Abstract
Bacterial infections are a major threat to the health of patients in healthcare facilities including hospitals. One of the major causes of patient morbidity is infection with Staphylococcus aureus. One of the the most dominant nosocomial bacteria, Methicillin Resistant Staphylococcus aureus (MRSA) have been reported to survive on hospital surfaces (e.g. privacy window glasses) for up to 5 months. None of the current anti-bacterial technology is efficient in eliminating Staphylococcus aureus. A novel transparent, immobilised and superhydrophilic coating of titanium dioxide, co-doped with fluorine and copper has been prepared on float glass substrates. Antibacterial activity has demonstrated (by using Staphylococcus aureus), resulting from a combination of visible light activated (VLA) photocatalysis and copper ion toxicity. Co-doping with copper and fluorine has been shown to improve the performance of the coating, relative to a purely fluorine-doped VLA photocatalyst. Reductions in bacterial population of log10 = 4.2 under visible light irradiation and log10 = 1.8 in darkness have been achieved, compared with log10 = 1.8 under visible light irradiation and no activity, for a purely fluorine-doped titania. Generation of reactive oxygen species from the photocatalytic coatings is the major factor that significantly reduces the bacterial growth on the glass surfaces.
The issue of healthcare acquired infections (HCAI) is of major concern across the globe1,2,3,4,5. The hospital environment is an important but often neglected factor in hospital cross-infection6 Data from the 2014 report of the European Centre for Disease Prevention and control showed resistance to methicilin in Staphylococcus aureus samples taken from hospitals at rates greater than 10% in in 19 out of 29 EEA countries. Resistance rates were 17.4% in France, 11.3% in Germany, 11.8% in the UK and as high as 56.0% in Romania7. Many studies have shown that these organisms can contaminate surfaces and equipment touched by hospital staff 8,9 including furniture and medical equipment10,11. In one study 92.9% of writing tools taken from 42 doctors showed bacterial contamination12. It is likely that the doctors in question adhered to appropriate hygiene practices but that fomites (inanimate objects that can harbour and serve in transmitting bacteria), such as door handles, table tops and any other contactable surfaces held bacterial reservoirs for transfer. Two of the most prevalent nosocomial bacteria, Methicillin Resistant Staphylococcus aureus (MRSA) and Clostridium difficile (C. diff ), have been shown to survive on such surfaces for up to 5 and 7 months respectively7. C. diff spores in particular are highly resistant to alcohol based cleaning and disinfection solutions13. As surface contamination can contribute to transmission of bacteria14, the development of surface enhanced coatings that causes sanitisation of the surface is an important development in reducing hospital acquired infections.
The use of photocatalysts for hygiene applications has been the subject of recent research, but is not a common practice as normally a high level of UV exposure is required to activate the catalysts15,16,17,18.
Existing hygiene coatings based on photocatalysts suffer from two principal drawbacks. Firstly, most rely on ultraviolet light to generate free electrons and subsequent reactive species. This limits their use to situations with a significant UV flux, typically relying on sunlight or else requiring deliberate exposure to artificial UV light sources, the latter having the obvious disadvantage of compromising human wellbeing. Secondly, a purely photocatalytic hygiene coating is inactive in darkness, apart from the very short time after exposure, during which the population of reactive species rapidly declines.
Previous studies have led to the development of VLA titania photocatalysts that form transparent conformal films, by the deposition of a precursor sol and subsequent heat treatment to crystallise the titania and fuse it to the substrate surface19,20.
It has been known for some time that several transition metals are toxic to a range of pathogenic microbial species and this has informed both widespread research on the use of such materials as practical antimicrobial agents and considerable commercial activity in this area. Work has been focussed principally on copper and silver as the active agents. While silver has been shown to be toxic to a wide range of pathogens21,22, with limited impact on human health, financial concerns have restricted its use to a minority constituent at low concentrations, principally as nanoparticle dispersions or a substituent for ion release from an inert and less costly matrix. Silver-substituted zeolites offer an attractive delivery vehicle, having a large surface area for controlled ion release, while requiring a low silver content and being easily dispersible23. Copper, its compounds and alloys are also the subject of considerable attention, both as dispersions and in the case of elemental copper and its alloys, for bulk fabrication and cladding24,25,26. In all form factors, copper and copper alloys have a considerable cost advantage over silver, particularly for fabrication, where the cost of silver is prohibitive27,28.
This paper reports the successful application of a visible light activated (VLA) anatase titanium dioxide photocatalyst to control and reduce populations of pathogenic bacteria.
This coating incorporates both a VLA photocatalyst and a metal ion release mechanism to ensure that it remains bactericidal in both light and darkness and is formed in a single deposition and heat treatment process. The copper ion release does not adversely affect the photocatalytic efficiency of the titanium dioxide and has only a limited impact on the transparency of the coating.
Materials and Methods
Materials and reagents
Titanium isopropoxide (purity > 97%), glacial acetic acid (>99.7%), trifluoroacetic acid (99%),) and. Copper (II) nitrate pentahemihydrate (98%) were obtained from Sigma Aldrich. All of the materials were used without any further purification.
Sol Synthesis
An aqueous sol of titanyl acetate and titanyl trifluoroacetate, optionally doped with copper nitrate was prepared according to the method of US Patent 9,210,93429 and UK Patent GB252140530.
