Abstract
This study describes a unique subset of ciliated, human papillomavirus (HPV) related, adenosquamous carcinomas (AsqCA) of the head and neck that in contrast to most AsqCA, often show areas with lower grade cytonuclear features. They are comprised of largely non-keratinizing squamous cell carcinoma components with cystic change, gland formation, mucin production, and cilia in tumor cells. Seven cases of ciliated AsqCA were retrieved. Site distribution was as follows: palatine tonsil—3/7, base of tongue—1/7, and neck (unknown primary site)—3/7. Despite the occasional resemblance to mucoepidermoid carcinoma (MEC), the tumors showed focal keratinizing morphology and atypia, and all tumors were negative for MAML2 rearrangements. Oropharyngeal and neck tumors were uniformly p16 positive and showed punctate staining by in situ hybridization for high risk HPV DNA. There were two distant metastases (lung), and one tumor related death. Thus, ciliated AsqCA are HPV-associated lesions that pose unique pitfalls, closely mimicking MEC and other salivary gland tumors. These tumors add to the list of those which defy the dogma that ciliated epithelium always equates to a benign process.
Keywords: Adenosquamous, Non-keratinizing, Ciliated, Human papillomavirus, MAML2
Introduction
Adenosquamous carcinoma (AsqCA) of the upper aerodigestive tract is generally considered a rare, aggressive variant of squamous cell carcinoma (SCC) with divergent glandular differentiation of any amount, as defined by the World Health Organization [1, 2]. The term originated in the literature in 1968, and was described as a distinct entity by Gerughty et al. [3]. Historically, some have considered AsqCA and mucoepidermoid carcinoma (MEC) to be interchangeable, both as terms and perhaps even as entities [4].
However, morphologic and biologic differences between MEC and AsqCA have since become increasingly recognized [1, 5]. AsqCA are more aggressive than MEC as a whole, and likely more aggressive even when comparing to high grade MEC alone [1, 2, 6]. Additionally, at least two reproducible translocations: CRTC1–MAML2 (previously known as MECT1–MAML2) [6, 7], and CRTC3–MAML2 [8] characterize the majority of MEC, whereas no reproducible genetic alterations exist for AsqCA. More recently, Alos et al. [9] have outlined specific criteria that are currently the standard to delineate AsqCA from MEC, namely: the presence of surface squamous dysplasia/carcinoma in situ, infiltrative growth pattern, pronounced keratinization, discrete adenocarcinomatous foci (often in deep portions of tumor), absence of intermediate cells, and pronounced nuclear atypia. In particular, AsqCA have classically been defined for having overt keratinizing squamous differentiation which should be minimal or lacking in MEC.
This approach works well for the majority of AsqCA for which the squamous components are cytomorphologically high grade and keratinizing. However, we have recently encountered cases of upper aerodigestive tract AsqCA in our files that defy this stereotype. Morphologically, such tumors have had relatively bland morphology, predominantly non-keratinizing squamous differentiation, and focal ciliated malignant cells. Furthermore, these have tended to arise in the oropharynx and harbor human papillomavirus (HPV). We herein report our experience with seven such cases that highlight some unique features and diagnostic pitfalls and demonstrate the utility of ancillary testing to differentiate them from MEC and other entities.
Materials and Methods
Case Selection
This study was approved by the University of Pittsburgh Institutional Review Board (IRB# PRO09040520). Seven cases (three from the University of Pittsburgh, two from Massachussetts General Hospital, one each from University of Michigan, and Washington University in St. Louis) were identified (2003–2014) from the subspecialty practices of dedicated head and neck pathologists at the respective institutions. These cases were flagged as demonstrating predominantly non-keratinizing squamous elements, and a mixture of glandular elements, mucous cells and ciliated cells intermingled with squamous elements in either the primary tumor or lymph node metastases. Clinical and pathologic parameters were obtained as available for each patient.
Immunohistochemistry for p16 and Chromogenic In Situ Hybridization for Human Papillomavirus
Immunohistochemical staining for p16 (clone E6H4, monoclonal mouse, prediluted, MTM Laboratories, West Borough MA) was performed using 5-µm tissue sections from formalin fixed paraffin embedded blocks at the respective submitting institutions. Slides were deparaffinized in xylene twice for 10 min, dehydrated twice with 100 % ethanol and stained for anti-p63 (Dako, Carpinteria, CA, dilution: 1/500). Labeling was performed using the I-view 2′-diaminobenzamide (DAB) detection kit (Ventana systems, Tucson, AZ) as the brown chromogen substrate. Positivity was defined as diffuse strong nuclear and cytoplasmic staining in >70 % of tumor cells [10]. All p16 results were evaluated by two authors (LR, RRS), either by review of the outside p16 stained slides or by their own p16 staining of received unstained slides.
