Abstract
Purpose/Objectives
Fibrosis is a late toxicity of thoracic irradiation that can result in substantial morbidity. Plasminogen activator inhibitor-1 (PAI-1) is a critical mediator of cellular senescence and fibrin stabilization. We sought to determine if the delivery of recombinant truncated PAI-1 protein (rPAI-123) would protect from the development of radiation-induced lung injury.
Methods and Materials
C57Bl/6 mice received intraperitoneal injections of rPAI-123 (5.4 μg/kg/day) or vehicle for 18 weeks beginning two days prior to radiation exposure (5 daily fractions of 6 Gy). Cohorts of mice were followed for survival (n=8 per treatment) and tissue collection (n=3 per treatment and time point). Fibrosis in lung was assessed with Masson-Trichrome staining and measurement of hydroxyproline content. Senescence was assessed with staining for beta-galactosidase activity in lung and primary pneumocytes.
Results
Hydroxyproline content in irradiated lung was significantly reduced in mice that received rPAI-123 compared to mice that received vehicle (IR+vehicle: 84.97, IR+rPAI-123: 56.2 μg/lung, p=0.001). C57Bl/6 mice exposed to IR+vehicle had dense foci of subpleural fibrosis at 19 weeks, whereas the lungs of mice exposed to IR+rPAI-123 were largely devoid of fibrotic foci. Cellular senescence was significantly decreased by rPAI-123 treatment in primary pneumocyte cultures and in lung at multiple time points after IR.
Conclusions
These studies identify that rPAI-123 is capable of preventing radiation-induced fibrosis in murine lungs. These anti-fibrotic effects are associated with increased fibrin metabolism, enhanced matrix metalloproteinase-3 (MMP-3) expression and reduced senescence in type II pneumocytes. rPAI-123 is a novel therapeutic option for radiation-induced fibrosis.
Keywords: PAI-1, radiation, fibrosis, senescence
BACKGROUND
One-half of cancer patients receive radiation therapy during the course of their illness. In most cases, normal tissue injury limits the radiation dose that can be administered, and as a result, the ability to locally cure malignancy (1,2). Fibrosis of irradiated lung is characterized by parenchymal cell depletion, inflammation, fibroblast proliferation, and excessive deposition of collagen (3-6).
Type II pneumocytes (airway epithelial cell, type II; AECII) function as the alveolar stem cell, repopulating the alveolar epithelium after injury. Recently, a time- and dose-dependent increase of AECII senescence and pneumocyte depletion after exposure to fibrogenic doses of irradiation (IR) was reported (7). Further, senescent AECII cells were capable of stimulating fibroblast proliferation and collagen secretion. These findings suggested that AECII senescence plays a causative role in IR-induced lung fibrosis and identified a novel target for intervention.
SERPINE-1, which encodes plasminogen activator inhibitor-1 (PAI-1), is a senescence-associated gene with increased expression in murine lung after fibrogenic doses of irradiation (7). PAI-1 is a serine protease that inhibits the activity of tissue plasminogen activator (tPA) and urokinase (uPA), and thus inhibits fibrinolysis and extracellular matrix (ECM) degradation (8,9). PAI-1 is up-regulated in fibrotic diseases and has been implicated in the progression of fibrosis (10-14), presumably through stabilization of the provisional ECM, increased epithelial to mesenchymal transition (EMT), and stimulation of fibrin-mediated influx of inflammatory and collagen producing cells (14). Importantly, PAI-1 has been described as a component of the senescence associated secretory phenotype (SASP) (15-17) and has further been described as a critical regulator of p53 mediated replicative senescence (17).
We hypothesized that modulation of PAI-1 activity would prevent radiation lung injury in a murine model through a reduction in AECII senescence and a reduction in collagen accumulation. We utilized a previously reported recombinant truncated PAI-1 protein, rPAI-123, to evaluate the role of PAI-1 in a murine model of radiation lung injury (18). Here, we report that treatment of irradiated mice with rPAI-123 prevented senescence of irradiated AECII, reduced collagen accumulation, and suppressed radiation-induced inflammation. These results demonstrate the potential of rPAI-123 as a strategy to prevent radiation-induced lung injury.
METHODS AND MATERIALS
Animal model
Animal studies were institutionally approved and in accordance with the guidelines of the Institute of Laboratory Animal Resources, National Research Council. rPAI-123 was prepared as previously described(19). Female C57Bl/6-Ncr mice, aged 10 weeks at the time of IR, received intraperitoneal injections of rPAI-123 (5.4 μg/kg/day) or vehicle (PBS) for 19 weeks beginning two days prior to IR. Irradiation was performed with mice restrained in a custom Lucite jig with lead shielding that allows for selective thoracic irradiation. Five daily fractions of 6 Gy were delivered to the thorax with a XRAD320 X-ray irradiator (Precision X-ray, Inc., North Branford, CT) using 2.0 mm Al filtration (300 kV peak) at a dose of 2.3 Gy/min. Dosimetry was verified with thermoluminescent dosimeters.
Lung was collected at 2, 4, 8, 16 and 19 weeks after irradiation (n≥3 per condition). Lung was snap frozen, inflated with neutral buffered formalin, or frozen in OCT compound. Formalin fixed lung was paraffin embedded and sectioned for histologic analysis. Primary pneumocytes were isolated from C57BL/6-Ncr mice as described previously (7) and detailed in the supplemental methods.
