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]



