Conspectus
The vast majority of the outstanding applications of metal nanoparticles (NPs) developed during the last two decades have arisen from their unique optical properties. Within this context, rational synthesis and assembly of gold NPs have been the main research focus, aiming at the design of nanoplasmonic devices with tailored optical functionalities. The progress made in this field is thus to be ascribed to the understanding of the origin of the interaction between light and such gold nanostructures, the dynamics of which have been thoroughly investigated with significant contributions from short and ultrashort pulse laser technologies.
We focus this Account on the potential of pulse lasers to provide new fundamental insights into the electron dynamics involved in the interaction of light with the free conduction electrons of Au NPs, that is, localized surface plasmon resonances (LSPRs). The excitation of LSPRs with a femtosecond pulse laser is followed by thermalization of the Au NP electrons and the subsequent relaxation of the nanocrystal lattice and the surrounding environment, which generally results in surface melting. By contrast, nanosecond irradiation usually induces AuNP fragmentation and uncontrolled melting due to overlapping excitation and relaxation phenomena. These concepts have been exploited toward the preparation of highly monodisperse gold nanospheres via pulse laser irradiation of polyhedral nanocrystal colloids, or in the fabrication of nanostructures with “written-in” optical properties. The applicability of pulsed coherent light has been extended toward the direct synthesis and manipulation of Au NPs. Through ablation of a gold target in a liquid with pulse lasers, spherical Au NPs can be synthesized with no need of stabilizing ligands, which is a great advantage in terms of reducing toxicity, rendering these NPs particularly suitable for medical applications. In addition, femtosecond laser irradiation has been proven a unique tool for the controlled welding of plasmonic gold nanostructures by electromagnetic field enhancement at the hot spots of assembled Au NPs. The combination of such nanostructures with pulse lasers promises significant chemical and biochemical advances, including the structural determination of organic reaction intermediates, the investigation of phase transitions in inorganic nanomaterials at mild reaction conditions, or the efficient photothermal destruction of cancer cells avoiding damage of surrounding tissue.
Introduction
The concept of light amplification by stimulated emission of radiation (laser) takes us back to the middle of the 20th century, when it was experimentally demonstrated for microwave radiation (maser).1,2 The first maser device radiated at 1 cm wavelength and approximately 10 nW of power. Soon thereafter, the first solid state pulse laser (the ruby laser) was constructed,3 and the femtosecond barrier was broken in 1974, when the first laser with a pulse width of 500 fs was reported,4 in the time scale of atoms and molecules in motion. Eventually, all these technological breakthroughs were brought together to establish the basis of a new field in modern chemistry dedicated to the study of the dynamics of bond formation and breaking during chemical reactions in real time: femtochemistry.5 Notwithstanding, the significance of lasers was beyond the study of temporal events in chemical reactions, providing a tremendous contribution to the development of novel spectroscopies. For example, only one decade after the fabrication of the first continuous wave laser, this type of radiation was used to produce enhanced Raman scattering from molecules adsorbed on a plamonic material.6 In this context, gold and silver nanoparticles as nanoplasmonic entities have attracted great interest due to their characteristic response to light, as well as simple synthesis and high chemical stability.7 The strong interaction of plasmonic gold nanoparticles with light arises from the coupling of conduction band electron oscillation with that of the incident electromagnetic radiation, which is localized on the surface of the nanocrystals.8 This phenomenon is known as localized surface plasmon resonance (LSPR), and has shown great potential for application in Au NPs synthesis, as well as in sensing and energy harvesting,10 among other applications.
In analogy to the investigations on femtochemistry,5 pulse lasers were used to study the mechanism of LSPR dynamics in Au NPs.11−13 In contrast to a continuous wave laser, pulsed coherent light enables irradiation with extremely high intensity (107–1012 W/cm2 vs 104–106 W/cm2) in ultrashort periods of time, allowing the deformation and even complete disintegration of the nanocrystals.14−16 In fact, such intensities proved suitable tools in the synthesis of colloidal nanoparticles via ablation of a target immersed in a liquid, the so-called pulse laser ablation in liquid method (PLAL).17,18 In the case of gold targets,19 energy deposited onto the surface induces the formation of electron plasma and excited atoms which, upon thermal equilibration with the surrounding environment, give rise to the nucleation and growth of Au NPs that are then released to the liquid.20−23 Through PLAL, the fabrication of plasmonic nanoparticles using picosecond and nanosecond pulse lasers occurs via thermal processes.21 On the other hand, for a femtosecond pulsed laser at low fluences, the synthesis occurs via a thermal-free ablation process.22
Control over the size and polydispersity of Au NPs can be achieved by modulation of the laser pulse width and fluence, but also through rational use of selected capping ligands with different affinity toward gold surface. Interestingly, the addition of capping agents such as the surfactant sodium dodecyl sulfate may quench the growth of pristine Au NPs by PLAL, resulting in improved monodispersity.21,23 Within this context, direct functionalization of the Au NP surface with various (bio)molecular ligands such as cyclodextrins,24 oligonucleotides,25 and proteins26 has been reported. In addition, PLAL in aqueous media provides access to the production of Au NPs with no need for reducing or molecular capping agents as required in wet chemistry synthesis, which is probably one of the main strengths of this method toward applications in, for example, biomedicine.27 The mechanism involved in the colloidal stability of “naked” Au NPs relies on the partial oxidation of surface atoms (Au–O, Au–Cl, Au–OH).28 The synthesis of Au NPs has also been extensively reported in a wide variety of organic solvents,29,30 ionic liquids,31 and supercritical fluids.32
With all these considerations in mind, we discuss in this Account the importance of nanosecond, but mainly femtosecond pulse lasers in the manipulation, modification, and assembly of plasmonic Au NPs (Table 1), highlighting some of the most interesting applications of such nanostructures.