HPV chromogenic in situ detection was performed on 5-µm tissue sections from formalin fixed paraffin embedded blocks. Sections were deparaffinized and digested with Proteinase K (Roche Diagnostics, Indianapolis, IN) and labeled by in situ hybridization using probes targeting a wide spectrum of HPV strains including 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, and 52 (Y1404, Dako, Carpinteria, CA). 2′-Diaminobenzamide (DAB) (Genpoint Kit, Dako, #K0620) was utilized as the chromogenic substrate for visualization. Nuclear staining was considered positive, and this was substratified into punctate, diffuse and mixed.
MAML2 Fluorescence In Situ Hybridization (FISH)
MAML2 FISH was performed on 5-µm tissue sections from formalin fixed paraffin embedded blocks. Slides were deparaffinized in xylene twice for 10 min, dehydrated twice with 100 % ethanol and then pretreated using the Vysis Paraffin Pretreatment Kit. Slides were digested for 28 min in protease solution (0.5 mg/ml) at 37º C. Dual-color FISH was performed using commercially available MAML2 break-apart probes (ZytoVision Ltd, Bremerhaven, Germany).
The slides were denatured at 75 °C for 5 min in 70 % formamide (Chemicon, Billerica, MA) and dehydrated in ethanol. The probe was denatured for 5 min at 75° C prior to hybridization. Slides were incubated with probe overnight at 37 °C in a humidified chamber. Post-hybridization washes were performed using 2XSSC/0.3 % Igepal (Sigma) at 72 °C for 2 min. Slides were air-dried in the dark and counterstained with DAPI (Vysis, Inc). Analysis was performed using an Applied Imaging Workstation equipped with Chroma Technology filters containing band excitors for SpectrumOrange, FITC, and DAPI. Only individual and well delineated cells were scored. Overlapping cells were excluded from the analysis. Approximately 60 cells were analyzed in the targeted region. A minimum of 20 % of cells with split signal was considered positive based on prior in-house validation [6].
Results
Clinical Features and Staging Parameters
Clinical features and staging parameters are summarized in Table 1. Site distribution was as follows: tonsil—3/7, base of tongue—1/7, and neck lymph nodes (unknown primary site)—3/7. All tumors with unknown primary site did have bilateral tonsillectomy with no evidence of tumor. The mean patient age was 58 years (range 52–68 years), and there was a 6:1 male predominance.
Table 1.
Clinical features and staging parameters
| Case number | Age (years) | Sex | Primary tumor site | T-size (cm) | N-size (cm) | Pathologic stage | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 68 | Female | L tonsil | 2.5 | 3.5 | pT2N2b | Primary resection, Chemo-XRT, selective neck dissection and parapharyngeal node excision | Radiologic evidence of lung metastasis at 9 months; DOD at 27 months |
| 2 | 54 | Male | R base of tongue | – | 3.5 | pTxN2a | Primary Chemo-XRT and selective neck dissection | NED at 15 months |
| 3 | 55 | Male | L tonsil | 1.2 | 2.2 | pT2N2a | Primary resection, Chemo-XRT and a modified radical left neck dissection | Biopsy proven lung metastasis at 38 months; AWD (lung) at 59 months |
| 4 | 55 | Male | L tonsil | 0.4 | 3.2 | pT1N2a | Primary resection, neck dissection, and postoperative XRT | NED at 68 months |
| 5 | 55 | Male | Unknown | – | 3.0 | pTxN1 | Chemo-XRT | NED at 31 months |
| 6 | 64 | Male | Unknown | – | 4.7 | pTxN2a | XRT | <6 months F/U |
| 7 | 52 | Male | Unknown | – | 2.5 | pTxN1 | – | <6 months F/U |
L left, R right, T tumor, N (lymph) node, DOD dead of disease, XRT radiotherapy, NED no evidence of disease, AWD alive with disease, F/U follow-up, LTF lost to follow-up
All oropharyngeal and “unknown primary site” cases presented with large cystic nodal metastases with a mean size (when available) of 3.2 cm (range 2.5–4.7). Primary oropharyngeal tumors were relatively small with a mean size of 1.4 cm (range 0.4–3.5 cm, n = 3).
Six patients had known treatment course. In all cases with known treatment, post-surgical treatment included radiotherapy or combined chemotherapy and radiotherapy (chemoradiation). Three patients with oropharyngeal primary tumors also underwent additional lymph node excision or some form of neck dissection in addition to what was initially performed. Two cases had <6 months of clinical follow up. One patient (case 1) developed a probable lung metastasis based on imaging studies at 9 months, and died of disease at 27 months. One patient (case 3) had a biopsy proven metastasis at 38 months, and is currently alive with lung disease. The remaining three patients were free of disease with a mean follow up of 38 months (range 15–68 months).
