Abstract
OBJECTIVE
We sought to determine if maternal weight or body mass index (BMI) modifies the effectiveness of 17-alpha hydroxyprogesterone caproate (17OHP-C).
STUDY DESIGN
We performed a secondary analysis of the Maternal-Fetal Medicine Units Network Trial for the Prevention of Recurrent Preterm Delivery by 17-Alpha Hydroxyprogesterone Caproate. Binomial regression models were estimated to determine the relative risk (RR) of preterm birth (PTB) in women randomized to 17OHP-C vs placebo according to BMI category and maternal weight. Adjusted models considered inclusion of potential confounders.
RESULTS
In all, 443 women with complete data were included. 17OHP-C is effective in preventing PTB <37 weeks only in women with prepregnancy BMI <30 kg/m2 (RR, 0.54; 95% confidence interval, 0.43–0.68). Above this BMI threshold there is a nonsignificant trend toward an increased risk of PTB (RR, 1.55; 95% confidence interval, 0.83–2.89) with 17OHP-C treatment. When analyzing by maternal weight, a similar threshold is observed at 165 lb, above which 17OHP-C is no longer effective.
CONCLUSION
The effectiveness of 17OHP-C is modified by maternal weight and BMI, and treatment does not appear to reduce the rate of PTB in women who are obese or have a weight >165 lb. This finding may be due to subtherapeutic serum levels in women with increased BMI or weight. Studies of adjusted-dose 17OHP-C in women who are obese or who weigh >165 lb are warranted, and current recommendations regarding the uniform use of 17OHP-C regardless of maternal BMI and weight may deserve reassessment.
Keywords: 17-alpha hydroxyprogesterone caproate, body mass index, obesity, prematurity, preterm birth, progesterone
The sequelae of preterm birth (PTB), including neonatal, childhood, and adult morbidity and mortality, remain the most critical pregnancy-related public health issues in the developed world.1–4 A history of PTB is the strongest risk factor known, with reported recurrence rates as high as 55%.5
Weekly administration of intramuscular 17-alpha hydroxyprogesterone caproate (17OHP-C) is the most effective modality currently available to prevent recurrent PTB.5 The 250-mg dosage of 17OHP-C used in the initial Maternal-Fetal Medicine Units (MFMU) Network Progesterone Trial was extrapolated from previous trials, and dose-finding studies have not been done. A recent study demonstrated that women with serum 17OHP-C levels below a threshold concentration experience higher levels of recurrent PTB than women with levels above the threshold.6 Pharmacokinetic simulations by the same group of investigators showed that serum 17OHP-C levels are inversely related to maternal body mass index (BMI).7 These observations raise the question of whether 17OHP-C is less effective at higher maternal weight or BMI. To address this question we performed a secondary analysis of the original Prevention of Recurrent Preterm Delivery by 17-Alpha Hydroxyprogesterone Caproate by Meis et al.5 Our hypothesis is that the effectiveness of 17OHP-C for prevention of recurrent PTB is modified by increased maternal weight and/or BMI.
Materials and Methods
Patient sample
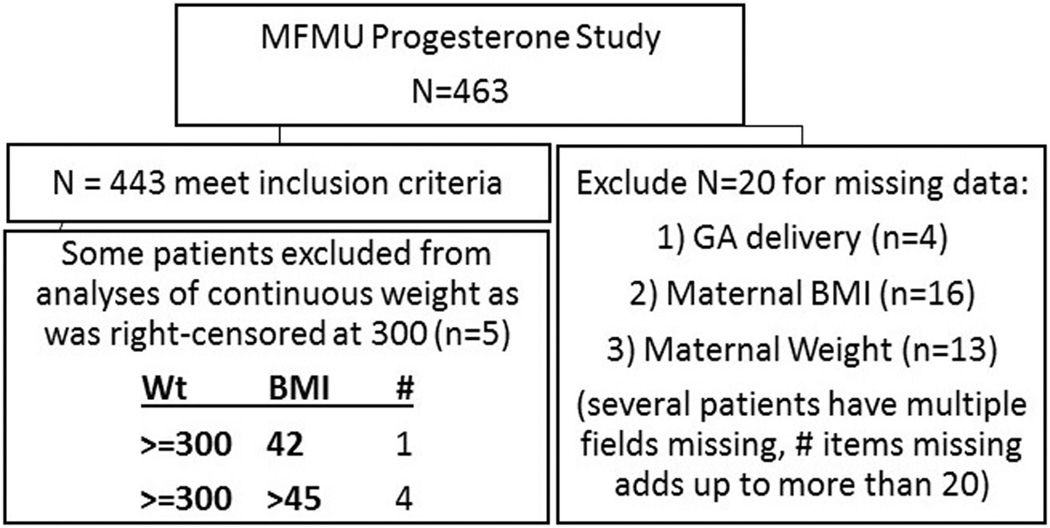
This is a secondary analysis of the MFMU Trial for the Prevention of Recurrent Preterm Delivery by 17-Alpha Hydroxyprogesterone Caproate.5 Full details are available in the original publication. Briefly, the original study was performed at 19 participating centers from 1999 through 2002. Women presenting for prenatal care were screened between 15–20 3/7 weeks’ gestation for a history of spontaneous PTB that occurred between 20–36 6/7 weeks. Eligible patients were randomized in a 2:1 fashion to weekly intramuscular injections of 250 mg of 17OHP-C or placebo, respectively, beginning at 16–20 6/7 weeks. There were 463 individual women in the original trial. For