In order to prepare the formulation, glacial acetic acid (24 mL) was added to a glass beaker while continuously stirring at room temperature. Next, titanium isopropoxide (12.5 mL) was added slowly in a drop-wise manner, and the mixture allowed to continue stirring for a period of 30 minutes. Then trifluoroacetic acid (4 mL) was added drop-wise and the solution was left to stir for 10 minutes. To prepare a VLA titanium dioxide precursor sol, 150 ml of water was added slowly to the previously prepared solution. At this stage a transparent, colourless solution was obtained which was left to mix for another 30 minutes. In order to remove any remaining agglomerates, the obtained formulation was filtered using 0.22 μm syringe filter and stored in the fridge prior to coating on a substrate.
To prepare a copper-doped VLA titanium dioxide precursor sol, glacial acetic acid (24 mL), titanium isopropoxide (12.5 mL) trifluoroacetic acid (4 mL) were mixed according to the protocol above. Meanwhile, the copper precursor, copper (II) nitrate pentahemihydrate (1.393 g) was added to water (150 ml), completely dissolved and then added slowly to the previously prepared solution. At this stage a transparent, blue coloured solution was obtained which was left to mix for another 30 minutes. This formulation was also filtered using 0.22 μm syringe filter and stored in the fridge prior to coating on a substrate.
Film Deposition and Crystallisation
The sol was deposited on a cleaned glass Plate, 50 mm × 55 mm, by means of dip coating. Substrates were immersed in a vessel of the sol at room temperature, then withdrawn vertically at a rate of 1.45 mms−1.
The coated glass was allowed to dry at room temperature for 30 minutes at room temperature, before being transferred to the furnace, and then heated for 90 minutes at 550 °C, to complete the transformation of the mixed titanyl acetate- titanyl trifluoroacetate precursor gel to titanium dioxide, its crystallisation to the anatase structure and the simultaneous partial substitution of fluorine for oxygen and in the copper-doped sols, copper for titanium, to achieve the desired doping.
After heat treatment, a 5 mm wide strip was cut from the edge that had been gripped by the sample holder during dip coating, leaving a fully coated 50 mm × 50 mm coupon for microbiological testing.
Microbiological Testing
Gram-positive Staphylococcus aureus (ATCC 6538) was chosen to conduct the pure culture studies. In order to prepare cultures for testing, 10 ml of sterile nutrient broth were inoculated with colonies of S.aureus and incubated overnight at 37 °C without shaking. Obtained stock cultures were centrifuged and washed with phosphate buffer saline (PBS) twice (15 min at 4,000 rpm). The concentration was adjusted accordingly to approx. 106 cfu/sample based on the calibration curve prepared for the organisms.
For the testing, each glass sample was aseptically placed in a sterile Petri dish containing moist filter paper. This was prepared by adding a sterilised moisture control paper filter in the bottom of a sterilised Petri dish. An adequate amount of sterile water was added to the paper filter (approximately 3–4 ml water). A glass plate was inserted in order to avoid contact between the test piece and the filter paper (Fig. 1). Next, 75 μl quantities of the adjusted bacterial suspensions (approx. 1 × 105 CFU/sample) were aseptically dispensed onto the centre of each sample and covered with transparent polymer film. The prepared samples were divided into two groups (test samples and controls). One of each was immediately placed in a cardboard box with cover (dark) and another set were exposed to T5 lighting (1,000 lux) for 24 hours at room temperature. For each set, the test was performed in triplicate.
Figure 1. Experimental setup – The “wet chamber”according to ISO27447.
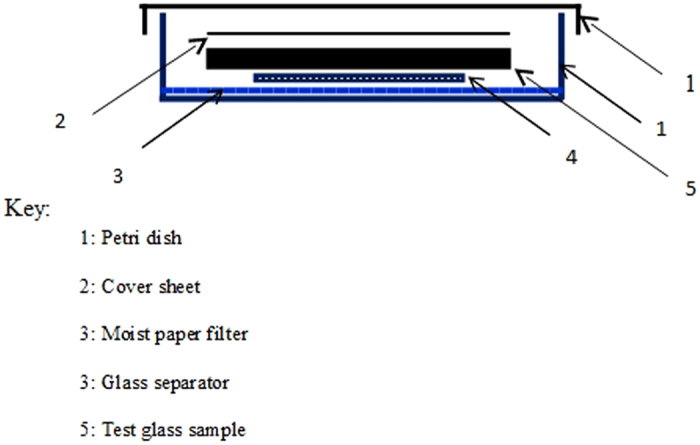
(1) Glass Petri dish, (2) Cover sheet, (3) Moist paper filter, (4) Glass separator, (5) Test glass sample.
Following 24 hour of incubation, all samples were processed to analyse for any viable bacteria remaining on the surface, post-exposure. Film was removed from sample surface first and aseptically transferred to a sterile stomacher bag containing 10 ml of sterile PBS. This was rubbed in order to remove remaining organisms from the surface. The test glass sample was then placed in the same stomacher bag and the same procedure was repeated. Serial dilutions were performed on the extraction solution accordingly. 1 ml aliquots of each dilution as well as the neat extraction solution were aseptically dispensed into sterile Petri dishes in duplicate. Approximately 15 ml of molten plate count agar was poured into each plate swirled and allowed to solidify. All plates were incubated aerobically at 37 °C for 24 hours after which a colony count was performed.
X-Ray Diffraction
Samples of the sol were dried at 120 °C, and then calcined in air at temperatures between 300 °C and 600 °C. X-Ray diffractometry was performed on the calcined powders, using a Siemens D500 diffractometer employing Cu-Kα radiation.
Optical Transmission
The optical transmission of heat treated films on glass was measured, using a Perkin Elmer Lambda 900 UV/Visible/NIR spectrometer over a wavelength range of 600 nm to 200 nm.