Pathologic Features
Pathologic features are summarized in Table 2. As stipulated in the inclusion criteria, all cases demonstrated a predominantly non-keratinizing morphology with an admixture of glandular elements (Fig. 1) in either the primary tumor or the lymph node metastasis and cilia.
Table 2.
Clinical features and staging parameters
| Case number | Keratinization | Surface component | Cilia | Predominant morphology | p16 | HPV in situ hybridization | HPV subtype | MAML2 translocation |
|---|---|---|---|---|---|---|---|---|
| 1 | Focal | Yes | Yes | Microcystic MEC-like | Positive | Positive, punctate | – | Negative |
| 2a | No | – | Yes | Mucin poor | Positive | Positive, punctate | – | Negative |
| 3 | Focal | No | Yes | Mucin poor | Positive | Positive, punctate | – | Negative |
| 4 | No | – | Yes | Mucin poor | Positive | Positive, punctate | – | Negative |
| 5 | Focal | – | Yes | Microcystic MEC-like | Positive | Positive, punctate | – | Negative |
| 6 | Focal | – | Yes | Mucin poor | Positive | Positive, punctate | – | Negative |
| 7 | Focal | – | Yes | Mucin poor | Positive | Positive, punctate | – | Negative |
MEC-like mucoepidermoid carcinoma-like, HPV human papillomavirus
aCase 2 did not have the primary tumor available for review; morphologic features are those of the lymph node metastasis
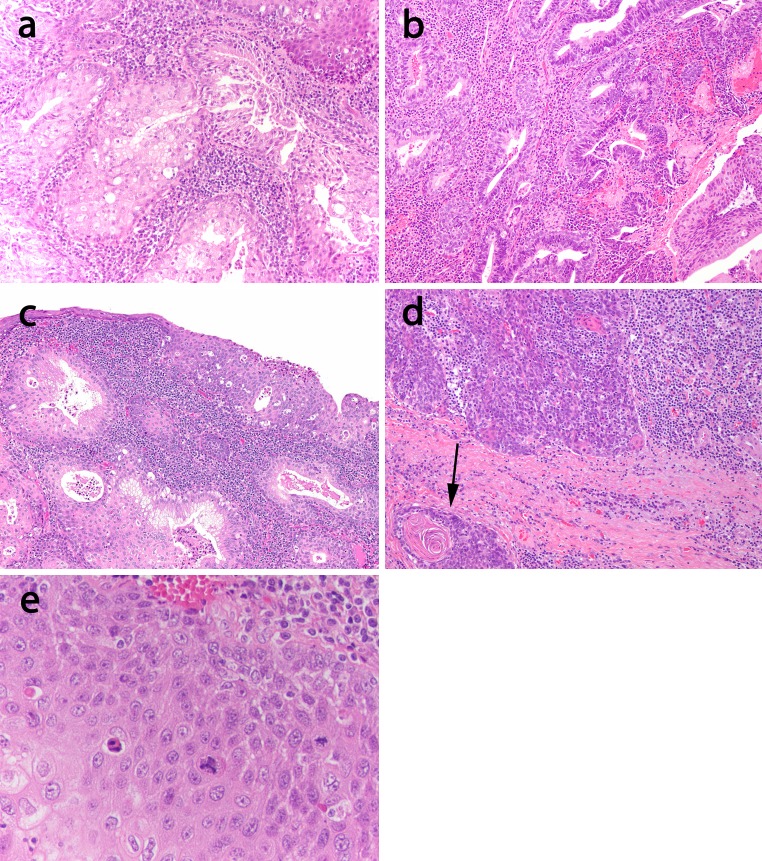
Fig. 1.
General histologic features. This tonsillar tumor (case 1) demonstrates an expansion of the tonsillar crypt by a relatively bland squamoglandular proliferation with micropapillary and vaguely cribriform spaces (×40 magnification). Inset there is a prominent mucous cell component (arrow, ×100 magnification)
Oropharyngeal Tumors
For one case (case 2), only the lymph node metastasis slides were available for review (as we will discuss below). For one of the four remaining oropharyngeal cases, the adenosquamous component consisted of microcystic areas within non-keratinizing squamous nests showing interspersed cuboidal to columnar cells and mucous cells reminiscent of MEC (Fig. 2a). In the remaining three cases, the adenosquamous components were relatively mucin poor and consisted of cuboidal to lightly eosinophilic columnar cells. In some areas, they demonstrated a distinctive bilayered tubuloglandular appearance with luminal columnar cells and abluminal non-keratinizing squamous elements (Fig. 2b). One of two primary tumors with surface mucosa in the specimen reviewed did indeed have an intra-mucosal component (Fig. 2c) that was squamoglandular. Despite the predominant non-keratinizing morphology in all cases, three cases did show focal keratinization (Fig. 2d). Finally, while most of the cases were somewhat monomorphic and cytologically bland, all cases did show at least focal atypia and mitotic activity (Fig. 2e). A rather unique finding was that all cases demonstrated ciliated cells as minor part of the glandular component. Recognition required scanning at high power magnification given their focality (Fig. 3).
