Abstract
Fatty acid composition profiles are important indicators of meat quality and tasting flavor. Metabolic indices of fatty acids are more authentic to reflect meat nutrition and public acceptance. To investigate the genetic mechanism of fatty acid metabolic indices in pork, we conducted genome-wide association studies (GWAS) for 33 fatty acid metabolic traits in five pig populations. We identified a total of 865 single nucleotide polymorphisms (SNPs), corresponding to 11 genome-wide significant loci on nine chromosomes and 12 suggestive loci on nine chromosomes. Our findings not only confirmed seven previously reported QTL with stronger association strength, but also revealed four novel population-specific loci, showing that investigations on intermediate phenotypes like the metabolic traits of fatty acids can increase the statistical power of GWAS for end-point phenotypes. We proposed a list of candidate genes at the identified loci, including three novel genes (FADS2, SREBF1 and PLA2G7). Further, we constructed the functional networks involving these candidate genes and deduced the potential fatty acid metabolic pathway. These findings advance our understanding of the genetic basis of fatty acid composition in pigs. The results from European hybrid commercial pigs can be immediately transited into breeding practice for beneficial fatty acid composition.
Fatty acids are basic energy sources and indispensable components for cellular regulation and metabolism in animals. Fatty acids also play critical roles in meat nutrition, sensory, tenderness and taste flavor1. For instance, polyunsaturated fatty acids (PUFA), such as linoleic acid (C18:2n6) and γ-linolenic acid (C18:3n6), are beneficial for human health by reducing low density lipoprotein cholesterol in blood2. Hence, a high ratio of polyunsaturated to monounsaturated fatty acids (PUFA/MUFA) and that of omega-3 to omega-6 fatty acid (n-3/n-6) are more preferable in terms of meat nutriology3. Although fatty acid composition is an important trait, it is usually not considered in pig breeding schemes as phenotypic measurement of this trait is time-consuming and costive. It is thus worthwhile to understand the genetic mechanisms underlying fatty acid composition in pork, which would allow us to establish novel molecular breeding tools for optimizing fatty acid composition in pork.
Recently, genome-wide association studies (GWAS) have made a substantial progress in identifying genetic factors underlying or associated with complex traits. In pigs, GWAS have detected a large number of genetic loci for a variety of phenotypic traits in divergent populations, including a list of significant loci for fatty acid composition in pork4,5,6,7. Many loci have pleiotropic effects on multiple fatty acid contents that show close phenotypic and genetic correlations with each other4,5,6,7. For these correlated traits, multivariate association models have been developed to detect and fine map the pleiotropic loci8.
Metabolic phenotypes are important proxies of many biological processes and pathways, including fatty acid composition. In human, GWAS have been conducted on phenotypic ratios in metabolomics9. To date, many loci are known to be associated with metabolic phenotypic ratios, such as serum metabolic ratios in humans10. It has been shown that the detection power can be raised several orders of magnitudes when metabolic ratios are used as quantitative traits in GWAS10.
The aim of this study was to detect genomic loci for fatty acid metabolic traits in five pig populations that remain unexplored in our previous studies5,6,7. We first tested phenotypic correlations between fatty acid metabolic traits. By applying GWAS, we then identified several novel pleiotropic loci for the tested traits and highlighted a list of candidate genes for the identified loci. We further built a potential network and pathway in which these candidate genes were involved. Our findings provide insight into the genetic mechanism of fatty acid composition in pork.
Materials and Methods
Ethics statement
All the experiments that involved animals were carried out in accordance with the approved guidelines by the Ministry of Agriculture of China. Approval was obtained from the ethics committee of Jiangxi Agricultural University before this study.
Animals and phenotypes
Five pig populations were used in this study, including one White Duroc × Erhualian F2 intercross (hereafter referred to as F2), one Duroc × (Landrace × Yorkshire) hybrid (hereafter referred to as DLY) population, and three Chinese pig populations (Sutai, Erhualian and Laiwu). The pedigree, management and phenotype recording information about the five populations has been described in our previous publications5,6,7,11,12. Briefly, two White Duroc founder boars and 17 Chinese Erhualian founder sows were mated to produce F1 animals, from which nine F1 boars and 59 F1 sows were intercrossed to produce 976 F2 males and 945 F2 females in six batches. Fatty acid composition traits were measured on longissimus dorsi muscle and abdominal fat samples of 677 F2 pigs. Sutai is a Chinese synthetic pig line that was originally generated from a cross between Chinese Erhualian/Meishan and Duroc pigs and has experienced artificial selection for prolificacy and growth for more than 18 generations13. A total of 281 Sutai pigs from five sires and 60 dams were measured for fatty acid composition in longissimus dorsi muscle as described previously5. Erhualian pigs that was originally located in Wuxi, Jiangsu Province, are famous for their high litter size of greater than 1613. Laiwu is a Chinese indigenous pig breed that was originally distributed in Laiwu, Shangdong Province. The Laiwu pig is known for its unusually high intramuscular fat content (average 9~12%)13. We measured fatty acid composition on longissimus dorsi muscle samples of 336 Erhualian pigs from 11 sires and 55 dams and 333 Laiwu pigs from 25 sires and 115 dams that covered the majority of sire and dam lines of the two Chinese breeds. During the fattening period (from ~90 days to the slaughter ages), F2, Sutai, Erhualian and Laiwu pigs were raised in a pig farm in Nanchang, Jiangxi Province, and were ad libitum fed the same diet containing 16% crude protein, 3100 kJ digestible energy and 0.78% lysine under standard management conditions. A total of 698 DLY pigs at 180 ± 3 days were purchased from a commercial pig farm in Xiushui, Jiangxi Province. DLY boars were castrated at day 25. All DLY pigs were raised under a consistent diet containing 16% crude protein, 2132 digestible energy and 0.85% lysine. Finally, F2 and Sutai pigs at 240 ± 3 days, DLY pigs at 180 ± 3 days, and Erhualian and Laiwu pigs at 300 ± 3 days were slaughtered in the same commercial abattoir. After slaughter, longissimus dorsi muscle samples from all individuals and abdominal fat tissues from the F2 pigs were collected to measure fatty acid composition as described previously5,6,7. In this study, we further calculated five fatty acid metabolic indices using the following equations14,15:
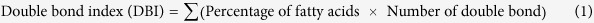 |
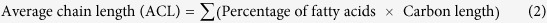 |
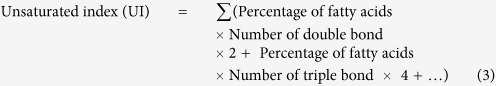 |
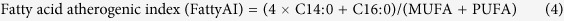 |
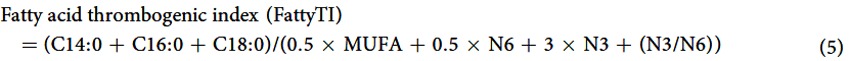 |
where MUFA is monounsaturated fatty acids including myristoleic acid (C14:1n5), palmitoleic acid (C16:1n7), oleic acid (C18:1n9) and eicosenoic acid (C20:1n9), and PUFA is polyunsaturated fatty acids including linoleic acid (C18:2n6), γ-linolenic acid (C18:3n6), α-linolenic acid (C18:3n3), eicosadienoic acid (C20:2n6), dihomo- γ-linolenic acid (C20:3n6), eicosatrienoic acid (C20:3n3), arachidonic acid (C20:4n6); N3 is omega-3 PUFA comprising C18:3n3, C20:3n3, and N6 is omega-6 PUFA including C18:2n6, C18:3n6, C20:2n6, C20:3n6 and C20:4n6.