Optical absorbance measurements were performed on mixtures of doped titania powders, formed by calcination at 330 °C and 550 °C at a concentration 0.5% in potassium bromide, compressed into transparent discs, using a Shimadzu 1600 UV/Visible/NIR spectrometer over a wavelength range of 600 nm to 200 nm. From these spectra, the band gap potential was calculated from the intercept tangents to the spectrum in the visible range and the transitional range from visible light transparency to ultraviolet opacity.
X-Ray Spectroscopy
XPS analyses were performed with a Thermo VG Scientific ESCALAB Mk II spectrometer and Alpha 110 analyser, using Al Kα radiation (hν = 1486.6 eV) at 300 W. All spectra were corrected for charging by reference to the C 1 s peak at 285 eV.
Surface Wetting and Contact Angle Measurement
The hydrophobic or hydrophilic nature of the surfaces of coated and uncoated substrates was characterised by means of a liquid contact angle measurement. Measurements were performed using a First Ten Angstroms Model 200 system and the wetting liquid was glycerol. Three measurements were performed on each surface and a mean calculated. The contact angles of the lower surface of the substrate that had been in contact with the tin bath during manufacturing and of the upper surface that had been exposed to air was measured separately.
Results and Discussion
Suitable choices of dopant elements and their concentration has enabled coatings to be developed in which the anatase to rutile transformation can be suppressed at temperatures of up to 900 °C31,32, allowing the application of VLA photocatalytic coatings to a wide range of substrates during existing manufacturing processes. Fluorine is an attractive dopant, since it has been found both to confer visible light activity and to stabilise anatase at elevated temperatures, thus fulfilling both roles with a single modification to the synthesis20,31. Addition of F is effective in titania doping as it can minimize the recombination of the electron hole pair due to charge compensation between F- and Ti4+ 33. In addition F-doping is reported to induce the creation of Ti3+ ions and thereby oxygen vacancies33.
X-Ray Diffraction
X-Ray diffraction spectra obtained from powders calcined at 300 °C and 600 °C are shown in Fig. 2, together with the spectrum of an anatase titania reference sample. As can be seen in Fig. 2, calcination at both temperatures results in the formation of a largely crystalline product, with a diffraction pattern characteristic of anatase. The peaks are relatively broad and less well differentiated than those in the reference spectrum, particularly at 300 °C, which we attribute to a combination of incomplete crystallisation at lower temperatures and peak spreading caused by the nanoparticulate nature of the material. Inhibition of titania condensation and crystallisation in fluorine-rich doped systems has already been noted by other workers31.
Figure 2. XRD Spectra of Anatase Reference Sample and Copper Doped Titania Calcined at 300 °C and 600 °C.
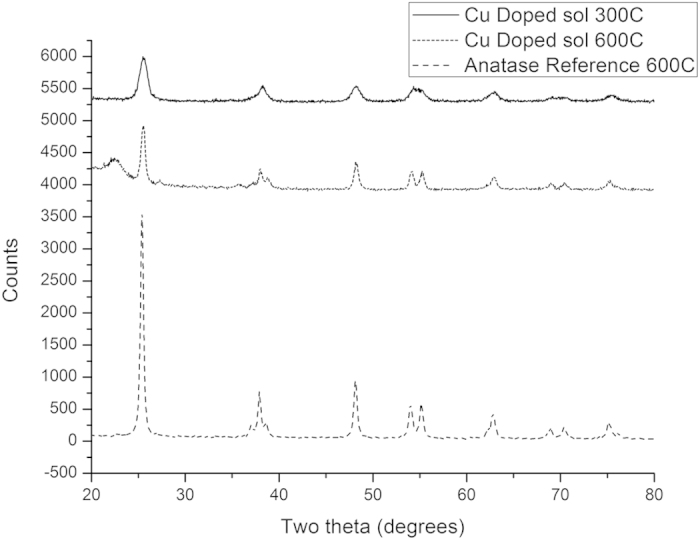
There are no additional peaks that could be attributed to copper oxides and from this we conclude that much of the copper is dissolved in the titania matrix, with any copper oxide being present at concentrations below the detection level of the technique.
Optical Transmission
The optical absorbance spectra of the glass substrate and glass coated with a two layer copper doped coating are shown in Fig. 3, below.
Figure 3. Optical Absorbance Spectra of Uncoated and Coated Glass.
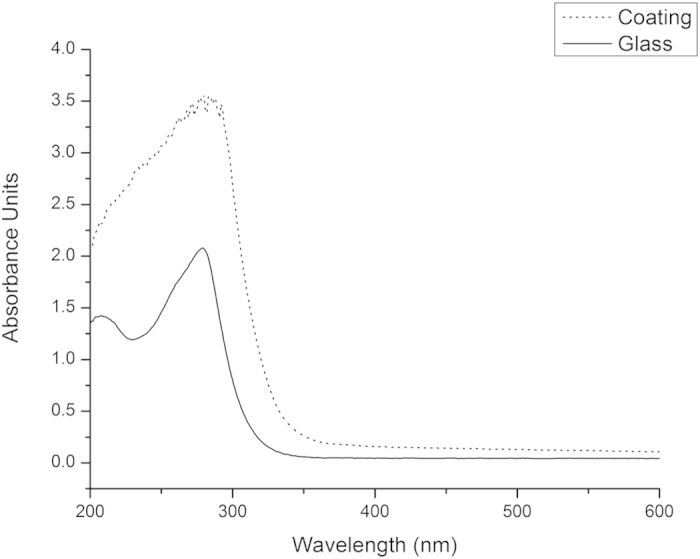
It can be seen that while the coating reduces the overall transparency of the glass, the absorbance spectrum in the visible range is still essentially flat, so the coated substrates do not have a colour cast. Absorption in the coated materials increases above that of the substrate at wavelengths below approximately 380 nm and rapidly increases at wavelengths below 300 nm, consistent with absorption of blue and particularly of ultraviolet light by the titanium dioxide.