this analysis, patients with missing gestational age at delivery, maternal BMI, and/or maternal weight were excluded, leaving 443 patients for this analysis (Figure 1). Reported maternal prepregnancy height and weight were obtained at the time of study enrollment and BMI was calculated. Actual weight at enrollment, total weight gain, and weight at delivery were not recorded. The MFMU study was approved by institutional review boards at each of the study sites with each subject providing written informed consent. This secondary analysis was considered exempt by the Colorado Multiple Institutional Review Board.
FIGURE 1. Patient selection from original cohort.
Selection of patients for this study from initial patient cohort.
BMI, body mass index; GA, gestational age; MFMU, Maternal-Fetal Medicine Units; Wt, weight.
Definitions and outcomes
For this analysis, we used the same primary outcome variable as the original study, namely preterm delivery <37 weeks. To investigate the interaction of maternal weight and BMI on 17OHP-C effectiveness, we used 2 standard categorical BMI definitions (3-category: <25, 25–<30, ≥30; 6-category: <18.5, 18.5–<25, 25–<30, 30–<35, 35–<40, ≥40). Weight was treated as a continuous variable. In the original data set, women with weight >300 lb were assigned a weight of 300 lb; women with these right-censored weights were excluded from further analyses using weight as a continuous variable, but remained in analyses using categorical BMI.
Statistical methods
Demographics and clinical characteristics of women randomized to 17OHP-C and placebo were summarized with mean and SE for continuous variables, and frequency and percentage for categorical measures. Differences between treatment groups were tested with 2-sample t test for continuous and χ2 for categorical measures. Demographics of women in each of 3 BMI categories were summarized similarly, and differences tested using analysis of variance for continuous and χ2 for categorical measures.
PTB rate and 95% confidence interval (CI) were calculated across BMI and weight categories according to treatment. To obtain unadjusted relative risks (RR), the probability of PTB was modeled using binomial regression, with treatment, BMI (3- and 6-category), or weight and their interactions in 3 separate models. To obtain adjusted RR, multivariable binomial regression models were estimated with an expanded set of potential covariates reported in the literature to be associated with the risk of PTB: race (black vs other), age >30 years, marital status, years of education, smoking during pregnancy, illicit drug and alcohol use during pregnancy, and >1 previous PTB. Parameters were eliminated through backwards selection separately for each model. In sensitivity analysis, models of continuous weight were estimated separately for black and non-black women. All analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
In all, 443 women with complete data records including gestational age at delivery and maternal BMI and weight were included in this analysis, and their demographics and clinical characteristics are detailed in Table 1. As reported in the original study, the average number of previous PTB per patient and the proportion of women with >1 previous PTB were higher in the placebo group than in the treatment group. There were no other significant differences between the 2 groups. Table 2 describes the characteristics of the treatment and placebo groups in terms of BMI and weight. There were no differences between the 2 groups. Table 3 describes the clinical and demographic variables by 3-category BMI. Race differed significantly across category. Other characteristics were not significantly different. The rate of PTB, spontaneous PTB, indicated PTB, and PTB before 35 and 32 weeks by BMI category is also included in Table 3.
TABLE 1.
