Abstract
Wnt signaling has recently emerged as an important regulator of cardiac progenitor cell proliferation and differentiation, but the exact mechanisms by which Wnt signaling modulates these effects are not known. Understanding these mechanisms is essential for advancing our knowledge of cardiac progenitor cell biology and applying this knowledge to enhance cardiac therapy. Here, we explored the effects of Sfrp2, a canonical Wnt inhibitor, in adult cardiac progenitor cell (CPC) differentiation and investigated the molecular mechanisms involved. Our data show that Sfrp2 treatment can promote differentiation of CPCs after ischemia-reperfusion injury. Treatment of CPCs with Sfrp2 inhibited CPC proliferation and primed them for cardiac differentiation. Sfrp2 binding to Wnt6 and inhibition of Wnt6 canonical pathway was essential for the inhibition of CPC proliferation. This inhibition of Wnt6 canonical signaling by Sfrp2 was important for activation of the non-canonical Wnt/Planar Cell Polarity (PCP) pathway through JNK, which in turn induced expression of cardiac transcription factors and CPC differentiation. Taken together, these results demonstrate a novel role of Sfrp2 and Wnt6 in regulating the dynamic process of CPC proliferation and differentiation, as well as providing new insights into the mechanisms of Wnt signaling in cardiac differentiation.
Keywords: Sfrp2, Cardiac Injury, Cardiac Progenitor Cells, Differentiation, Wnt Signaling
1. Introduction
Over the past decade, multiple studies have identified and characterized various populations of cardiac progenitor cells (CPCs) that may be involved in cardiac regeneration [1–5]. Within the adult heart, CPCs reside in niches with supplementary cells that provide a specialized microenvironment, which can promote the replacement of mature cells lost during injury or turnover [6]; albeit at levels insufficient to achieve functional repair of the myocardium. In an effort to unlock the clinical potential of these endogenous processes, recent studies have focused on identification of factors and molecular pathways that promote ex vivo expansion of CPCs and/or enhance regenerative processes in vivo [7, 8].
Modulation of paracrine factors of the Wnt signaling pathway at different stages of cardiomyogenesis controls progenitor cell specification, expansion, and differentiation through a time-dependent and dose-dependent dynamic process [9]. Notably, blockade of canonical Wnt/β-Catenin signaling during cardiac injury reduces infarct size and induces differentiation of adult Sca-1+αMHC+ cardiac progenitors [10, 11]. Still, the connection between Wnt signaling and cardiac progenitor differentiation is complex, and the mechanisms involved remain unclear.
The Wnt antagonist Secreted Frizzled Related Protein 2 (Sfrp2) is one of the most upregulated Wnt signaling modulators during cardiac injury [12]. Sfrp2 has been shown to play a key paracrine role in enhancing cardiac function after injury [13–16] by modulating cardiac repair through cytoprotection [13], fibrosis [12, 15] and angiogenesis [17]. Here we show that Sfrp2 can also promote differentiation of CPCs after ischemia-reperfusion injury. In vitro, treatment of CPCs with Sfrp2 inhibited CPC proliferation and primed them for cardiac differentiation. Sfrp2 binding to Wnt6 and inhibition of the Wnt6 canonical pathway was essential for the inhibition of CPC proliferation. This inhibition of Wnt6 canonical signaling by Sfrp2 was important for activation of the non-canonical Wnt/Planar Cell Polarity (PCP) pathway through JNK, which in turn induced expression of cardiac transcription factors and CPC differentiation. Taken together, these results demonstrate a novel role of Sfrp2 and Wnt6 in regulating the process of CPC proliferation and differentiation by modulation of both canonical and non-canonical Wnt signaling pathways.
2. Materials and Methods
Detailed Material and Methods are presented in Supplementary Information.
2.1. Animal studies
All animal procedures were approved by the Duke University Institutional Animal Care and Use Committee. Myocardial ischemia-Reperfusion was performed in 10–12 week old mice as described previously (13) and further details are provided in the Supplementary.
2.2. Mouse Cardiac Progenitor Cell Isolation and Differentiation
CPCs were isolated from 8–16 week old FVB mice. Single cell clones were isolated by limiting dilutions and cultured in the media (DMEM/F12-K 1:1, 20% ES cell qualified FBS, 10 ng/mL bFGF, 20 ng/mL EGF, 100U LIF, and 1x ITS (insulin-transferrin-selenium)). CPCs were plated at >80% confluence on gelatin-coated glass bottom tissue culture plates. 24-hours later, the media was changed to Differentiation Media (αMEM with 2% FBS) or Ascorbic Acid Media (Advanced DMEM /F12, 0.2% BSA, 2 mM L-glutamine, 1x ITS, 250 μM ascorbic acid) with or without SFRP2 for 14–21 days.
2.3. Co-immunoprecipitation
CPCs were grown for 48 hours in Reduced Growth Factor Media prior to harvest of conditioned media. Conditioned media was separated from cell debris by filtration using a 0.45 μm low protein binding filter (Milipore, Billerica, MA). 6xHis tagged Sfrp2 (R&D System, Minneapolis, MN) was diluted to 10 nM and incubated in conditioned or fresh media for 1 hour at 37 °C, 5% CO2. Sfrp2-bound prey was incubated with Dynabead anti-His Pulldown beads (Invitrogen, Carlsbad, CA) per manufacturer’s protocol.
2.4
qRT-PCR, FACS, BrdU Cell Cycle analysis, Western Blot and Immunostaining were performed using standard protocols.
2.5. Data analysis
Statistical comparison was performed using 2-tailed Student’s t-test for single comparisons (animal study, assuming unequal variance) or two-way analysis of variance with Bonferroni Correction for multiple comparisons.