Table 1. Main Aspects and Applications of the Interaction of Pulse Lasers and Au NPs.
| laser pulse width | laser mediated application | ref |
|---|---|---|
| nanosecond | reshaping of Au NPs | (16, 34, 41, 46, 48) |
| fragmentation of Au NPs | (16, 33, 34, 38, 39, 41) | |
| assembly of Au NPs | (50, 51, 57) | |
| picosecond | Au NP electron dynamics | (12, 14) |
| femtosecond | reshaping of Au NPs | (16, 34, 40, 41) |
| fragmentation of Au NPs | (16, 34, 36, 40, 41) | |
| Au NP electron dynamics | (12, 13, 40) | |
| assembly of Au NPs | (52, 54) |
Pulse Laser-Induced Reshaping and Fragmentation
Shape modification of Au NPs with pulse lasers involves the interaction of laser radiation in resonance with the LSPR of the plasmonic nanocrystals and/or interband transitions. The first investigations on the effect of nanosecond laser pulses on aqueous colloidal dispersions of spherical Au NPs showed that fragmentation and reshaping were the two main effects at high and low laser fluences, respectively.33 Later on, the influence of nanosecond and femtosecond laser pulses at different energies on gold nanorods (Au NRs) colloids, as well as the corresponding structural transformations, were studied.16 Femtosecond laser pulses at 800 nm led to reshaping of the anisotropic NPs into spheres while keeping constant the initial volume, whereas nanosecond pulses resulted predominantly in fragmentation of the Au NRs.34 The energy required for complete reshaping of a single Au NR, with average size of 92 nm × 30 nm, has been measured using white light scattering spectroscopy and electron microscopy, and found to be 260 fJ (Figure 1).35 Additionally, the effect of thermal heating on Au NRs, compared to that produced by femtosecond laser irradiation,36 has shown that at 250 °C the anisotropic nanocrystals reshaped into spheres within 1 h, while laser irradiation induced heating up to 700 ± 50 °C did not affect their morphology. These phenomena were attributed to relaxation processes with the surrounding medium, which in the case of the latter were favored by the larger temperature difference between Au NRs and their environment.
Figure 1.
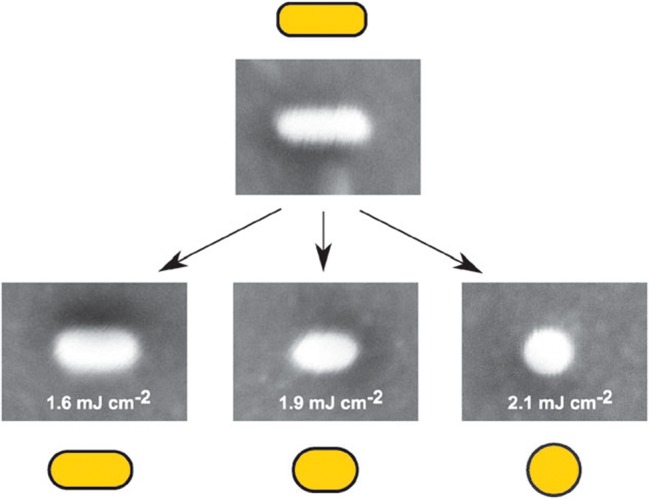
Observed effect of different pulse energy densities on the reshaping of a gold nanorod. The dimensions of all SEM images are 200 nm × 150 nm. Reproduced with permission from ref (35). Copyright 2009 Royal Society of Chemistry.
All these reshaping and fragmentation phenomena are governed by the electron dynamics of the nanocrystals and the relaxation processes after laser irradiation. When subjected to laser excitation, photons are absorbed producing fast thermalization of electrons (electron–electron coupling, 500 fs), followed by electron–lattice energy transfer (electron–phonon coupling, <10 ps) and heat transfer from the lattice to the surrounding solvent (phonon–phonon coupling, 100 ps).12,14,33 In the case of femtosecond pulse laser excitation, the fast energy deposition rate leads to a high electronic temperature, which is transferred to the nanocrystal lattice via electron–phonon relaxation processes, heating the nanoparticles above the melting temperature of bulk gold and therefore inducing reshaping and melting of the nanostructure.12,14 On the other hand, in nanosecond pulse laser experiments, the absorption of photons continues when the relaxation processes have already started and the lattice is still hot, yielding an increase of the lattice energy that induces subsequent fragmentation of the nanocrystals.16 However, since these effects are strongly related not only to the pulse width but also to the pulse energy, high energy femtosecond pulses and low energy nanosecond pulses can also produce fragmentation and melting, respectively.16,34,37
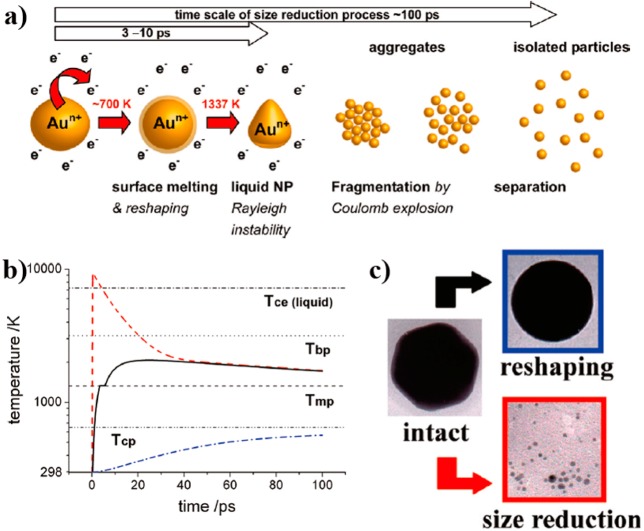
Some investigations were carried out to disclose the mechanisms involved in size reduction and fragmentation of Au NPs via laser irradiation. For example, the photothermal evaporation model ascribed the shape change and size reduction of Au NPs during 532 nm nanosecond pulsed excitation to melting and vaporization phenomena, respectively.38 The determination of nanocrystal temperature was based on thermodynamic calculations, in which nearly no heat transfer to the surrounding water was assumed. In contrast to the photothermal evaporation theory, this Coulomb explosion model implies that the thermionic emission of electrons occurs simultaneously to electron–phonon relaxation, as confirmed by the detection of hydrated electrons via transient-absorption measurements and the presence of positively charged gold ions by mass spectrometry.39,40 When the lattice temperature reaches 700 K, surface melting of the NPs gives rise to reshaping phenomena. If the temperature of the structure increases up to 1337 K, Au NPs melt. Further temperature increments are accompanied by thermionic emission of conduction electrons, which may go beyond the critical charge of the liquid nanoparticle, which in turn becomes unstable and breaks up into smaller droplets (Figure 2). The time scale of the entire process is about 100 ps, but may be faster if the laser fluence is increased.40
Figure 2.