We also determined the ratios of UFA to SFA (UFA/SFA), PUFA to MUFA (PUFA/MUFA), MUFA to SFA (MUFA/SFA), PUFA to SFA (PUFA/SFA) and N6 to N3 (N6/N3), where UFA refers to unsaturated fatty acids including MUFA and PUFA, and SFA is saturated fatty acids including myristic acid (C14:0), palmitic acid (C16:0), stearic acid (C18:0) and arachidic acid (C20:0). Lastly, we calculated all possible products/substrates ratios in fatty acid metabolic pathway, including C14:1n5/C14:0, C16:0/C14:0, C18:0/C16:0, C16:1n7/C16:0, C18:1n9/C16:1n7, C18:1n9/C18:0, C18:3n6/C18:2n6, C20:0/C18:0, C20:1n9/C18:1n9, C20:1n9/C20:0, C20:2n6/C18:2n6, C20:3n6/C18:2n6, C20:3n6/C18:3n6, C20:3n3/C18:3n3, C20:4n6/C18:2n6, C20:4n6/C18:3n6, C20:4n6/C20:2n6 and C20:4n6/C20:3n6 (Supplementary Table 1). Before the association analysis, 33 fatty acid metabolic traits were log2-transformed owing to the relationship of log (a/b) = −log (b/a), so that the association of a ratio and of its inverse would obtain identical results.
Genotypes and quality control
Genomic DNA was extracted from ear tissue using a standard phenol-chloroform method. As reported previously5,7,11,12, animals from the DLY, Erhualian and Laiwu populations were genotyped with PorcineSNP60 BeadChips (v2), and those from the F2 and Sutai populations were genotyped with BeadChip (v1)16 on an iScan system (Illumina, USA) following the manufacture’s instruction. SNPs with call rates > 0.90 and minor allele frequencies > 0.01, samples with genotyping call rates > 0.90 were retained for further statistical analyses. To compare the current results to our previously reported loci for fatty acid contents, we re-conducted GWAS for fatty acid composition using the same above-mentioned quality control procedures across the five populations. A meta-analysis of GWAS was conducted for each fatty acid metabolic index by using a common set of qualified SNPs across the five populations.
Statistical analyses
To investigate phenotypic correlations between the metabolic traits measured, we corrected phenotypes by treating sex and slaughter batch as fixed effects, and polygenic effects as random effects under a linear mixed model implemented in polygenic function in R package GenABEL17. The phenotypic correlation matrix was calculated by Pearson product-moment correlation coefficients using “corrplot” package in R. The polygenic function of GenABEL package was applied to estimate heritabilities of fatty acid metabolic traits17. The phenotypic variance explained by each top GWAS SNP was calculated by (Vreduce − Vfull)/Vreduce, where Vreduce and Vfull are residual variances of ordinary linear models with and without including SNP genotypes as predictor variables, respectively.
For GWAS, single-marker association was conducted using polygenic and mmscore function of GenABEL. A generalized linear mixed model (see equation (6)) was explored to evaluate the association between qualified SNPs and phenotypic values.
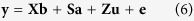 |
where y is the vector of phenotypes; b is the vector of fixed effects including sex and batch effects; a is the allelic substitution effects; u is the vector of random additive genetic effects following the multinormal distribution u ~ N (0, Gσα2), in which G is the genomic relationship matrix that was constructed based on qualified SNPs, σα2 is the polygenetic additive variance. X and Z are the incidence matrices for b and u, respectively; S is the incidence vector for a, and e is a vector of residual errors with a distribution of N (0, Iσe2); where I is the identity matrix and σe2 is the residual variance. Population stratification was accounted by adjusting the kinship covariance matrix estimated from SNP data using “ibs” function in GenABEL. Genomic inflation factors (λ) were calculated as median (obsPvals)/median (expPvals), where “obsPvals” and “expPvals” represent the observed and expected P values in the association analysis, respectively. Sex and batch were fitted as fixed effects in the model. Bonferroni methods were used to correct the genome-wide significant (0.05/N) and suggestive (1/N) thresholds, where N is the number of SNPs used for analyses.
A meta-analysis was conducted using a Z-score method implemented in METAL software18, which combined the P-value and allelic effect of each SNP. A total of 28,124 common SNPs across the five populations were analyzed in the meta-analysis.
Gene function, pathway and network analyses
To highlight functionally plausible candidate genes at genome-wide significant loci, we operationally searched for annotated genes within a 1 Mb region centering each top SNP on the pig reference genome assembly (Build 10.2). The homologous segments in other mammals including mouse, cattle and human were investigated to characterize unannotated genes in target regions. LASTZ, a sequence alignment program19, was explored to align pairwise genomic sequences of different species. Candidate genes were determined by their functions related to fatty acid metabolism through literature mining. Enriched functional connections and networks involving the highlighted candidate genes were investigated by using GeneMANIA20. A potential fatty acid metabolic pathway was deduced from the known biochemical functions of the highlighted candidate genes.
Results
Summary of phenotype and genotype statistics
Supplementary Table 1 shows phenotypic values of 33 fatty acid metabolic traits measured in the five populations, including number of individuals, mean, standard deviation and estimated heritabilities (h 2). Most (F2 longssimus muscle, 70%; F2 abdominal fat, 85%; Sutai, 42%; Erhualian, 82%; Laiwu, 82%; DLY, 55%) of the metabolic indices displayed moderate to high heritability estimates ranging from 0.25 to 0.65, indicating that genetic components significantly contribute to fatty acid metabolic traits. We estimated phenotypic correlations between the 33 analyzed traits (Supplementary Fig. 1). SFA, FattyAI and FattyTI were highly correlated with UFA/SFA, MUFA/SFA, DBI and UI because of the definitive calculation methods of these traits. After the same quality control process, a total of 47,953, 49,109, 56,126, 35,974 and 49,442 SNPs were qualified for 597 F2, 281 Sutai, 610 DLY, 336 Erhualian and 319 Laiwu pigs. The average adjacent distances between markers were 62.56, 61.08, 53.45, 83.39 and 60.67 kilo bases (kb) in the F2, Sutai, DLY, Erhualian and Laiwu populations, respectively. Bonferroni-corrected genome-wide significant thresholds were 1.04 × 10−6 (0.05/47,935), 1.01 × 10−6 (0.05/49,109), 8.91 × 10−7 (0.05/56,126), 1.39 × 10−6 (0.05/35,974) and 1.01 × 10−6 (0.05/49,442) in these populations, respectively.
Single-population GWAS
We conducted single-marker association studies on the 33 fatty acid metabolic traits measured in the five populations. In total, we detected 11 genome-wide significant loci on nine chromosomes (Table 1) and 12 suggestive regions on nine chromosomes (Table 2). Of the 11 significant loci, four were population-specific and novel loci that were not identified in previous studies5,6,7, including the Sus scrofa chromosome (SSC) 2 locus (9.13–12.86 Mb) for C20:3n6/C18:2n6 and C20:4n6/C20:3n6 in Erhualian, the SSC12 locus (57.80–61.49 Mb) for C20:4n6/C20:3n6, PUFA/SFA and SFA in Laiwu, the SSC13 locus (28.52 Mb) for C16:1n7/C16:0 in DLY and the SSCX locus (124.40–125.87 Mb) for C20:2n6/C18:2n6 and C20:4n6/C20:2n6 in Laiwu. The other seven loci corresponded to previously identified loci for fatty acid composition5,6,7,21,22. These loci were distributed on SSC2, 7, 8, 12, 14, 16 and X and showed pleiotropic effects on more than one fatty acid metabolic trait (Table 1). Three of these loci were also evidenced as pleiotropic QTL for fatty acid composition in both abdominal fat and longissimus dorsi tissues in the F2 population in our previous studies5, including the SSC7 locus (27.45–80.45 Mb), the SSC8 locus (119.42–129.56 Mb) and the SSC16 locus (21.23–63.72 Mb) (Table 1). Moreover, we detected several loci simultaneously affecting the same metabolic index in a single population. For instance, the loci at 27.45–80.45 Mb, 134.27–134.68 Mb on SSC7 and 21.23–63.72 Mb on SSC16 affected C20:1n9/C20:0 in abdominal fat tissue in the F2 population, and the loci at 119.72–121.47 Mb on SSC8, 28.52 Mb on SSC13 and 120.21–123.29 Mb on SSC14 affected C16:1n7/C16:0, apparently indicating the polygenic basis of these traits.