3. Results
3.1. Sfrp2 enhances CPC differentiation in vivo
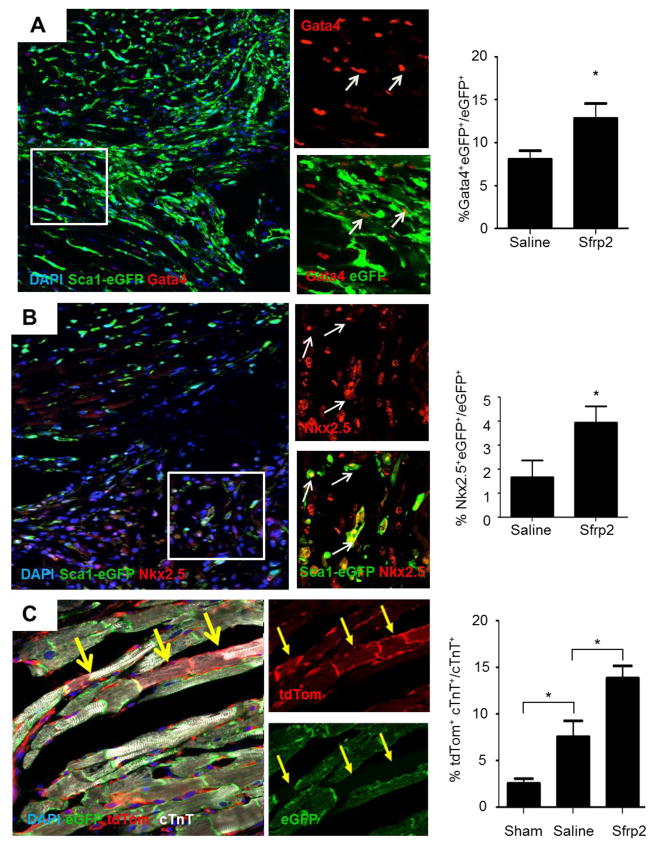
To investigate the effects of treatment with the Sfrp2 protein treatment in CPC differentiation in vivo, we utilized transgenic animals with eGFP under the control of the Sca-1 (Ly6a) promoter [18] to track the cell fate of the Sca-1+ cells. Sca-1+ CPCs, a sub-population of the Sca-1 population in the heart, are defined as non-hematopoietic cells (CD45−) that express a variety of mesenchymal stem cells markers [2, 4, 19]. These cells have been shown to participate in cardiomyocyte repair [19, 20]. Ischemia-reperfusion injury resulted in an increase in the number of Sca1-eGFP+ cells present in the myocardium (Sham 23.4 ± 2.2% vs Saline 38.2 ± 3.1%; P<0.05) (Supplementary Figure S1A and S1B). Sfrp2 treatment 2 days after ischemia-reperfusion injury did not affect the numbers of Sca1-eGFP+ in the myocardium (Supplementary Figure S1B). However, it led to an 2–3 fold increase in the number of Sca1-eGFP+ also co-expressing cardiac markers Gata4 (Saline 8.1 ± 1.0% vs Sfrp2 12.8 ± 1.8%; P<0.05) (Figure 1A) or Nkx2.5 (Saline 1.7± 0.7 vs 4.0 ± 0.7%; P<0.05) (Figure 1B) in the border zone of the infarct (Figure 1B). Of note, while a subset of Sca-1+ cells also expressed cKit, all cKit+ cells were positive for Sca-1 (Supplementary Figure S2A). Neither the total number nor the number of proliferating cKit+ cells within the myocardium increased in response to Sfrp2 treatment after injury (Supplementary Figure S2B). Co-expression of Gata4, Nkx2.5, and cTnT in cKit+ cells was never detected (Supplementary Figure S2C), In addition, Sca1-eGFP cells positive for the hematopoietic cells marker CD45 existed in the myocardium, cells co-expressing Sca-1 with Gata4 and CD45 were never detected (Supplementary Figure S2D), indicating that the Sca1-eGFP+Gata4+ cells were derived from Sca1-eGFP+CD45− cardiac progenitors. These results were associated with the formation of new cardiomyocytes (Figure 1C and Supplementary Figure S3A–C) as shown by the use of an inducible αMHC-MerCreMer transgene and tdTomato-eGFP reporter (αMHC-MerCreMer+/−x ROSA-mTmG+/−). In these mice, tamoxifen-induced recombination results in cardiomyocyte-specific eGFP expression and any newly generated cardiomyocytes derived from non-myocyte (αMHC−) source after injury express tdTomato (tdTom)[21]. Following cardiac ischemia-reperfusion injury, Sfrp2 increased the number of newly regenerated cardiomyocytes by 2-fold in the border zone (Saline 7.5 ± 2.5% vs Sfrp2 13.8 ± 1.8%; P<0.05) (Figure 1C and Supplementary Figure S3A–C). No tdTom+eGFP+ double positive cardiomyocytes were detected excluding the possibility that the tdTom+ cardiomyocytes were the result of cell fusion. Parallel experiments confirmed that injection of Sfrp2, compared to saline control, reduced infarct size and improved cardiac function at 2 months (Supplementary Figure S4). Overall, these results are consistent with the notion that Sfrp2 treatment after cardiac injury has pleiotropic reparative effects, which include priming of CPCs for differentiation.
Figure 1. Sfrp2 promotes differentiation of CPCs in vivo.
(A and B) Representative images and quantification of (A) Sca1-eGFP+ and Gata4+ cells and (B) Sca1-eGFP+ and Nkx2.5+ cells 7 days after ischemia-reperfusion injury (arrows point to Sca1-eGFP+Gata4+ and Sca1-eGFP+Nkx2.5+ cells respectively); n=7 saline, n=9 Sfrp2. * P≤0.05. (C) Representative images of immunofluorescence staining of newly formed tdTomato+cTnT+ cardiomyocytes in the infarct border zone of Sfrp2 treated heart with quantification. *P<0.05, compared to PBS treated animals; n=6 Sham, n=5 PBS, n=6 Sfrp2.
3.2. Sfrp2 primes CPC for cardiac differentiation by inducing cell cycle arrest and upregulation of cardiac transcription factors
To gain insights into the potential mechanism by which Sfrp2 mediates CPC differentiation, we established single cell CPC clones for in vitro studies. For this, CPCs were isolated from the non-cardiomyocyte fraction of the adult mouse heart (Supplementary Figure S5A). Cultured cells appeared homogeneous and exhibited a spindle-like shape. (Supplementary Figure S5B). These cells were positive for Sca-1 expression and typical mesenchymal markers such as CD44, CD105, CD73, CD90 and CD54 [3, 4]. Initially they also expressed the c-Kit, but they lost this expression upon passaging in culture (Supplementary Figure S5C and S5D). As expected for CPCs (4), the cultured cells showed the capacity for differentiation towards the cardiomyocyte lineage (Supplementary Figure 6A).
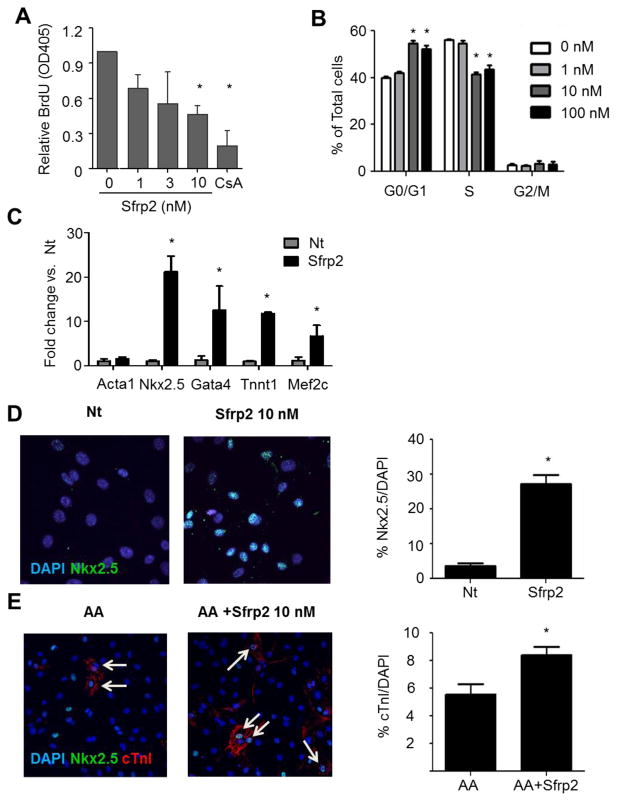
To test for the direct effects of Sfrp2 in these CPCs, the cells were treated with increasing doses of Sfrp2 (0–10 nM) for 16 hours and cell proliferation was measured by ELISA for BrdU incorporation. Treatment with 10 nM Sfrp2 resulted in significant decrease in CPC proliferation (Figure 2A). To further define the role of Sfrp2 in CPC cell cycle, cells were treated with 1–100 nM of Sfrp2 for 8 hours and subjected to FACS analysis using BrdU and 7-AAD staining to determine the percent of cells in S-phase and cell ploidy, respectively. An 8 hour incubation in this assay to prevent double-labelling of the cells which precludes analysis of the data. In accordance with the ELISA data, Sfrp2 treatment resulted in 16 ± 7% reduction of CPCs in S phase accompanied by 43 ± 13% increase of cells at the G1/G0 stage compared to no treatment (Figure 2B). No significant changes were seen in the G2-M phase. These data suggest that Sfrp2 treatment induces cell cycle arrest of CPCs.