Histopathology and histochemistry
Detailed methods are included in the supplemental methods. Briefly, deparaffinized and rehydrated tissue sections were stained using Masson's trichrome technique. Stained slides were examined on a Leica DM LB2 microscope (Wetzlar, Germany). Digital micrographs were captured at 40× magnification and imported into QCapture (Quantitative Imaging Corporation, Surrey, BC, Canada).
For immunohistochemical staining, blocking in 2.5% horse serum was performed after citrate buffer-based antigen retrieval and blocking of endogenous peroxidase activity. Primary antibodies were applied for 1 hour at room temperature followed by incubation with a peroxidase-conjugated secondary antibody. Impact 3,3’-diaminobenzidine (Vector Laboratories, Burlingame, CA), was used as the chromogen with hematoxylin counterstaining (Sigma Aldrich, St Louis, MO). The number of macrophages, neutrophils, or CD3+ cells was counted in five high power fields (40×) in each lung (15 fields per group).
The β-galactosidase activity assay (Abcam, Cambridge, MA) was performed according to the manufacturer's instructions in frozen sections or primary pneumocyte cultures to assess for senescence. To determine if senescent cells were AECII, co-staining was performed with an anti-prosurfactant-C antibody (Pro-SP-C, Abcam, Cambridge, MA), a marker of AECII, and a compatible secondary antibody conjugated to Alexa Flour 594 (Life technologies, Grand Island, NY). Slides were mounted with ProLong antifade reagent containing DAPI (Life technologies, Grand Island, NY).
Hydroxyproline Assay
At the time of collection, the right lung (n=3 mice per cohort) was weighed, mechanically homogenized, and snap frozen. Hydroxyproline content was measured after hydrolysis of lung in 1 ml of 6 N HCl at 110°C for 18 hours. Hydrolysate was analyzed with the Biovision Hydroxyproline assay kit (Milpitas, CA) per manufacturer's instructions. Hydroxyproline content was calculated based upon total lung weight and expressed as micrograms in the lung.
For assessment of hydroxyproline content in fibroblast cultures, adherent cells were scraped in ice cold PBS and pelleted by centrifugation. Cell pellets were hydrolyzed with 6 N HCl at 110°C for 18 hours, and analyzed as above.
Murine fibroblast proliferation assay
NIH-3T3 cells (ATCC, Manassas, VA) were maintained with DMEM containing 10% FCS and 0.1% β-mercaptoethanol, and cultured with reduced serum media (1% FCS) overnight prior to use. A total of 5×103 cells were cultured with 200 μl complete media containing rPAI-123 (0, 0.6 and 10 nM). Cell proliferation was assessed using a BrdU cell proliferation kit (EMD Millipore, Billerica, MA), per manufacturer's instructions.
Western Blotting
Lung extracts were prepared using radioimmunoprecipitation assay buffer (Pierce) containing protease (Roche Applied Science) and phosphatase inhibitors (Sigma-Aldrich), followed by measurement of protein concentrations by the Bradford method (Bio-Rad). Equal amounts of protein were subjected to western blot analysis. Primary antibodies included an anti-fibrin antibody (Dako, Carpinteria, CA), anti-MMP-3 antibody (EMD Millipore, Billerica, MA), and anti-actin antibody (EMD Millipore). Horseradish peroxidase conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX). Specific bands for each protein were detected by ImageQuant LAS4000 (GE Healthcare Life Science, Pittsburgh, PA) using the SuperSignal Chemiluminescence kit (Thermo Scientific, Rockford, IL). Densitometric analysis of expression was performed using ImageJ software (NIH, Bethesda, MD). The expression of each molecule was normalized to actin.
Real-time Quantitative RT-PCR
Total RNA was extracted using the RNeasy plus mini kit (Qiagen, Valencia, CA). After genomic DNA elimination, 1 μg of total RNA was reverse transcribed into first-strand cDNA using a QuantiTect Reverse Transcription kit (Qiagen). Quantitative real-time PCR was performed using TaqMan® Gene Expression assay primers and reagents (Applied Biosystems, Foster City, CA) with a 7500 real-time PCR system (Applied Biosystems, Foster City, CA) using 0.5 μg of cDNA in a 20 μL reaction volume. Reactions were incubated for 2 minutes at 50 °C, 10 minutes at 95°C, followed by 50 cycles of 95°C for 15 seconds and 60°C for 1 minute. Cytokine expression was normalized to the endogenous control (18s).
Statistical Analysis
Comparisons between conditions were conducted with ANOVA with Tukey correction for multiple comparisons. A p value of less than 0.05 was considered statistically significant. In vitro studies were performed in duplicate and validated in three separate experiments. Assessment of tissue included at least 3 mice per treatment.