Clinical and demographic characteristics of women randomized to 17-alpha hydroxyprogesterone caproate or placebo
| Description | Value | 17OHP-C N = 294 (%) |
Placebo N = 149 (%) |
P value |
|---|---|---|---|---|
| No. of previous preterm deliveries | Mean (SE) | 1.40 (0.04) | 1.61 (0.07) | .016 |
| >1 Previous preterm delivery | >1 | 83 (28.23) | 63 (42.28) | .004 |
| ≥1 Previous term deliveries | ≥1 | 145 (49.32) | 69 (46.31) | .615 |
| Gestational age at randomization, wk | Mean (SE) | 18.9 (0.09) | 18.8 (0.12) | .581 |
| Maternal age, y | Mean (SE) | 26.1 (0.31) | 26.6 (0.43) | .382 |
| Race | Black | 177 (60.20) | 89 (59.73) | .862 |
| Caucasian | 74 (25.17) | 34 (22.82) | ||
| Hispanic | 38 (12.93) | 23 (15.44) | ||
| Other/unknown | 5 (1.70) | 3 (2.01) | ||
| Marital status | Married/living with partner | 149 (50.68) | 69 (46.31) | .629 |
| Divorced/widowed/separated | 29 (9.86) | 18 (12.08) | ||
| Never married | 116 (39.46) | 62 (41.61) | ||
| Years of school completed | Mean (SE) | 11.8 (0.13) | 12.0 (0.19) | .379 |
| Smoked during pregnancy | Yes | 65 (22.11) | 30 (20.13) | .713 |
| Drank alcohol during pregnancy | Yes | 26 (8.84) | 10 (6.71) | .469 |
17OHP-C, 17-alpha hydroxyprogesterone caproate.
TABLE 2.
Maternal body mass index and weight characteristics of women randomized to 17-alpha hydroxyprogesterone caproate or placebo
| Description | Value | 17OHP-C N = 294 (%) |
Placebo N = 149 (%) |
P value |
|---|---|---|---|---|
| Maternal BMI (continuous) |
Mean (SE) | 26.5 (0.41) | 25.5 (0.51) | .147 |
| Maternal BMI (3-category) |
Normal/under (BMI <25 kg/m2) |
137 (46.60) | 80 (53.69) | .291 |
| Preobese (BMI 25–29.9 kg/m2) |
64 (21.77) | 32 (21.48) | ||
| Obese (BMI ≥30 kg/m2) | 93 (31.63) | 37 (24.83) | ||
| Maternal BMI (6-category) |
Underweight | 25 (8.50) | 10 (6.71) | .437 |
| Normal BMI | 112 (38.10) | 70 (46.98) | ||
| Preobese | 64 (21.77) | 32 (21.48) | ||
| Obese I | 46 (15.65) | 16 (10.74) | ||
| Obese II | 22 (7.48) | 12 (8.05) | ||
| Obese III | 25 (8.50) | 9 (6.04) | ||
| Maternal weight (continuous) |
Mean (SE) | 154 (2.49) | 148 (3.24) | .149 |
17OHP-C, 17-alpha hydroxyprogesterone caproate; BMI, body mass index.
TABLE 3.
