Abstract
Intercalated duct lesions (IDL) of the salivary glands are recently described, and encompass both hyperplasia and benign neoplasms that remain incompletely understood. IDLs have been linked to various benign and low-grade malignant salivary gland neoplasms. We herein present a case of a 77 year old woman with an IDL of the parotid composed of both a hyperplastic and an adenomatous component and report, for the first time, the fine needle aspiration findings of such a lesion. This case illustrates the morphologic spectrum of an IDL, as well as challenges in rendering an accurate cytological and histologic diagnosis. The potential diagnostic pitfalls presented by the hybrid pattern of this lesion are also discussed.
Keywords: Intercalated duct lesion, Intercalated duct hyperplasia, Intercalated duct adenoma, Salivary gland, Fine needle aspiration cytology, Parotid
Case Report
A 77 year old Malay woman presented with an asymptomatic subcentimeter nodule in the superficial parotid gland. The patient had a history of metastatic papillary thyroid carcinoma (PTC) 17 years prior to presentation that was treated with total thyroidectomy and adjuvant radioactive iodine. At the time of presentation she was undergoing radioactive iodine treatment following surgery for local and nodal recurrent PTC, during which a magnetic resonance imaging (MRI) scan showed an ovoid, enhancing nodule in the left superficial parotid measuring 9 mm (Fig. 1).
Fig. 1.
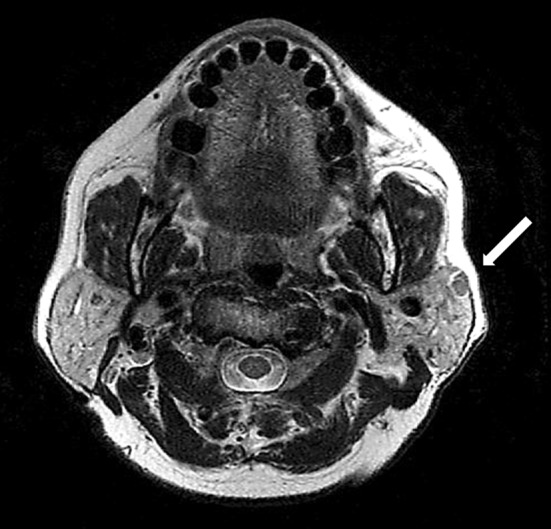
Axial MRI image showing an ovoid, enhancing nodule in the left superficial parotid (arrow)
On examination, a vaguely palpable nodule was present in the left parotid gland. Facial nerve function was preserved. A thyroidectomy scar and previously documented right vocal cord paralysis was noted. There was no palpable cervical lymphadenopathy.
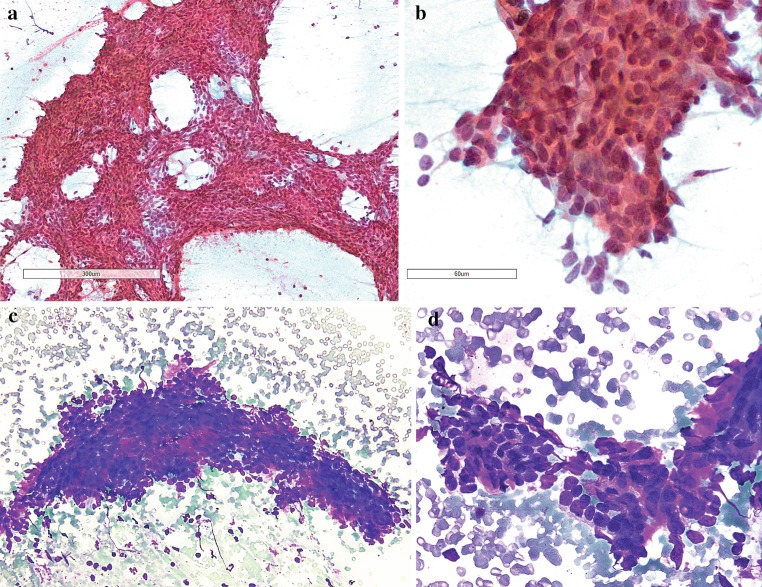
Fine needle aspiration biopsy of the parotid nodule was performed. This showed sheets and clusters of basaloid epithelial cells with high nuclear to cytoplasmic ratio (Fig. 2a, c); on high power magnification, the epithelial cells displayed fairly uniform, round to ovoid nuclei (Fig. 2b, d). Small amounts of dense stromal material were seen at the periphery of a few cell clusters (Fig. 2c, d). No plasmacytoid or spindled myoepithelial cells were identified. There was no evidence of high-grade cytological atypia, nuclear features of papillary thyroid carcinoma, or necrotic debris. A cytological diagnosis of a salivary gland basaloid neoplasm was rendered and excision was recommended.