RESULTS
Radiation-induced lung fibrosis is reduced by rPAI-123
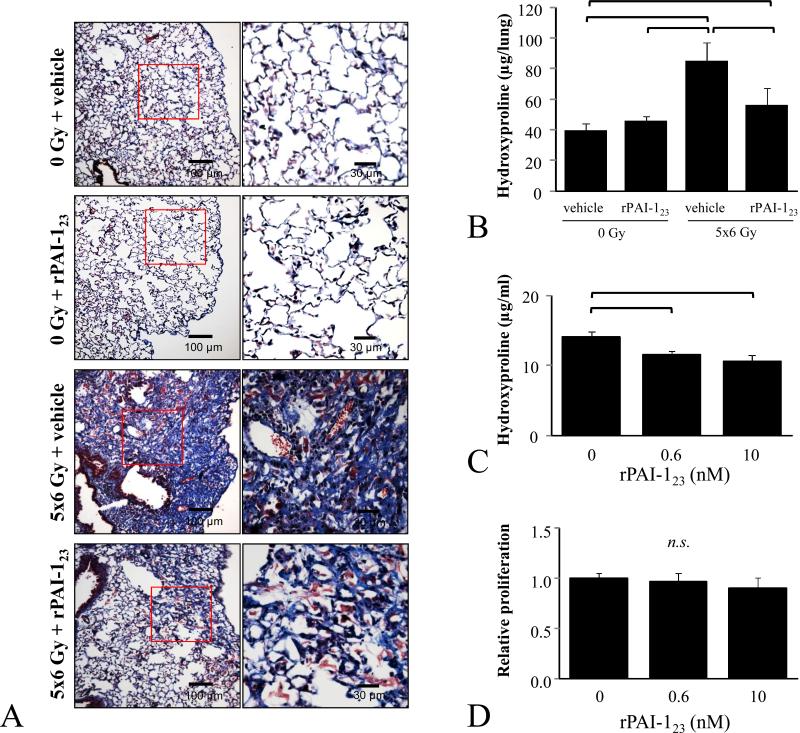
Masson-trichrome staining of lung at 19 weeks after IR revealed extensive sub-pleural collagen accumulation in the lung of vehicle+IR mice, but not in that of rPAI-123+IR mice (Figure 1A, Supplemental Figure 1). Similarly, lung hydroxyproline content of vehicle+IR mice was significantly higher than that measured in unirradiated mice (85.0 vs. 39.8 μg/lung, p=0.001) and rPAI-123+IR mice (57.0 vs. 85.0 g per lung, p<0.001, Figure 1B). Indeed, irradiated mice treated with vehicle died rapidly beginning at 16 weeks, whereas there were no deaths in the PAI-123 treated group (Supplemental Figure 2)
Figure 1. Effects of rPAI-123 on radiation-induced lung injury.
C57Bl/6NCr mice were exposed to 5×6 Gy of thoracic IR and treated with rPAI-123 or vehicle. A) Masson trichrome staining of lung at 19 weeks after IR. The lungs of vehicle-treated mice exhibited extensive foci of subpleural fibrosis while those of rPAI-123 treated mice exhibited minimal fibrosis. Collagen: blue, nuclei: purple, cytoplasm/epithelia: pink. B) Lung collected at 19 weeks after IR was analyzed for hydroxyproline content. C-D) NIH-3T3 cells were cultured in reduced serum media (1% FBS) overnight, treated with rPAI-123 (0, 0.6, 10 nM), and incubated for 72 hours. Hydroxyproline content of the NIH-3T3 cell/matrix (cell pellet and scrapings of matrix adherent to culture plastic) was assessed (C). Proliferation was evaluated by BrdU cell proliferation kit and normalized to the proliferation of the vehicle treated control (D). Columns: mean, error bars: SD, brackets: p<0.05 by ANOVA.
To determine if treatment with rPAI-123 had direct effects on fibroblasts, the hydroxyproline content of NIH-3T3 cultures was assessed after treatment with rPAI-123 (Figure 1C). Increasing concentrations of rPAI-123 only modestly reduced the accumulation of hydroxyproline in cultured fibroblasts. To confirm that the rPAI-123 mediated reduction in hydroxyproline content was not due to toxicity alone, proliferation was assessed under identical conditions. No significant effects of rPAI-123 treatment on fibroblast proliferation were detected.
rPAI-123 treatment reduces fibrin accumulation and stabilization
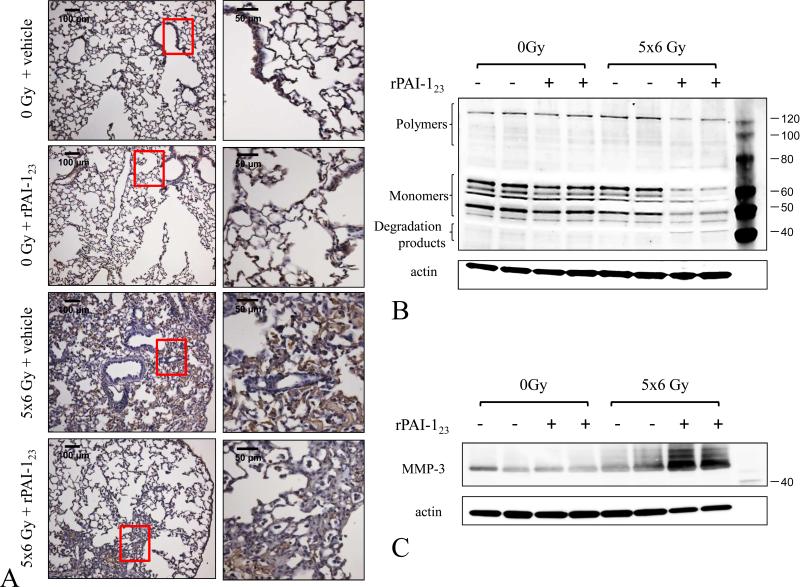
PAI-1 is known to play a role in fibrin stabilization (20,21), a critical component of lung fibrosis (14,22). To determine if treatment with rPAI-123 altered fibrin metabolism after IR, lung was probed for fibrin by immunohistochemistry and western blotting. Irradiation resulted in accumulation of fibrin in lung, especially in subpleural areas, an effect that was markedly reduced with rPAI-123 treatment (Figure 2A). As expected, treatment with rPAI-123+IR resulted in increased fibrin degradation products compared to that observed after vehicle+IR (Figure 2B). Densitometry confirmed an increase in fibrin metabolism in rPAI-123 treated mice (Supplemental Figure 3).