Clinical and demographic characteristics of women across 3-category body mass index class
| Description | Value | BMI < 25 N = 217 |
BMI 25<30 N = 96 |
BMI ≥30 N = 130 |
P value |
|---|---|---|---|---|---|
| No. of previous preterm deliveries | Mean (SE) | 1.50 (0.06) | 1.46 (0.08) | 1.43 (0.07) | .711 |
| >1 Previous preterm delivery | >1 | 73 (33.64) | 33 (34.38) | 40 (30.77) | .813 |
| Maternal age, y | Mean (SE) | 26.1 (0.38) | 26.2 (0.60) | 26.6 (0.46) | .638 |
| Race, N (%) | Black | 120 (55.30) | 51 (53.13) | 95 (73.08) | .009 |
| Caucasian | 65 (29.95) | 27 (28.13) | 16 (12.31) | ||
| Hispanic | 28 (12.90) | 16 (16.67) | 17 (13.08) | ||
| Other/unknown | 4 (1.84) | 2 (2.08) | 2 (1.54) | ||
| Marital status, N (%) | Married/living with partner | 110 (50.69) | 46 (47.92) | 62 (47.69) | .873 |
| Divorced/widowed/separated | 25 (11.52) | 10 (10.42) | 12 (9.23) | ||
| Never married | 82 (37.79) | 40 (41.67) | 56 (43.08) | ||
| Years of school completed | Mean (SE) | 12.1 (0.15) | 11.5 (0.25) | 11.6 (0.20) | .086 |
| Smoked during pregnancy (baseline), N (%) | Yes | 49 (22.58) | 24 (25.00) | 22 (16.92) | .292 |
| Alcohol during pregnancy (baseline), N (%) | Yes | 15 (6.91) | 9 (9.38) | 12 (9.23) | .657 |
| Illicit drug use during pregnancy (baseline), N (%) | Yes | 6 (2.76) | 5 (5.21) | 4 (3.08) | .53 |
| Gestational age at randomization, wk | Mean (SE) | 18.8 (0.10) | 18.9 (0.14) | 18.9 (0.13) | .835 |
| PTB <37 wk, N (%) | Yes | 100 (46.08) | 44 (45.83) | 44 (33.85) | .062 |
| Spontaneous PTB <37 wk, N (%) | Yes | 88 (40.55) | 34 (35.42) | 32 (24.62) | .010 |
| Indicated PTB <37 wk, N (%) | Yes | 12 (5.53) | 10 (10.42) | 12 (9.23) | .238 |
| PTB <35 wk, N (%) | Yes | 56 (25.81) | 25 (26.04) | 26 (20.00) | .420 |
| PTB <32 wk, N (%) | Yes | 34 (15.67) | 15 (15.63) | 15 (11.54) | .533 |
BMI, body mass index; PTB, preterm birth.
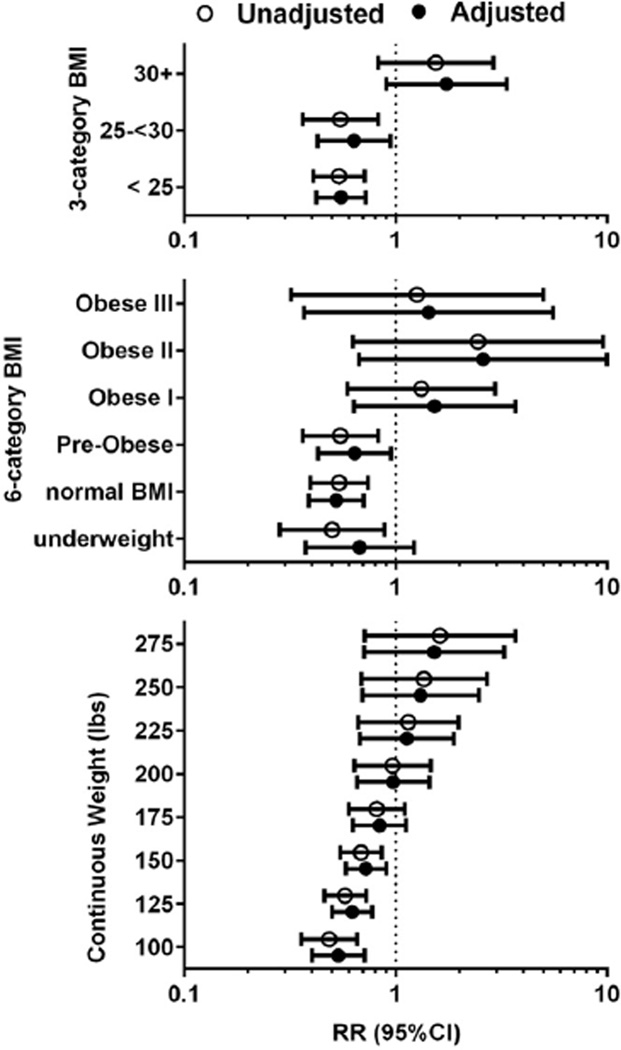
Figure 2 depicts both unadjusted and adjusted RR of PTB for women randomized to 17OHP-C vs placebo as a function of 3-category and 6-category BMI class and for weight. In adjusted multivariable modeling, only >1 previous PTB remained in each model as a covariate after backwards selection as described; all unadjusted and adjusted results are similar. The interaction term between 17OHP-C and maternal habitus (expressed as 3- and 6-category BMI and as weight), were significant in each unadjusted and adjusted model: For 3-category BMI, P = .0023 (adjusted .0011); for 6-category BMI, P = .025 (adjusted .0110); for weight, P = .0180 (adjusted .0257). This demonstrates that the effectiveness of 17OHP-C to prevent PTB was significantly modified by 3-category BMI, 6-category BMI, and weight.