Table 1. Summary of significant loci for fatty acid metabolic indices in five pig populations.
| Chr | Trait | Pop | Nsnp | Range of SNP (Mb) | Top SNP | Position (bp) | P-Value | Candidate genes |
|---|---|---|---|---|---|---|---|---|
| 2 | C20:3n6/C18:2n6, C20:4n6/C20:3n6 | Erhualian | 11 | 9.13–12.86 | ASGA0008884 | 9139348 | 3.55E-11 | FADS2 |
| 7 | A20:1n9/A18:1n9, A20:1n9/A20:0, A20:2n6/A18:2n6, A20:4n6/A18:2n6, A20:4n6/A18:3, A20:4n6/A20:3, A22:5/A20:4n6, AMUFA, APUFA, APUFA/SFA, C20:1n9/C18:1n9, C20:4n6/C18:3, | F2 | 210 | 27.45–80.45 | ASGA0033714 | 53659203 | 2.31E-13 | PLA2G7 |
| A20:1n9/A18:1n9, A20:1n9/A20:0, A20:2n6/A18:2n6, A20:4n6/A18:2n6, A20:4n6/A18:3, C20:1/C18:1n9 | F2 | 4 | 134.27–134.68 | MARC0060950 | 134313767 | 1.52E-12 | ELOVL5 | |
| C20:2n6/C18:2n6 | DLY | 3 | 52.80–53.56 | MARC0034834 | 53102034 | 5.42E-08 | ACSBG1 | |
| C20:1n9/C18:1n9, C20:1n9/C20:0, C20:2n6/C18:2n6, C20:3n3/C18:3n3, C20:3n6/C18:3n6, | Erhualian | 42 | 32.23–59.70 | INRA0025107 | 41743168 | 1.03E-09 | ||
| C20:1n9/C18:1n9, C20:1n9/C20:0, C20:2n6/C18:2n6, C20:3n3/C18:3n3, | Erhualian | 6 | 134.15–134.54 | ALGA0114746 | 134540651 | 2.84E-20 | ||
| C16:1n7/C16:0, C18:1n9/C16:1n7, C20:1n9/C18:1n9, C20:1n9/C20:0, C20:2n6/C18:2n6, C20:3n3/C18:3n3 | Laiwu | 70 | 28.07–76.29 | INRA0026056 | 58234493 | 1.30E-16 | ||
| 8 | A18:0/A16:0, A18:1n9/A16:1n7, C16:0/C14:0, C18:1n9/C16:1n7 | F2 | 18 | 119.42–129.56 | MARC0114654 | 126831850 | 1.43E-10 | ELOVL6 |
| C14:1n5/C14:0, C18:1n9/C16:1n7 | Erhualian | 2 | 127.07–138.50 | DIAS0004322 | 138501500 | 6.43E-10 | MTTP | |
| C16:1n7/C16:0, C18:0/C16:0, C18:1n9/C16:1n7 | DLY | 8 | 119.72–121.47 | H3GA0025321 | 119887525 | 6.40E-17 | ||
| 9 | C16:1n7/C14:0 | DLY | 1 | 14.21 | MARC0100725 | 14217867 | 4.73E-08 | |
| 12 | C14:1n5/C14:0, C16:0/C14:0, C16:1n7/C16:0, C18:1n9/C16:1n7, DBI, FattyAI, UI | Erhualian | 10 | 0.4–8.29 | MARC0063090 | 1779278 | 2.09E-13 | FASN |
| C16:0/C14:0 | Laiwu | 8 | 0.2–8.37 | ASGA0099260 | 248014 | 2.06E-10 | ||
| C20:4n6/C20:3n6, PUFA/SFA, SFA, | Laiwu | 6 | 57.80–61.49 | ALGA0067099 | 57950908 | 1.08E-07 | SREBF1 | |
| 13 | C16:1n7/C16:0 | DLY | 1 | 28.52 | ASGA0104338 | 28524131 | 2.21E-07 | |
| 14 | C16:1n7/C16:0, C18:0/C16:0, C18:1n9/C18:0, MUFA/SFA, UFA/SFA | F2 | 23 | 119.51–123.40 | ALGA0081025 | 119955763 | 3.47E-10 | SCD |
| C18:0/C16:0, C18:1n9/C18:0, FattyTI, SFA, UFA/SFA | Sutai | 12 | 116.35–119.96 | MARC0111695 | 116694325 | 1.59E-09 | ||
| C16:1n7/C16:0, C18:0/C16:0, C18:1n9/C16:1n7, C18:1n9/C18:0, DBI, FattyAI, FattyTI, MUFA, MUFA/SFA, SFA, UFA/SFA, UI | DLY | 38 | 120.21–123.29 | ALGA0081091 | 120986865 | 1.19E-21 | ||
| 16 | A20:0/A18:0, A20:1n9/A20:0, C20:0/C18:0, C20:1n9/C20:0 | F2 | 162 | 21.23–63.72 | ALGA0090423 | 41393886 | 6.51E-24 | ELOVL7 |
| C20:0/C18:0 | Sutai | 79 | 21.71–50.25 | MARC0073781 | 28101697 | 2.79E-09 | ||
| C20:0/C18:0, C20:1n9/C20:0, C20:1n9/C18:1n9 | DLY | 62 | 35.24–49.17 | DRGA0016155 | 43534471 | 7.69E-37 | ||
| C20:0/C18:0, C20:1n9/C20:0 | Erhualian | 51 | 26.89–55.96 | ASGA0072949 | 34715842 | 3.92E-18 | ||
| C20:0/C18:0 | Laiwu | 61 | 24.67–62.24 | ASGA0072968 | 35190026 | 3.50E-17 | ||
| X | C20:2n6/C18:2n6, C20:4n6/C20:2n6 | Laiwu | 2 | 124.40–125.87 | H3GA0052009 | 125876415 | 2.69E-07 |
Chr: chromosome; Pop, population; F2, the White Duroc × Erhualian F2 intercross; DLY, Duroc × (Landrace × Yorkshire) hybrid pigs; Nsnp, the total number of significant SNPs associated with the traits; Top SNP, the most significantly associated SNP for a given trait; Position, genomic position of the top SNP on the Sscrofa 10.2 pig genome assembly; P-value, the P-value of the top SNP; P-values were calculated by the use of the GenABEL package in R.
Table 2. Summary of suggestive loci for fatty acid metabolic indices in five pig populations.
| Chr | Trait | Pop | Nsnp | Range of SNP (Mb) | Top SNP | Position (bp) | P-value | Candidate genes |
|---|---|---|---|---|---|---|---|---|
| 2 | C20:4n6/C18:2n6, C20:4n6/C20:2n6 | Sutai | 10 | 107.47–114.96 | MARC0080651 | 109657735 | 1.30E-06 | PAM |
| 4 | A_UI, A_DBI, APUFA/SFA, APUFA | F2 | 3 | 89.00–91.69 | ALGA0026229 | 89038458 | 7.02E-06 | PBX1 |
| 5 | C20:0/C18:0 | Laiwu | 8 | 17.32–17.92 | H3GA0015868 | 17786755 | 3.71E-06 | SCN8A |
| 6 | C20:2/C18:2n6 | F2 | 7 | 5.92–9.14 | M1GA0026870 | 8453407 | 1.78E-06 | HSDL1 |
| ACL, N6/N3, C16:1n7/C16:0, C18:0/C16:0, C18:1n9/C16:1n7 | Laiwu | 4 | 65.19–71.01 | ASGA0106005 | 71011913 | 1.11E-06 | AADACL3 | |
| 8 | C16:1n7/C16:0, C18:1n9/C16:1n7, N3, N6, PUFA, PUFA/MUFA | Erhualian | 4 | 28.85–33.74 | H3GA0056028 | 33740711 | 1.87E-06 | TBC1D1 |
| C20:1n9/C20:0, C20:3n6/C18:3n6, MUFA | DLY | 4 | 17.53–18.14 | ASGA0037971 | 17530855 | 4.99E-06 | ||
| 9 | C16:0/C14:0, C16:1n7/C16:0, C18:1n9/C16:1n7 | Laiwu | 6 | 11.48–14.51 | ALGA0051583 | 14447589 | 2.28E-06 | DGAT2 |
| C16:0/C14:0, C20:1n9/C20:0 | DLY | 2 | 11.34–14.30 | MARC0046646 | 14306925 | 6.20E-06 | ||
| 10 | C16:0/C14:0, C20:1n9/C20:0 | F2 | 2 | 68.62–70.25 | ASGA0048928 | 70250117 | 1.29E-06 | |
| C20:4n6/C20:2n6 | Laiwu | 2 | 69.82–70.55 | M1GA0014342 | 69820079 | 2.49E-06 | ||
| 13 | C14:1n5/C14:0 | Sutai | 3 | 71.04–72.23 | DRGA0012717 | 72216697 | 1.55E-08 | |
| 15 | C20:4n6/C18:2n6, C20:4n6/C20:2n6 | Laiwu | 6 | 13.26–17.10 | CASI0010463 | 15140520 | 6.82E-06 | |
| C20:4n6/C20:2n6, N6/N3 | Laiwu | 3 | 127.72–130.59 | ALGA0086940 | 130249305 | 1.17E-06 | ||
| C14:1n5/C14:0, C20:1n9/C20:0, C20:1n9/C18:1n9, C20:4n6/C20:2n6 | Laiwu | 11 | 144.54–147.38 | H3GA0045535 | 147384455 | 3.03E-06 |
Chr: chromosome; Pop, population; Nsnp, the total number of significant SNPs associated with the traits; F2, the White Duroc × Erhualian F2 intercross; DLY, Duroc × (Landrace × Yorkshire) hybrid pigs; Top SNP, the most significantly associated SNP for a given trait; Position, genomic position of the top SNP on the Sscrofa 10.2 pig genome assembly; P-value, the P-value of the top SNP; P-values were calculated by the use of the GenABEL package in R.