(a) Proposed femtosecond-laser-induced fragmentation process. (b) Electron temperature temporal evolution (dashed red curve); lattice temperature (solid black curve); and maximum water temperature at the NP surface-water interface (dash-dot blue curve), for a 60 nm diameter gold nanosphere and a single 400 nm laser pulse of 150 fs and fluence of 12.3 mJ/cm2. (c) TEM images representing reshaping and size reduction at low and high fluences for a 60 nm quasi-sphere. Reproduced with permission from ref (40). Copyright 2011 American Chemical Society.
Additionally, the interaction of pulsed laser beams with gold nanoparticles not only depends on the laser parameters but also on particle features. Both light absorption and thermodynamics of phase transitions are highly sensitive to Au NP size and shape. The melting and evaporation thresholds are almost constant for gold nanospheres (Au NSs) with sizes ranging from 10 to 100 nm, whereas a marked increase of the fluence is necessary for an Au NP diameter of 600 nm.41 The effect of particle shape can be illustrated for the particular case of single crystal Au NRs. The structural transformation that occurs upon irradiation with femtosecond and nanosecond pulse lasers with energy below the melting threshold shows different reshaping mechanisms. Although single crystal AuNRs are defect free, analysis of the crystalline structure after irradiation reveals the presence of twins and planar defects in the body of nanocrystals, which together with the diffusion of surface Au atoms acts as a nucleating point to drive the conversion of {110} facets into the more stable {100} and {111} facets (Figure 3).42 An additional mechanism has been proposed for extremely high femtosecond pulse laser fluences, in which the ejection of material takes place far below the melting threshold due to near-field enhancement on the particles.43 On the other hand, the effect of Au NPs functionalization has been shown to modify the electron dynamics process. For instance, functionalization with cysteine and cystine amino acid derivatives produces subpicosecond delays in the relaxation times after excitation.44
Figure 3.
High resolution transmission electron microscopy images of single crystal AuNRs after irradiation with femtosecond and nanosecond pulse of 1 mJ/cm2 (0.5 μJ per pulse) and 250 mJ/cm2 (20 μJ per pulse), respectively. (a,b) Point defects and twinned particles after femtosecond laser irradiation. (c) Twinned particles after nanosecond laser irradiation. (d) Scheme of the proposed mechanism for shape transformation. Adapted with permission from ref (42). Copyright 2000 American Chemical Society.
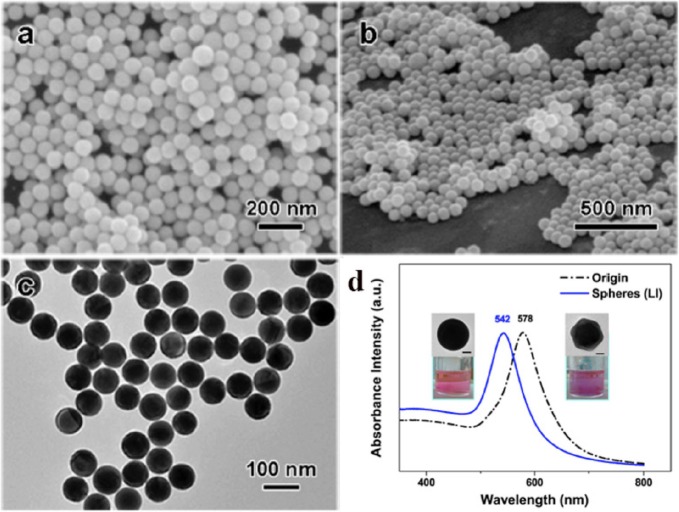
Apart from mechanistic considerations, the photothermal reshaping, fragmentation and melting effects of laser irradiation on Au NPs have been exploited toward potential technological uses. A nanostructured system has been recently designed to create a spectral transmission window resulting from selective reshaping of Au NRs with an LSPR in resonance with a femtosecond laser.45 Similar nanostructures with “written-in” optical properties were fabricated by dispersion and alignment of Au NRs within poly(vinyl alcohol) films,46 in which irradiation with a Nd:YAG nanosecond laser (1064 nm, 6 ns, 850 mJ/pulse) led to selective reshaping of nanorods into spheres. The modification of the optical properties through reshaping enabled micropatterning of optical structures. On the other hand, preparation of highly monodisperse Au NSs (Figure 4) has been reported via reshaping of colloidal gold nano-octahedra using 532 nm nanosecond laser irradiation (nonfocused Nd:YAG laser operated at 20 Hz with a wavelength of 532 nm and pulse duration of 10 ns, 5.50 mJ/cm2).47 Using the same concept, a narrow size distribution of naked Au NSs was obtained by 532 nm nanosecond irradiation (8 ns, total energy fluence per pulse ranging from 658 mJ/cm2 to 334 mJ/cm2) of a drop of polydisperse gold nanoprisms and nanospheres previously synthesized by reduction of HAuCl4 with hydrogen peroxide. The key advantage of the designed protocol lies on the rapid functionalization of the pristine irradiated Au NSs with different ligands when gold colloid and capping solutions are mixed, proving the versatility of the method for production of AuNPs with custom derivatization.48
Figure 4.
Gold nanospheres obtained by nonfocused nanosecond pulse laser irradiation of pristine octahedral nanoparticles (wavelength, 532 nm; fluence, 3.84 mJ/cm2; irradiation time, 60 s). (a,b) SEM images and (c) TEM image of the gold nanospheres. (d) UV–vis spectra of pristine nano-octahedra (black dash-dot line) and the resulting nanospheres (blue solid line) after laser irradiation. Inset: TEM images and photographs of colloidal solutions of AuNPs (scale bars are 20 nm). Adapted with permission from ref (47). Copyright 2015 Nature.
Pulse Laser-Controlled Assembly and Welding
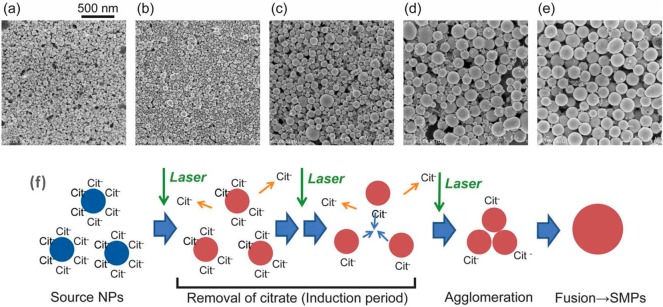
The use of light to guide the self-assembly of gold nanoparticles41 has been explored by various groups by means of tailored functionalization of the nanocrystal surface with photosensitive organic molecules.49 However, only recently have the optical properties of Au NPs been explored as a way to manipulate and direct the interaction between nanoparticles in solution. When Au NSs stabilized with citrate ions are irradiated by 10 ns pulses of a 532 nm Nd:YAG laser, with typical fluences of 60 mJ/cm2, decomposition and removal of the ligand molecules induces agglomeration of the AuNPs. Consequently, the laser causes melting and welding within the aggregates, giving rise to submicrometer spherical particles (Figure 5).50 This type of submicrometer-sized Au NSs have also been produced by laser-induced melting with low-toxicity stabilizing reagents, with potential biocompatible applications.51
Figure 5.