Fig. 2.
Morphologic spectrum. a This tonsillar tumor (case 1) shows a mucoepidermoid like morphology with prominent mucous cells admixed with bland squamous cells mimicking the appearance of epidermoid and intermediate cells of MEC (×100). b This tonsillar tumor (case 3) on the other hand shows a glandular component relatively devoid of mucous cells but instead consists of a stratified columnar glandular component surrounded by non-keratinizing squamous cells in a bilayered fashion (×100). c One oropharyngeal tumor showed a surface component. This tumor (case 1) demonstrates squamoglandular morphology even in this component (×100). Despite the predominant bland non-keratinizing appearance in this group of AsqCA, d focal keratinization (case 3, ×100), and e atypia (case 1, ×200) were noted
Fig. 3.
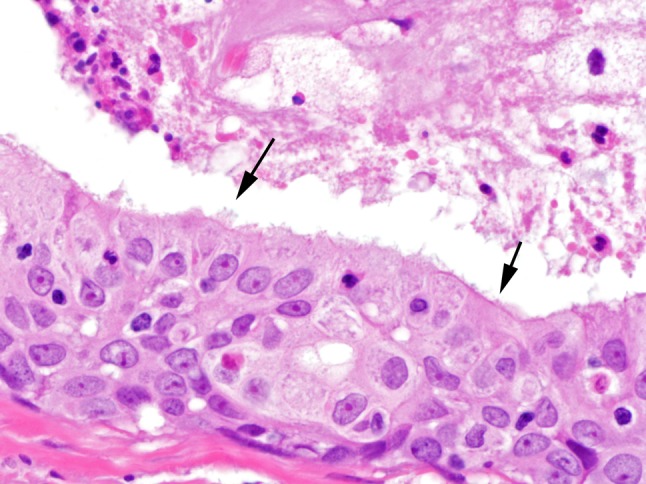
Ciliated epithelium, though sparsely distributed, defined this group of tumors (case 3, ×400, arrows)
Lymph Nodes/Unknown Primary
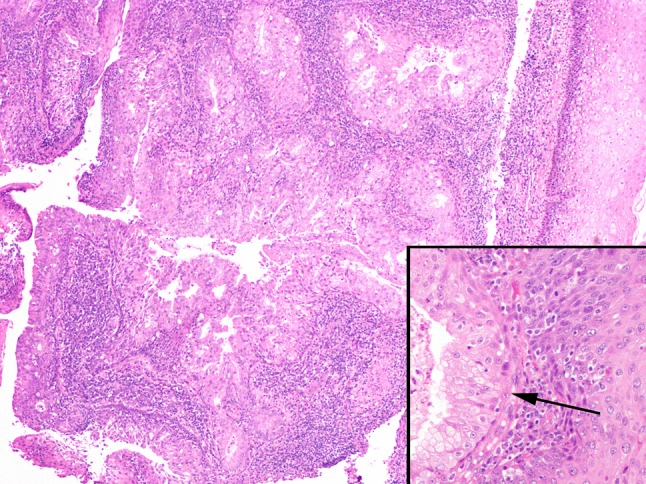
Lymph node metastases, whether from a known or unknown primary, demonstrated a multilocular, macrocystic configuration (Fig. 4). The majority (2/3 (67 %)) of lymph node metastases from unknown primary sites showed a mucin poor morphology, but all showed ciliated epithelium (Fig. 4 inset). Similarly, the lymph node metastases in case 2, for which the primary tumor was unavailable, also showed a mucin poor appearance.
Fig. 4.