Surface Wetting and Contact Angle Measurements
The results of the contact angle measurements are shown in Table 1, below.
Table 1. Contact Angles of Glass Substrates and Titanium Dioxide Coatings.
| Sample | Glass, tin bath side | Glass, air side | Titania coated glass |
|---|---|---|---|
| Contact angle | 56.46° | 52.34° | 8.51° |
Relatively little difference in contact angle was seen between the two sides of the uncoated glass, though the surface that had been in contact with the tin bath had a marginally higher contact angle (56.46°) than the upper surface that had not been exposed to the tin (52.34°).
The application of the titanium dioxide coating significantly reduced the contact angle to a mean value of 8.51° (superhydrophilic nature).
The reduction in contact angle on the application of the titanium dioxide coating is illustrated by the change in macroscopic wetting behaviour of water droplets applied by pipette as sen in Fig. 4. The droplet placed on the uncoated glass displays modest spreading over the surface, while that on the titanium dioxide layer spreads extensively. This is consistent with results found in the literature and is an important factor in the antimicrobial activity of the coating16. Photocatalytic degradation by titanium dioxide proceeds principally through the formation of oxidative intermediates, produced by holes in the titania valence band oxidising surface water. Thus, it is advantageous for the surface to be strongly hydrophilic, maximising the opportunity for these reactive peroxide and oxygen radicals to form16. The substantial reduction in contact angle also illustrates the importance of obtaining the most uniform coating possible from the first stages of deposition. Since the sol is aqueous and the titania coating is more hydrophilic than the glass substrate, subsequent layers will tend to wet out and adhere preferentially in areas where a partial coating is already present. It is therefore important to ensure that the substrate is uniformly clean and free of contaminants that could increase the water contact angle. Dip coating has been used to prepare the materials in this study, since it ensures complete coverage in a single step.
Figure 4. Behaviour of Water Droplet on Coated and Uncoated Glass, Illustrating Superhydrophilic Nature of the Doped Titanium Dioxide Coating.
Left side – control, right side – coated glass.
Microbiological Testing
The results of microbiological testing of films with S. aureus are shown in Table 2, below.
Table 2. Performance of Fluorine Doped and Copper-Fluorine Doped VLA Titanium Dioxide Coating Against S. aureus Culture.
| Sample | % Reduction (light) | % Reduction (dark) | Log Reduction (light) | Log Reduction (dark) |
|---|---|---|---|---|
| Fluorine doped coating | 98.40% | 0 | 1.8 | 0 |
| Copper – Fluorine doped coating | 100.00% | 98.30% | 4.2 | 1.8 |
The microbiological testing results show the efficacy of the copper-doped coating both in light and darkness, with at least a substantial reduction in the bacterial population and at best, reducing the bacterial load below detectable levels.
The distinction between the coatings prepared with and without copper doping illustrates the dramatic improvement in performance resulting from the addition of copper to the formulation. The precise degree to which this improved performance is the result of the toxicity of copper to bacteria and how much is potentially caused by increased photocatalytic activity above the solely fluorine-doped material is the subject of ongoing study, but the persistent effectiveness of the copper-doped coatings in darkness implies that the former mechanism is dominant.
In the present study, the negligible photocatalytic activity of undoped titanium dioxide in the absence of ultraviolet radiation is taken as established. This was previously reported in the literature19,33.
The photocatalytic activity of undoped stoichiometric anatase titania depends on the presence of a significant ultraviolet flux. Previous work by Fisher et al.19 on photocatlytic activity of titania for water decontamination, in an undoped state and with dopants including copper and nitrogen has demonstrated that the undoped material brings no significant reduction in populations of E. coli or Enterococcus faecalis in the absence of ultraviolet radiation19. In that work, it was found that doping with copper increased the activity slightly, but it was acknowledged that this improved activity could have been caused by a combination of VLA photocatalysis and copper ion toxicity.
X-Ray Photoelectron Spectroscopy
Selected energy range scans covering the O1s, Ti2p, Cu2p and F1s regions of the XPS spectra are shown in figures 5, 6, 7 and 8, respectively.
Figure 5. High Resolution Scan of O1s Peak in XPS Spectra.
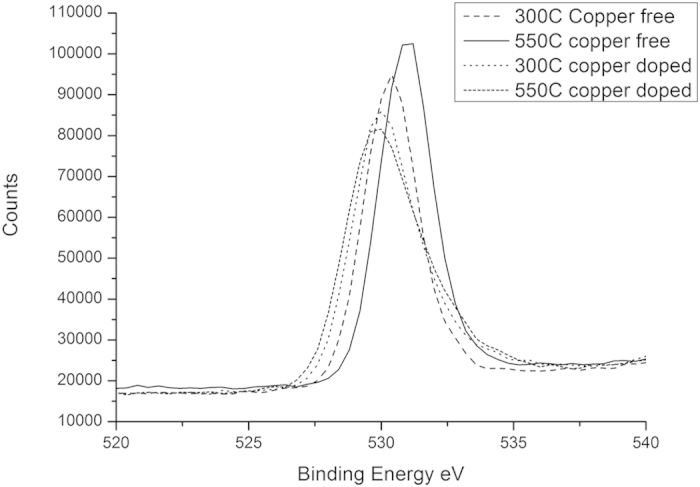
Figure 6. High Resolution Scan of Ti2p Peak in XPS Spectra.