Fig. 2.
a, c At low magnification, fine needle aspiration cytologic smears showed cellular sheets and clusters of epithelial cells; b, d at high magnification, the epithelial cells exhibited a high nuclear to cytoplasmic ratio, and fairly uniform, ovoid nuclei. c, d Small stromal fragments were admixed with the epithelial cells (a, b—PAP, c, d—HC)
A left near-total parotidectomy was performed. On gross examination, the cut surface of the specimen revealed a mostly well circumscribed, firm, homogenously tan-white nodule measuring 0.7 cm (Fig. 3a). Multiple unremarkable intra- and peri-parotid lymph nodes ranging from 0.2 to 0.6 cm were present. Microscopically, there was a partially encapsulated lesion with two components: a nodular component and an adjacent irregular component. The nodular component was composed of closely packed, well-formed tubules with little intervening stroma (Fig. 3b). The tubules were lined by a single layer of cuboidal cells with cytologically bland, rounded nuclei and moderate amounts of pale eosinophilic cytoplasm (Fig. 3c). The luminal spaces were small, and some contained PAS-positive eosinophilic secretions (Fig. 3d). No obvious bilayering was detected, except focally at the periphery of the lesion where a rim of myoepithelial cells was vaguely discernible around more widely separated tubules (Fig. 3e). The above described nodule was juxtaposed to an area with irregular borders composed of similarly appearing tubules and scattered serous acinar cells (Fig. 3f). No necrosis or perineural infiltration was identified. The background parotid parenchyma showed areas of mild acinar atrophy and focal mild hyperplasia of intercalated-type ducts (Fig. 3g). Of the 12 intra- and peri-parotid lymph nodes, one contained a salivary gland inclusion composed of fine caliber ducts that were contiguous with extranodal salivary gland tissue (Fig. 3h).
Fig. 3.
a Grossly, a mostly well-circumscribed nodule with a tan-white cut surface was seen; b low power view of IDA/IDH hybrid lesion, showing a partially encapsulated proliferation juxtaposed with an irregular area of intercalated duct hyperplasia; c IDA composed of closely packed, well-formed tubules lined by a single layer of cuboidal cells; d PAS positive material was present in some tubules; e discernible rim of myoepithelial cells focally at the periphery; f presence of acinar cells in the IDH component that was not seen in the IDA component; g focus of intercalated duct hyperplasia in the background parenchyma; h a salivary gland inclusion composed of fine caliber intercalated-type ducts that were contiguous with extranodal salivary gland tissue
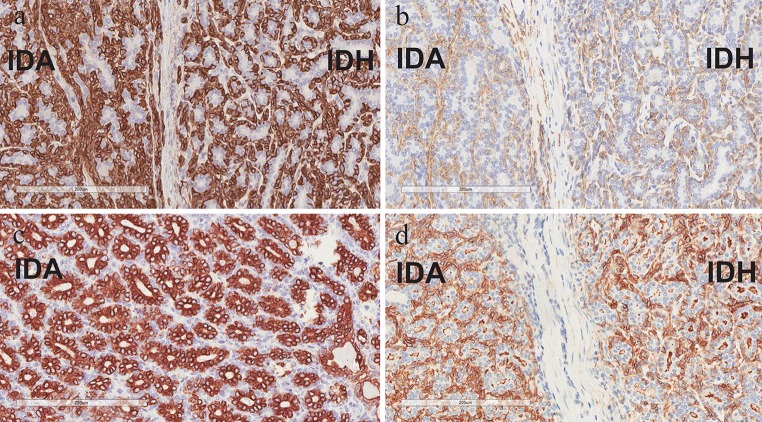
An immunohistochemical study was performed (Fig. 4): CK14 and smooth muscle actin (SMA) highlighted a distinct outer layer of abluminal myoepithelial cells surrounding the tubules in both the encapsulated and irregular parts of the lesion. The luminal cells exhibited diffuse positivity for CK7 and there was patchy reactivity (nuclear and cytoplasmic) for S-100 protein in the luminal cells and focally in the abluminal myoepithelial cells. No significant reactivity for S-100 was detected in the stromal cells. DOG-1 highlighted the apical border of the luminal cells and showed patchy circumferential staining of the abluminal cells. The Ki-67 proliferative index was low (<5 %).