Figure 2. Sfrp2 primes CPC for cardiac differentiation.
(A) BrdU incorporation ELISA of CPCs treated for 16 hours with Sfrp2. Cyclosporin A (CsA) was used as control for decreased proliferation. Data shown as mean ± SE of 5 independent experiments. *P<0.01 compared to no treatment; n=6.
(B) FACS analysis of CPCs cultured for 8 hours in the presence or absence of Sfrp2. Ploidy was identified by 7-AAD (DNA) staining and S-phase was identified by BrdU. Data from a representative experiment are presented as mean ± SD (n=3).* P<0.05, compared to no treatment.
(C) qRT-PCR analysis for cardiac markers in non-treated (Nt) and CPCs treated with 10nM Sfrp2 for 10 days. Relative mRNA levels are expressed as fold change over control Nt cells. Data shown as mean ± SE of 4 independent experiments. * P<0.05; n=4.
(D) Representative immunofluorescence staining images for nuclear Nkx2.5 in CPCs after 14 days of continuous treatment with 10nM of Sfrp2. Data shown as mean ± SE. *P<0.05 compared to Nt; 5 images x n=3.
(E) Representative immunofluorescence staining images for cTnI in CPCs after 21 days of continuous treatment with ascorbic acid (AA) with or without 10 nM Sfrp2. Data shown as mean ± SE. *P<0.05, compared to Nt: 4–5 images x n=3. In D and E arrows denote positive cells.
Accumulating evidence in recent years has linked cell cycle regulation with stem cell maintenance and differentiation [22]. To test if Sfrp2 treatment also affected CPC differentiation, the cells were continuously treated for 14 days with 10 nM of Sfrp2. Gene expression analysis by qRT-PCR showed that the levels of cardiac-specific markers, such as Nkx2.5, Gata4, Mef2c and cTnI, were all increased after Sfrp2 treatment (Figure 2C). Additional analysis using immunofluorescence microscopy confirmed the induction of Nkx2.5 expression (Nt: 3.6 ± 0.9 % vs. Sfrp2: 27.2 ± 3.2%; P<0.05) (Figure 2D with controls shown in Supplementary Figure S7); though treatment with Sfrp2 alone rarely induced the expression of more mature cardiac contractile markers (cTnI, α-MHC, and α-Actinin) at the protein level. To investigate if Sfrp2 could induce expression of mature cardiomyocyte markers in combination with other treatments, CPCs were treated with Sfrp2 and 250 μM ascorbic acid, a known inducer of cardiac differentiation [23, 24]. Continuous treatment with Sfrp2 enhanced the cardiomyogenic potential of ascorbic acid by increasing the percent of cells expressing cTnI (Nt: 5.5 ± 2.3% vs. Sfrp2: 8.4 ± 1.7%; P<0.05) (Figure 2E). Collectively, these data indicate that treatment with 10 –100 nM Sfrp2 primes CPCs for cardiac differentiation by inducing cell cycle arrest and upregulation of cardiac transcription factors.
3.3. Sfrp2 affects both Wnt canonical and non-canonical pathways in CPCs
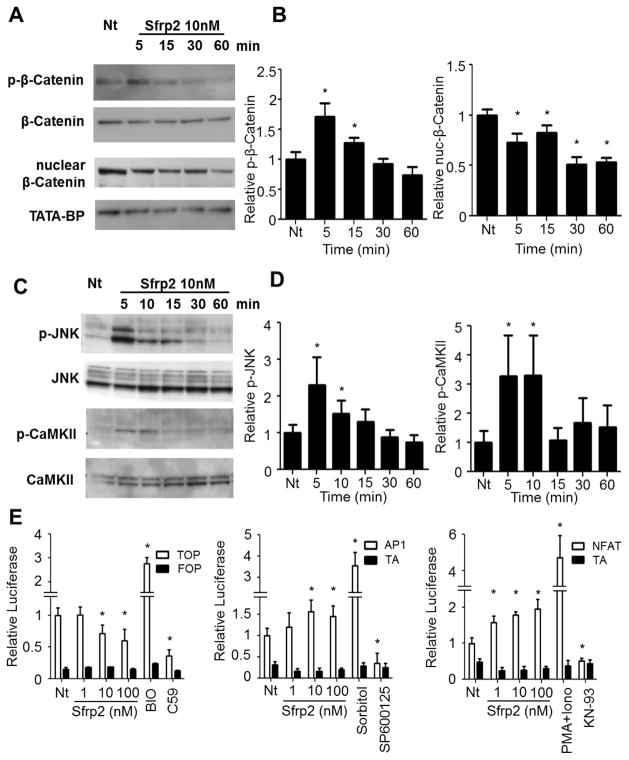
Sfrp2 is a well-known inhibitor of canonical Wnt signaling, however recent evidence suggests it can also act through modulation of non-canonical Wnt pathways [17]. To define the downstream effects of Sfrp2 on Wnt pathways in CPCs, cells were treated with 10 nM of Sfrp2 for 5–60 minutes. Activation of the canonical pathway was then evaluated by western blot analysis of nuclear and phosphorylated β-Catenin, whereas activation of non-canonical pathways was assessed by phosphorylation of JNK (Planar Cell Polarity (PCP) pathway) [25], or CaMKII (Wnt/Ca+ pathway) [26, 27]. Treatment with Sfrp2 resulted in increased phosphorylation of β-Catenin, thereby targeting free β-Catenin for proteasomal degradation (Figure 3A and 3B. Immunoblot controls provided in Supplemental Figure S8). Accordingly, Sfrp2 decreased nuclear localization of β-Catenin, effectively inhibiting the canonical pathway (Figure 3A and 3B). In contrast, Sfrp2 activated both non-canonical Wnt pathways as shown by increased phosphorylation of both JNK and CaMKII (Figure 3C and 3D). To validate these observations, CPCs were transduced with luciferase reporter constructs that allow monitoring of transcriptional activation of Wnt signaling through β-Catenin/TCF (TOP-Flash), PCP (AP1-Flash) or Wnt/Ca+ (NFAT-Flash), and treated with 1–100 nM of Sfrp2. After 24 hours of treatment, Sfrp2 reduced canonical β-Catenin/TCF signaling and activated JNK-AP1 and NFAT signaling at a concentration equal or greater than 10 nM (Figure 3E). Altogether, the above data suggest that in CPCs, Sfrp2 acts by modulating both the inhibition of canonical and activation of the non-canonical Wnt pathways.
Figure 3. Sfrp2 modulates both canonical and non-canonical Wnt signaling in CPCs.
(A) Canonical Wnt pathway activation assessed by western blotting for p-β-Catenin or nuclear β-Catenin at various time points after treatment with 10 nM Sfrp2. Representative blots are shown.