In the F2 population, a total of 441 SNPs on seven chromosomal regions were significantly associated with 25 fatty acid metabolic traits. We detected a genome-wide significant locus for abdominal fat weight in the F2 population23. Considering that fatty acid composition in abdominal fat tissues was correlated with abdominal fat weight in this population24, we herein treated abdominal fat weight as a fixed effect in the GWAS model and found no obviously different results (data not shown). This indicates that the loci affecting abdominal fat weight have no significant effect on fatty acid composition in abdominal fat tissues. In our previous study, we detected only one suggestive locus at 52.35 Mb on SSC16 for C16:0 content and did not find any significant loci for C18:0 content in abdominal fat tissues in the F2 population5. When we used the ratio of C18:0/C16:0 as a phenotype, we herein detected a region at 126.83–129.56 Mb on SSC8 that showed genome-wide significant (P < 8.91E-7) association with the metabolic trait with the top SNP (ASGA0039796) at 129.44 Mb on this chromosome (Fig. 1a). We previously identified a significant (P = 5.07E-08) locus around 34.80 Mb on SSC7 for C18:1n9 but not for C16:1n7 content in abdominal fat5. However, when GWAS was re-conducted for the ratio of C18:1n9/C16:1n7, all the significant SNPs associated with C18:1n9 on SSC7 disappeared. Instead, a significant region at 128.84–129.56 Mb on SSC8 with the top SNP (ALGA0049404, P = 1.81E-07) was detected for the metabolic ratio (Fig. 1b). At 134.27–134.68 Mb on SSC7, we identified a significant locus for the ratio of C20:2n6/C18:2n6 in abdominal fat tissues in the F2 population (Fig. 1c). This region was not associated with C18:2n6 or C20:2n6 content in the F2 population. A marked locus containing one significant and five suggestive SNPs around 129 Mb on SSC8 have been associated with C16:1n7 content in longissimus dorsi muscle in the F2 population5. The top SNP (H3GA0025376, P = 3.97E-07) at 126.88 Mb explained 6.24% of phenotypic variance5. Here we conducted GWAS for C18:1n9/C16:1n7, resulting in more associated SNPs (N = 14) in the 119–130 Mb region on SSC8 and an increased association strength with the top SNP (MARC0114654, P = 1.45E-10) at 126.83 Mb explaining 11.48% of phenotypic variance (Fig. 1d). We noted that two SNPs at 63.84 Mb (INRA0046679) and 63.98 Mb (MARC0049861) on SSC4 were significantly associated with C16:1n7/C16:0, C18:1n9/C18:0, UFA/SFA and MUFA/SFA. However, by applying linear homologous comparison via LASTZ19, we found that the genomic segment containing the two significant SNPs was inaccurately assembled, and the most likely location of this segment was in the vicinity of the 121.35 Mb region on SSC14 that was also significantly associated with the four metabolic traits mentioned above (Supplementary Fig. 2).
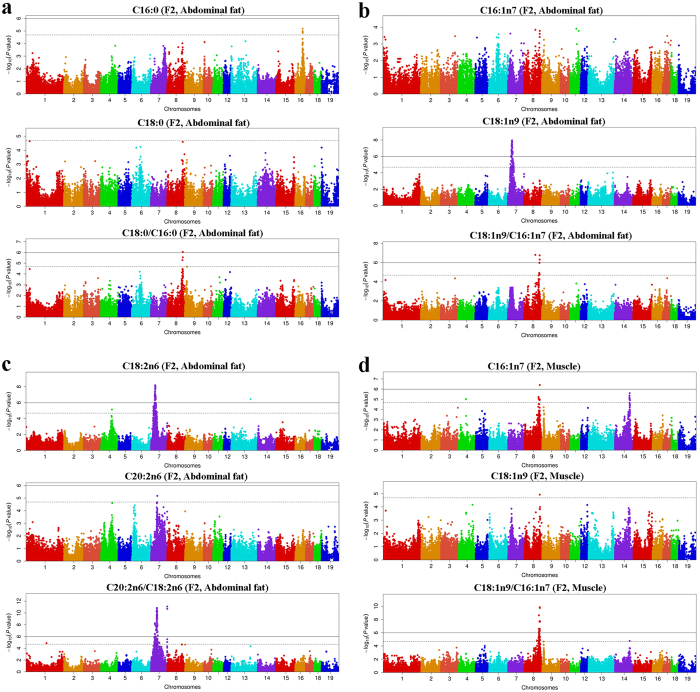
Figure 1. Manhattan plots of significant loci for fatty acid metabolic traits in the White Duroc × Erhualian F2 population.
(a) GWAS results for C16:0, C18:0 and C18:0/C16:0 in abdominal fat; (b) GWAS results for C16:1n7, C18:1n9 and C18:1n9/C16:1n7 in abdominal fat; (c) GWAS results for C18:2n6, C20:2n6 and C20:2n6/C18:2n6 in abdominal fat; (d) GWAS results for C16:1n7, C18:1n9 and C18:1n9/C16:1n7 in longissimus dorsi muscle. Negative log10 P values of the qualified SNPs (N) are plotted against their genomic positions. SNPs on different chromosomes are denoted by different colors. The solid line represents the genome-wide threshold (0.05/N). The dash line indicates the suggestive threshold (1/N).
In the Erhualian population, we identified seven genome-wide significant loci for 17 fatty acid metabolic traits on five chromosomes. By applying GWAS for fatty acid contents in this population, we have previously detected only one significant SNP (DIAS0001596) at 32.15 Mb on SSC8 for C18:2n6 content and did not identify any significant signals for C20:2n6 or C20:3n6 content7. In this study, a novel locus at 9.13–12.86 Mb on SSC2 containing 11 significant SNPs was detected for C20:3n6/C18:2n6 and C20:4n6/C20:3n6 (Fig. 2a). The top SNP (ASGA0008884, P = 3.55E-11) locates in the fifth intron of the FADS2 (fatty acid desaturase 2) gene. This Erhualian-specific locus has not ever been reported in any association studies for fatty acids in pigs. Moreover, we identified two regions (36–56 Mb and 134.1–134.5 Mb) on SSC7 and one region (0.4–4 Mb) on SSC12 that were associated with C20:2n6/C18:2n6 at the genome-wide significant and suggestive levels, respectively (Fig. 2b). The 0.4–4 Mb region on SSC12 has been reported to be associated with multiple fatty acid contents, such as C14:0, C16:0, C16:1n7, C18:1n9, C20:1n9, but not with C18:2n6 or C20:2n6 content7. The locus at 134.1–134.5 Mb on SSC7 affects both fatty acid composition traits and metabolic traits in F2 and Erhualian pigs.