Fig. 4.
Immunohistochemistry: a CK14 and b SMA highlighted myoepithelial cells that were indistinct on light microscopy; c CK7 highlighted luminal cells; d DOG-1 highlighted the apical border of the luminal cells and showed patchy circumferential staining of the abluminal cells
Based on these features, the diagnosis of an intercalated duct lesion with “hybrid” features of intercalated duct adenoma (encapsulated part) and intercalated duct hyperplasia (irregular part) was made. The patient underwent no further treatment for this lesion.
Discussion
Intercalated duct lesions (IDLs) of salivary glands are recently described, apparently rare proliferative entities that encompass a spectrum of lesions from hyperplasia to adenoma [1]. IDLs occur mainly in the parotid gland, with a slight female predominance, and are mostly small, subcentimeter lesions that may be unifocal (85 %) or multi-focal [1, 2].
The features of an intercalated duct lesion on fine needle aspiration biopsy have not been previously described. In our case, the findings were that of a salivary gland basaloid neoplasm with several differential diagnoses ranging from benign neoplasms such as basal cell adenoma and cellular pleomorphic adenoma to low-grade malignant tumors including basal cell adenocarcinoma and the tubular variant of adenoid cystic carcinoma. The paucity of metachromatic fibrillary stroma and absence of spindled or plasmacytoid myoepithelial cells rendered the diagnosis of cellular pleomorphic adenoma unlikely. The large metachromatic stromal globules and angulated hyperchromatic nuclei of adenoid cystic carcinoma were not seen either. The nuclear features of papillary thyroid carcinoma were also not identified on the smears, which was a relevant finding given the clinical history of recurrent/metastatic papillary thyroid carcinoma. Thus, basal cell adenoma/adenocarcinoma were the main diagnostic considerations based on fine needle aspiration findings alone.
In retrospect, an intercalated duct lesion is a rare, but worthwhile differential to be considered in the evaluation of a small, asymptomatic parotid lesion with basaloid features. However, as the distinction of several benign lesions from low grade malignant salivary gland neoplasms is based primarily upon invasion, a definitive diagnosis cannot be made on fine needle aspiration cytology, thus warranting surgical excision.
The architectural features of intercalated duct lesions are varied, with three described patterns: Intercalated duct hyperplasia (IDH) appears as an unencapsulated, irregular focus containing occasional acinar cells while an intercalated duct adenoma (IDA) forms a discrete, partially to completely encapsulated nodule without admixed native acinar cells. The third pattern is that of a hybrid of both IDH and IDA [1]. In our case, we observed both an encapsulated component as well as a juxtaposed irregular focus containing occasional acinar cells (which were completely lacking in the encapsulated adenomatous component), which hence conforms to those of a hybrid IDH-IDA lesion.