SEM images showing the morphological changes of citrate gold nanoparticles after nanosecond pulse laser irradiation at 650 μJ/cm2 fluence for (a) 0 min, (b) 5 min, (c) 9 min, (d) 11 min, and (e) 14 min. (f) Scheme of the laser-induced assembly and fusion of citrate stabilized particles. Adapted with permission from ref (50). Copyright 2013 Royal Society of Chemistry.
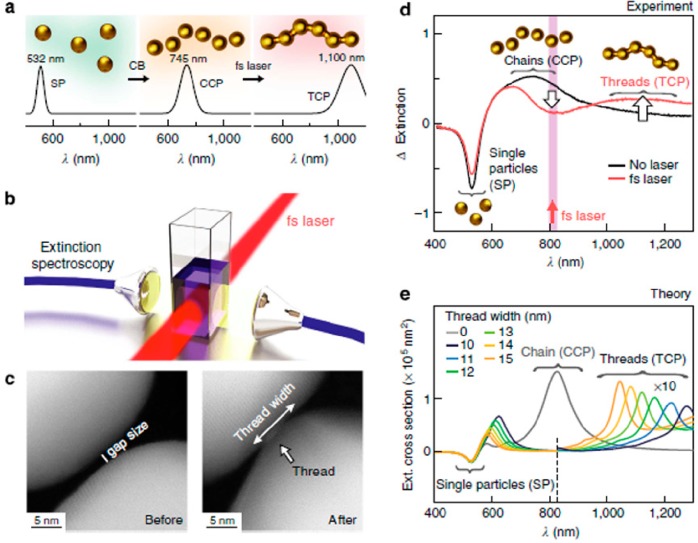
As a remarkable example, citrate stabilized 50 nm Au NSs were assembled using barrel-shaped cucurbituril molecules, into aggregates with rigid 0.9 nm gaps (Figure 6).52 Using UV–vis-NIR spectroscopy, the assembly of Au NSs could be monitored in real time, by the decrease in the extinction band at 532 nm and formation of a new band at 745 nm due to coupling of the electron clouds of individual NPs during the induced aggregation, which was termed capacitative chain plasmon (CCP) resonance. Once the threads were formed, and since they absorb radiation with wavelength around 800 nm, treatment with unfocused 805 nm, 200 fs laser pulses of 90 mW/cm2 led to coupling of the CCP resonances with the laser. The extremely high field enhancements generated at interparticle gaps, where plasmon coupling leads to formation of hot spots, ultimately induced welding of the gold nanocrystals as confirmed by electron microscopy, and a new threaded chain plasmon (TCP) resonance was found at 1100 nm due to charge transfer between the Au NPs.53 Far-field extinction spectra, near-field distribution, and phase map simulations were carried out for thread widths ranging from 10 to 14 nm, and it was found that the position of the resonance modes depends on the thread width. Three main peaks were identified: one associated with a rod-like mode in the mid-IR, a second one in the NIR originated from a hybrid chain/rodlike mode, and a third one in the visible related to a modified optical chain plasmon mode (Figure 6).52
Figure 6.
(a) Schematic representation of Au NP chain assembly. Single NPs exhibit a plasmon resonance (SP) at 532 nm. Nanoparticle chains display CCP resonances at 745 nm. Irradiation with femtosecond laser pulses (b) weld the AuNPs into strings, producing TCP resonances at 1100 nm. (c) TEM images of NP chain interparticle gap before and after laser irradiation. (d) Spectra of the AuNP chains with/without femtosecond laser irradiation (single NP response subtracted from the spectra). (e) Numerical simulations of TCP modes in six-NP-long chains and the effect of the nanothread widths contributing to the signal in (d). Reproduced with permission from ref (52). Copyright 2014 Nature.
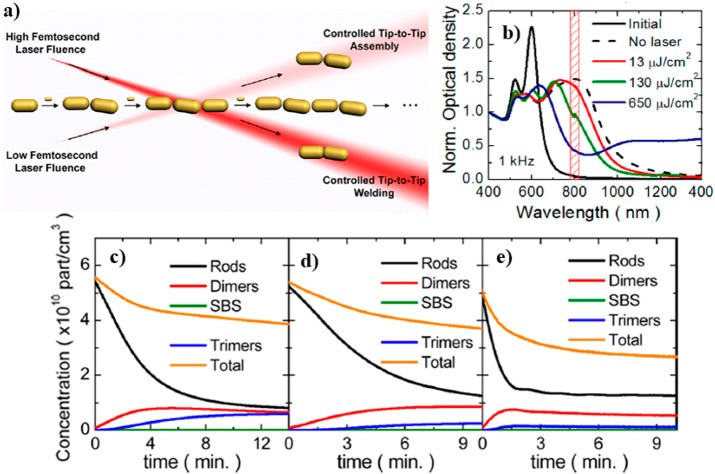
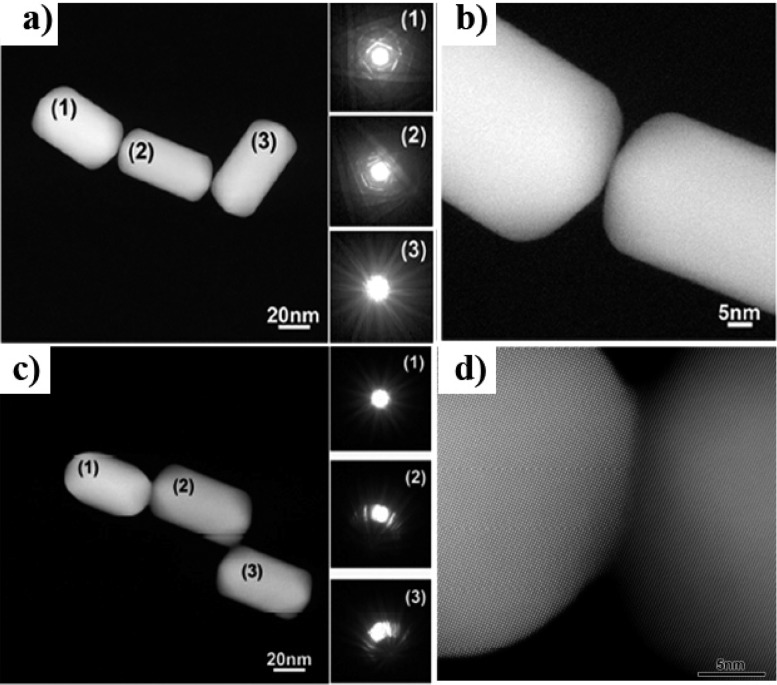
Directed binding of Au NPs through molecular linkers results in highly efficient methodologies for morphologically controlled nanocrystal assembly. However, akin to molecular polymerization synthesis, chain growth control is difficult to achieve, which hampers the preparation of one single type of oligomer and makes this methodology hard to use for practical application in nanoplasmonics. To overcome these limitations, ultrafast NIR femtosecond laser pulses at relatively low fluences were used to irradiate Au NR aggregates and control their population with respect to dimeric species.54 By irradiating with 800 nm Ti:sapphire low fluence (130 μJ/cm2) 50 fs laser pulses during dithiolated molecule induced (1,8-octanedithiol) tip-to-tip assembly of Au NRs, the formation of longer oligomers such as trimers, with longitudinal LSPRs in resonance with the laser wavelength, could be selectively disrupted. Therefore, dimers with LSPR at 700 nm (out of resonance) were obtained in high yield (7Figure 7). The proposed mechanism reveals the importance of highly efficient hot spots generated at interparticle gaps, which are responsible for the photothermal decomposition of the dithiolated molecular linker (1,8-octanedithiol). Again, welding of the plasmonic oligomers was achieved by increasing the laser fluence (650 μJ/cm2). The welded oligomers displayed a new broad band ranging from 900 to 1400 nm. A systematic analysis of the influence of laser parameters (pulse fluence, frequency and average fluence) revealed that the most important parameter toward controlling the kinetics of NR assembly was the pulse fluence.54 The relative concentration of the main plasmonic species and the characterization of welded and assembled oligomers were determined by electron microscopy. The yield of dimers at 130 μJ/cm2 was found to be twice that obtained without irradiation, meaning that femtosecond laser irradiation can trap the intermediate dimer. High-resolution HAADF-STEM analysis of the welded oligomers showed that the nanocrystals were oriented in the same crystallographic direction (Figure 8) but with a slight misorientation, probably due to the extreme concentration of energy in this region and/or to the fact that energy deposition occurs in such short times that welding is essentially a nonthermal process.