FIGURE 2. RR of PTB for women randomized to 17OHP-C vs placebo.
Relative risk (RR) of preterm birth (PTB) (<37 weeks) for progesterone vs placebo. Unadjusted RR (open circles) and adjusted RR (closed circles) from binomial regression. Each adjusted model is result of backward selection process that considered: race (black vs otherwise), smoking cigarettes during pregnancy, marital status, maternal age >30 years, years of education, and illicit drug or alcohol use during pregnancy, as well as interactions between smoking and weight/body mass index (BMI), and smoking and treatment as potential covariates. For each adjusted binomial model, >1 previous PTB was only predictor to remain as covariate. Integer weight at which confidence intervals (CI) begin to include RR = 1 was 166 lb in unadjusted model, and 164 lb in model including previous PTB.
In the BMI analysis, no benefit of 17OHP-C was noted with prepregnancy BMI categories ≥30 in unadjusted or adjusted models. In the analysis using maternal weight, 17OHP-C was no longer significantly associated with a lower rate of PTB compared to placebo with maternal weight >165 lb in the unadjusted model. Among all women with BMI <30, the RR for PTB with 17OHP-C vs placebo was 0.54 (95% CI, 0.43–0.68) compared with 1.6 (95% CI, 0.83–2.89) for women with a BMI ≥30.
To further understand how BMI modifies the effect of 17OHP-C on PTB, we also calculated RR for PTB according to assignment to 17OHP-C vs placebo with PTB categorized as spontaneous vs indicated. These findings are summarized in Table 4. The lack of effect of 17OHP-C in obese women persists when the analysis is limited to just those women with recurrent spontaneous PTB.
TABLE 4.
Relative risk of spontaneous and indicated preterm birth by body mass index category
| PTB category | Entire cohort, N = 443 |
BMI <25, N = 217 |
BMI 25–30, N = 96 |
BMI >30, N = 130 |
|---|---|---|---|---|
| PTB <37 wk | 0.66 (0.53–0.81) | 0.54 (0.41–0.71) | 0.55 (0.36–0.83) | 1.55 (0.83–2.89) |
| Spontaneous PTB <37 wk |
0.64 (0.50–0.82) | 0.51 (0.37–0.70) | 0.63 (0.37–1.07) | 1.72 (0.77–3.84) |
| Indicated PTB <37 wk |
0.72 (0.38–1.39) | 0.82 (0.27–2.49) | 0.33 (0.10–1.10) | 1.19 (0.34–4.17) |
All entries expressed as relative risk (95% confidence interval).
BMI, body mass index; PTB, preterm birth.
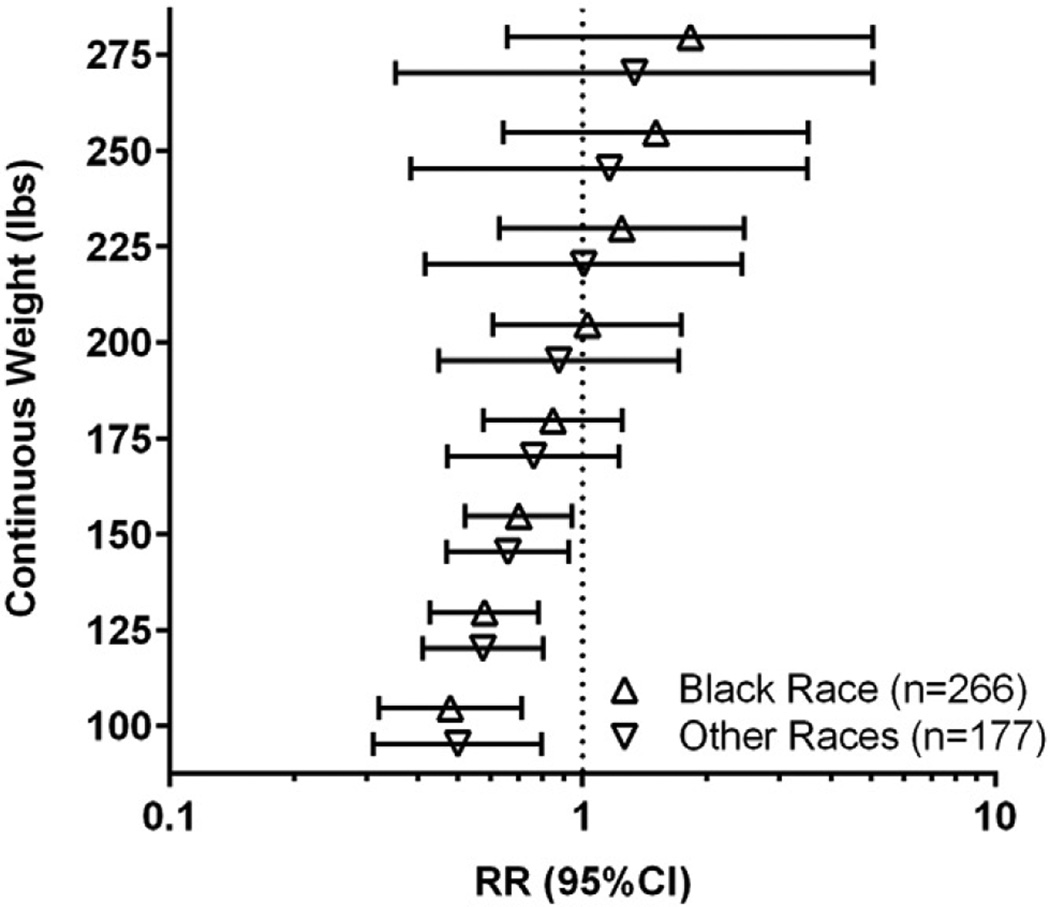
Figure 3 depicts unadjusted RR of PTB by assignment to 17OHP-C vs placebo for black and other races. No significant differences by race were found.
FIGURE 3. RR of PTB according to treatment and race.
Unadjusted relative risk (RR) of preterm birth (<37 weeks) for progesterone vs placebo for black race (open triangle) and other race (open inverted triangle).
CI, confidence interval.
Comment
