Abstract
The endocytotic c-type lectin receptor DEC-205 is highly expressed on immature dendritic cells. In previous studies, it was shown that antigen-targeting to DEC-205 is a useful tool for the induction of antigen-specific Foxp3+ regulatory T cells and thereby can prevent inflammatory processes. However, whether this approach is sufficient to mediate tolerance in mucosal tissues like the gut is unknown. In this study, we established a new mouse model in which the adoptive transfer of naive hemagglutinin (HA)-specific CD4+Foxp3– T cells into VILLIN-HA transgenic mice leads to severe colitis. To analyze if antigen-targeting to DEC-205 could protect against inflammation of the gut, VILLIN-HA transgenic mice were injected with an antibody–antigen complex consisting of the immunogenic HA110–120 peptide coupled to an α-DEC-205 antibody (DEC-HA) before adoptive T cell transfer. DEC-HA-treated mice showed significantly less signs of intestinal inflammation as was demonstrated by reduced loss of body weight and histopathology in the gut. Strikingly, abrogated intestinal inflammation was mediated via the conversion of naive HA-specific CD4+Foxp3– T cells into HA-specific CD4+Foxp3+ regulatory T cells. In this study, we provide evidence that antigen-targeting to DEC-205 can be utilized for the induction of tolerance in mucosal organs that are confronted with large numbers of exogenous antigens.
Keywords: DEC-205, antigen-targeting, regulatory T cells, experimental colitis, inflammatory bowel disease
Introduction
Immunological tolerance is fundamental for the control of immune responses towards self or harmless antigens. Dysregulation of processes that mediate and maintain tolerance leads to the onset of autoimmune disorders. One prominent example is that of inflammatory bowel diseases (IBD) in humans that comprises Crohn’s disease and ulcerative colitis. Both diseases are characterized by a relapsing–remitting inflammatory state of the gut. Due to the complex etiology of IBD, to date, treatment options only imply symptomatic therapeutics. Immunologically, it is widely accepted that loss of tolerance towards commensal gut bacteria and/or dietary antigens is an initial event for the manifestation of IBD [1, 2]. Therefore, the directed induction of immunological tolerance in the gut represents a promising therapeutic approach for the treatment of inflammatory disorders including IBD.
Regulatory T cells (Treg) expressing the transcription factor Foxp3 represent a subpopulation of CD4+ T cells that is able to repress immune responses of different inflammatory immune cells [3]. Due to these features, Foxp3+ Treg are in the spotlight of many studies evaluating the potential for therapeutic intervention in inflammatory diseases. In fact, in a mouse model of intestinal inflammation, it was demonstrated that adoptive transfer of Foxp3+ Treg could not only prevent the onset of disease but also cure mice from an established colitis, demonstrating their potential therapeutic benefit in IBD [4, 5].
Since the ex vivo expansion of Treg for therapeutic application is complex and costly [6], it is desirable to induce and expand Treg in vivo. One promising approach for this task is the utilization of dendritic cells (DCs) as professional antigen-presenting cells of the immune system. In addition to their capability to induce potent immune responses towards pathogens, DCs are efficient inducers of immune tolerance, i.e., by the induction of Foxp3+ Treg [7]. Among intestinal CD103+ DCs that are able to generate Foxp3+ Treg in the gut in a TGF-β/retinoic acid-dependent manner [8, 9], in recent years, the population of CD8α+ DEC-205+ DCs has been identified as potent Treg inducers. DEC-205 is a c-type lectin receptor that is highly expressed on immature DCs and different epithelial cell types [10]. It has been demonstrated extensively that targeting antigens to DEC-205 by specific antibody–antigen complexes leads to the efficient uptake and presentation of antigens on MHC I and MHC II complexes resulting in the activation of antigen-specific CD8+ and CD4+ T cells, respectively [10, 11]. When applied to steady-state DCs, this approach leads to the induction of tolerance via different mechanisms, one of which being the efficient induction of Foxp3+ Treg [12], whereas pro-inflammatory immune responses are initiated in the presence of mature DCs [13]. Previously, we and others have demonstrated that inflammatory disorders can be prevented by this means in different autoimmune mouse models, i.e., in autoimmune diabetes or a mouse model of multiple sclerosis [14, 15]. However, in these studies, induction and expansion of Treg were achieved in organs like the pancreas or the central nervous system, which are not exposed to exogenous antigens. The gastrointestinal tract, on the other hand, is the organ of the body that is confronted most with factors not only from the outer environment but also by a vast number of antigens from commensal gut bacteria. Due to these factors, a state of “physiological inflammation” predominates in the gut [16]. Whether antigen-targeting to DEC-205 can lead to the induction of tolerance instead of immunity in the gut under these unideal conditions has not been addressed so far. Therefore, in the present study, we investigated the potential of DEC-205-mediated antigen-targeting for the prevention of intestinal inflammation.