Figure 7.
(a) Schematic representation of the effect of femtosecond laser pulses on the tip-to-tip assembly of gold nanorods. (b) Extinction spectra during Au NR tip-to-tip assembly in ethanol, using 1,8-octanedithiol as linker, upon 10 min irradiation with 800 nm 50 fs laser pulses at 1 kHz and different pulse fluences of 13, 130, and 650 μJ/cm2; intervals of 90 s. (d,e) Concentration of single Au NRs (black line), dimers (red line), side-by-side dimers (green line), trimers (blue line), and the total concentration (orange line) for no laser (c) and pulse fluences of 130 μJ/cm2 (d) and 650 μJ/cm2 (e). Adapted with permission from ref (54). Copyright 2015 American Chemical Society.
Figure 8.
HAADF-STEM and high-resolution HAADF-STEM images of a trimer (a,b) and a welded trimer (c,d) of Au NRs obtained without laser irradiation and at 650 μJ/cm2 laser pulses, respectively. Insets in panels (a,c) show the respective electron diffraction patterns. Adapted with permission from ref (54). Copyright 2015 American Chemical Society.
Conclusions and Outlook
The interaction of pulse lasers with metal nanoparticles has been presented in a sequential manner, from the synthesis of Au NPs by ablation of a gold target via pulse laser irradiation, to reshaping, fragmentation and assembly of nanocrystals due to electromagnetic field and temperature enhancements upon excitation of LSPRs with laser pulses. We additionally focused our efforts on the description of electron dynamics in nanocrystals and relaxation processes following laser irradiation. Real technological applications such as the fabrication of plasmonic nanostructures with “written-in” optical properties have been detailed. It is important however to keep in mind that a number of promising chemical and biochemical uses of Au NPs in combination with laser pulses are still under development. For example, the wide range of high temperatures attained via excitation of Au NPs with ultrashort pulse lasers may allow new advances in practical organic chemistry and catalysis, in which unconventional reaction conditions of temperature can be reached by local heating.55 Combination of such chemical control with the potential of SERS spectroscopy, and specially time-resolved SERS,56 can also be envisaged to determine the chemical structure of elusive reaction intermediates hitherto unknown. For instance, reactions involving nondetected zwitterioinc or biradical intermediates would be excellent candidates for these studies. Additionally, such thermal assisted reactivity may find application in the synthesis of Janus Au NP dimers of nanocrystals functionalized with different reactive functional groups. Moreover, the complexity of reactivity induced by Au NPs can increase upon application on inorganic materials, in which local phase transitions of such inorganic nanoparticles (e.g., metal oxides) directly linked to Au NPs may be induced.57 We finally anticipate the combination of irradiation with low fluence NIR ultrashort laser pulses on assembled AuNPs, acting as nanolenses that are able to confine light at the interparticle gaps, for efficient photothermal destruction of cancer cells avoiding damage of tissues in regenerative medicine.58
Acknowledgments
This research has been funded by the Spanish MINECO (MAT2014-59678-R and MAT2013-46101-R) and the European Research Council (ERC Advanced Grant Number 267867, Plasmaquo). A.G.-M. and G.G.-R., respectively, acknowledge receipt of Ramón y Cajal and FPI Fellowships from the Spanish MINECO.
Biographies
Guillermo González-Rubio is a Ph.D. student under cosupervision by Andrés Guerrero-Martínez (Universidad Complutense) and Luis M. Liz-Marzán (CIC Biomagune). He obtained a master degree in Chemistry at the Universidad Complutense in 2012. Currently his research interests are focused on novel aspects of the synthesis, characterization, and application of gold nanorods.