Figure 2. Fibrin degradation is enhanced by rPAI-123.
C57Bl/6NCr mice were exposed to 5×6 Gy of thoracic IR and treated with vehicle or rPAI-123. Lung was collected at 16 weeks after IR and evaluated by immunohistochemistry for fibrin(ogen) content (brown) with a hematoxylin (blue) counterstain. (A) Lung homogenates were subjected to western blotting for fibrin (B) and MMP-3 expression (C). Western blotting for actin was used as a loading control.
MMPs can degrade a number of proteins that constitute the ECM (23). MMP-3 is known to degrade fibrinogen and cross-linked fibrin (24), an effect enhanced by treatment with rPAI-123 (25). Indeed, MMP-3 expression was increased markedly in the lungs of rPAI-123+IR mice compared to vehicle+IR mice (Figure 2C).
rPAI-123 treatment prevents radiation-induced AECII senescence
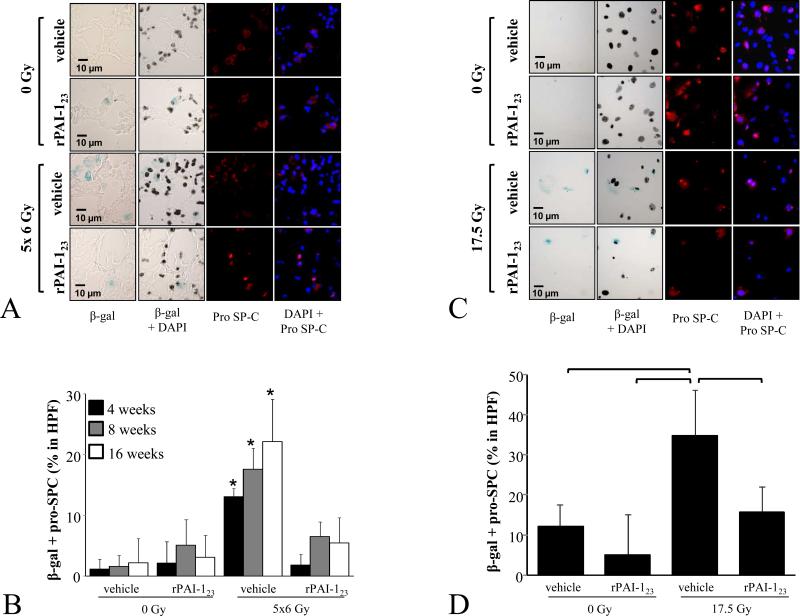
PAI-1 is well-known as both a marker of senescence and as a critical mediator of p53-mediated senescence (17,26,27). To determine if rPAI-123 was capable of reducing AECII senescence after IR, senescence associated β-Galactosidase expression was evaluated in lung and in primary pneumocyte cultures. As previously reported (7), radiation-induced senescence occurs in AECII in a time-dependent fashion (Figure 3A). A marked reduction in AECII senescence was observed in rPAI-123 treated mice, with levels indistinguishable from unirradiated mice.
Figure 3. AECII senescence after irradiation is reduced with rPAI-123 treatment.
C57Bl/6NCr mice were exposed to 5×6 Gy of thoracic IR. A, B) The percentage AECII in lung with senescence associated β-galactosidase activity was scored by dose and time point (A: representative images at 16 weeks after IR). Bars: mean; error bars: standard error; *p < 0 .05 for the comparison to rPAI-123 + 5×6 Gy treatment at the same time point by ANOVA. C, D) Primary pneumocyte cultures were treated with rPAI-123 (0, 0.6, 10 nM) 1 hour before IR (17.5 Gy). Cells were fixed and co-stained for β-galactosiade activity and pro-SP-C at 5 days after IR.
A number of cytokines and oxidant molecules are elaborated by pneumocytes, stromal cells, and inflammatory cells after irradiation that may contribute to senescence (28-31). To confirm that rPAI-123 was capable of directly inhibiting pneumocyte senescence in the absence of non-pneumocyte lineages, primary pneumocyte cultures were treated with rPAI-123 (Figure 3B). A similar pattern was observed with an increase in AECII senescence after IR and protection from radiation-induced pneumocyte senescence afforded by rPAI-123 treatment.
rPAI-123 treatment reduces expression of inflammatory and senescence associated cytokines in irradiated lungs
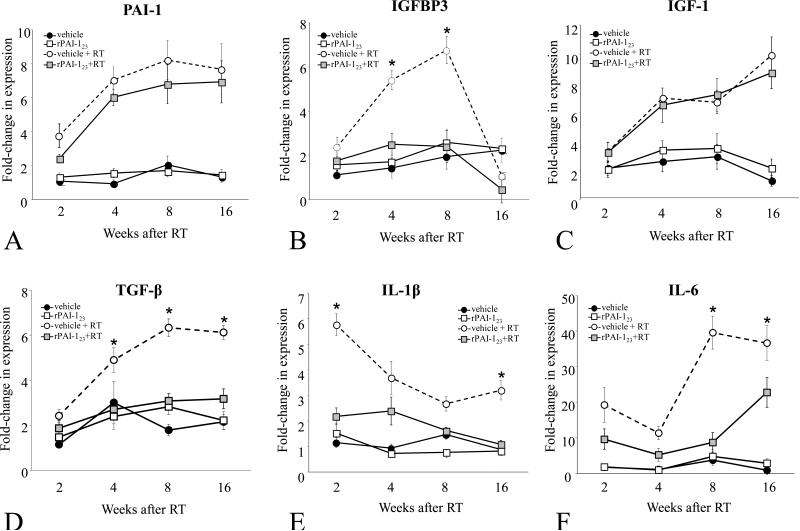
Senescence is associated with the elaboration of a number of secreted pro-inflammatory factors (32-34). Several of these pro-inflammatory cytokines, such as IL-6, IL-1β, and TGF-β are known to be elevated in irradiated lung (35-38). To determine if the rPAI-123 mediated reduction in senescence was accompanied by a reduction in senescence associated signaling, RNA isolated from lung of treated mice was subjected to quantitative real-time PCR (Figure 4A-F). The expression levels of PAI-1 and IGF-1 were increased after vehicle+IR, with no evidence of an effect of rPAI-123 treatment. In contrast, treatment with rPAI-123 significantly reduced the radiation-induced expression of IGFBP-3, TGF-β, IL-1β, and IL-6.