(B) Densitometry of p-β-Catenin (n=4) or nuclear β-Catenin (n=4) immunoblots from (A). *P<0.05, compared to Nt.
(C) Non-canonical Wnt pathway assessed by western blotting for p-JNK or p-CaMKII at various time points after treatment with 10 nM Sfrp2. Representative blots are shown.
(D). Densitometry of p-JNK or p-CaMKII immunoblots from (C). *P<0.05, compared to Nt.
(E) Assessment of canonical and non-canonical Wnt pathway activity by relative Luciferase expression in CPCs transduced with TOP-Flash (β-Catenin), AP1-Flash (PCP pathway), and NFAT-Flash (Wnt/Ca+ pathway) 24 hrs after treatment with 10 nM Sfrp2. FOP-Flash (empty vector) was used as control for TOP-FLASH and TA-Luc (empty vector) was used as control for AP1-Flash and NFAT-Flash. Data shown as mean ± SD. *P<0.05, compared to Nt; n=6 per condition. The compounds BIO and C59 were used at 10 μM each as positive and negative controls for canonical Wnt signaling. Sorbitol at a concentration 0.5M and 10 μg/mL PMA + 10 nM Ionophore were used as positive controls for the JNK and CaMKII non canonical pathway activation respectively. The compounds SP600125 at 5 μM and KN-93 at 10 μM were used as negative controls for JNK and CaMKII non canonical pathway activation respectively. Additional tests for the controls used are presented in Supplementary Figure 9. Nt: No treatment.
3.4. Sfrp2 promotes CPC priming via inhibition of Canonical/β-Catenin and activation of Non-canonical/PCP Wnt Pathway
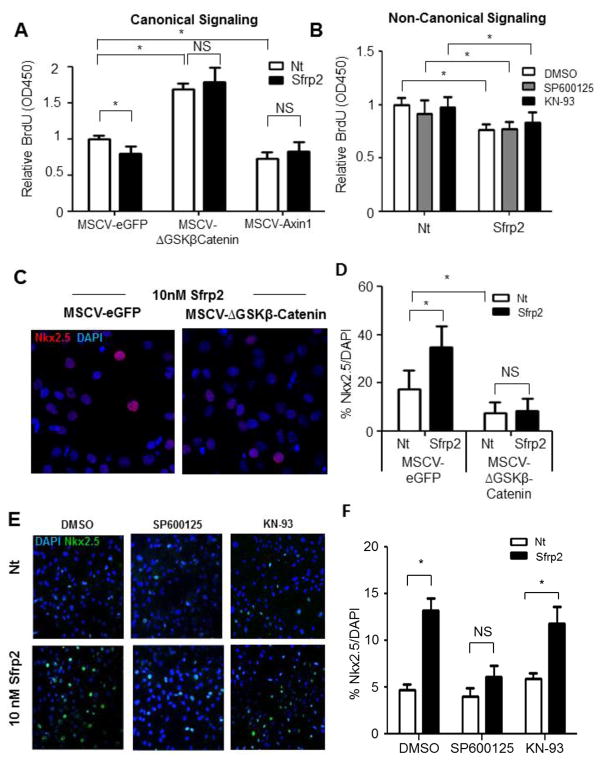
Next, we tested if the above observations had a functional association with CPC proliferation and differentiation. For this we first used CPCs with constitutively active β-Catenin (MSCV-ΔGSKβCatenin). At baseline, these cells had increased proliferation compared to control CPCs (MSCV-eGFP) (Figure 4A). Sfrp2 was no longer able to reduce proliferation in these cells, suggesting that inhibition of the canonical pathway by Sfrp2 is crucial for mediating its effect on CPC proliferation (Figure 4A). CPCs with dominant negative β-Catenin (MSCV-Axin1) showed reduced proliferation (Figure 4A), providing corroborating evidence for the importance of Wnt canonical pathway inhibition in CPC proliferation. Sfrp2 addition did not further reduce the proliferation in these cells strengthening the hypothesis that its effects are canonical pathway dependent. To also test if the non-canonical/PCP and Wnt/Ca+ pathways were also involved, we treated CPCs with the JNK inhibitor SP600125 or the CaMKII inhibitor KN-93. However, no effect on Sfrp2’s anti-proliferation effect was observed (Figure 4B). These data support the hypothesis that Sfrp2 inhibits CPC proliferation through inhibition of the Wnt canonical pathway alone.
Figure 4. The effects of Sfrp2 on CPC proliferation and differentiation are mediated by inhibition of canonical and activation of non-canonical Wnt pathways.
(A) BrdU ELISA in CPCs transduced with constitutively β-Catenin (MSCV-ΔGSKβCatenin) and treated for 16 hours with 10 nM Sfrp2. MSCV-eGFP transduced cells were used as negative control. MSCV-Axin transduced cells with constitutive inhibition of β-Catenin served as positive controls. Data shown as mean ± SD. * P<0.05; n=6.
(B) BrdU ELISA of CPCs treated with 10 nM Sfrp2 for 16 hours in the presence or absence of JNK inhibitor SP600125 or CAMKII inhibitor KN-93. Data shown as mean ± SD. * P<0.05; n=6.
(C) Immunofluorescence staining for Nkx2.5 in CPCs transduced with constitutively active β-Catenin (MSCV-ΔGSKβCatenin) and treated for 14 days with 10 nM Sfrp2. MSCV-eGFP transduced cells were used as negative control. *P<0.05; 5 images x n=3.
(D) Quantitative analysis of (C).
(E) Immunofluorescence staining for Nkx2.5 after 14 days continuous treatment with Sfrp2 in the presence or absence of JNK inhibitor SP600125 or CamKII inhibitor KN-93. DMSO was used as a vehicle.
(F) Quantitative analysis of Figure 4E. Data shown as mean ± SE. *P<0.05; 5 images x n=3, analysis performed on data from 3 independent experiments. Nt: No treatment; NS: Not significant.
To further investigate the downstream Wnt pathways involved in Sfrp2-mediated priming of CPC for cardiomyocyte differentiation, CPCs with constitutively active β-Catenin were treated for 14 days with and without Sfrp2 and immunostained for Nkx2.5 expression. CPCs with constitutively active β-Catenin not only showed lower baseline expression of Nkx2.5, but they also did not respond to Sfrp2 treatment (Figure 4C and 4D). Similar experiments to investigate the role of the non-canonical Wnt pathways showed that inhibition of JNK by AP600125, but not CaMKII by KN-93, prevented Sfrp2 from upregulating Nkx2.5 expression (Figure 4E with quantification provided in Figure 4F. Immunostaining controls Supplementary Figure S9A), suggesting that Sfrp2 affects CPC differentiation via activation of the non-canonical/PCP pathway through JNK. We further validated our findings with AS601245 and Autocamtide-2-related inhibitory peptide (AIP), a JNK and CaMKII inhibitor respectively (Supplementary Figure 9B). Collectively, these data indicate that both downregulation of canonical signaling and activation JNK by Sfrp2 are crucial in priming CPCs for differentiation.