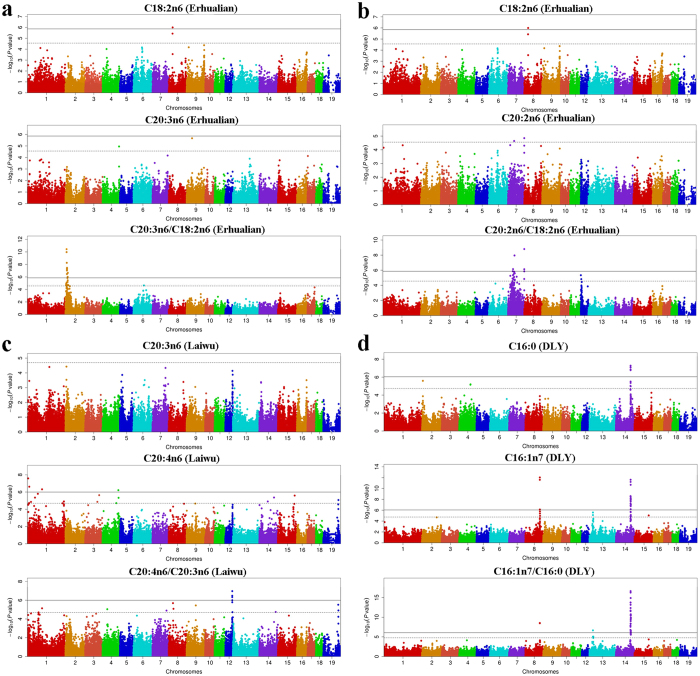
Figure 2. Manhattan plots of significant loci for fatty acid metabolic traits in the Erhualian, Laiwu and DLY populations.
(a) GWAS results for C18:2n6, C20:3n6 and C20:3n6/C18:2n6 in the Erhualian population; (b) GWAS results for C18:2n6, C20:2n6 and C20:2n6/C18:2n6 in the Erhualian population; (c) GWAS results for C20:3n6, C20:4n6, and C20:4n6/C20:3n6 in the Laiwu population; (d) GWAS results for C16:0, C16:1n7 and C16:1n7/C16:0 in the DLY population; Negative log10 P values of the qualified SNPs (N) are plotted against their genomic positions. SNPs on different chromosomes are denoted by different colors. The solid line represents the genome-wide threshold (0.05/N). The dash line indicates the suggestive threshold (1/N).
In the Laiwu population, we detected seven significant loci affecting 10 traits, which confirmed five previously reported QTL and revealed two novel loci. One novel chromosomal region at 57.80–61.49 Mb on SSC12 was associated with multiple metabolic traits, where six genome-wide SNPs for C20:4n6/C20:3n6, FattyTI, SFA and seven suggestive SNPs for PUFA/SFA, UFA/SFA, DBI and UI were found (Fig. 2c and Table 1). This pleiotropic locus was observed only in the Laiwu population. At 124.40–125.87 Mb on SSCX, we identified another novel locus associated with C20:2n6/C18:2n6 and C20:4n6/C20:2n6 (Table 1). This locus exists only in Laiwu pigs and have not been reported to associate with any fatty acid traits before this study.
In the DLY population, six chromosomal regions containing 114 SNPs were associated with 17 fatty acid metabolic traits. We have previously detected a significant locus (Top SNP: ASGA0066120, P = 2.42E-12) around 121.51 Mb on SSC14 for C16:1n7 content and a suggestive locus (28.52–31.47 Mb, P = 2.64E-06) on SSC137. In this study, the association strength at the suggestive locus on SSC13 surpassed the genome-wide significant threshold for C16:1n7/C16:0 (P = 2.21E-07, Fig. 2d). The SSC13 locus is DLY-specific in the five populations. Moreover, the association strength at the SSC14 locus increased from 2.42E-12 for C16:1 to 2.24E-17 for C16:1n7/C16:0 (Fig. 2d).
Meta-analysis of GWAS
We conducted a meta-analysis of GWAS for each fatty acid metabolic trait across the five populations. Genomic inflation factors (λ) in the meta-analysis were between 0.961 and 1.047, indicating that population stratification was properly adjusted. The meta-analysis integrates all association signals from the five populations. Therefore, when two or more populations show consistent association directions with target traits, the detection power for these traits will be improved by the meta-analysis of GWAS. By applying this analysis, we did not identify new loci but observed enhanced association strength at five loci, especially at the locus around 121 Mb on SSC14 and the locus around 43 Mb on SSC16 (Fig. 3). The most significant SNP for C18:1n9/C18:0 was ALGA0081091 (P = 1.19E-21) around 121 Mb on SSC14 in the DLY population. In the meta-analysis, the top SNP for C18:1n9/C18:0 was CASI0010164 (P = 7.07E-33) at 121.31 Mb on SSC14. Around 43 Mb on SSC16, the lead SNP for C20:0/C18:0 was DRGA0016155 (P = 7.69E-37) in the DLY population. We detected the same top SNP for the same trait at this locus with a much lower P value of 3.38E-52 (Fig. 3) in the meta-analysis. Two lead SNPs which were 12.69 Mb on SSC9 and 1.16 Mb on SSC12 showed consistent effects on C16:0/C14:0 across the five populations, conceivably resulting in an enhanced association signal in the meta-analysis (Fig. 3 and Supplementary Table 2). Moreover, more significant SNPs were detected at the 11–13 Mb region on SSC9 for C16:0/C14:0, C16:1n7/C16:0 and C18:1n9/C16:1n7 (Supplementary Table 2). The meta-analysis also characterized three new genome-wide significant loci for C16:1n7/C16:0 and C18:1n9/C16:1n7, which were located around 119.72 Mb on SSC8, 11.83 Mb on SSC9 and 121.30 Mb on SSC14 (Supplementary Table 2). Association signals at some significant loci, such as the Laiwu-specific locus at 57.80–61.49 Mb on SSC12 and the Erhualian-specific locus around 134 Mb on SSC7, were weakened or vanished in the meta-analysis (Supplementary Table 2). This could be due to population heterogeneity across the F2, Sutai, DLY, Laiwu and Erhualian populations.
Figure 3. Manhattan plot of the GWAS meta-analysis across the five populations.
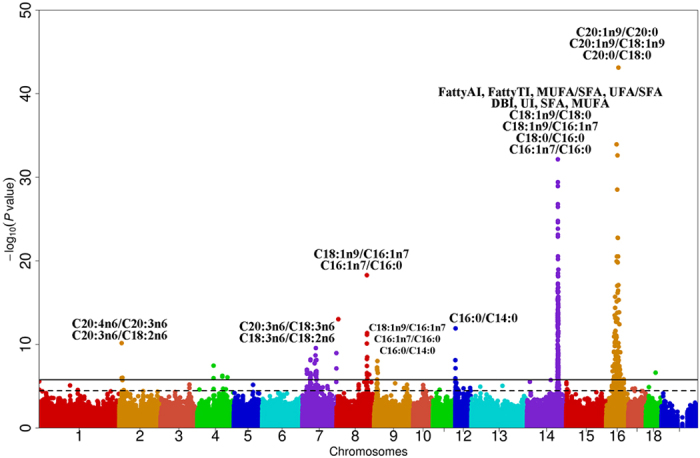
The associated traits are indicated above the corresponding loci. Negative log10 P values of the qualified SNPs are plotted against their genomic positions. The solid line represents the genome-wide threshold (0.05/N). The dash line indicates the suggestive threshold (1/N). N stands for the common SNPs across the five populations.