Andrés Guerrero-Martínez received his Ph.D. degree in Chemistry under the supervision of Prof. G. Tardajos at the University Complutense in 2006. He then joined the group of Prof. L. De Cola at the University of Münster as a “Marie Curie” postdoctoral fellow, from 2007 to 2009. Then, he held a “Juan de la Cierva” Postdoctoral Fellowship in the group of Prof. L. M. Liz-Marzán at the University of Vigo. Since 2012, he holds a “Ramón y Cajal” researcher position at the Universidad Complutense, where he is investigating nanostructured plasmonic materials based on molecular concepts.
Luis M. Liz-Marzán obtained a Ph.D. from University of Santiago de Compostela in 1992. After a postdoctoral stay at Utrecht University, he moved to University of Vigo, where he held several positions, becoming Full Professor in 2006. Since 2012, he is Ikerbasque Professor and Scientific Director at CIC biomaGUNE, in San Sebastian, Spain. He has been Visiting Professor at various institutions worldwide and received numerous awards. His research interests are in the synthesis and assembly of nanoparticles and the application of nanoplasmonics in biomedical sciences.
The authors declare no competing financial interest.
References
- Bertolotti M.The History of the Laser; Taylor & Francis: London, UK, 1999. [Google Scholar]
- Gordon J. P.; Zeiger H. J.; Townes C. H. The Maser—New Type of Microwave Amplifier, Frequency Standard, and Spectrometer. Phys. Rev. 1955, 99, 1264–1274. 10.1103/PhysRev.99.1264. [DOI] [Google Scholar]
- Maiman T. H. Optical and Microwave-Optical Experiments in Ruby. Phys. Rev. Lett. 1960, 4, 564–566. 10.1103/PhysRevLett.4.564. [DOI] [Google Scholar]
- Shank C. V. Subpicosecond Kilowatt Pulses from a Mode-Locked Cw Dye Laser. Appl. Phys. Lett. 1974, 24, 373. 10.1063/1.1655222. [DOI] [Google Scholar]
- Zewail A. H. Femtochemistry: Atomic-Scale Dynamics of the Chemical Bond. J. Phys. Chem. A 2000, 104, 5660–5694. 10.1021/jp001460h. [DOI] [PubMed] [Google Scholar]
- Fleischmann M.; Hendra P. J.; McQuillan A. J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. 10.1016/0009-2614(74)85388-1. [DOI] [Google Scholar]
- Liz-Marzán L. M. Nanometals: Formation and Color. Mater. Today 2004, 7, 26–31. 10.1016/S1369-7021(04)00080-X. [DOI] [Google Scholar]
- Willets K. A.; Van Duyne R. P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. 10.1146/annurev.physchem.58.032806.104607. [DOI] [PubMed] [Google Scholar]
- Grzelczak M.; Liz-Marzán L. M. The relevance of light in the formation of colloidal metal nanoparticles. Chem. Soc. Rev. 2014, 43, 2089–2097. 10.1039/C3CS60256G. [DOI] [PubMed] [Google Scholar]
- Guerrero-Martínez A.; Grzelczak M.; Liz-Marzán L. M. Molecular Thinking for Nanoplasmonic Design. ACS Nano 2012, 6, 3655–3662. 10.1021/nn301390s. [DOI] [PubMed] [Google Scholar]
- Logunov S. L.; Ahmadi T. S.; El-Sayed M. A.; Khoury J. T.; Whetten R. L. Electron Dynamics of Passivated Gold Nanocrystals Probed by Subpicosecond Transient Absorption Spectroscopy. J. Phys. Chem. B 1997, 101, 3713–3719. 10.1021/jp962923f. [DOI] [Google Scholar]
- Link S.; El-Sayed M. A. Optical Properties and Ultrafast Dynamics of Metallic Nanocrystals. Annu. Rev. Phys. Chem. 2003, 54, 331–366. 10.1146/annurev.physchem.54.011002.103759. [DOI] [PubMed] [Google Scholar]
- Ahmadi T. S.; Logunov S. L.; El-Sayed M. A. Picosecond Dynamics of Colloidal Gold Nanoparticles. J. Phys. Chem. 1996, 100, 8053–8056. 10.1021/jp960484e. [DOI] [Google Scholar]
- Setoura K.; Okada Y.; Hashimoto S. CW-Laser-Induced Morphological Changes of a Single Gold Nanoparticle on Glass: Observation of Surface Evaporation. Phys. Chem. Chem. Phys. 2014, 16, 26938–26945. 10.1039/C4CP03733B. [DOI] [PubMed] [Google Scholar]
- Link S.; Burda C.; Mohamed M. B.; Nikoobakht B.; El-Sayed M. A. Laser Photothermal Melting and Fragmentation of Gold Nanorods: Energy and Laser Pulse-Width Dependence. J. Phys. Chem. A 1999, 103, 1165–1170. 10.1021/jp983141k. [DOI] [Google Scholar]
- Usui H.; Shimizu Y.; Sasaki T.; Koshizaki N. Photoluminescence of ZnO nanoparticles prepared by laser ablation in different surfactant solutions. J. Phys. Chem. B 2005, 109, 120–124. 10.1021/jp046747j. [DOI] [PubMed] [Google Scholar]
- Zeng H.; Du X.-W.; Singh S. C.; Kulinich S. A.; Yang S.; He J.; Cai W. Nanomaterials via Laser Ablation/Irradiation in Liquid: A Review. Adv. Funct. Mater. 2012, 22, 1333–1353. 10.1002/adfm.201102295. [DOI] [Google Scholar]
- Henglein A. Physicochemical properties of small metal particles in solution: ″microelectrode″ reactions, chemisorption, composite metal particles, and the atom-to-metal transition. J. Phys. Chem. 1993, 97, 5457–5471. 10.1021/j100123a004. [DOI] [Google Scholar]
- Goekce B.; van’t Zand D. D.; Menendez-Manjon A.; Barcikowski S. Ripening Kinetics of Laser-Generated Plasmonic Nanoparticles in Different Solvents. Chem. Phys. Lett. 2015, 626, 96–101. 10.1016/j.cplett.2015.03.010. [DOI] [Google Scholar]