Figure 4. rPAI-123 modulates radiation-induced inflammatory cytokine expression.
Mice were exposed to 5×6 Gy of thoracic IR and treated with vehicle or rPAI-123. Quantitative real time PCR was performed on RNA isolated from lung to determine the expression of PAI-1 (A), IGFBP3 (B), IGF-1 (C), TGF-β (D), IL-1β (E), and IL-6 (F). Expression was normalized to the expression of 18s. Bars: mean. Error bars: standard error. *p < 0.05 for the comparison of each treatment to controls.
rPAI-123 treatment reduces radiation-induced inflammation
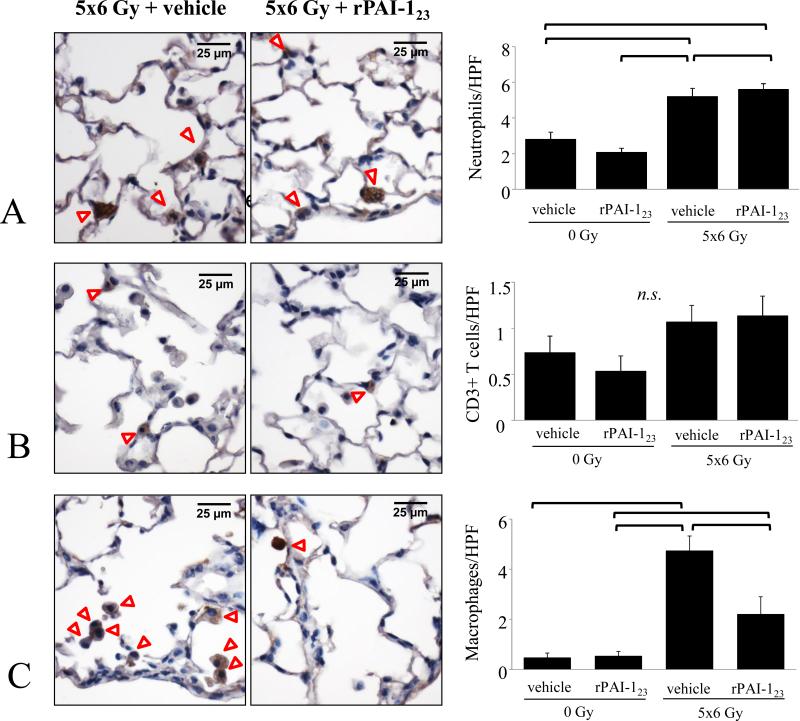
To determine if the reduction in pro-inflammatory cytokine expression in irradiated lung after treatment with rPAI-123 correlated with a reduction in inflammatory cell infiltration, lung was subjected to immunohistochemistry for markers of neutrophils, macrophages, and T-cells (Figure 5C). Treatment of irradiated mice with rPAI-123 reduced macrophage accumulation after irradiation. No significant differences in neutrophil and T-cell accumulation in lungs of vehicle+IR mice and rPAI-123+IR mice were observed (Figure 5A and B).
Figure 5. rPAI-123 decreases macrophage accumulation in irradiated lung.
Mice were exposed to 5×6 Gy of thoracic IR and treated with vehicle or rPAI-123. The number of neutrophils (A), T-cells (B), and macrophages (C) in lung at 16 weeks after IR were compared. DAB (brown) was used as the chromogen with hematoxylin (blue) counterstain. Columns: mean, error bars: SD, brackets: p< 0.05 by ANOVA, HPF: high power field (63×).
DISCUSSION
The present study demonstrates that delivery of rPAI-123 prevents radiation-induced lung fibrosis in Bl6/NcR mice. Treatment with rPAI-123 resulted in increased MMP-3 expression and reduced fibrin accumulation in irradiated lungs. Further, the irradiated lungs of mice treated with rPAI-123 exhibited a marked reduction in AECII senescence, inflammation, and collagen accumulation. In vitro studies confirmed that rPAI-123 was capable of directly inhibiting AECII senescence after IR. The ability of rPAI-123 to inhibit radiation injury and radiation-induced stem cell senescence is novel.
Irradiation results in alveolar epithelial injury, which in turn activates the fibrinolytic activities of plasminogen activators (tPA, uPA) and matrix metalloproteinases (MMPs) (22). Activation of MMPs and plasminogen activators promotes fibroblast migration, proliferation, and ECM deposition during tissue remodeling (39,40). Although critical in wound healing, prolonged activation of coagulation factors and fibrin accumulation may contribute to excessive matrix deposition, resulting in fibrosis (14).