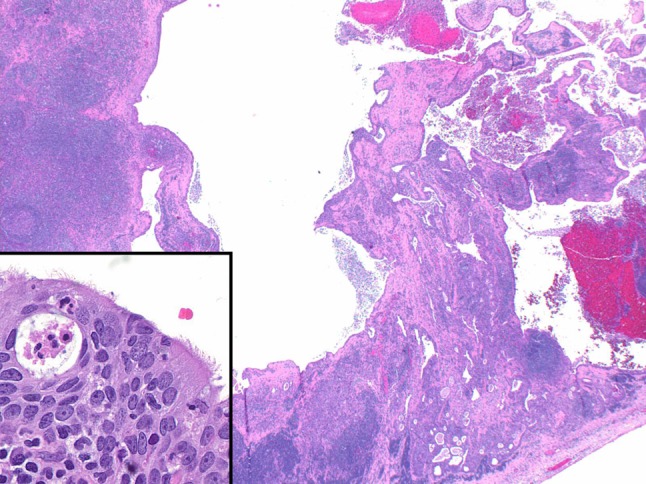
Lymph node metastases were typically multilocular and cystic mimicking the architectural appearance of a low grade MEC (case 5 metastasis, ×20). Inset lymph node metastases still showed ciliated epithelium (case 8, ×400)
Two cases (oropharyngeal: cases 1, 4) had paired primary and nodal metastases available for review. All cases showed adenosquamous morphology in both primary tumors and lymph node metastases. Remarkably, in case 1, a lymph node that was excised after chemotherapy, there was extensive keratinization with only rare glandular elements (Fig. 5).
Fig. 5.

Treated AsqCA metastasis. The metastasis (case 1, ×40) showed keratinizing maturation with only sparse glandular elements (inset ×200)
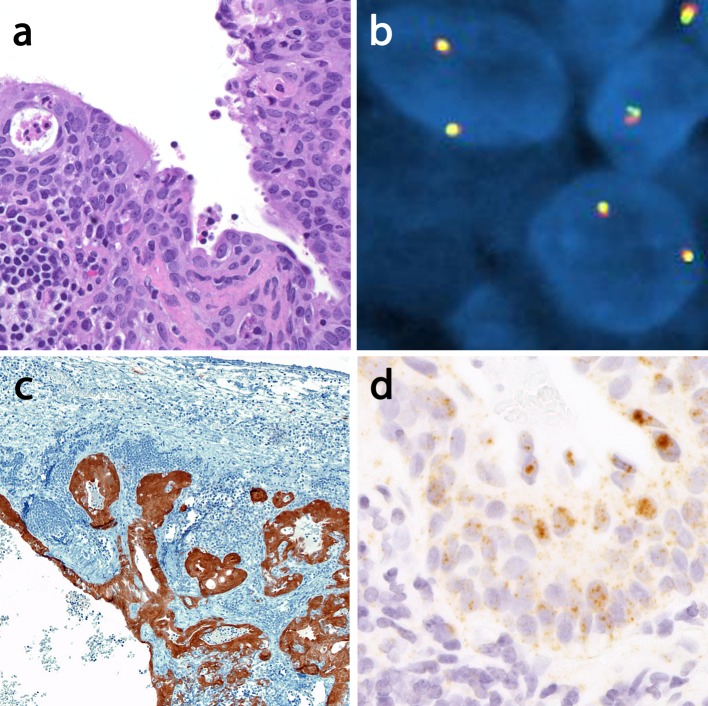
Ancillary Studies
All cases were negative for MAML2 rearrangements by FISH (Fig. 6a, b). All oropharyngeal cases and tumors of unknown primary were p16 positive (Fig. 6c) and showed a punctate positivity for HPV DNA by in situ hybridization using a pan-selective probe set. Positivity was noted in both glandular and squamous elements (Fig. 6d).
Fig. 6.
Ancillary testing profile. a Neck AsqCA of unknown primary site (case 5) with ciliated epithelium (×200). b MAML2 FISH shows two intact (yellow) signals and thus no evidence of translocation. c However, this case is diffusely strongly p16 positive in all components (×100) and d HPV DNA in situ hybridization using a pan selective probe set show punctate signals in the ciliated glandular component. Note the negative lymphoid stroma on the bottom left (×400). AsqCA adenosquamous carcinoma, MEC mucoepidermoid carcinoma, FISH fluorescence in situ hybridization, HPV human papillomavirus
Discussion
This series illustrates an unusual set of tumors within the spectrum of AsqCA, some of which truly test the standard criteria for AsqCA including those used to make the distinction from MEC. Two of 7 cases showed several common morphologic features that mimic MEC: lower grade morphology than expected in the prototypical AsqCA, a prominence of non-keratinizing squamoproliferative areas resembling epidermoid and even intermediate cells of MEC, admixed mucocytes and cystic spaces reminiscent of MEC (rather than discrete tubuloglandular structures), and scattered ciliated columnar cells. Of the criteria for AsqCA recommended by Alos et al. [9], three of them are focal to absent in these cases, including:
Pronounced keratinization
Discrete adenocarcinomatous foci (often in deep portions of tumor)
Pronounced nuclear atypia
Additionally, since the non-keratinizing components closely mimic the epidermoid or intermediate cells of MEC, reliance on this criterion does not appear ‘fool-proof’ either. Also, only one case with MEC like morphology happened to demonstrate intramucosal involvement. This is may be useful when present, but given the limited cases for which the surface mucosa could be assessed, the ultimate utility of surface involvement in the context of a tonsillar tumor type that is traditionally thought to evolve from the tonsillar crypt rather than surface mucosa is unclear.