Here we demonstrate evidence that 250 mg of intramuscular 17OHP-C weekly is ineffective in the prevention of recurrent PTB in obese women, and that this finding pertains to spontaneous PTB as well as total PTB. It is important to note that this finding is from the original data set that forms the contemporary rationale for 17OHP-C administration and not from another study that conflicts with the original study’s findings. These findings have the potential to affect a substantial number of pregnant women. One in 3 US women of reproductive age are obese,8 and 29% of patients in this study were obese.
A corollary of these findings is that at lower BMI and weight, 17OHP-C may be more effective than originally reported. The RR for recurrent PTB for women in this cohort with a BMI <30 treated with 17OHP-C was 0.54 (95% CI, 0.43–0.68), a stronger effect than the effect noted in the study population as a whole (RR, 0.66; 95% CI, 0.54–0.81).5 This presents the hope that the ultimate potential for 17OHP-C treatment may not yet have been realized.
As noted in Table 3, women with higher BMI in this cohort were proportionately more often of black race. Accordingly it was important to exclude race as a confounder in our findings. This possibility was perhaps unlikely since the original study demonstrated that 17OHP-C is equally effective in black and non-black women.5 Additionally, black race did not have a significant effect in multivariable modeling, and was eliminated from the final reported adjusted models. Further we showed in Figure 3 that the pattern of decreasing 17OHP-C effectiveness with increasing weight is present in both black and non-black women, and is not significantly different between women who are black and women of other races.
The strengths of this study are the prospective data collection that occurred at the time of the original trial by trained research nurses. The cohort is large and diverse, and includes many of the population groups at increased risk of PTB. PTB was the primary outcome of the original trial and allowed us to make rigorous comparisons between women who did and did not deliver early during the original trial.
A weakness of this analysis is that prepregnancy weights were self-reported by patients and not measured. Also, weight gain during pregnancy is not known, and so it is not known how that might affect these findings. In practice, clinicians must choose whether or not to initiate 17OHP-C early in pregnancy before pregnancy weight gain is known. As an unplanned post hoc analysis these results should be interpreted cautiously. However, we believe that the use of standard BMI classes, the known impact of BMI on 17OHP-C serum levels, and the strong inverse dose-response relationship between weight and 17OHP-C effectiveness make these results credible.