3.5. Sfrp2 promotes CPC priming and differentiation by blocking canonical Wnt6
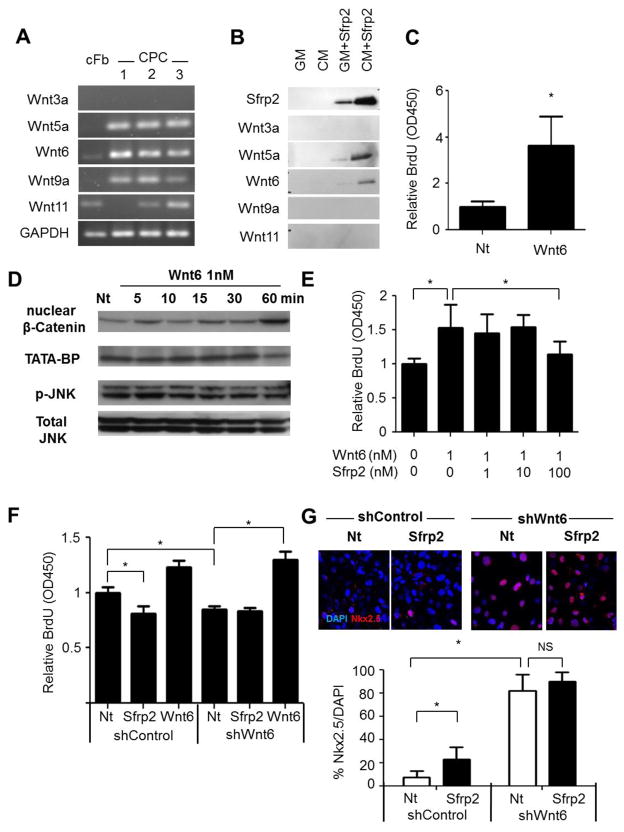
Sfrps are traditionally known to bind to Wnt proteins and inhibit these Wnts from acting [28]. To determine the Wnt protein that Sfrp2 acts upon to modulate the effects on CPCs observed above, we assessed for Wnt gene expression in CPCs by RT-PCR. CPCs expressed canonical Wnt9a [29], the non-canonical Wnt5a [30] and Wnt11 [31, 32], as well as Wnt6, which has been associated with both canonical and non-canonical pathways [33, 34] (Figure 5A). Therefore, we hypothesized that Sfrp2 modulates downstream pathways in CPCs by directly interacting with any of these Wnts. To test this, 10 nM 6x-HIS tagged Sfrp2 was added to conditioned media from CPCs and was used as bait to pull-down Sfrp2 binding partners. Under the conditions examined, Sfrp2 was found to interact with Wnt5a and Wnt6, but not Wnt9a or Wnt11 (Figure 5B).
Figure 5. Sfrp2 promotes CPC priming and differentiation by blocking canonical Wnt6.
(A) RT-PCR analysis of three different CPC isolations for expression of Wnts compared to adult cardiac fibroblasts [cFb].
(B) Western blot protein analysis for Wnts binding to HIS-Tagged Sfrp2 after Ni+ bead pull down from control Growth Media [GM] or CPC Conditioned Media [CM].
(C) BrdU ELISA of CPCs treated for 16 with 1 nM Wnt6. *P<0.05; n=6.
(D) Western blot analysis for nuclear β-Catenin and p-JNK in CPCs at various time points after treatment with 1 nM Wnt6.
(E) BrdU ELISA of CPCs treated with 1nM Wnt6 in the presence of increasing amounts of Sfrp2 (0–100). *P<0.05; n=6.
(F) BrdU ELISA of shControl and shWnt6 CPCs in the presence or absence of 10nM Sfrp2 or 1nM Wnt6. *P<0.05; n=6. All data shown as mean ± SD of representative experiments. Nt: No treatment.
(G) Representative images of immunofluorescence staining (top) and quantification (bottom) for Nkx2.5 expression (Red) in shControl or shWnt6 CPCs after 14 days of continuous treatment with 10nM Sfrp2. * P<0.05; 4–5 images x n=3. All data are shown as mean ± SD of a representative experiment. Nt: No treatment.
To define the potential roles of Wnt6 and Wnt5a in regulating the effects of Sfrp2 on CPCs, cells were treated with recombinant Wnt6 or Wnt5a protein. Cell proliferation and differentiation was then assessed as before. Wnt5a had no effect on either CPC proliferation or differentiation (Supplementary Figure 10). In contrast, treatment of CPCs for 16 hours with 1 nM Wnt6 induced a 3.95 ± 0.95 fold increase in proliferation (Figure 5C). Moreover, treatment of CPCs with 1 nM of Wnt6 was able to increase nuclear β-Catenin signaling, but not p-JNK non-canonical signaling in these cells (Figure 5D). To further validate the effect of Wnt6 on canonical and non-canonical Wnt signaling in CPCs, CPCs transduced with luciferase reporter constructs β-Catenin/TCF (TOP-Flash), PCP (AP1-Flash) or Wnt/Ca+ (NFAT-Flash), and treated with 0.1–10 nM of Wnt6. After 24 hours, Wnt6 activated canonical β-Catenin/TCF signaling at 1 nM but did not affect JNK-AP1 or NFAT signaling at greater than 10 nM (Supplementary Figure S11A–C), suggesting in CPCs, Wnt6 acts as a canonical Wnt. Co-incubation of CPCs with 1 nM of Wnt6 with increasing concentrations of Sfrp2 achieved neutralization of both the Wnt6-induced β-Catenin activation (Supplementary Figure S11D) and the Wnt6-induced proliferative effects (Figure 5E), suggesting a functional importance between the interaction of Sfrp2 and Wnt6 in modulating CPC proliferation.
To further determine the importance of interaction of Sfrp2 and Wnt6 in CPC proliferation and differentiation, Wnt6 was knocked down in CPCs using a lentiviral shRNA construct (shWnt6) (Supplementary Figure S11E). As expected, shWnt6 CPCs had reduced proliferation compared to shControl CPCs and addition of Wnt6 restored proliferation (Figure 5F). Importantly, the addition of Sfrp2 did not change proliferation compared to non-treated shWnt6 CPCs (Figure 5F), demonstrating that the antiproliferative effects of Sfrp2 are mediated solely through the interaction of Sfrp2 and Wnt6. Accordingly, CPC differentiation as measured by Nkx2.5 expression was increased in shWnt6 CPCs compared to shControl CPCs (Figure 5G). In the absence of Wnt6, addition of Sfrp2 did not have any effect on Nkx2.5 expression (Figure 5G). Altogether, these results support the hypothesis that Sfrp2 promotes CPC priming and differentiation by binding and blocking Wnt6 canonical signaling.
3.6. Sfrp2 activates non-canonical Wnt/PCP pathway by inhibiting canonical Wnt6
Based on the finding that activation of the non-canonical/PCP pathway through JNK was important for the Sfrp2 mediated CPC differentiation; we next directed our attention to the mechanism by which Sfrp2 increases p-JNK. Two potential mechanisms by which Sfrp2 may be acting include: a) directly, by binding a Frizzled receptor, promoting activation in a Wnt-independent manner [28]; b) indirectly, by inhibition of the canonical Wnt pathway, activating the non-canonical JNK signaling [35].