Furthermore, it does appear that even when focal, nuclear anaplasia, and keratinization in a squamoglandular neoplasm are useful in separating a predominantly non-keratinizing AsqCA from MEC, particularly in the context of cystic morphology. Cystic MEC are, as a rule, low grade, and comprised largely of bland monomorphic mucous/columnar cells and intermediate cells with only focal epidermoid components and no keratinizing squamous differentiation. Thus, a juxtaposition of high grade features with low grade architecture would represent ‘discordant’ morphologic features that argue against a diagnosis of MEC even within a lymph node metastasis [6, 11, 12].
Ultimately, several situations (i.e. limited sampling, ulcerated tumors without surface component, nodal metastases) exist in which the distinction between a MEC and a relatively bland non-keratinizing AsqCA may be greatly facilitated by ancillary testing, since common translocations exist that are fairly specific for MEC and are not seen in AsqCA. The t(11;19)(q21;p13) translocation results in a CRTC1–MAML2 fusion (previously known as MECT1–MAML2) [7], and the t(11;15)(q21;q26) results in a fusion of CRTC3–MAML2 [8]. These are noted in 70 % of MEC overall, and in recent studies may be present in a considerable proportion of even high grade MEC, assuming rigorous exclusion of other entities (like AsqCA) [6]. FISH for MAML2 rearrangements in all three cases described here was negative, providing evidence against MEC.
The concern still exists that 30 % of MEC may still be negative for a translocation, thus a ‘positive marker’ to support AsqCA is desirable. As mentioned before, there are no reproducible genetic alterations in AsqCA on which to capitalize for resolving this differential diagnosis, however, conceptually, as these are surface-derived tumors, an approach that supports a surface (or crypt) derived phenotype may prove useful. Given the non-keratinizing morphology seen in these AsqCA, it appears reasonable to expect that, similar to oropharyngeal and papillary sinonasal non-keratinizing SCC, these may also harbor HPV DNA. Thus, HPV testing and/or p16 immunostaining can be used as ancillary data to further support a surface (or tonsillar crypt epithelium) derivation for a tumor, and in the example of cases 5–7, suggest the oropharynx as the primary site for a nodal metastasis [13–17]. Given limited materials on many of our cases, further subtyping of the HPV present was not possible. However, the prominent p16 immunoreactivity in addition to the punctate (integrated) HPV DNA signal strongly suggests a transcriptionally active high risk HPV subtype. Indeed the initial clinical work-up of two cases (cases 6 and 7) demonstrated HPV16 by a PCR based methodology (data not shown). Most recently, it has been demonstrated on the other hand that salivary MEC do not harbor transcriptionally active HPV regardless of translocation status [18].
Most of the cases did not raise consideration for MEC since they were mucin poor and in some cases showed a bilayered tubuloglandular configuration. In such cases however, ‘basaloid’ salivary gland tumor considerations are plausible. However, most basaloid salivary gland tumors tend to be monomorphic and show a myoepithelial component. Interestingly, in case 7, a cystic lymph node metastasis, where the glandular component was more oncocytoid, a diagnosis of malignant transformation of Warthin tumor was entertained given this bilayered appearance in areas.
Perhaps the most unanticipated finding here is the presence of ciliated cells in this series. Thus, this subset of HPV-related non-keratinizing AsqCA is yet another exception to the ‘cardinal rule’ that ciliated epithelium equates to a benign process. We considered the possibility that these represent residual entrapped ciliated cells from normal structures such as excretory ducts or patches of native respiratory epithelium that may be noted even in palatine tonsillar areas. However, these cells showed a similar spectrum of cytonuclear atypia as well as positivity for HPV by in situ hybridization. More importantly, these ciliated cells were noted in lymph nodes metastases. Hence, the assumption that the ciliated epithelium represents a benign process is not always accurate. The ciliated appearance does however perhaps represent an extreme form of differentiation recapitulating normal elements occasionally seen even in normal tonsil as noted above. The presence of ciliated carcinoma cells has precedent in a variety of adenocarcinomas [19, 20], and has in fact been described in the veterinary pathology literature in one case of adenosquamous carcinoma of the nasal cavity! [21].