- Tomko J.; Naddeo J. J.; Jimenez R.; Tan Y.; Steiner M.; Fitz-Gerald J. M.; O’Malley S. M.; Bubb D. M. Size and Polydispersity Trends Found in Gold Nanoparticles Synthesized by Laser Ablation in Liquids. Phys. Chem. Chem. Phys. 2015, 17, 16327–16333. 10.1039/C5CP01965F. [DOI] [PubMed] [Google Scholar]
- Kabashin a. V.; Meunier M. Synthesis of Colloidal Nanoparticles during Femtosecond Laser Ablation of Gold in Water. J. Appl. Phys. 2003, 94, 7941–7943. 10.1063/1.1626793. [DOI] [Google Scholar]
- Fong Y.; Gascooke J.; Visser B. R.; Metha G. F.; Buntine M. A. Laser-Based Formation and Properties of Gold Nanoparticles in Aqueous Solution: Formation Kinetics and Surfactant-Modified Particle Size Distributions†. J. Phys. Chem. C 2010, 114, 15931–15940. 10.1021/jp9118315. [DOI] [Google Scholar]
- Kabashin A. V; Meunier M.; Kingston C.; Luong J. H. T. Fabrication and Characterization of Gold Nanoparticles by Femtosecond Laser Ablation in an Aqueous Solution of Cyclodextrins. J. Phys. Chem. B 2003, 107, 4527–4531. 10.1021/jp034345q. [DOI] [Google Scholar]
- Petersen S.; Barcikowski S. In Situ Bioconjugation: Single Step Approach to Tailored Nanoparticle-Bioconjugates by Ultrashort Pulsed Laser Ablation. Adv. Funct. Mater. 2009, 19, 1167–1172. 10.1002/adfm.200801526. [DOI] [Google Scholar]
- Petersen S.; Barchanski a.; Taylor U.; Klein S.; Rath D.; Barcikowski S. Penetratin-Conjugated Gold Nanoparticles - Design of Cell-Penetrating Nanomarkers by Femtosecond Laser Ablation. J. Phys. Chem. C 2011, 115, 5152–5159. 10.1021/jp1093614. [DOI] [Google Scholar]
- Rehbock C.; Jakobi J.; Gamrad L.; van der Meer S.; Tiedemann D.; Taylor U.; Kues W.; Rath D.; Barcikowski S. Current state of laser synthesis of metal and alloy nanoparticles as ligand-free reference materials for nano-toxicological assays. Beilstein J. Nanotechnol. 2014, 5, 1523–1541. 10.3762/bjnano.5.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre J.-P.; Poulin S.; Kabashin A. V.; Sacher E.; Meunier M.; Luong J. H. T. Surface Chemistry of Gold Nanoparticles Produced by Laser Ablation in Aqueous Media. J. Phys. Chem. B 2004, 108, 16864–16869. 10.1021/jp047134+. [DOI] [Google Scholar]
- Amendola V.; Polizzi S.; Meneghetti M. Laser Ablation Synthesis of Gold Nanoparticles in Organic Solvents. J. Phys. Chem. B 2006, 110, 7232–7237. 10.1021/jp0605092. [DOI] [PubMed] [Google Scholar]
- Boyer P.; Meunier M. Modeling Solvent Influence on Growth Mechanism of Nanoparticles (Au, Co) Synthesized by Surfactant Free Laser Processes. J. Phys. Chem. C 2012, 116, 8014–8019. 10.1021/jp2092994. [DOI] [Google Scholar]
- Wender H.; Andreazza M. L.; Correia R. R. B.; Teixeira S. R.; Dupont J. Synthesis of Gold Nanoparticles by Laser Ablation of an Au Foil inside and Outside Ionic Liquids. Nanoscale 2011, 3, 1240–1245. 10.1039/c0nr00786b. [DOI] [PubMed] [Google Scholar]
- Saitow K. I.; Okamoto Y.; Yano Y. F. Fractal of Gold Nanoparticles Controlled by Ambient Dielectricity: Synthesis by Laser Ablation as a Function of Permittivity. J. Phys. Chem. C 2012, 116, 17252–17258. 10.1021/jp304109h. [DOI] [Google Scholar]
- Kurita H.; Takami A.; Koda S. Size Reduction of Gold Particles in Aqueous Solution by Pulsed Laser Irradiation. Appl. Phys. Lett. 1998, 72, 789. 10.1063/1.120894. [DOI] [Google Scholar]
- Link S.; Burda C.; Nikoobakht B.; El-Sayed M. A. Laser-Induced Shape Changes of Colloidal Gold Nanorods Using Femtosecond and Nanosecond Laser Pulses. J. Phys. Chem. B 2000, 104, 6152–6163. 10.1021/jp000679t. [DOI] [Google Scholar]
- Zijlstra P.; Chon J. W. M.; Gu M. White Light Scattering Spectroscopy and Electron Microscopy of Laser Induced Melting in Single Gold Nanorods. Phys. Chem. Chem. Phys. 2009, 11, 5915–5921. 10.1039/b905203h. [DOI] [PubMed] [Google Scholar]
- Petrova H.; Perez Juste J.; Pastoriza-Santos I.; Hartland G. V; Liz-Marzán L. M.; Mulvaney P. On the Temperature Stability of Gold Nanorods: Comparison between Thermal and Ultrafast Laser-Induced Heating. Phys. Chem. Chem. Phys. 2006, 8, 814–821. 10.1039/B514644E. [DOI] [PubMed] [Google Scholar]
- Baffou G.; Rigneault H. Femtosecond-pulsed optical heating of gold nanoparticles. Phys. Rev. B: Condens. Matter Mater. Phys. 2011, 84, 035415. 10.1103/PhysRevB.84.035415. [DOI] [Google Scholar]
- Takami A.; Kurita H.; Koda S. Laser-Induced Size Reduction of Noble Metal Particles. J. Phys. Chem. B 1999, 103, 1226–1232. 10.1021/jp983503o. [DOI] [Google Scholar]
- Yamada K.; Miyajima K.; Mafuné F. Thermionic Emission of Electrons from Gold Nanoparticles by Nanosecond Pulse-Laser Excitation of Interband. J. Phys. Chem. C 2007, 111, 11246–11251. 10.1021/jp0730747. [DOI] [Google Scholar]