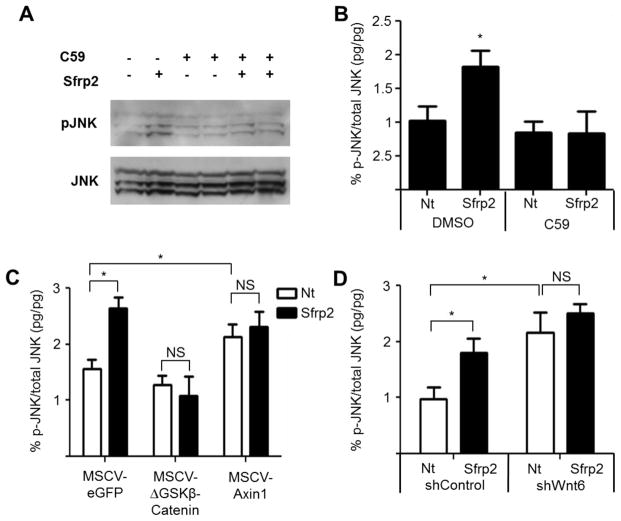
To test the first possibility, CPCs were treated with a pan-Wnt inhibitor (C59 Porcupine inhibitor) [36, 37] to block secretion of all Wnts, followed by addition of Sfrp2 to measure the effects on non-canonical/PCP signaling using both western blot analysis and ELISA assay for p-JNK (Figure 6A–B). As expected, cells treated with Sfrp2 had increased p-JNK relative to non-treated cells. However, this effect was abrogated when the cells were pre-treated with C59, suggesting that Sfrp2 does not activate JNK via a direct interaction with Frizzled receptors and requires a Wnt to function.
Figure 6. Sfrp2 activates non-canonical Wnt/PCP pathway by inhibiting canonical Wnt6.
(A) Western blot protein analysis of p-JNK in CPCs after overnight treatment with 10 μM C59 Porcupine inhibitor with or without 10 nM Sfrp2.
(B) p-JNK activation in CPCs as assessed by ELISA in the presence or absence of 10 μM C59 Porcupine inhibitor with and without 10 nM Sfrp2 treatment. *P<0.05 compared to Nt; n=6.
(C) p-JNK activation in Control (MSCV-eGFP), constitutively active β-Catenin (MSCV-ΔGSKβCatenin) or dominate negative β-Catenin (MSCV-Axin1) CPCs as assessed by ELISA with and without 10 nM Sfrp2.
(D) p-JNK activation in shControl and shWnt6 CPCs assessed by ELISA in the presence or absence of 10 nM Sfrp2. *P<0.05; n=4. Nt: no treatment.
To inquire whether Sfrp2 increased p-JNK and activated non-canonical/PCP downstream pathways indirectly by its inhibition of the canonical Wnt pathway, we used CPCs with constitutively active (MSCV-ΔGSKβCatenin) or dominant negative β-Catenin (MSCV-Axin1), and assessed for JNK phosphorylation as before (Figure 6C). As expected, in MSCV-eGFP CPCs, Sfrp2 increased p-JNK similar to non-transduced cells. When the canonical pathway was constitutively active, addition of Sfrp2 could not activate JNK (Figure 6C). In agreement, CPCs with dominant negative β-Catenin (MSCV-Axin1), whereby the canonical pathway is suppressed, showed increased JNK activation (Figure 6C). Previous studies have shown that such β-Catenin dependent activation of non-canonical pathways is possible by regulation of the expression of non-canonical Wnts, in particular Wnt11 [35]. To address this possibility, we treated CPCs with Sfrp2 for 8h and measured the levels of non-canonical Wnts such as Wnt5a and Wnt11; however we did not observe any changes (Supplementary Figure S11F). To determine if Wnt6 plays a role in these effects, we next used CPCs transduced with either control shRNA (shControl) or shRNA against Wnt6 (shWnt6). Wnt6 knockdown doubled the levels of JNK activation compared to shControl (Figure 6D). However, Sfrp2 treatment did not have an additive effect (Nt-shWnt6 2.16 ± 0.36% vs. Sfrp2-shWnt6 2.51 ± 0.1 7%; P=0.874), suggesting that inhibition of canonical Wnt6 signaling by Sfrp2 is essential in mediating the Sfrp2 effects on JNK activation.
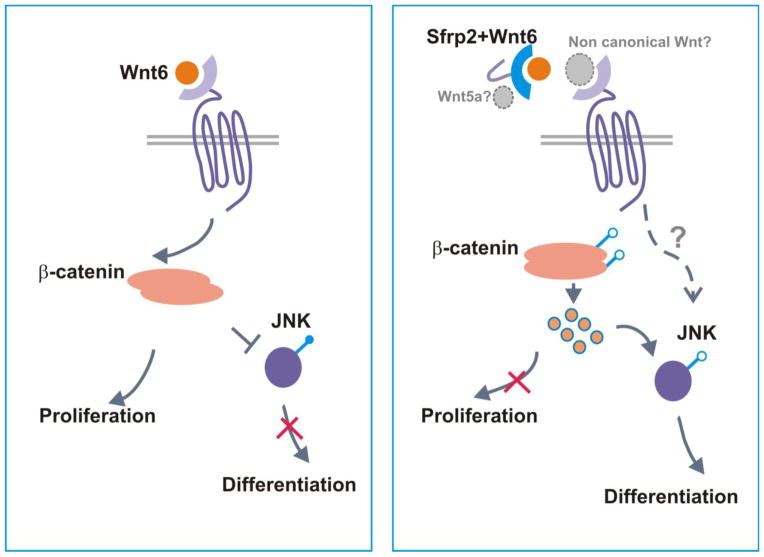
Overall, the above data support the notion that Sfrp2 activation of JNK non-canonical pathway and differentiation does involve an indirect component through its inhibition of Wnt6 canonical signaling (Figure 7).
Figure 7. Proposed mechanism of Sfrp2/Wnt6/β-Catenin signaling action on CPC proliferation and differentiation.
Sfrp2 binds and inhibits Wnt6 signaling through the canonical pathway leading to cell cycle arrest. This inhibition of canonical pathway activates JNK and non–canonical/PCP downstream pathways which in turn induce expression of cardiac transcription factors priming CPC for differentiation. Sfrp2 binding to Wnt6 could further facilitate binding and activation of non-canonical Wnt signaling (grey font and arrows).
4. Discussion
Current studies support an important role for Wnt signaling in cardiac function and repair [11, 38–40]. The overarching conclusion from these studies is that inhibition of Wnt/β-Catenin signaling is beneficial and results in improved cardiac function and regeneration after injury (Reviewed in [10, 41, 42]). However, the mechanisms and exact Wnt pathway factors involved remain elusive.
Here, we identified a novel role for Wnt6 signaling in maintaining CPCs in their proliferative state preventing their differentiation. Wnt6 inhibition plays a role in Xenopus heart development by regulating cardiogenic mesoderm differentiation into heart muscle [34, 43], however its importance for mammalian cardiac differentiation and function is unknown. Our data highlight a role for Wnt6 in adult CPC differentiation, inferring its potential as a novel therapeutic target for cardiac regeneration.