Inhibition of plasminogen activators by PAI-1 results in a sequential inhibition of plasminogen-to-plasmin conversion as well as plasmin dependent MMP activation (14). Therefore, PAI-1 activity contributes to elevated levels of fibrin and enhanced migration of inflammatory cells and collagen producing cells (41-43). Stabilization of the fibrin matrix and stimulation of collagen production by fibroblasts have been described as critical methods of PAI-1 mediated fibrosis (44-46).
The systemic delivery of rPAI-123 is capable of reducing PAI-1 activity, increasing plasmin activity, and promoting fibrin proteolysis through MMP3 activation, resulting in ECM degradation (25). In this study, systemic delivery of rPAI-123 resulted in an increase in MMP-3 expression in irradiated lungs and enhanced fibrin proteolysis. This was accompanied by rPAI-123 mediated reduction in fibrin matrix deposition by immunohistochemical assessment and a significant reduction of collagen accumulation in irradiated lung. Further, treatment of murine fibroblasts with rPAI-123 decreased collagen deposition without effects on fibroblast proliferation.
AECII function as the alveolar stem cell, giving rise to AECII and type I pneumocytes after toxic insults (7,47,48). Depletion of pneumocytes has been described in models of lung fibrosis (49,50), and causality is suggested by the finding that targeted AECII depletion is sufficient to cause pulmonary fibrosis (51). It has been theorized that AECII senescence plays an important role in radiation lung fibrosis by depleting the stem cell reserve in irradiated lung (7). Here, we have shown that systemic treatment with rPAI-123 is capable of preventing AECII senescence after irradiation and reducing radiation lung fibrosis. The ability of rPAI-123 to prevent AECII senescence in cultured pneumocytes suggests a direct effect that was not merely due to a reduction in inflammation, oxidative stress, or other paracrine interactions.
Aside from its role in senescence, PAI-1 has been described as a component of the SASP, a group of molecules secreted by senescent cells, many of which are pro-inflammatory and mitogenic in nature (17,29,30,52). In this study, the expression of six members of the SASP was evaluated. Three cytokines (TGF-β, IL-1β, and IL-6) were chosen for analysis based on their recognized importance in radiation fibrosis. For each of these three cytokines, IR resulted in an increase in expression in lung, which was at least partially prevented with rPAI-123 treatment.
Although reduced expression of these pro-inflammatory cytokines may be a direct result of reduced AECII senescence, senescent pneumocytes are not the only source of pro-inflammatory cytokines in irradiated lung. For example, many cell types contribute to the expression of TGF-β in irradiated lung, including pneumocytes, fibroblasts, and macrophages (53). Systemic administration of rPAI-123 significantly inhibited macrophage accumulation in irradiated lungs. It is likely that a reduction in the elaboration of the pro-inflammatory SASP after treatment with rPAI-123 is at least partially responsible for this effect.
Conversely, IGF-1 and PAI-1 expression in irradiated lungs was not different between groups treated with vehicle and rPAI-123. Evidence from transgenic animal models suggests that increased IGF-1 secretion alone has no effect on the evolution of fibrosis (54). It has been suggested that the pro-fibrotic effect of IGF-1 expression may require a co-factor, such as IGFBP-3 (55). rPAI-123 treatment was capable of preventing radiation-induced expression of IGFBP-3. This, in turn, may interfere with the ability of IGF-1 to enhance fibrosis, providing another potential mechanism of reduced lung fibrosis after rPAI-123 treatment.
IGFBP-3 appears to be the downstream mediator of PAI-1 mediated senescence (56). IGFBP-3 activation is inhibited by tPA, thus, PAI-1 activity decreases tPA activation and results in increased IBFBP-3 activity and senescence (56). In irradiated lungs, we observed an increase in PAI-1 expression, consistent with our prior study (7). Treatment with rPAI-123 did not alter the expression of PAI-1, as is expected, however it was capable of reducing IGFBP-3 expression. The reduction in IGFBP-3 after rPAI-123 treatment was accompanied by a reduction in the number of senescent AECII after irradiation.
The involvement of PAI-1 in many facets of lung fibrosis provides an ability to interfere with multiple pro-fibrotic pathways simultaneously. Treatment with rPAI-123 was capable of inhibiting fibrin accumulation and inhibiting pneumocyte senescence. As a result of these two processes, collagen accumulation and pro-inflammatory signaling was reduced. Collectively, these studies identify the systemic delivery of rPAI-123 as a promising strategy for the prevention of radiation-induced pulmonary fibrosis.
Supplementary Material
SUMMARY.
This article describes the use of a recombinant truncated plasminogen activator inhibitor-1 protein (rPAI-123) as a method to mitigate radiation lung fibrosis in a murine model. PAI-1 is a protein involved in the activation of plasminogen to plasmin and plays an important role in fibrin matrix stabilization and senescence. The use of rPAI-123 in this mouse model prevents pneumocyte senescence, reduces collagen accumulation, and alters fibrin metabolism after thoracic irradiation.