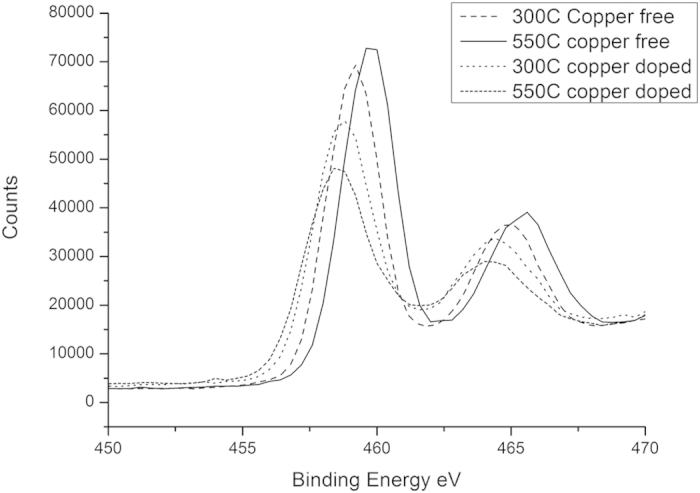
Figure 7. High Resolution Scan of Cu2p Peak in XPS Spectra.
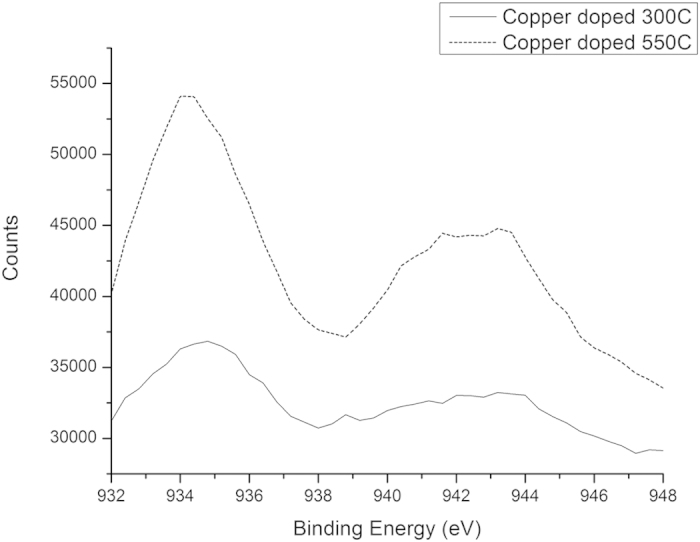
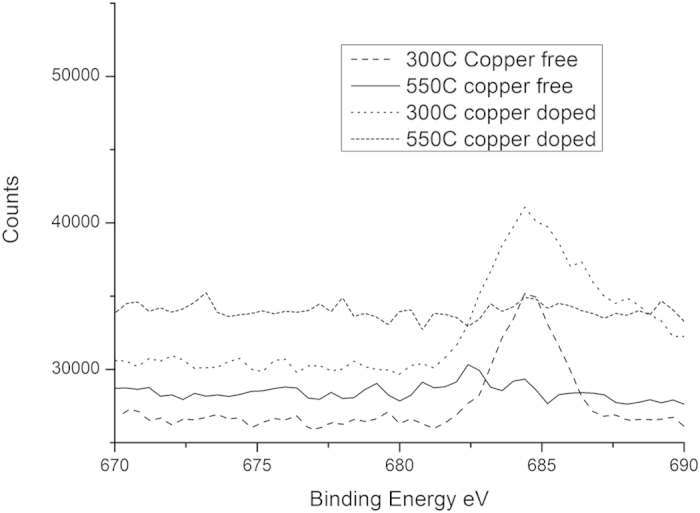
Figure 8. High Resolution Scan of F1s Peak in XPS Spectra.
Addition of copper to the sol substantially modifies the binding energies of both the Ti2p and O1s electrons in the materials after heat treatment.
As seen in Fig. 5, a consistent downward shift of 0.5 to 0.7 eV in the O1s binding energy occurs on the addition of copper to the titanium dioxide matrix. This is consistent the oxygen depletion of the titanium dioxide measured in the composition quantification, with a reduction in oxygen content of 1.5 to 1.8 atomic percent in the co-doped material, relative to that doped with fluorine only. Similar shifts in binding energy were reported by Nolan et al.34, on the addition of silver to a titanium dioxide matrix. This is consistent with the binding energy of the Cu2p3/2 peak in the sample calcined at 300 °C, at 933.74 eV closely matcheing that of CuO, as seen in Fig. 7 35. The absence of any CuO phase detected by XRD suggests incorporation of the copper in the +2 oxidation state into the titanium dioxide matrix, with consequent oxygen depletion of the matrix, relative to stoichiometric titanium dioxide.
The Cu2p2/3 binding energy in the copper doped sample heat treated at 550 °C displays an upward peak shift of 0.8 eV to 934.54 eV, greater than the expected deviation in measured values in CuO. This is accompanied by an increase in the oxygen content from 58.54% to 64.69% and is consistent with a super-saturation of oxygen relative to that found in CuO, with a consequent increase in the binding energy caused by the more electronegative environment than would be found in CuO.
The Ti2p binding energy displays downward shifts of 0.6 eV and 0.74 eV in the copper-doped material after treatment at 300 °C and 550 °C respectively, accompanied by a peak broadening of 0.5 eV (Fig. 6), Again, this is consistent with oxygen depletion in the titania and the formation of a minor Ti2+ component. Lower Ti2p3/2 and O s binding energies for copper doped titania are direct measures of the formation of Ti 3+ due to the generation of oxygen18. Such a downward shift indicates the existence of oxygen vacancies and Ti 3+ in the Cu doped samples. The overall composition is thus oxygen depleted with respect to stoichiometric TiO2 and oxygen super-saturated with respect to stoichiometric CuO.