Conditional GWAS for pleiotropic, linked or common QTL
In the single-population GWAS, the significant loci that we identified were usually associated with more than one fatty acid metabolic trait. To test if there is only one pleiotropic QTL or more linked QTL at these loci, we conducted GWAS by including the most significant SNPs (shown in Table 1) as fixed effects in the model. When conditional on the effects of the top SNPs, most of association signals disappeared (Supplementary Fig. 3), supporting that common pleiotropic variants underlie these loci. For instance, the 119–123 Mb region on SSC14 was associated with multiple metabolic traits including C16:1n7/C16:0, C18:1n9/C18:0 and C18:0/C16:0. When we treated the top SNP (ALGA0081025 in F2, MARC0111695 in Sutai and ALGA0081091 in DLY) as a fixed effect, no association was evidenced for any traits around this region on SSC14, suggesting a real pleiotropic QTL in these populations. In contrast, after fixing the top population-specific SNPs (ASGA0033714 in F2, MARC0034834 in DLY, and INRA0025107 in Erhualian and INRA0026056 in Laiwu) on SSC7 in the GWAS model, association signals for all traits disappeared in F2 and DLY populations but retained in Laiwu and Erhualian pigs. In Laiwu pigs, another region around 28.06–37.39 Mb on this chromosome showed association with C16:0/C14:0, C16:1n7/C16:0, C18:1n9/C16:1n7, C20:1n9/C20:0 and FattyAI with the top SNP (DRGA0007448, P = 1.36E-09) at 31.62 Mb. In the Erhualian population, three SNPs were still significantly (P = 5.30E-09) associated with C18:1n9/C16:1n7, C20:1n9/C18:1n9, C20:1n9/C20:0 and C20:3n3/C18:3n3 even conditional on the effect of the top SNP. Moreover, when fixed the top SNPs (MARC0063090 and ASGA0099260) in Erhualian and Laiwu populations respectively, association signals of the 0.21–8.37 Mb region on SSC12 for C16:0/C14:0 disappeared in the Laiwu population, but a significant SNP (ASGA0052511, P = 6.13E-07) at 2.62 Mb together with seven suggestive variants in an adjacent region were still associated with C18:1n9/C16:1n7 in Erhualian pigs (Supplementary Fig. 3). This could be explained by the existence of multiple linked QTL in this region. Thus, higher-density markers or sequence data should be explored to clarify this issue in the near future.
Of note, the association signals for C20:0/C18:0 on SSC16 vanished in Erhualian and Laiwu populations when corrected for the effect of the top GWAS SNPs (ASGA0072949 in Erhualian and ASGA0072968 in Laiwu). These SNPs were located in an adjacent region of ~500 kb (Table 1). This leads us to assume that a common causal variant at the SSC16 locus is responsible for C20:0/C18:0 in the two populations. Given short linkage disequilibrium (LD) extents across Chinese pig populations25, the lead SNPs are most likely very close to the causal variant.
Plausible candidate genes at significant loci
A list of strong candidate genes have been highlighted by our and other previous studies4,5,6,7,21,22, such as SCD on SSC14 for C18:0, ELOVL5 on SSC7 for C20:1n9, ELOVL6 on SSC8 for C16:1n7, ELVOL7 on SSC16 for C20:0, FASN on SSC12 for C16:0, ACSBG1 on SSC7 for C20:2n6 and MTTP on SSC8 for C14:1n5. By conducting GWAS on fatty acid metabolic traits, we herein proposed three novel candidate genes at the genome-wide significant loci.
At 9.13–12.86 Mb on SSC2, we detected a significant locus comprising 11 consecutive SNPs for C20:3n6/C18:2n6 and C20:4n6/C20:3n6 in Erhualian pigs. The top SNP (ASGA0008884) at this locus locates in the fifth intron of fatty acid desaturase 2 (FADS2) gene. FADS2 is a rate-limiting enzyme in the synthesis of long-chain polyunsaturated fatty acids through the introduction of double bond into delta-6 carbons in mammals. C20:3n6 and C18:2n6 are delta-6 unsaturated fatty acids. A nucleotide insertion in the transcriptional regulatory region of FADS2 causes human fatty acid delta-6-desaturase deficiency26. Variants of FADS2 are known to affect omega-6 and omega-3 milk fatty acids in cows27. Thus, FADS2 is a promising candidate gene for the SSC2 locus.
At 57.80–61.49 Mb on SSC12, a significant locus for C20:4n6/C20:3n6, PUFA/SFA and SFA was found in the Laiwu population. The top SNP (ALGA0067099) locates between the MYH13 and MYH1 genes that have no obvious function related to fatty acid metabolism. However, comparative genomic analysis between human and pig shows that an interesting gene, SREBF1 (sterol regulatory element binding transcription factor 1), is located in a homologous region (around 63.20 Mb) 1.8 Mb upstream of the locus. SREBF1 is a transcription factor involved in fatty acid synthesis regulation. It initiates the transcription of more than 33 target genes that participate in synthesis of cholesterol, fatty acids, triglycerides, and phospholipids in mice28. So further studies are worthwhile for the SREBF1 gene as a candidate for the SSC12 locus.
The 27–52 Mb region on SSC7 contains 150 significant SNPs associated with C20:4n6/C18:2n6, C20:4n6/C18:3 and C20:4n6/C20:3 in abdominal fat in the F2 population. The top SNP for C20:4n6/C18:2n6 and C20:4n6/C18:3 is ALGA0041153 at 48.90 Mb. PLA2G7 that locates at 48.15 Mb on SSC7 is one of the arachidonic acid (C20:4n6) pathway members29 and could be a candidate gene for the three fatty acid metabolic traits.
Gene networks and metabolic pathway
We explored GeneMANIA20 to characterize enriched functional processes and gene networks involving the candidate genes highlighted for both significant (Table 1) and suggestive (Table 2, also see below) loci. Most of candidate genes were enriched in biological processes related to fatty acid metabolism, such as fatty acid metabolic process (FDR = 1.17E-18), acylglycerol and neutral lipid metabolic process (FDR = 3.16E-16), triglyceride metabolic process and long-chain fatty-acyl-CoA metabolic process (FDR = 6.68E-15), fatty-acyl-CoA metabolic (FDR = 1.46E-14) and fatty-acyl-CoA biosynthesis process (FDR = 9.02E-14, Supplementary Table 3). A total of 404 links were observed in the gene networks related to fatty acid metabolism, which included additional 19 genes that were not identified in this study (Supplementary Fig. 4, Supplementary Tables 3 and 4). The 19 genes are also functionally related to fatty acid metabolism. Moreover, many genes co-occurred in multiple networks. For example, the ELOVL family genes and FADS family genes appeared in many pathways, including fatty acid metabolic and biosynthetic process, carboxylic acid biosynthetic process and long-chain fatty acid metabolic process. This emphasizes the importance of the ELOVL and FADS family genes as key driver enzymes in fatty acid elongation and desaturation. Altogether, the interactive analysis of candidate genes gives us a visually understanding of metabolic pathway for fatty acid composition (Fig. 4).
Figure 4. Graphical illustration of the potential fatty acid metabolism pathway and plausible candidate genes.
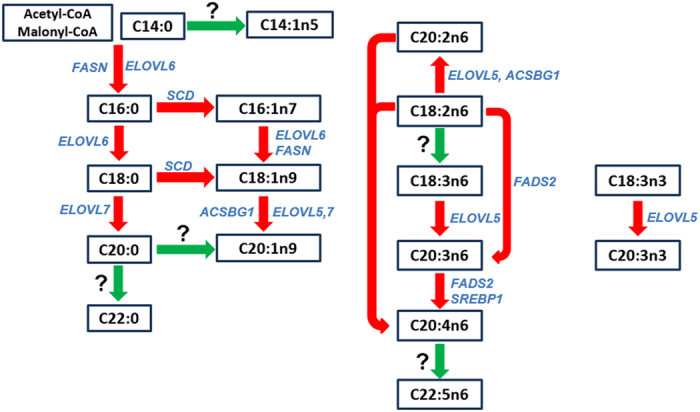
The illustration shows the step-by-step synthesis of fatty acids. Significant loci have been identified for the ratios of substrates to products linked by red arrows. Plausible candidate genes that we highlighted for significant loci in this study are indicated alongside red arrows. For fatty acids linked by blue arrows, the loci for the metabolic ratios between these fatty acids remain unexplored.
Suggestive loci
The Bonferroni-corrected threshold is too stringent to detect a significant locus with moderate or small effects30. Thus, we defined 12 suggestive loci on nine chromosomes (Table 2) for the tested traits at a less strict level of 1/N, where N represents the number of qualified SNPs in each population. To our knowledge, many of suggestive loci are reported for the first time, such as the locus at 107.47–114.96 Mb on SSC2 for C20:4n6/C18:2n6 in the Sutai population, the locus at 17.32–17.92 Mb on SSC5 for C20:0/C18:0 in the Laiwu population and the locus at 5.92–9.14 Mb on SSC6 for C20:2/C18:2n6 in the F2 population. At five suggestive loci (107.47–114.96 Mb on SSC2, 5.92–9.14 Mb on SSC6, 65.19–71.01 Mb on SSC6, 28.85–33.74 Mb on SSC8 and 11.48–14.51 Mb on SSC9), we found functionally plausible candidate genes, including PAM, AADACL3, TBC1D1, and DGAT2. These genes have been associated with fatty acid contents in previous studies31,32,33,34 and warrant further investigations.