We also highlight a novel role for Sfrp2 as multifaceted regulator of Wnt pathways in CPCs. Binding of Wnt6 by Sfrp2 primes Sca-1+ CPCs for differentiation through blocking of canonical β-Catenin signaling. This is accompanied by Sfrp2/Wnt6/canonical β-Catenin dependent activation of the non-canonical Wnt/PCP signaling through JNK, which is an important step in promoting upregulation of early cardiac transcription factors. One potential mechanism for the inhibition of the non-canonical/PCP pathway by β-Catenin would be the indirect regulation of non-canonical Wnt expression [35], however our data do not support this hypothesis. Further investigations to determine the intracellular signals that regulate β-Catenin dependent activation of JNK and non-canonical/PCP downstream pathways would be of great value in elucidating the complex molecular networks involved in Wnt signaling effects in cardiogenesis and cardiac repair. One should note that from our analysis we cannot exclude the possibility that binding of Sfrp2 to Wnt6 alters the stoichiometry of non-canonical activating Wnts present, which could also act to reinforce the activation of non-canonical downstream pathways (Figure 6C). In line with the in vitro studies, Sfrp2 treatment in Sca1-eGFP transgenic mice after cardiac injury provided direct evidence that Sfrp2 promotes priming of a fraction of CPCs for differentiation as measured by increased Gata4 or Nkx2.5 expression in Sca-1+ cells. Although the Sca1-eGFP mouse model does not allow marking of Sca-1 derived mature cardiomyocytes ([19] and internal data), the use of the αMHC-MerCreMer+/−x Rosa-mTmG+/− mouse model suggested that the enhancement of CPC differentiation by Sfrp2 was associated with enhanced cardiac regeneration in vivo. Co-staining with cKit+ marker, did not detect any Sca1+cKit+ cells expressing cardiomyocyte markers. This might be explained by a possible loss of the cKit expression during differentiation as reported previously. Still we cannot exclude that in vivo Sfrp2 only affects the subfraction of Sca1+ CPCs that are c-Kit negative[4, 44]. The very recent development of Cre recombinase based CPC lineage tracing models [5, 19] will be useful to address this question directly. Similarly, the validation of our in vitro findings regarding the role of Wnt6/Sfrp2 interaction will require the development of appropriate cell and time specific models of Wnt6 or canonical Wnt/β-Catenin inhibition, which are not currently available.
Nevertheless, our data provide additional evidence for the potential importance of Sfrps in cardiac disease. Studies involving exogenous delivery or overexpression of Sfrps in the heart suggest that the different Sfrp isofroms might have overlapping but distinct contributions to cardiac repair [45]. Overexpression of Sfrp1 in transgenic animals improved cardiac function by modulating Wnt/Ca2+ pathway and the inflammatory response after injury [46, 47]. Interestingly, Sfrp1 overexpression in endothelial cells or cardiomyocytes did not have any effect suggesting that cell specific expression of the Sfrps is crucial for determining the effects in cardiac repair. Sfrp2 has been shown to inhibit cardiomyocyte apoptosis [13, 48] and promote neovascularization [14, 17]. While the anti-apoptotic effects of Sfrp2 were attributed to blockade of Wnt3a induced canonical signaling [48], the pro-angiogenic effects are related to the activation of the non-canonical Wnt/Ca+ pathway [17]. Moreover, Sfrp2 can act as profibrotic [12] or antifibrotic [15] depending on its concentration (10–20 nM pro-fibrotic, 100–200 nM antifibrotic) highlighting that Sfrp2 effects are highly dependent on timing, cellular context and concentration [49]. The current study provides new information about a robust role of Sfrp2 (10–100nM in vitro) on CPC differentiation and the indirect activation of non-canonical/PCP pathway in CPCs. These data suggest that Sfrp2 might have a time dependent cell specific role in the autocrine/paracrine regulatory pathways of the cardiac niche; the future development of cell specific conditional models of Sfrp2 cardiac overexpression or ablation could aid in addressing these questions directly.
In conclusion, the present study demonstrates that Sfrp2 primes CPCs for cardiac differentiation by blockade of Wnt6 canonical/β-Catenin pathway and activation of non-canonical/PCP signaling through JNK (Figure 7). These data provide further support for Sfrp2 as a key paracrine factor with multiple effects in vital mechanisms of cardiac repair. This information is important for understanding how the Wnt signaling pathway can affect cardiac repair and reinforce the potential of Sfrp2 as a prime therapeutic candidate for cardiac injury.
Conclusion
The mechanisms that regulate cardiac progenitor cell fate after injury remain largely elusive. Understanding of these mechanisms is important for cardiac therapy. Here, we show that administration of Sfrp2, a canonical Wnt pathway inhibitor, promoted differentiation of adult cardiac progenitor cells (CPCs) in vivo. In vitro, Sfrp2 treatment induced CPCs to exit the cell cycle and primed them for cardiac differentiation. This was achieved by inhibition of Wnt6 canonical signaling and activation of Wnt non-canonical pathways. Our results provide mechanistic insights on how the Wnt signaling pathway can regulate CPC cell biology and potentially affect cardiac repair after injury.
Supplementary Material
Highlights.
Sfrp2 promotes CPC differentiation in vivo and in vitro.
Sfrp2 inhibits CPC proliferation in vitro.
Sfrp2 binds to Wnt6.
Inhibition of Wnt6 by Sfrp2 activates JNK.
Acknowledgments
Research conducted in these studies was supported by National Heart, Lung, and Blood Institute grants RO1 HL35610, HL81744, HL72010, and HL73219 (to V.J.D.); the Edna Mandel Foundation (to V.J.D. and M.M.); M.M. is also supported by an American Heart Association National Scientist Development Award (10SDG4280011). AV was also supported by the Duke University Department of Medicine Eugene Stead Research Scholarship. Sincere thanks to Drs Conrad Hodgkinson and Jose Gomez for their critical reading of this manuscript and Dr. David Virshup at Duke-NUS Graduate Medical School, Singapore for kindly providing the C59 pan-Wnt inhibitor.