It is also notable that the fluorine content decreases rapidly with heat treatment temperature, in both compositions, falling by factors of 4.51 and 17.87 in the fluorine doped and copper-fluorine co-doped materials respectively. This is illustrated clearly by the near disappearance of the F1s peak in Fig. 8, following heat treatment at 550 °C. After treatment at 550 °C, it can be expected that the properties of the copper-fluorine co-doped material to be dominated by the copper, rather than fluorine doping. This behaviour matches that observed by Padmanabhan et al.31, in which the fluorine content of titania prepared by the hydrolysis and calcination of a pure titanyl trifluoroacetate was reduced from 0.5 atomic percent after calcination at 550 °C to 0.3 atomic percent after calcination at 700 °C.
The mechanism for the antimicrobial activity of oxide semiconductor catalysts has been established as cell membrane rupture, caused by reactive oxidising species including hydroxyl radicals and peroxides generated by the oxidation of water and oxygen by holes in the valence band that are created during the photonic excitation of electrons to the conduction band (Fig. 9)17.
Figure 9. Schematic representation of the photocatalytic anti-bacterial action.
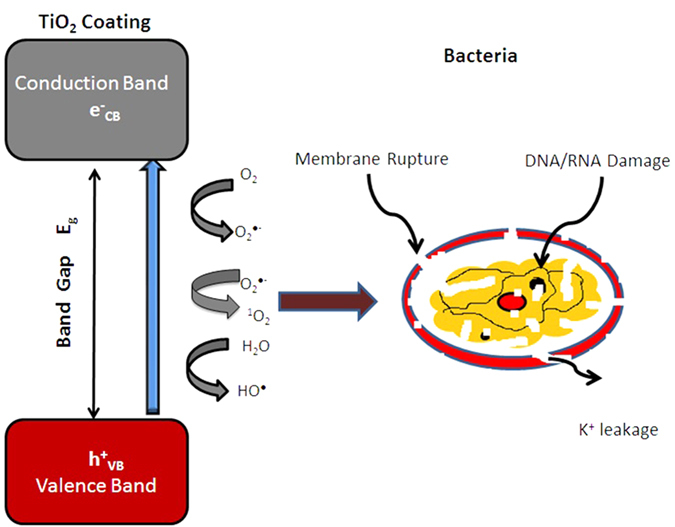
The superior activity of the copper-doped materials and their continued activity in darkness is the result of a mechanism of a bactericidal action that is independent of that of the photocatalyst, but which has the potential to reinforce it under conditions of illumination.
Copper and its compounds achieve their antimicrobial activity by degrading cell membranes36. Previous studies have shown that rapid accumulation of copper ions in Staphylococcus haemolyticus was observed on both dry and moist metallic copper surfaces, while no significant mutagenic effects were observed, indicating that absorption of copper does not cause lethal DNA damage, but instead kills by fatally compromising the cell membrane, evidence for which is found from the rapid accumulation of copper ions within the cell, on a scale of minutes. This mechanism is further expanded by Lemire et al.37 and Macomber et al.38 who demonstrated that the formation of Cu2+ ions is associated with an accelerated formation of reactive oxygen species and this accelerates the consumption of antioxidants, reducing the capacity of the cell wall to absorb damage from these species and to self-repair.
These reactive species are generated by a Fenton reaction as described in Equation 1, thus39.
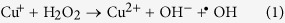 |
Hydrogen peroxide is a transient metabolic by-product of cellular respiration37,39, so this mechanism is active whenever cellular respiration is taking place, but it is noteworthy that in the present study, the dissolved copper in the doped titanium dioxide is in a particularly strongly oxidising environment, as indicated by the XPS data, and can thus accelerate the cell wall damage intrinsic to the photocatalytic action.
Similar increases in the bactericidal efficacy of titanium dioxide occurring on the addition of copper have been observed in TiO2: Cu films co-sputtered on polyester fabric40,41. In these experiments, solar spectrum and actinic lights with low UV flux were used. High levels of self-cleaning activity were observed, attributed to the increased generation of reactive oxygen species from a combination of hydroxyl radicals generated by Fenton chemistry40 and visible light activated photocatalysis resulting from the presence of intra-band gap energy levels created by the copper doping41. Evidence for the effectiveness of copper in inducing VLA photocatalytic activity comes from the substantial increase in decomposition rate of methylene blue in copper doped films, relative to sputtered undoped titanium dioxide under solar spectrum irradiation, where the UV component is relatively minor, which correlated well with a reduction in the estimated band gap41.
Closely related systems, also comprising co-sputtered TiO2: Cu films have demonstrated antibacterial activity on PET fabric42. Evidence for copper-moderated O2 reduction as a mechanism for bactericidal activity was provided by the increased activity in aerobic, relative to anaerobic conditions.
Taken together, these results support the argument that copper doping of titanium dioxide increases its photocatalytic and antimicrobial activity by a combination of band-gap modification and hydrogen peroxide splitting through Fenton chemistry.