Discussion
GWAS on metabolic traits identifies novel loci for fatty acid composition
To our knowledge, although QTL for fatty acid indices have been reported in previous studies4,6,21,35,36, this is the first GWAS on fatty acid metabolic traits in multiple diverse pig populations. In total, we identified 867 genome-wide significant SNPs, 97.25% of which locate in noncoding regions, indicative of complex regulatory mechanisms for fatty acid metabolism. Of note, our findings not only replicated the previously reported loci for fatty acid composition, but also detected four novel significant loci, including the SSC2 locus (9.13–12.86 Mb) for C20:3n6/C18:2n6 in Erhualian, the SSC12 (57.80–61.49 Mb) locus for C20:4n6/C20:3n6 in Laiwu, the SSC13 locus (28.52–31.47 Mb) for C16:1n7/C16:0 in DLY and the SSCX locus (124.40–125.87 Mb) for C20:4n6/C20:2n6 in Laiwu. The four loci are population specific loci (Figs 1 and 2). This supports the assumption that GWAS on metabolic ratios can increase the power of GWAS compared to that of association of individual metabolites. As pointed by Petersen et al.37, if two metabolites are a product-substrate of an enzymatic reaction, then ratios between their concentrations are potential proxies of the enzymatic reaction rate. When the SNPs in an enzyme-coding gene show much stronger (ten times or more) association with the metabolic ratio than with two individual metabolites in GWAS, then the enzyme is likely the one responsible for conversion of substrate into product, even if the molecular function of that enzyme was not already known. So using metabolic ratios as quantitative traits for GWAS may allow us to deduce new enzymatic activities. For example, variants proximal to the FADS2 gene are significantly associated with the ratio of C20:4n6/C20:3n6. The findings enable us to deduce that FADS2 may catalyze the conversion of C20:4n6 from C20:3n6, which remained unknown before this study.
GWAS on metabolic traits increases association strength at several significant loci
In this study, GWAS on fatty acid metabolic traits increased association strength at several significant loci for fatty acid contents. For example, we have previously identified a significant locus on SSC16 for C20:0 content across the five populations. The top SNPs at 43.53 Mb (DRGA0016155, P = 4.32E-31), 34.71 Mb (ASGA0072949, P = 6.97E-13) and 45.31 Mb (DRGA0016169, P = 7.41E-14) on SSC16 explained 31.79%, 12.07% and 25.50% phenotypic variance in the DLY, Erhualian and Laiwu populations, respectively. Interestingly, by conducting the GWAS for C20:0/C18:0, more SNPs on SSC16 (N = 62, 51 and 61) were detected in the three populations. The top SNPs at 43.53 Mb (DRGA0016155, P = 7.69E-37), 34.71 Mb (ASGA0072949, P = 3.92E-18) and 35.19 Mb (ASGA0072968, P = 3.50E-17) explained 39.97%, 20.21% and 31.16% of phenotypic variance in these populations, respectively. This again illustrates that GWAS on fatty acid metabolic traits could result in more associated SNPs with stronger association strength explaining more phenotypic variance. This is consistent with previous reports that GWAS on metabolic ratios as intermediate phenotypes can reduce the variance of the dataset and yield enhanced association statistics10,37.
The deduced metabolic pathway of fatty acids advances our understanding of the genetic mechanism of fatty acid composition
GWAS on fatty acid metabolic traits provide us important clues to deduce the fatty acid metabolic pathway (Fig. 4), in which different genes are responsible for the step-by-step synthesis of fatty acids. We noted that several loci were population specific, indicating that variants in some genes in the pathway may exert their effects on fatty acid metabolism in a population-specific manner. For example, the locus at 134.54 Mb on SSC7 containing ELOVL5 was detected for C20:1n9/C18:1n9 and C20:2n6/C18:2n6 in F2 and Erhulian pigs. So the ELVOL5 is more likely to elongate C18:1n9 and C18:2n6 acyl-CoAs to C20:1n9 and C20:2n6 acyl-CoAs in the two populations. In Erhualian population, the same locus was also associated with C20:3n3/C18:3n3; thus, ELOVL5 could specifically elongate C18:3n3 acyl-CoA to C20:3n3 acyl-CoA in this population. Some genes appeared to exhibit pleiotropic effects in multiple metabolic steps. For example, the ELOVL6 gene could not only catalyzes two carbons to C16:0 acyl-CoA to synthesize C18:0 acyl-CoA, but also mediates the synthesis of C18:1n9 acyl-CoA from C16:1n7 acyl-CoA. This is in conflict with the previous assumption that ELOVL6 is only involved in the elongation of C12-16 saturated fatty acid to C18 saturated fatty acid in mammals38. Although the deduced metabolic pathway allows us to better understand substrate-product relationships and the genetic mechanism of fatty acid compositions, more experimental assays are needed to validate the deduced pathway.
Biological, mediate and spurious pleiotropy
Pleiotropy widely exists in species. In the human genome, 16.9% of genes and 4.6% of SNPs show pleiotropic effects39. Generally, there are three kinds of pleiotropy: biological, mediate and spurious pleiotropy40. We detected a significant locus at 121.35 Mb on SSC14 for C16:1n7/C16:0 and C18:1n9/C18:0 in the F2 and DLY populations. Interestingly, the candidate gene SCD within this region catalyzes conversion of both C16:0 and C18:0 to C16:1n7 and C18:1n9. Thus, it is likely that a common causative mutation with the SCD gene underlie the pleiotropic locus on SSC14. The locus at 27.45–80.45 Mb on SSC7 containing 210 SNPs influences 12 metabolic traits in abdominal fat and longissimus dorsi muscle in the F2 population (Table 1). We found high LD extents within the chromosome region harboring these 210 SNPs in this population (Supplementary Fig. 5a). Thus, it is conceivable to observe that the association signals of all these 210 SNPs disappeared when conditional on the effect of the top SNP (ASGA0033714) at this locus by treating it as a fixed effect in the GWAS model (Supplementary Fig. 3). This observation support the locus as a biological pleiotropy. However, we cannot rule out the possibility that different causative mutations having strong LD with the top SNP may co-localize at this locus. We note that the top SNP has moderate LD (r 2 < 0.5) with these 210 SNPs (Supplementary Fig. 5a), indicating that the causative mutation underlying the SSC7 locus is likely located in the vicinity of the top SNP. The pleiotropic QTL with a beneficial allelic effect on more than one trait warrant further investigations to characterize the underlying mutations, which would enable us to develop novel molecular breeding tools for optimizing fatty acid compositions in pork.
The 0.4–8.29 Mb region on SSC12 was significantly associated with seven metabolic traits in the Erhualian population. When the top SNP (MARC0063090) was fixed in the GWAS model, the association signal for C18:1n9/C16:1n7 still surpassed the genome-wide significant threshold with the top SNP (ASGA0052511) at 2.62 Mb on this chromosome (Supplementary Fig. 3). We found that the LD between the two top SNPs (MARC0063090 and ASGA0052511) is relatively low (r2 = 0.38, Supplementary Fig. 5b). This supports our assumption that the locus is a spurious pleiotropic one and different causative variants are in LD with the lead SNPs.
It is difficult to distinguish mediate pleiotropy from biological and spurious pleiotropy. In this study, the locus at 9.13–12.86 Mb on SSC2 was associated with C20:3n6/C18:2n6 and C20:4n6/C20:3n6 in Erhualian pigs. C20:3n6 can be converted to C20:4n6 by fatty acid delta-desaturase, while C18:2n6 cannot be directly converted to C20:3n6, which must be converted to C18:3n6 by fatty acid delta-desaturase and then transformed to C20:3n6 by ELOVL541. By treating C20:3n6/C18:2n6 as a fixed effect in GWAS model, the top SNP changed from ASGA0008884 (P = 3.54E-11) to ALGA0011877 (P = 7.53E-07). Thus, the locus may be a mediate pleiotropic locus and C20:3n6/C18:2n6 is likely an intermediate phenotype.