Acknowledgement
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none
References
- 1.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 2.Bhide SA, Nutting CM. Recent advances in radiotherapy. BMC Med. 2010;8:25. doi: 10.1186/1741-7015-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quarmby S, Kumar P, Kumar S. Radiation-induced normal tissue injury: role of adhesion molecules in leukocyte-endothelial cell interactions. Int J Cancer. 1999;82:385–395. doi: 10.1002/(sici)1097-0215(19990730)82:3<385::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets. 2013;14:1347–1356. doi: 10.2174/13894501113149990198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015 doi: 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97:149–161. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Citrin DE, Shankavaram U, Horton JA, Shield W, 3rd, Zhao S, Asano H, White A, Sowers A, Thetford A, Chung EJ. Role of type II pneumocyte senescence in radiation-induced lung fibrosis. J Natl Cancer Inst. 2013;105:1474–1484. doi: 10.1093/jnci/djt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hertig A, Berrou J, Allory Y, Breton L, Commo F, Costa De Beauregard MA, Carmeliet P, Rondeau E. Type 1 plasminogen activator inhibitor deficiency aggravates the course of experimental glomerulonephritis through overactivation of transforming growth factor beta. FASEB J. 2003;17:1904–1906. doi: 10.1096/fj.03-0084fje. [DOI] [PubMed] [Google Scholar]
- 9.Tucker T, Idell S. Plasminogen-plasmin system in the pathogenesis and treatment of lung and pleural injury. Semin Thromb Hemost. 2013;39:373–381. doi: 10.1055/s-0033-1334486. [DOI] [PubMed] [Google Scholar]
- 10.Dong C, Zhu S, Wang T, Yoon W, Li Z, Alvarez RJ, ten Dijke P, White B, Wigley FM, Goldschmidt-Clermont PJ. Deficient Smad7 expression: a putative molecular defect in scleroderma. Proc Natl Acad Sci U S A. 2002;99:3908–3913. doi: 10.1073/pnas.062010399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Wei J, Ghosh AK, Sargent JL, Komura K, Wu M, Huang QQ, Jain M, Whitfield ML, Feghali-Bostwick C, Varga J. PPARgamma downregulation by TGFss in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLoS One. 2010;5:e13778. doi: 10.1371/journal.pone.0013778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest. 1996;97:232–237. doi: 10.1172/JCI118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazar MH, Christensen PJ, Du M, Yu B, Subbotina NM, Hanson KE, Hansen JM, White ES, Simon RH, Sisson TH. Plasminogen activator inhibitor-1 impairs alveolar epithelial repair by binding to vitronectin. Am J Respir Cell Mol Biol. 2004;31:672–678. doi: 10.1165/rcmb.2004-0025OC. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol. 2012;227:493–507. doi: 10.1002/jcp.22783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein S, Moerman EJ, Fujii S, Sobel BE. Overexpression of plasminogen activator inhibitor type-1 in senescent fibroblasts from normal subjects and those with Werner syndrome. J Cell Physiol. 1994;161:571–579. doi: 10.1002/jcp.1041610321. [DOI] [PubMed] [Google Scholar]
- 16.Lafferty-Whyte K, Bilsland A, Cairney CJ, Hanley L, Jamieson NB, Zaffaroni N, Oien KA, Burns S, Roffey J, Boyd SM, Keith WN. Scoring of senescence signalling in multiple human tumour gene expression datasets, identification of a correlation between senescence score and drug toxicity in the NCI60 panel and a pro-inflammatory signature correlating with survival advantage in peritoneal mesothelioma. BMC Genomics. 2010;11:532. doi: 10.1186/1471-2164-11-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulligan-Kehoe MJ, Wagner R, Wieland C, Powell R. A truncated plasminogen activator inhibitor-1 protein induces and inhibits angiostatin (kringles 1-3), a plasminogen cleavage product. J Biol Chem. 2001;276:8588–8596. doi: 10.1074/jbc.M006434200. [DOI] [PubMed] [Google Scholar]
- 19.Drinane M, Walsh J, Mollmark J, Simons M, Mulligan-Kehoe MJ. The anti-angiogenic activity of rPAI-1(23) inhibits fibroblast growth factor-2 functions. J Biol Chem. 2006;281:33336–33344. doi: 10.1074/jbc.M607097200. [DOI] [PubMed] [Google Scholar]
- 20.Bergheim I, Guo L, Davis MA, Duveau I, Arteel GE. Critical role of plasminogen activator inhibitor-1 in cholestatic liver injury and fibrosis. J Pharmacol Exp Ther. 2006;316:592–600. doi: 10.1124/jpet.105.095042. [DOI] [PubMed] [Google Scholar]
- 21.Wagner OF, de Vries C, Hohmann C, Veerman H, Pannekoek H. Interaction between plasminogen activator inhibitor type 1 (PAI-1) bound to fibrin and either tissue-type plasminogen activator (t-PA) or urokinase-type plasminogen activator (u-PA). Binding of t-PA/PAI-1 complexes to fibrin mediated by both the finger and the kringle-2 domain of t-PA. J Clin Invest. 1989;84:647–655. doi: 10.1172/JCI114211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu RM. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid Redox Signal. 2008;10:303–319. doi: 10.1089/ars.2007.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129:307–321. doi: 10.1111/j.1365-2141.2005.05444.x. [DOI] [PubMed] [Google Scholar]
- 24.Bini A, Itoh Y, Kudryk BJ, Nagase H. Degradation of cross-linked fibrin by matrix metalloproteinase 3 (stromelysin 1): hydrolysis of the gamma Gly 404-Ala 405 peptide bond. Biochemistry. 1996;35:13056–13063. doi: 10.1021/bi960730c. [DOI] [PubMed] [Google Scholar]