Conclusions
A purely fluorine-doped titania displays bactericidal activity under conditions of visible light irradiation, but this is inferior to the activity of a fluorine-copper co-doped material, illustrating the synergistic effect of the combined doping. Co-doping of titanium dioxide with fluorine and copper provides significantly improved antimicrobial activity relative to doping with fluorine alone, both under illumination at visible wavelengths and in darkness. This is attributed to the known toxicity of copper to many species of pathogenic bacteria. The performance of the copper-fluorine co-doped material remains greater under visible light irradiation than in darkness, indicating that the copper does not critically degrade the photocatalytic activity of the titania and we propose that the copper has the potential to increase the yield of highly reactive hydroxyl radicals from photocatalytic oxidation of water. Furthermore, the purely fluorine-doped titania is ineffective in darkness, indicating that its activity is solely photocatalytic.
Additional Information
How to cite this article: Leyland, N. S. et al. Highly Efficient F, Cu doped TiO2 anti-bacterial visible light active photocatalytic coatings to combat hospital-acquired infections. Sci. Rep. 6, 24770; doi: 10.1038/srep24770 (2016).
Acknowledgments
The authors would like to thank Dr. Tim Yeomans and Dr. Tanya Beletskaya of Shannon Applied Biotechnology Centre, Limerick Institute of Technology, for carrying out the microbiological testing. The authors also thank CREST, Focas DIT (Dr. Darragh Ryan, Dr. Brendan Duffy, Dr. Hugh Hyaden, Dr. John Colreavy, Prof. Declan E. McCormack and Prof. Hugh Byrne) for scientific support and providing the laboratory facilities. The authors would also like to thank Enterprise Ireland for Funding. The current experimental procedure has been awarded both US and UK patents in December 2015 (US Patent No. 9,210,934; UK Patent No. GB2521405 B).
Footnotes
Author Contributions N.L. and J.C. carried out the formulation preparation. N.L. wrote the main manuscript and prepared Figures 2–3 and 5–8. J.C. and B.Q. developed the anti-microbial protocol. J.B. assisted in developing microbial testing and materials preparation. J.C. prepared Figures 1 and 4. S.P. designed the experiments and S.P. prepared the Figure 9. S.H. has carried out XPS analysis. All authors reviewed the manuscript.
References
- Lobdell K. W., Stamou S. & Sanchez J. A. Surg Clin N. Amer. 92, 65–77 (2012). [DOI] [PubMed] [Google Scholar]
- Haley R. W., Culver D. H., White J. W., Morgan W. M. & Emori T. G. The Nationwide Nosocomial Infection Rate: A New Need For Vital Statistics. Amer. J. Epidemiol. 121(2), 159–167 (1985). [DOI] [PubMed] [Google Scholar]
- Martone W. J., Culver D. H. & Haley R. W. In Hospital infections. (eds. Bennett J. V., Brachman P. S.) 577–96 (Little, Brown, and Company (1992). [Google Scholar]
- Ferreira L. & Zumbuehl A. Non-leaching surfaces capable of killing microorganisms on contact. Journal of Materials Chemistry 19(42), 7796–7806 (2009). [Google Scholar]
- Zielnik A. The green revolution enters the antimicrobial fray. Journal of Architectural Coatings. 4(8), 31–35 (2009). [Google Scholar]
- Kramer A., Schwebke I. & Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infectious Diseases. 6(1), 130 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susceptibility of Staphylococcus aureus Isolates to Methicillin in Participating Countries in 2014, European Centre for Disease Prevention and Control http://ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/database/Pages/table_reports.aspx (Last accessed on 13 January 2016).
- Devine J., Cooke R. P. D. & Wright E. P. Is methicillin-resistant Staphylococcus aureus (MRSA) contamination of ward-based computer terminals a surrogate marker for nococomial MRSA transmission and handwashing compliance? Journal of Hospital Infection. 48(1), 72–75 (2001). [DOI] [PubMed] [Google Scholar]
- Oie S., Hosokawa I. & Kamiya A. Contamination of room door handles by methicillin-sensitive/methicillin-resistant Staphylococcus aureus. Journal of Hospital Infection. 51(2), 140–143 (2002). [DOI] [PubMed] [Google Scholar]
- Rampling A. et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. Journal of Hospital Infection. 49(2), 109–116 (2001). [DOI] [PubMed] [Google Scholar]
- Boyce J. M., Potter-Bynoe G., Chenevert C. & King T. Environmental Contamination Due to Methicillin-Resistant Staphylococcus aureus: Possible Infection Control Implications. Infection Control and Hospital Epidemiology. 18(9), 622–627 (1997). [PubMed] [Google Scholar]
- Datz C., Jungwirth A., Dusch H., Galvan G. & Weiger T. What’s on doctors’ ball point pens? The Lancet, 350(9094), 1824–1824 (1997). [DOI] [PubMed] [Google Scholar]
- Rutala W. Environmental Control to Reduce GI Illness. Presented at the Society of Health Care Epidemiology Scientific Meeting (2006).
- Teare E. L., C. B., French G., Gould D., Jenner E. & McCulloch J. Hand washing. A modest measure with big effects. BMJ 318, 686 (1999).10073995 [Google Scholar]
- Podporska-Carroll J. et al. Antimicrobial Properties of Highly Efficient Photocatalytic TiO2 Nanotubes. Applied Catalysis B: Environmental 176, 70–75 (2015). [Google Scholar]
- Banerjee S., Dionysiou D. D. & Pillai S. C. Self-Cleaning Applications of TiO2 by Photo-Induced Hydrophilicity and Photocatalysis. Applied Catalysis B: Environmental 176, 396–428 (2015). [Google Scholar]
- Fagan R., McCormack D. E., Dionysiou D. D. & Pillai S. C. A Review of Solar and Visible Light Active TiO2 Photocatalysis for Treating Bacteria, Cyanotoxins and Contaminants of Emerging Concern. Materials Science in Semiconductor Processing 42, 2–14 (2016). [Google Scholar]
- Etacheri V., Di Valentin C., Schneider J., Bahnemann D. & Pillai S. C. Visible-Light Activation of TiO2 Photocatalysts: Advances in Theory and Experiments. Journal of Photochemistry and Photobiology 25, 1–29 (2015). [Google Scholar]
- Fisher M. B. et al. Nitrogen and copper doped solar light active TiO2 photocatalysts for water decontamination Applied. Catalysis B: Environmental 130–131, 8–13 (2013). [Google Scholar]
- Etacheri V., Colreavy J. & Pillai S. C. inventors; Dublin Institute of Technology, assignee. Visible Light Activatable Photocatalyst. United States Patent US 8,551,909 (2013).