- Werner D.; Furube A.; Okamoto T.; Hashimoto S. Femtosecond Laser-Induced Size Reduction of Aqueous Gold Nanoparticles: In Situ and Pump-Probe Spectroscopy Investigations Revealing Coulomb Explosion. J. Phys. Chem. C 2011, 115, 8503–8512. 10.1021/jp112262u. [DOI] [Google Scholar]
- Pyatenko A.; Wang H.; Koshizaki N.; Tsuji T. Mechanism of pulse laser interaction with colloidal nanoparticles. Laser Photon. Rev. 2013, 7 (4), 596–604. 10.1002/lpor.201300013. [DOI] [Google Scholar]
- Link S.; Wang Z. L.; El-Sayed M. A. How Does a Gold Nanorod Melt?. J. Phys. Chem. B 2000, 104, 7867–7870. 10.1021/jp0011701. [DOI] [Google Scholar]
- Plech A.; Kotaidis V.; Lorenc M.; Boneberg J. Femtosecond Laser near-Field Ablation from Gold Nanoparticles. Nat. Phys. 2006, 2, 44–47. 10.1038/nphys191. [DOI] [Google Scholar]
- Falamas A.; Tosa N.; Tosa V. Dynamics of laser excited colloidal gold nanoparticles functionalized with cysteine derivatives. J. Quant. Spectrosc. Radiat. Transfer 2015, 162, 207–212. 10.1016/j.jqsrt.2015.03.011. [DOI] [Google Scholar]
- DeSantis C. J.; Huang D.; Zhang H.; Hogan N. J.; Zhao H.; Zhang Y.; Manjavacas A.; Zhang Y.; Chang W.-S.; Nordlander P.; Link S.; Halas N. J. Laser-Induced Spectral Hole-Burning through a Broadband Distribution of Au Nanorods. J. Phys. Chem. C 2015, 10.1021/acs.jpcc.5b08290. [DOI] [Google Scholar]
- Pérez-Juste J.; Rodríguez-González B.; Mulvaney P.; Liz-Marzán L. M. Optical Control and Patterning of Gold-Nanorod-Poly(vinyl Alcohol) Nanocomposite Films. Adv. Funct. Mater. 2005, 15, 1065–1071. 10.1002/adfm.200400591. [DOI] [Google Scholar]
- Liu D.; Li C.; Zhou F.; Zhang T.; Zhang H.; Li X.; Duan G.; Cai W.; Li Y. Rapid Synthesis of Monodisperse Au Nanospheres through a Laser Irradiation -Induced Shape Conversion, Self-Assembly and Their Electromagnetic Coupling SERS Enhancement. Sci. Rep. 2015, 5, 7686. 10.1038/srep07686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno-Alejo C. J.; D’Alfonso C.; Pacioni N. L.; González-Béjar M.; Grenier M.; Lanzalunga O.; Alarcon E. I.; Scaiano J. C. Ultraclean Derivatized Monodisperse Gold Nanoparticles through Laser Drop Ablation Customization of Polymorph Gold Nanostructures. Langmuir 2012, 28, 8183–8189. 10.1021/la3010689. [DOI] [PubMed] [Google Scholar]
- Klajn R.; Bishop K. J. M.; Grzybowski B. a. Light-Controlled Self-Assembly of Reversible and Irreversible Nanoparticle Suprastructures. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 10305–10309. 10.1073/pnas.0611371104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T.; Yahata T.; Yasutomo M.; Igawa K.; Tsuji M.; Ishikawa Y.; Koshizaki N. Preparation and investigation of the formation mechanism of submicron-sized spherical particles of gold using laser ablation and laser irradiation in liquids. Phys. Chem. Chem. Phys. 2013, 15, 3099–3107. 10.1039/c2cp44159d. [DOI] [PubMed] [Google Scholar]
- Tsuji T.; Higashi Y.; Tsuji M.; Ishikawa Y.; Koshizaki N. Preparation of submicron-sized spherical particles of gold using laser-induced melting in liquids and low-toxic stabilizing reagent. Appl. Surf. Sci. 2015, 348, 10–15. 10.1016/j.apsusc.2015.02.057. [DOI] [Google Scholar]
- Herrmann L. O.; Valev V. K.; Tserkezis C.; Barnard J. S.; Kasera S.; Scherman O. a.; Aizpurua J.; Baumberg J. J. Threading Plasmonic Nanoparticle Strings with Light. Nat. Commun. 2014, 5, 4568. 10.1038/ncomms5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Ma L.; Hou M.; Xie Z.; Zhang Z. Gradual plasmon evolution and huge infrared near-field enhancement of metallic bridged nanoparticle dimers. Phys. Chem. Chem. Phys. 2016, 18, 2319–2323. 10.1039/C5CP07185B. [DOI] [PubMed] [Google Scholar]
- González-Rubio G.; González-Izquierdo J.; Bañares L.; Tardajos G.; Rivera A.; Altantzis T.; Bals S.; Peña-Rodríguez O.; Guerrero-Martínez A.; Liz-Marzán L. M. Femtosecond Laser-Controlled Tip-to-Tip Assembly and Welding of Gold Nanorods. Nano Lett. 2015, 15, 8282–8288. 10.1021/acs.nanolett.5b03844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanles-Sobrido M.; Pérez-Lorenzo M.; Rodríguez-González B.; Salgueiriño V.; Correa-Duarte M. A. Highly Active Nanoreactors: Nanomaterial Encapsulation Based on Confined Catalysis. Angew. Chem., Int. Ed. 2012, 51, 3877–3882. 10.1002/anie.201105283. [DOI] [PubMed] [Google Scholar]
- Stacy A. M.; Van Duyne R. P.. Time Resolved Vibrational Spectroscopy; Academic Press, Inc.: New York, 1983; pp 377–385. [Google Scholar]
- Lau M.; Ziefuss A.; Komossa T.; Barcikowski S. Inclusion of supported gold nanoparticles into their semiconductor support. Phys. Chem. Chem. Phys. 2015, 17, 29311–29318. 10.1039/C5CP04296H. [DOI] [PubMed] [Google Scholar]
- Chen J.; Wang D.; Xi J.; Au L.; Siekkinen A.; Warsen A.; Li Z.-Y.; Zhang H.; Xia Y.; Li X. Immuno Gold Nanocages with Tailored Optical Properties for Targeted Photothermal Destruction of Cancer Cells. Nano Lett. 2007, 7, 1318–1322. 10.1021/nl070345g. [DOI] [PMC free article] [PubMed] [Google Scholar]