- 25.Mollmark J, Ravi S, Sun B, Shipman S, Buitendijk M, Simons M, Mulligan-Kehoe MJ. Antiangiogenic activity of rPAI-1(23) promotes vasa vasorum regression in hypercholesterolemic mice through a plasmin-dependent mechanism. Circ Res. 2011;108:1419–1428. doi: 10.1161/CIRCRESAHA.111.246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kortlever RM, Bernards R. Senescence, wound healing and cancer: the PAI-1 connection. Cell Cycle. 2006;5:2697–2703. doi: 10.4161/cc.5.23.3510. [DOI] [PubMed] [Google Scholar]
- 27.Eren M, Boe AE, Klyachko EA, Vaughan DE. Role of plasminogen activator inhibitor-1 in senescence and aging. Semin Thromb Hemost. 2014;40:645–651. doi: 10.1055/s-0034-1387883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 29.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29:273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fumagalli M, d'Adda di Fagagna F. SASPense and DDRama in cancer and ageing. Nat Cell Biol. 2009;11:921–923. doi: 10.1038/ncb0809-921. [DOI] [PubMed] [Google Scholar]
- 33.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Ren JL, Pan JS, Lu YP, Sun P, Han J. Inflammatory signaling and cellular senescence. Cell Signal. 2009;21:378–383. doi: 10.1016/j.cellsig.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rube CE, Uthe D, Wilfert F, Ludwig D, Yang K, Konig J, Palm J, Schuck A, Willich N, Remberger K, Rube C. The bronchiolar epithelium as a prominent source of pro-inflammatory cytokines after lung irradiation. Int J Radiat Oncol Biol Phys. 2005;61:1482–1492. doi: 10.1016/j.ijrobp.2004.12.072. [DOI] [PubMed] [Google Scholar]
- 36.Chiang CS, Liu WC, Jung SM, Chen FH, Wu CR, McBride WH, Lee CC, Hong JH. Compartmental responses after thoracic irradiation of mice: strain differences. Int J Radiat Oncol Biol Phys. 2005;62:862–871. doi: 10.1016/j.ijrobp.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 37.Ao X, Zhao L, Davis MA, Lubman DM, Lawrence TS, Kong FM. Radiation produces differential changes in cytokine profiles in radiation lung fibrosis sensitive and resistant mice. J Hematol Oncol. 2009;2:6. doi: 10.1186/1756-8722-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nature reviews. Molecular cell biology. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovascular research. 2000;46:214–224. doi: 10.1016/s0008-6363(00)00003-1. [DOI] [PubMed] [Google Scholar]
- 41.Henke C, Marineili W, Jessurun J, Fox J, Harms D, Peterson M, Chiang L, Doran P. Macrophage production of basic fibroblast growth factor in the fibroproliferative disorder of alveolar fibrosis after lung injury. Am J Pathol. 1993;143:1189–1199. [PMC free article] [PubMed] [Google Scholar]
- 42.Henke C, Fiegel V, Peterson M, Wick M, Knighton D, McCarthy J, Bitterman P. Identification and partial characterization of angiogenesis bioactivity in the lower respiratory tract after acute lung injury. J Clin Invest. 1991;88:1386–1395. doi: 10.1172/JCI115445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svee K, White J, Vaillant P, Jessurun J, Roongta U, Krumwiede M, Johnson D, Henke C. Acute lung injury fibroblast migration and invasion of a fibrin matrix is mediated by CD44. J Clin Invest. 1996;98:1713–1727. doi: 10.1172/JCI118970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeshita K, Yamamoto K, Ito M, Kondo T, Matsushita T, Hirai M, Kojima T, Nishimura M, Nabeshima Y, Loskutoff DJ, Saito H, Murohara T. Increased expression of plasminogen activator inhibitor-1 with fibrin deposition in a murine model of aging, “Klotho” mouse. Semin Thromb Hemost. 2002;28:545–554. doi: 10.1055/s-2002-36699. [DOI] [PubMed] [Google Scholar]
- 45.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106:1341–1350. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tuan TL, Wu H, Huang EY, Chong SS, Laug W, Messadi D, Kelly P, Le A. Increased plasminogen activator inhibitor-1 in keloid fibroblasts may account for their elevated collagen accumulation in fibrin gel cultures. Am J Pathol. 2003;162:1579–1589. doi: 10.1016/S0002-9440(10)64292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leslie KO. Idiopathic pulmonary fibrosis may be a disease of recurrent, tractional injury to the periphery of the aging lung: a unifying hypothesis regarding etiology and pathogenesis. Arch Pathol Lab Med. 2012;136:591–600. doi: 10.5858/arpa.2011-0511-OA. [DOI] [PubMed] [Google Scholar]
- 50.Gifford AH, Matsuoka M, Ghoda LY, Homer RJ, Enelow RI. Chronic inflammation and lung fibrosis: pleotropic syndromes but limited distinct phenotypes. Mucosal Immunol. 2012;5:480–484. doi: 10.1038/mi.2012.68. [DOI] [PubMed] [Google Scholar]
- 51.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, Thannickal VJ, Moore BB, Christensen PJ, Simon RH. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A, Willich N, Rube C. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000;47:1033–1042. doi: 10.1016/s0360-3016(00)00482-x. [DOI] [PubMed] [Google Scholar]
- 54.Frankel SK, Moats-Staats BM, Cool CD, Wynes MW, Stiles AD, Riches DW. Human insulin-like growth factor-IA expression in transgenic mice promotes adenomatous hyperplasia but not pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L805–812. doi: 10.1152/ajplung.00420.2004. [DOI] [PubMed] [Google Scholar]
- 55.Pilewski JM, Liu L, Henry AC, Knauer AV, Feghali-Bostwick CA. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am J Pathol. 2005;166:399–407. doi: 10.1016/S0002-9440(10)62263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elzi DJ, Lai Y, Song M, Hakala K, Weintraub ST, Shiio Y. Plasminogen activator inhibitor 1--insulin-like growth factor binding protein 3 cascade regulates stress-induced senescence. Proc Natl Acad Sci U S A. 2012;109:12052–12057. doi: 10.1073/pnas.1120437109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.