Materials and methods
Mice
BALB/c mice were obtained from Harlan (Harlan Winkelmann, Borchen, Germany). VILLIN-HA transgenic mice express the A/PR/8/34 influenza hemagglutinin under the enterocyte-specific villin promotor [17]. T cell receptor (TCR)-HA transgenic mice that harbor CD4+ T cells expressing an α/β-TCR specific for the MHC class II H2Ed:HA110–120-restricted epitope of the HA protein [18] were bred to Foxp3-GFP mice expressing both Foxp3 and GFP under the endogenous regulatory sequence of the Foxp3 locus; Foxp3-GFP mice were obtained from Charles River Laboratories (Sulzfeld, Germany). All animal experiments were performed in accordance with institutional, state and federal guidelines (approved by the LandesamtfürNatur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, Germany).
Conjugation of the HA110–120 peptide to a DEC-205-specific mAb
Conjugation of the HA110–120 peptide to the DEC-205-specific monoclonal antibody (mAb) was performed as previously described [12]. In brief, α-DEC-205 (clone NLDC-145) or an IgG control antibody (clone GL117, kindly provided by Prof. Dr. Karsten Mahnke, University of Heidelberg) was purified from hybridoma supernatants and coupled to an activated HA110–120 peptide using the heterobifunctional cross-linker SMCC (succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-car-boxylate) (Pierce, Thermo Scientific, Bonn, Germany) according to the manufacturer’s protocol, resulting in the conjugates DEC-HA and GL117-HA, respectively.
Antibodies and flow cytometry
Flow cytometric analysis was performed using an antibody against α-mouse CD4 (RM4-5) (BD Bioscience, Heidelberg, Germany) as pacific blue conjugate. The mAb 6.5 (binds to the HA-specific TCR of CD4+ T cells from TCR-HA mice) [18] was purified from hybridoma supernatants and coupled to the fluorochrome AlexaFluour647 using the AlexaFluor647 Protein Labeling Kit (Molecular Probes, Invitrogen, Karlsruhe, Germany) according to the manufacturer’s recommendation. Dead cell exclusion was achieved by the addition of 7AAD to the antibody solution (eBioscience, San Diego, CA, USA). Flow cytometric analysis was performed on an LSRII using FACS DIVA software (BD Bioscience, Heidelberg, Germany).
RNA isolation and quantitative real-time PCR
RNA isolation from biopsies of murine colons was performed using the RNeasy Fibrous Tissue Kit according to the manufacturer’s recommendation (Qiagen, Hilden, Germany). Afterwards, 1 µg of whole RNA was reversely transcribed using M-MLV reverse transcriptase (H-), point mutant (Promega, Mannheim, Germany). Quantitative real-time PCR analysis was performed in an ABI PRISM cycler using the Fast SYBR Green Master Mix (both Life Technologies, Darmstadt, Germany) and specific primers for IFN-γ (5′-AGG AAC TGG CAA AAG GAT GGT GA-3′ and 5′-TGT TGC TGA TGG CCT GAT TGT CTT-3′), TNF-α (5′-CAA TGC ACA GCC TTC CTC ACA G-3′ and 5′-CCC GGC CTT CCA AAT AAA TAC AT-3′), and RPS-9 (5′-CTG GAC GAG GGC AAG ATG AAG C-3′ and 5′-TGA CGT TGG CGG ATG AGC ACA-3). Relative mRNA levels were calculated by using included standard curves for each individual gene and further normalization to the housekeeping gene RPS-9.
Isolation, flow cytometry, and FACS-sorting of lymphocytes
Spleens and MLN were mashed through 70 µm cell strainers and washed with erythrocyte lysis buffer or PBS containing 2% FCS and 2 mM EDTA, respectively. Lymphocytes from the lamina propria of the colon were isolated as described previously [17]. In brief, colons were rinsed extensively with ice cold PBS and cut into small pieces followed by washing steps in PBS/2 mM EDTA and cell culture media under constant stirring at 37 °C. Afterwards, colons underwent digestion with collagenase IV (Sigma, Bonn, Germany) for 90 min at 37 °C. Afterwards, suspensions were passed through 70 µm cell strainers to obtain single cell suspensions.
CD4+ T cells were enriched from spleens of TCR-HA/Foxp3-GFP mice using autoMACS technology according to the manufacturer’s recommendation (Miltenyi Biotec, Bergisch-Gladbach, Germany). To obtain a pure population of HA-specific CD4+Foxp3- T cells, cells were stained with α-CD4 and an antibody against the HA-specific TCR (6.5) and sorted for CD4+ 6.5+ (HA-specific TCR) Foxp3– (GFP–) T cells on an ARIA II cell sorter (BD Bioscience, Heidelberg, Germany). Purity of sorted cells was ≥95%.