Another issue with ciliated components pertaining specifically to the cystic lymph node metastases is the re-emergence of the hypothetical (almost mythical) consideration of so-called ‘branchiogenic carcinoma’ arising from a second branchial cleft cyst. This diagnosis stirred controversy even at the time of its original description and has continued to do so to this day. Current consensus is that essentially all such lesions are cystic metastases rather than true malignancies arising from branchial cleft cysts. However, authors such as Khafif et al. [22] allow for the possibility of the existence of branchial cleft cyst carcinoma assuming that strict criteria were employed:
The tumor located in the anatomic region of the branchial cleft cyst or sinus.
The tumor consistent with its origin from branchial vestiges (i.e. squamous cell carcinoma).
Carcinoma is noted within the lining of an identifiable cyst.
There is a transition from the benign squamous or respiratory epithelium of the cyst to carcinoma.
There is no identifiable primary malignant tumor after exhaustive evaluation of the patient; this must include endoscopy (nasopharyngoscopy, laryngoscopy, bronchoscopy, and esophagoscopy), radiographic examinations, full CT scan of the head and neck, and appropriate biopsies. Many authors also advocate a minimum of 5 years follow-up without evidence of a primary tumor [23].
In our cases with unknown primary sites, a proponent of the branchiogenic carcinoma theory may argue that the ciliated columnar epithelium noted in the cases represents ‘normal’ cyst epithelium with the proliferative areas representing transition to tumor. However, akin to the oropharyngeal cases in this study, p16 and HPV positivity argue for a mucosal derivation, once again relegating ‘branchial cleft cyst carcinoma’ to ‘pathologic folklore’ status. Another point of contention is the failure to identify a primary tumor despite bilateral tonsillectomy. However, there is an increasing recognition that the lingual tonsillar tissue of base of tongue along with other parts of the Waldeyer ring may harbor occult primary as well. While traditionally difficult to access, base of tongue resections are now more common with the advent of transoral robotic surgery, which can identify occult primary tumors in a good proportion of patients failing conventional work-up [24]. Thus, for our cases with unknown primary, other subsites such as base of tongue remain far more plausible than invoking ‘branchial cleft cyst carcinoma.’
The occurrence of cilia in this subgroup of AsqCA do raise the question of whether this is the defining phenotype for HPV associated AsqCA of oropharynx. However, non-ciliated tumors with a similar appearance have been reported [2]. Another question that arises is whether ciliated epithelium, when present, can be used to distinguish AsqCA from MEC. However, though this is not documented in the literature to our knowledge, it is plausible that MEC can demonstrate ciliated components since MEC are thought to recapitulate an excretory duct phenotype [25], and ciliated epithelium is a component of excretory ducts. Thus, we would be reluctant to rely on ciliated epithelium in this context.
The small size of this series precludes strong statements regarding clinical outcome, but it does appear that unlike the prototypical AsqCA, the ciliated AsqCA do not necessarily have a uniformly aggressive outcome. This may be attributable to HPV status as seen previously in the study by Masand et al. [2].
In summary, we describe a subset of ciliated HPV-associated AsqCA that display a deceptively bland appearance and occasionally mimicking MEC, and raising consideration for other salivary gland tumor types. However, MAML2 rearrangements are uniformly negative, and in conjunction with surface components (when present), even focal atypia and keratinization may be useful excluding MEC. When seen in neck lymph nodes, the metastases are often cystic, and the theoretical consideration of branchiogenic carcinoma resurfaces given the ciliated component. However, the presence of HPV DNA argues for origin from a surface/crypt epithelium and may thus suggest possible mucosal sites of origin.
Acknowledgments
The authors would like to thank the University of Pittsburgh Pathology Research Immunohistochemistry and In situ Hybridization Laboratory for their excellent work in performing the ancillary testing for this study.