- Kaali P. et al. Antimicrobial properties of Ag loaded zeolite polyester polyurethane and silicone rubber and long-term properties after exposure to in-vitro ageing. Polymer Degradation and Stability 95, 1456–1465 (2010). [Google Scholar]
- Roberto Monteiro D. et al. The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. International Journal of Antimicrobial Agents 34, 103–110 (2009). [DOI] [PubMed] [Google Scholar]
- Sabbani S. et al. Synthesis of silver-zeolite films on micropatterned porous alumina and its application as an antimicrobial substrate. Microporous and Mesoporous Materials 135, 131–136 (2010). [Google Scholar]
- Ren G. et al. Characterisation of copper oxide nanoparticles for antimicrobial applications. International Journal of Antimicrobial Agents 33, 587–590 (2009). [DOI] [PubMed] [Google Scholar]
- Ruparelia J. P., Chatterjee A. K., Duttagupta S. P. & Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticle. Acta Biomaterialia 4, 707–716 (2008). [DOI] [PubMed] [Google Scholar]
- Yoon K.-Y., Byeon J. H., Park J.-H. & Hwang J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Science of the Total Environment 373, 572–575 (2007). [DOI] [PubMed] [Google Scholar]
- Faúndez G., Troncoso M., Navarrete P. & Figueroa G. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiology 4, 19 doi: 10.1186/1471-2180-4-19 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehtar S., Wiid. I. & Todorov S. D. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: an in-vitro study. Journal of Hospital Infection 68, 45–51 (2008). [DOI] [PubMed] [Google Scholar]
- Ryan D., Pillai S. C. & Carroll J. inventors; Dublin Institute of Technology, assignee. A Surface Coating. United States patent US 9,210,934 (2015).
- Ryan D., Pillai S. C. & Carroll J. inventors; Dublin Institute of Technology, assignee. A Surface Coating. United Kingdom patent GB2521405 (2015).
- Padmanabhan S. C. et al. A Simple Sol-Gel Processing for the Development of High-Temperature Stable Photoactive Anatase Titania. Chem. Mater. 19, 4474–4481 (2007). [Google Scholar]
- Etacheri V., Seery M. K., Hinder S. J. & Pillai S. C. Oxygen Rich Titania: A Dopant Free, High Temperature Stable, and Visible-Light Active Anatase Photocatalyst. Adv. Funct. Mater. 21, 3744–3752 (2011). [Google Scholar]
- Li N. Ohashi S. Hishita T. Kolodiazhnyi H. & Haneda H. Origin of visible-light-driven photocatalysis: A comparative study on N/F-doped and N–F-codoped TiO2 powders by means of experimental characterizations and theoretical calculations. J. Solid State Chem. 178, 3293–3302 (2005). [Google Scholar]
- Nolan N. T., Seery M. K., Hinder S. J., Healy L. F., Pillai S. C. & Systematic A. Study of the Effect of Silver on the Chelation of Formic Acid to a Titanium Precursor and the Resulting Effect on the Anatase to Rutile Transformation of TiO2. J. Phys. Chem. C 114, 13026–13034 (2010). [Google Scholar]
- McIntyre N. S. & Cook M. G. X-ray photoelectron studies on some oxides andhydroxides of cobalt, nickel, and copper. Analytical Chemistry, 47, 2208–2213 (1975). [Google Scholar]
- Espirito Santo C., Quaranta D. & Grass G., Antimicrobial metallic copper surfaces kill Staphylococcus haemolyticus via membrane damage. MicrobiologyOpen 1(2), 46–52 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire A., Harrison J. & Turner R. J. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nature Reviews Microbiology 11, 371–384 (2013). [DOI] [PubMed] [Google Scholar]
- Macomber L., Rensing C. & Imlay J. A. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J.Bacteriol 189, 1616–1626 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G., Rensing C. & Solioz M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 77(5), 1541–1546 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghriche O., Rtimi S., Pulgarin C., Sanjines R. & Kiwi J. Effect of the Spectral Properties of TiO2, Cu, TiO2/Cu sputtered films on the bacterial inactivation under low intensity actinic light. Journal of Photochemistry and Photobiology A: Chemistry 251, 50–56 (2013). [Google Scholar]
- Rtimi S., Pulgarin C., Sanjines R. & Kiwi J. Accelerated self-cleaning by Cu promoted semiconductor binary-oxides under low intensity sunlight irradiation. Applied Catalysis B: Environmental, 180, 648–655 (2016). [Google Scholar]
- Rtimi S., Giannakis S., Sanjines R., Pulgarin C. & Bensimon M., Kiwi, Insight on the photocatalytic bacterial inactivation by co-sputtered TiO2-Cu in aerobic and anaerobic conditions J. Applied Catalysis B: Environmental 182, 277–285 (2016). [Google Scholar]