Footnotes
Disclosure
Maria Mirotsou is currently employed by Capricor Therapeutics, a biotechnology company working to develop and commercialize cardiac stem cell therapies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 2.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. The Journal of biological chemistry. 2004;279:11384–91. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 4.Chong JJ, Chandrakanthan V, Xaymardan M, Asli NS, Li J, Ahmed I, et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell stem cell. 2011;9:527–40. doi: 10.1016/j.stem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–42. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 6.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, et al. Stem cell niches in the adult mouse heart. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9226–31. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noseda M, Peterkin T, Simoes FC, Patient R, Schneider MD. Cardiopoietic factors: extracellular signals for cardiac lineage commitment. Circulation research. 2011;108:129–52. doi: 10.1161/CIRCRESAHA.110.223792. [DOI] [PubMed] [Google Scholar]
- 8.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. Journal of molecular and cellular cardiology. 2011;50:280–9. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gessert S, Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circulation research. 2010;107:186–99. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann MW. WNT signaling in adult cardiac hypertrophy and remodeling: lessons learned from cardiac development. Circulation research. 2010;107:1198–208. doi: 10.1161/CIRCRESAHA.110.223768. [DOI] [PubMed] [Google Scholar]
- 11.Zelarayan LC, Noack C, Sekkali B, Kmecova J, Gehrke C, Renger A, et al. Beta-Catenin downregulation attenuates ischemic cardiac remodeling through enhanced resident precursor cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19762–7. doi: 10.1073/pnas.0808393105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi K, Luo M, Zhang Y, Wilkes DC, Ge G, Grieskamp T, et al. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nature cell biology. 2009;11:46–55. doi: 10.1038/ncb1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, et al. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18366–71. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He W, Zhang L, Ni A, Zhang Z, Mirotsou M, Mao L, et al. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21110–5. doi: 10.1073/pnas.1004708107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfaro MP, Vincent A, Saraswati S, Thorne CA, Hong CC, Lee E, et al. sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair. The Journal of biological chemistry. 2010;285:35645–53. doi: 10.1074/jbc.M110.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courtwright A, Siamakpour-Reihani S, Arbiser JL, Banet N, Hilliard E, Fried L, et al. Secreted frizzle-related protein 2 stimulates angiogenesis via a calcineurin/NFAT signaling pathway. Cancer research. 2009;69:4621–8. doi: 10.1158/0008-5472.CAN-08-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma X, Robin C, Ottersbach K, Dzierzak E. The Ly-6A (Sca-1) GFP transgene is expressed in all adult mouse hematopoietic stem cells. Stem cells. 2002;20:514–21. doi: 10.1634/stemcells.20-6-514. [DOI] [PubMed] [Google Scholar]
- 19.Uchida S, De Gaspari P, Kostin S, Jenniches K, Kilic A, Izumiya Y, et al. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem cell reports. 2013;1:397–410. doi: 10.1016/j.stemcr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey B, Fransioli J, Gude NA, Alvarez R, Jr, Zhang X, Gustafsson AB, et al. Sca-1 knockout impairs myocardial and cardiac progenitor cell function. Circulation research. 2012;111:750–60. doi: 10.1161/CIRCRESAHA.112.274662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nature medicine. 2007;13:970–4. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends in cell biology. 2002;12:432–8. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 23.Burridge PW, Thompson S, Millrod MA, Weinberg S, Yuan X, Peters A, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PloS one. 2011;6:e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, et al. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107:1912–6. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
- 25.Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–18. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- 26.Crespi F, Croce AC, Fiorani S, Masala B, Heidbreder C, Bottiroli G. Autofluorescence spectrofluorometry of central nervous system (CNS) neuromediators. Lasers in surgery and medicine. 2004;34:39–47. doi: 10.1002/lsm.10240. [DOI] [PubMed] [Google Scholar]
- 27.Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. The Journal of biological chemistry. 2000;275:12701–11. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- 28.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. Journal of cell science. 2008;121:737–46. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto K, Miki R, Nakayama M, Tatsumi N, Yokouchi Y. Wnt9a secreted from the walls of hepatic sinusoids is essential for morphogenesis, proliferation, and glycogen accumulation of chick hepatic epithelium. Developmental biology. 2008;319:234–47. doi: 10.1016/j.ydbio.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Koyanagi M, Iwasaki M, Haendeler J, Leitges M, Zeiher AM, Dimmeler S. Wnt5a increases cardiac gene expressions of cultured human circulating progenitor cells via a PKC delta activation. PloS one. 2009;4:e5765. doi: 10.1371/journal.pone.0005765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandur P, Maurus D, Kuhl M. Increasingly complex: new players enter the Wnt signaling network. BioEssays : news and reviews in molecular, cellular and developmental biology. 2002;24:881–4. doi: 10.1002/bies.10164. [DOI] [PubMed] [Google Scholar]
- 32.Pandur P, Lasche M, Eisenberg LM, Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–41. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt C, McGonnell IM, Allen S, Otto A, Patel K. Wnt6 controls amniote neural crest induction through the non-canonical signaling pathway. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236:2502–11. doi: 10.1002/dvdy.21260. [DOI] [PubMed] [Google Scholar]
- 34.Lavery DL, Martin J, Turnbull YD, Hoppler S. Wnt6 signaling regulates heart muscle development during organogenesis. Developmental biology. 2008;323:177–88. doi: 10.1016/j.ydbio.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afouda BA, Martin J, Liu F, Ciau-Uitz A, Patient R, Hoppler S. GATA transcription factors integrate Wnt signalling during heart development. Development. 2008;135:3185–90. doi: 10.1242/dev.026443. [DOI] [PubMed] [Google Scholar]
- 36.Proffitt KD, Virshup DM. Precise regulation of porcupine activity is required for physiological Wnt signaling. The Journal of biological chemistry. 2012;287:34167–78. doi: 10.1074/jbc.M112.381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA, et al. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer research. 2013;73:502–7. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- 38.Laeremans H, Hackeng TM, van Zandvoort MA, Thijssen VL, Janssen BJ, Ottenheijm HC, et al. Blocking of frizzled signaling with a homologous peptide fragment of wnt3a/wnt5a reduces infarct expansion and prevents the development of heart failure after myocardial infarction. Circulation. 2011;124:1626–35. doi: 10.1161/CIRCULATIONAHA.110.976969. [DOI] [PubMed] [Google Scholar]
- 39.Hahn JY, Cho HJ, Bae JW, Yuk HS, Kim KI, Park KW, et al. Beta-catenin overexpression reduces myocardial infarct size through differential effects on cardiomyocytes and cardiac fibroblasts. The Journal of biological chemistry. 2006;281:30979–89. doi: 10.1074/jbc.M603916200. [DOI] [PubMed] [Google Scholar]
- 40.Saraswati S, Alfaro MP, Thorne CA, Atkinson J, Lee E, Young PP. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PloS one. 2010;5:e15521. doi: 10.1371/journal.pone.0015521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zelarayan L, Gehrke C, Bergmann MW. Role of beta-catenin in adult cardiac remodeling. Cell cycle. 2007;6:2120–6. doi: 10.4161/cc.6.17.4632. [DOI] [PubMed] [Google Scholar]
- 42.Daskalopoulos EP, Hermans KC, Janssen BJ, Matthijs Blankesteijn W. Targeting the Wnt/frizzled signaling pathway after myocardial infarction: a new tool in the therapeutic toolbox? Trends in cardiovascular medicine. 2013;23:121–7. doi: 10.1016/j.tcm.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Gibb N, Lavery DL, Hoppler S. sfrp1 promotes cardiomyocyte differentiation in Xenopus via negative-feedback regulation of Wnt signalling. Development. 2013;140:1537–49. doi: 10.1242/dev.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fransioli J, Bailey B, Gude NA, Cottage CT, Muraski JA, Emmanuel G, et al. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem cells. 2008;26:1315–24. doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dawson K, Aflaki M, Nattel S. Role of the Wnt-Frizzled system in cardiac pathophysiology: a rapidly developing, poorly understood area with enormous potential. The Journal of physiology. 2013;591:1409–32. doi: 10.1113/jphysiol.2012.235382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barandon L, Couffinhal T, Ezan J, Dufourcq P, Costet P, Alzieu P, et al. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation. 2003;108:2282–9. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- 47.Barandon L, Casassus F, Leroux L, Moreau C, Allieres C, Lamaziere JM, et al. Secreted frizzled-related protein-1 improves postinfarction scar formation through a modulation of inflammatory response. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:e80–7. doi: 10.1161/ATVBAHA.111.232280. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Deb A, Zhang Z, Pachori A, He W, Guo J, et al. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. Journal of molecular and cellular cardiology. 2009;46:370–7. doi: 10.1016/j.yjmcc.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xavier CP, Melikova M, Chuman Y, Uren A, Baljinnyam B, Rubin JS. Secreted Frizzled-related protein potentiation versus inhibition of Wnt3a/beta-catenin signaling. Cellular signalling. 2014;26:94–101. doi: 10.1016/j.cellsig.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.