References
- 1.Keelawat S, Liu CZ, Roehm PC, Barnes L. Adenosquamous carcinoma of the upper aerodigestive tract: a clinicopathologic study of 12 cases and review of the literature. Am J Otolaryngol. 2002;23(3):160–168. doi: 10.1053/ajot.2002.123462. [DOI] [PubMed] [Google Scholar]
- 2.Masand RP, El-Mofty SK, Ma XJ, Luo Y, Flanagan JJ. Lewis Jr JS. Adenosquamous carcinoma of the head and neck: relationship to human papillomavirus and review of the literature. Head Neck Pathol. 2011;5(2):108–116. doi: 10.1007/s12105-011-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerughty RM, Hennigar GR, Brown FM. Adenosquamous carcinoma of the nasal, oral and laryngeal cavities. A clinicopathologic survey of ten cases. Cancer. 1968;22(6):1140–1155. doi: 10.1002/1097-0142(196811)22:6<1140::AID-CNCR2820220610>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Damiani JM, Damiani KK, Hauck K, Hyams VJ. Mucoepidermoid-adenosquamous carcinoma of the larynx and hypopharynx: a report of 21 cases and a review of the literature. Otolaryngol Head Neck Surg. 1981;89(2):235–243. doi: 10.1177/019459988108900218. [DOI] [PubMed] [Google Scholar]
- 5.Evans HL. Mucoepidermoid carcinoma of salivary glands: a study of 69 cases with special attention to histologic grading. Am J Clin Pathol. 1984;81(6):696–701. doi: 10.1093/ajcp/81.6.696. [DOI] [PubMed] [Google Scholar]
- 6.Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34(8):1106–1121. doi: 10.1097/PAS.0b013e3181de3021. [DOI] [PubMed] [Google Scholar]
- 7.Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33(2):208–213. doi: 10.1038/ng1083. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama T, Miyabe S, Okabe M, Sakuma H, Ijichi K, Hasegawa Y, et al. Clinicopathological significance of the CRTC3–MAML2 fusion transcript in mucoepidermoid carcinoma. Mod Pathol. 2009;22(12):1575–1581. doi: 10.1038/modpathol.2009.126. [DOI] [PubMed] [Google Scholar]
- 9.Alos L, Castillo M, Nadal A, Caballero M, Mallofre C, Palacin A, et al. Adenosquamous carcinoma of the head and neck: criteria for diagnosis in a study of 12 cases. Histopathology. 2004;44(6):570–579. doi: 10.1111/j.1365-2559.2004.01881.x. [DOI] [PubMed] [Google Scholar]
- 10.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 11.Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82(7):1217–1224. doi: 10.1002/(SICI)1097-0142(19980401)82:7<1217::AID-CNCR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25(7):835–845. doi: 10.1097/00000478-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9(17):6469–6475. [PubMed] [Google Scholar]
- 14.El-Mofty SK, Zhang MQ, Davila RM. Histologic identification of human papillomavirus (HPV)-related squamous cell carcinoma in cervical lymph nodes: a reliable predictor of the site of an occult head and neck primary carcinoma. Head Neck Pathol. 2008;2(3):163–168. doi: 10.1007/s12105-008-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldenberg D, Begum S, Westra WH, Khan Z, Sciubba J, Pai SI, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30(7):898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 16.El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):339–345. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Chernock RD, El-Mofty SK, Thorstad WL, Parvin CA, Lewis JS., Jr HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3(3):186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop JA, Yonescu R, Batista D, Yemelyanova A, Ha PK, Westra WH. Mucoepidermoid carcinoma does not harbor transcriptionally active high risk human papillomavirus even in the absence of the MAML2 translocation. Head Neck Pathol. 2014;8(3):298–302. doi: 10.1007/s12105-014-0541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendrickson MR, Kempson RL. Ciliated carcinoma—a variant of endometrial adenocarcinoma: a report of 10 cases. Int J Gynecol Pathol. 1983;2(1):1–12. doi: 10.1097/00004347-198301000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Imai T, Suga M, Kaimori M, Hiyama M, Yokoyama K, Kurotaki H. Peripheral pulmonary papillary adenocarcinoma with prominent cilia: report of a rare case that was difficult to diagnose preoperatively. Acta Cytol. 2010;54(5 Suppl):949–957. [PubMed] [Google Scholar]
- 21.Fukui D, Bando G, Ishikawa Y, Kadota K. Adenosquamous carcinoma with cilium formation, mucin production and keratinization in the nasal cavity of a red fox (Vulpes vulpes schrencki) J Comp Pathol. 2007;137(2–3):142–145. doi: 10.1016/j.jcpa.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Khafif RA, Prichep R, Minkowitz S. Primary branchiogenic carcinoma. Head Neck. 1989;11(2):153–163. doi: 10.1002/hed.2880110209. [DOI] [PubMed] [Google Scholar]
- 23.Gourin CG, Johnson JT. Incidence of unsuspected metastases in lateral cervical cysts. Laryngoscope. 2000;110(10 Pt 1):1637–1641. doi: 10.1097/00005537-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Byrd JK, Smith KJ, de Almeida JR, Albergotti WG, Davis KS, Kim SW, et al. Transoral robotic surgery and the unknown primary: a cost-effectiveness analysis. Otolaryngol Head Neck Surg. 2014;150(6):976–982. doi: 10.1177/0194599814525746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhry AP, Cutler LS, Leifer C, Labay G, Satchidanand S, Yamane GM. Ultrastructural study of the histogenesis of salivary gland mucoepidermoid carcinoma. J Oral Pathol Med. 1989;18(7):400–409. doi: 10.1111/j.1600-0714.1989.tb01572.x. [DOI] [PubMed] [Google Scholar]