Additional Information
How to cite this article: Zhang, W. et al. Genome-wide association studies for fatty acid metabolic traits in five divergent pig populations. Sci. Rep. 6, 24718; doi: 10.1038/srep24718 (2016).
Supplementary Material
Acknowledgments
This work was supported by funds from National Natural Science Foundation of China (31525023, 31230069 and 31572379). We thank all the members in our lab for enrollment in the animal management, sample collection, and 60K genotyping affairs.
Footnotes
Author Contributions L.H. conceived and designed the experiment. J.R. and B.Y. supervised the experiment and data analyses. W.Z. analyzed the data, prepared the tables and figures. J.R. wrote the manuscript. L.H. revised the draft. B.Y., J.Z., L.C., J.M., C.C., H.A. and S.X. contributed to experimental materials. All authors read and approved the final manuscript.
References
- Wood J. D. et al. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci 78, 343–358 (2008). [DOI] [PubMed] [Google Scholar]
- Fats and fatty acids in human nutrition. Report of an expert consultation. FAO Food Nutr Pap 91, 1–166 (2010). [PubMed] [Google Scholar]
- Jimenez-Colmenero F., Ventanas J. & Toldra F. Nutritional composition of dry-cured ham and its role in a healthy diet. Meat Sci 84, 585–93 (2010). [DOI] [PubMed] [Google Scholar]
- Munoz M. et al. Genome-wide analysis of porcine backfat and intramuscular fat fatty acid composition using high-density genotyping and expression data. BMC Genomics 14, 845 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B. et al. Genome-wide association analyses for fatty acid composition in porcine muscle and abdominal fat tissues. PLos One 8, e65554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T. et al. Quantitative trait loci for fatty acid composition in longissimus dorsi and abdominal fat: results from a White Duroc x Erhualian intercross F2 population. Anim Genet 40, 185–191 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang W. et al. Genetic architecture of fatty acid composition in the longissimus dorsi muscle revealed by genome-wide association studies on diverse pig populations. Genet Sel Evol 48, 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. & Stephens M. Efficient multivariate linear mixed model algorithms for genome-wide association studies. Nat Methods 11, 407–409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhre K. & Gieger C. Genetic variation in metabolic phenotypes: study designs and applications. Nat Rev Genet 13, 759–769 (2012). [DOI] [PubMed] [Google Scholar]
- Gieger C. et al. Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLos Genet 4, e1000282 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. et al. Genome-wide association analyses for meat quality traits in Chinese Erhualian pigs and a Western Duroc x (Landrace x Yorkshire) commercial population. Genet Sel Evol 47, 44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X. et al. Genome-wide association analysis reveals genetic loci and candidate genes for meat quality traits in Chinese Laiwu pigs. Mamm Genome 26, 181–190 (2015). [DOI] [PubMed] [Google Scholar]
- Wang L. et al. In Animal Genetic Resources in China: Pigs (ed. China National Commission of Animal Genetic Resource) 2–16 (China Agricultural Press, 2011). [Google Scholar]
- Pamplona R. et al. Mitochondrial membrane peroxidizability index is inversely related to maximum life span in mammals. J Lipid Res 39, 1989–1994 (1998). [PubMed] [Google Scholar]
- Ulbricht T. L. & Southgate D. A. Coronary heart disease: seven dietary factors. Lancet 338, 985–992 (1991). [DOI] [PubMed] [Google Scholar]
- Ramos A. M. et al. Design of a high density SNP genotyping assay in the pig using SNPs identified and characterized by next generation sequencing technology. PLos One 4, e6524 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulchenko Y. S., Ripke S., Isaacs A. & van Duijn C. M. GenABEL: an R library for genome-wide association analysis. Bioinformatics 23, 1294–1296 (2007). [DOI] [PubMed] [Google Scholar]
- Willer C. J., Li Y. & Abecasis G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. Improved pairwise alignment of genomic DNA. PhD Thesis, The Pennsylvania State University (2007).
- Warde-Farley D. et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 38, W214–W220 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramayo-Caldas Y. et al. Genome-Wide Association Study for Intramuscular Fatty Acid Composition in an Iberian x Landrace Cross. J Anim Sci 90, 2883–2893 (2012). [DOI] [PubMed] [Google Scholar]
- Uemoto Y. et al. Genome-wide mapping for fatty acid composition and melting point of fat in a purebred Duroc pig population. Anim Genet 43, 27–34 (2012). [DOI] [PubMed] [Google Scholar]
- Qiao R. et al. Genome-wide association analyses reveal significant loci and strong candidate genes for growth and fatness traits in two pig populations. Genet Sel Evol 47, 17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K. et al. Genetic correlations among fatty acid compositions in different sites of fat tissues, meat production, and meat quality traits in Duroc pigs. J Anim Sci 84, 2026–2034 (2006). [DOI] [PubMed] [Google Scholar]
- Ai H. et al. Adaptation and possible ancient interspecies introgression in pigs identified by whole-genome sequencing. Nat Genet 47, 217–225 (2015). [DOI] [PubMed] [Google Scholar]
- Nwankwo J. O., Spector A. A. & Domann F. E. A nucleotide insertion in the transcriptional regulatory region of FADS2 gives rise to human fatty acid delta-6-desaturase deficiency. J Lipid Res 44, 2311–2319 (2003). [DOI] [PubMed] [Google Scholar]
- Ibeagha-Awemu E. M., Akwanji K. A., Beaudoin F. & Zhao X. Associations between variants of FADS genes and omega-3 and omega-6 milk fatty acids of Canadian Holstein cows. BMC Genet 15, 25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. D. et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA 100, 12027–12032 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio P. et al. Arachidonic acid pathway members PLA2G7, HPGD, EPHX2, and CYP4F8 identified as putative novel therapeutic targets in prostate cancer. Am J Pathol 178, 525–536 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham P. C. & Purcell S. M. Statistical power and significance testing in large-scale genetic studies. Nat Rev Genet 15, 335–346 (2014). [DOI] [PubMed] [Google Scholar]
- Puig-Oliveras A. et al. A co-association network analysis of the genetic determination of pig conformation, growth and fatness. PLos One 9, e114862 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent D. & Xu Y. Sphingosine-1-phosphate: characterization of its inhibition of platelet aggregation. Platelets 11, 226–232 (2000). [DOI] [PubMed] [Google Scholar]
- Lee J. & Chung W. Y. The role played by the peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) activator, GW501516, in control of fatty acid metabolism: a new potential therapeutic target for treating metabolic syndrome. Endocrinology 152, 1742–1744 (2011). [DOI] [PubMed] [Google Scholar]
- Maher A. C., McFarlan J., Lally J., Snook L. A. & Bonen A. TBC1D1 reduces palmitate oxidation by inhibiting beta-HAD activity in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 307, R1115–R1123 (2014). [DOI] [PubMed] [Google Scholar]
- Clop A. et al. Detection of QTL affecting fatty acid composition in the pig. Mamm Genome 14, 650–656 (2003). [DOI] [PubMed] [Google Scholar]
- Sanchez M. P. et al. Identification of QTL with effects on intramuscular fat content and fatty acid composition in a Duroc x Large White cross. BMC Genet 8, 55 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A. K. et al. On the hypothesis-free testing of metabolite ratios in genome-wide and metabolome-wide association studies. BMC Bioinformatics 13, 120 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson A., Westerberg R. & Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res 45, 237–249 (2006). [DOI] [PubMed] [Google Scholar]
- Sivakumaran S. et al. Abundant pleiotropy in human complex diseases and traits. Am J Hum Genet 89, 607–618 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovieff N., Cotsapas C., Lee P. H., Purcell S. M. & Smoller J. W. Pleiotropy in complex traits: challenges and strategies. Nat Rev Genet 14, 483–495 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon Y. A., Hammer R. E. & Horton J. D. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J Lipid Res 50, 412–423 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.