As originally reported in the trial of Meis et al5 weekly administration of 17OHP-C beginning at 16–20 weeks’ gestation leads to a marked reduction in the incidence of PTB in women with a prior PTB. This treatment is currently endorsed by both the American Congress of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine for all women with a history of spontaneous PTB between 20–36 6/7 weeks.9,10 The dosage of 17OHP-C used in the original MFMU study was chosen based on dosages used in prior studies and no dose-finding studies have been conducted. Recent studies have suggested that serum levels may be reduced in women with increased BMI and that if a lower serum threshold is not reached, 17OHP-C may have reduced or absent effectiveness.6,7 Accordingly it is plausible that 17OHP-C at a fixed 250-mg per week dose may be ineffective in women in higher BMI categories or at heavier weights.
Of note is the consistent trend for increased risk of recurrent PTB in women with higher BMI and weight who receive 17OHP-C, although this never reaches statistical significance. We were limited in exploring the upper range of incremental differences in weight and BMI, as the data were right-censored at 300 lb and BMI 40. Better articulation of this trend may be of concern as the number of obese pregnant women continues to increase. Even if 17OHP-C is merely neutral in its effect on PTB in these cases, we suggest that the recommendation that it be used in all women with a prior spontaneous PTB regardless of weight or BMI may warrant reconsideration. The 17OHP-C formulation currently Food and Drug Administration approved is expensive and weekly administration requires both patient and health care resources.
Perhaps the most apparent explanation of our findings is that at higher maternal BMI categories and heavier weights, a fixed dose of 17OHP-C is insufficient to achieve a therapeutic serum level. No serum progesterone levels or stored serum samples were obtained as part of this study so this hypothesis cannot be tested directly from the existing study data. If the problem lies with subtherapeutic serum levels, perhaps an increased dose of 17OHP-C will correct this deficit. As mentioned above if all women could benefit from the risk reduction achieved by women in this cohort with BMI <30, this would present a substantial advance in PTB prevention. Accordingly, we believe that a study of adjusted-dose 17OHP-C is urgently needed to address these questions.
Footnotes
The contents of this report represent the views of the authors, not those of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network or the National Institutes of Health, which made the database from the Prevention of Recurrent Preterm Delivery by 17-Alpha Hydroxyprogesterone Caproate Trial available for secondary analysis.
The authors report no conflict of interest.
REFERENCES
- 1.Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and mortality in young adulthood. JAMA. 2011;306:1233–1240. doi: 10.1001/jama.2011.1331. [DOI] [PubMed] [Google Scholar]
- 2.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359:262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 3.EXPRESS Group. Fellman V, Hellström-Westas L, Norman M, et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009;301:2225–2233. doi: 10.1001/jama.2009.771. [DOI] [PubMed] [Google Scholar]
- 4.McCormick MC, Litt JS, Smith VC, Zupancic JA. Prematurity: an overview and public health implications. Annu Rev Public Health. 2011;32:367–379. doi: 10.1146/annurev-publhealth-090810-182459. [DOI] [PubMed] [Google Scholar]
- 5.Meis PJ, Klebanoff M, Thom E, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 6.Caritis SN, Venkataramanan R, Thom E. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network and Obstetric-Fetal Pharmacology Research Units Network. Relationship between 17-alpha hydroxyprogesterone caproate concentration and spontaneous preterm birth. Am J Obstet Gynecol. 2014;210:128.e1–128.e6. doi: 10.1016/j.ajog.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caritis SN, Sharma S, Venkataramanan R. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Pharmacokinetics of 17-hydroxyprogesterone caproate in multifetal gestation. Am J Obstet Gynecol. 2011;205:40.e1–40.e8. doi: 10.1016/j.ajog.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 9.Committee on Practice Bulletins–Obstetrics, American College of Obstetricians and Gynecologists. Prediction and prevention of preterm birth. Practice bulletin no. 130. Obstet Gynecol. 2012;120:964–973. doi: 10.1097/AOG.0b013e3182723b1b. [DOI] [PubMed] [Google Scholar]
- 10.Society for Maternal-Fetal Medicine Publications Committee, with assistance of Vincenzo Berghella. Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206:376–386. doi: 10.1016/j.ajog.2012.03.010. [DOI] [PubMed] [Google Scholar]