Importantly, a potential diagnostic pitfall was presented by the juxtaposition of both components, as the irregular edges of the IDH component could be misinterpreted as an invasive focus arising from the larger IDA, with the presence of intermixed acinar cells incorrectly perceived as parenchymal infiltration by a basal cell adenoma, i.e. a low-grade basal cell carcinoma. The overall cytological benignity of the lesion, coupled with awareness of the hybrid pattern of IDL, are important in avoiding this diagnostic pitfall. The presence of other foci of mild intercalated ductal hyperplasia in the background parotid parenchyma (as in the case above) is another clue to avoiding a misdiagnosis of a tubular variant of basal cell adenocarcinoma. In addition, the presence of lymph node inclusions composed of fine caliber “intercalated-type” ducts contiguous with extranodal salivary gland tissue (as seen in the case above) may be mistakenly interpreted as lymph node metastasis of a malignant neoplasm. This difficulty is further compounded by the fact that IDLs have been occurring in conjunction with various low-grade malignant salivary gland tumors such as basal cell adenocarcinomas and epithelial-myoepithelial carcinomas [1, 3–5].
Features distinguishing intercalated duct lesions from other low grade salivary neoplasms with ductal differentiation have been previously examined [1, 6, 7]. The tubular variant of basal cell adenoma is more a histological than a clinically relevant differential diagnosis. Both lesions are benign and closely related. This is not only reflected in their very similar cellular composition, but there have also been described cases of IDLs occurring in direct continuity with basal cell adenomas [7]. As stated above, the lack of obvious bilayering on routine haematoxylin and eosin stain, as seen in our case, is an important factor distinguishing IDA from the tubular variant of basal cell adenoma, reflecting a smaller number of myoepithelial cells in the former [1, 7]. The lack of a significant stromal spindle cell component that shows immunoreactivity for S-100 is another feature in favour of intercalated duct adenoma over basal cell adenoma [1]. In both entities, the immunoprofiles of luminal and myoepithelial cells are similar to that of the normal intercalated duct [7]. A study has found that DOG-1 expression is present on the apical membrane of the luminal cells of both entities, but exhibits more focal positivity in the myoepithelial component of IDL compared to BCA [7]. Other differential diagnoses to be considered include the cellular variant of pleomorphic adenoma and the tubular pattern of adenoid cystic carcinoma. The former may be distinguished from IDL by the presence of chondromyxoid stroma, which was not observed in this case, as well as positivity for PLAG1 immunostain [8, 9]. The tubular variant of adenoid cystic carcinoma features an infiltrative pattern of growth frequently associated with perineural invasion, which was not observed in the case described above. Assessing the presence of MYB expression, which occurs in a subset of adenoid cystic carcinomas, via immunohistochemical study may be helpful in ruling out this differential [10, 11].
As stated above, IDLs have been associated with a range of benign and malignant salivary gland neoplasms that include basal cell adenocarcinomas and epithelial-myoepithelial carcinomas [1, 3–5, 7]. The co-existence of IDLs with these neoplasms has raised the question of whether IDLs are precursor lesions for these neoplasms. Apart from basal cell adenomas, with which IDLs share several morphological and immunohistochemical features, and may belong within the same spectrum, the biological basis of possible neoplastic links between IDLs and other neoplasms remains unexplored and warrants further investigation.
Early studies have documented proliferation of intercalated ducts in the context of chronic parotitis, atrophy, and radiation therapy, in what is deemed as a reactive process [5]. Interestingly, our patient had a history of radioactive iodine therapy for metastatic papillary thyroid carcinoma. Apart from the thyroid gland, radioactive iodine hones in on the salivary glands where it is concentrated and causes dose dependent damage to the salivary parenchyma [12]. In our case, there were patchy areas of atrophy and mild chronic inflammation in the background parotid parenchyma, focal areas of mild intercalated duct hyperplasia, and a dominant hybrid intercalated duct lesion with an adenomatous component. The pathological spectrum described herein may thus be illustrative of the interplay between environmental, age-associated, and genetic factors in determining the switch from reactive to neoplastic ductal proliferations of the salivary gland.