Induction of intestinal inflammation and therapeutic treatment of VILLIN-HA transgenic mice
For the induction of an acute colonic inflammation, HA-specific CD4+Foxp3– T cells were FACS sorted from spleens of TCR-HA/Foxp3-GFP mice and 2.75 × 106 cells were adoptively transferred intravenously into VILLIN-HA transgenic mouse. Mice were monitored daily for signs of sickness (i.e., body weight loss) and were sacrificed on day 6 post cell transfer for analysis.
For the in vivo antigen-targeting to DEC-205, VILLIN-HA transgenic mice were injected i.p. with 1 μg of DEC-HA, GL117-HA, or 200 μl sterile PBS on days –2 and –1 before adoptive transfer of HA-specific CD4+ T cells.
Histopathological analysis
Colons were rinsed extensively with ice-cold PBS and immersion fixed with 4% buffered formalin, embedded in paraffin, sectioned at 4 μm thickness and stained with hematoxylin and eosin (H&E). The severity of histopathology was determined by scoring inflammation markers like infiltration of the colonic lamina propria with immune cells, atrophy and fusion of epithelial cells, cell hyperplasia, and necrosis from 0 (= no signs of inflammation) to 3 (= severe signs of inflammation) in a blinded fashion.
Statistical analysis
One-way ANOVA followed by Bonferroni’s multiple comparison or Student’s t test were used to determine statistical significance on Prism software (GraphPad, La Jolla, CA, USA). p values of ≤0.05 were considered significant.
Ethics statement
Animal experiments were performed in accordance with institutional, state, and federal guidelines. The animal protocol was approved by the Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen. Animals were handled with appropriate care and welfare, and all efforts were made to minimize suffering.
Results
Adoptive transfer of HA-specific CD4+Foxp3– T cells into VILLIN-HA transgenic mice leads to severe colonic inflammation
A large number of mouse models are available for the analysis of intestinal inflammatory disorders. However, only few models exist in which T cells with defined antigen-specificity are the driving force for the onset of inflammation. In VILLIN-HA transgenic mice, it was demonstrated that adoptive transfer of HA-specific CD8+ T cells leads to severe inflammation of the small intestine and the cecum [19]. However, adoptive transfer of HA-specific CD4+ T cells into VILLIN-HA transgenic mice does not result in inflammation of the gut [20]. From these findings, we hypothesized that CD4+Foxp3+ Treg that were cotransferred as part of the entire CD4+ T cell population suppressed T cell responses, thereby preventing inflammation in VILLIN-HA transgenic mice. To test this hypothesis, we FACS sorted HA-specific CD4+Foxp3– T cells from TCR-HA/Foxp3-GFP mice (Fig. 1a) and adoptively transferred these cells into VILLIN-HA transgenic mice or nontransgenic littermates. From day 4 post cell transfer, VILLIN-HA transgenic mice lost body weight reaching its peak on day 6 post cell transfer with about 15% less weight compared to their initial weight. Nontransgenic littermates were not affected by T cell transfer (Fig. 1b). Histopathological analysis of colonic sections revealed severe inflammation of the colon with epithelial cell hyperplasia, crypt loss, and increased immune cell infiltration of the lamina propria in VILLIN-HA transgenic mice (Fig. 1c). Again, nontransgenic control mice did not show any signs of inflammation in the gut. Hence, by the adoptive transfer of HA-specific CD4+Foxp3– T cells into VILLIN-HA transgenic mice, we have established a new mouse model that allows for the analysis of antigen-specific CD4+ T cell responses during intestinal inflammation.
Fig. 1.