Conclusion
In conclusion, we have presented an instructive case of a hybrid intercalated duct lesion, with fine needle aspiration findings described for the first time. Awareness of the varied architectural features of this entity is helpful in avoiding potential diagnostic pitfalls and the use of select immunohistochemical markers may help to differentiate this lesion from closely related entities.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Weinreb I, Seethala RR, Hunt JL, Chetty R, Dardick I, Perez-Ordonez B. Intercalated duct lesions of salivary gland: a morphologic spectrum from hyperplasia to adenoma. Am J Surg Pathol. 2009;33(9):1322–1329. doi: 10.1097/PAS.0b013e3181a55c15. [DOI] [PubMed] [Google Scholar]
- 2.Naunheim MR, Lin HW, Faquin WC, Lin DT. Intercalated duct lesion of the parotid. Head Neck Pathol. 2012;6(3):373–376. doi: 10.1007/s12105-012-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chetty R. Intercalated duct hyperplasia: possible relationship to epithelial-myoepithelial carcinoma and hybrid tumours of salivary gland. Histopathology. 2000;37(3):260–263. doi: 10.1046/j.1365-2559.2000.00976.x. [DOI] [PubMed] [Google Scholar]
- 4.Di Palma S. Epithelial-myoepithelial carcinoma with co-existing multifocal intercalated duct hyperplasia of the parotid gland. Histopathology. 1994;25(5):494–496. doi: 10.1111/j.1365-2559.1994.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 5.Yu GY, Donath K. Adenomatous ductal proliferation of the salivary gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(2):215–221. doi: 10.1067/moe.2001.110419. [DOI] [PubMed] [Google Scholar]
- 6.Weinreb I, Simpson RH, Skalova A, Perez-Ordonez B, Dardick I, Chetty R, et al. Ductal adenomas of salivary gland showing features of striated duct differentiation (‘striated duct adenoma’): a report of six cases. Histopathology. 2010;57(5):707–715. doi: 10.1111/j.1365-2559.2010.03682.x. [DOI] [PubMed] [Google Scholar]
- 7.Montalli VA, Martinez E, Tincani A, Martins A, Abreu Mdo C, Neves C, et al. Tubular variant of basal cell adenoma shares immunophenotypical features with normal intercalated ducts and is closely related to intercalated duct lesions of salivary gland. Histopathology. 2014;64(6):880–889. doi: 10.1111/his.12339. [DOI] [PubMed] [Google Scholar]
- 8.Martins C, Fonseca I, Roque L, Pereira T, Ribeiro C, Bullerdiek J, et al. PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: a combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod Pathol. 2005;18(8):1048–1055. doi: 10.1038/modpathol.3800386. [DOI] [PubMed] [Google Scholar]
- 9.Rotellini M, Palomba A, Baroni G, Franchi A. Diagnostic utility of PLAG1 immunohistochemical determination in salivary gland tumors. Appl Immunohistochem Mol Morphol. 2014;22(5):390–394. doi: 10.1097/PAI.0b013e3182936ea7. [DOI] [PubMed] [Google Scholar]
- 10.Brill LB, 2nd, Kanner WA, Fehr A, Andren Y, Moskaluk CA, Loning T, et al. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011;24(9):1169–1176. doi: 10.1038/modpathol.2011.86. [DOI] [PubMed] [Google Scholar]
- 11.West RB, Kong C, Clarke N, Gilks T, Lipsick JS, Cao H, et al. MYB expression and translocation in adenoid cystic carcinomas and other salivary gland tumors with clinicopathologic correlation. Am J Surg Pathol. 2011;35(1):92–99. doi: 10.1097/PAS.0b013e3182002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandel SJ, Mandel L. Radioactive iodine and the salivary glands. Thyroid. 2003;13(3):265–271. doi: 10.1089/105072503321582060. [DOI] [PubMed] [Google Scholar]