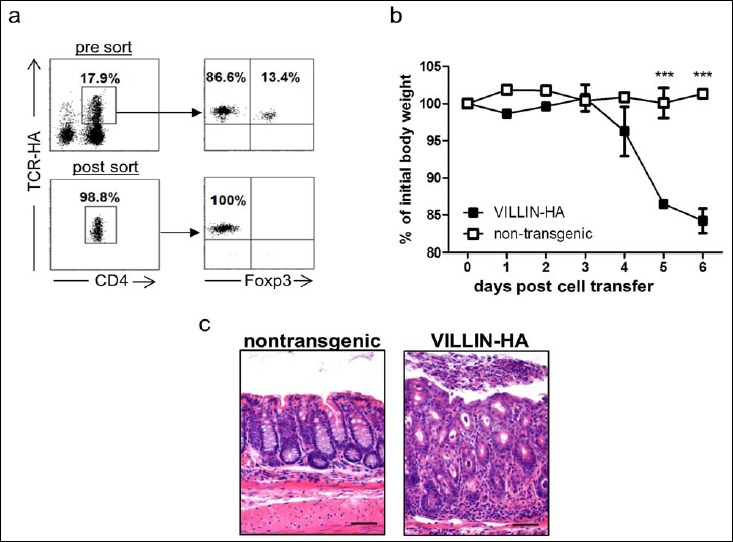
Adoptive transfer of HA-specific CD4+Foxp3– T cells into VILLIN-HA transgenic mice leads to severe intestinal inflammation. HA-specific CD4+Foxp3– T cells were FACS-sorted from spleens of TCR-HA/Foxp3-GFP mice (a) and were adoptively transferred into VILLIN-HA transgenic mice or nontransgenic littermates. Mice were monitored daily for signs of sickness, i.e., body weight (b). On day 6 post cell transfer, histopathological analysis of colonic sections was performed (c). Scale bars represent 50 μm. Representative data from one out of three independent experiments with similar results are shown as means ± SEM. Statistical analysis was performed using Student’s t test (***p < 0.001)
VILLIN-HA transgenic mice are protected from CD4+ T-cell-mediated intestinal inflammation by antigen-targeting to DEC-205
Next, we assessed the potential of antigen-targeting to DEC-205 for the prevention of intestinal inflammation in the newly established colitis model. Therefore, VILLIN-HA transgenic mice were injected i.p. with an antigen–antibody conjugate consisting of the HA110–120 epitope coupled to a DEC-205-specific antibody (DEC-HA) on days –2 and –1 prior to T cell transfer. As controls, mice received a conjugate of the HA110–120 epitope coupled to the nonbinding isotype control antibody GL117 (GL117-HA) or sterile PBS (Fig. 2a). On day 0, adoptive transfer of HA-specific CD4+Foxp3– T cells was performed; mice were analyzed on day 6 post cell transfer. GL117-HA- and PBS-treated mice showed loss of body weight of 5–10% on day 6 post cell transfer compared to their initial weight. In contrast, DEC-HA-treated animals exhibited a constant body weight over the observed period of time, which was significantly higher than that of the control groups on day 6 post cell transfer (Fig. 2b). Histopathological analysis revealed massive inflammation in the colon of GL117-HA- and PBS-treated groups with severe destruction of the epithelial layer and strong infiltration of the lamina propria with immune cells. Strikingly, DEC-HA treatment clearly abrogated inflammation of the colon with significant lower histological scores than control animals (Fig. 2c).
Fig. 2.
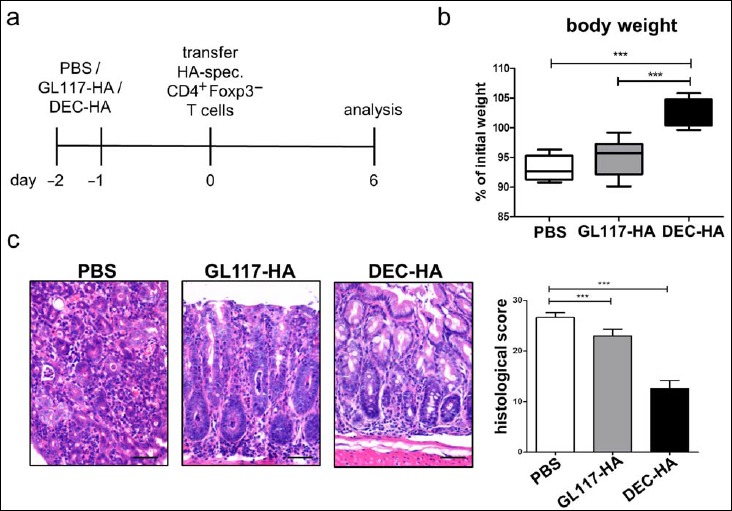
Antigen-targeting to DEC-205 leads to less severe histopathology in the colon of VILLIN-HA transgenic mice. VILLIN-HA transgenic mice were injected i.p. twice with DEC-HA, GL117-HA, or PBS, followed by adoptive transfer of HA-specific CD4+Foxp3– T cells (a). Mice were monitored for 6 days for body weight (b) and clinical signs of sickness. On day 6 post cell transfer, histopathological analysis of the colons was performed (c). Scale bars represent 50 μm. Data from three independent experiments are shown as means ± SEM. Statistical analysis was performed using one-way ANOVA (***p < 0.001)
To further characterize the severity of inflammation in the different groups, we performed quantitative real-time PCR (qPCR) analysis on colonic biopsies evaluating the expression of pro-inflammatory mediators such as IFN-γ and TNF-α. Both cytokines were highly upregulated in the gut of GL117-HA- and PBS-treated mice. In contrast, expression of IFN-γ and TNF-α in the colons of the DEC-HA group was only slightly above detection limit of the qPCR, suggesting a strong protection in the gut of these mice (Fig. 3). In summary, these data clearly show that targeting the HA-antigen to DEC-205 leads to a significant reduction of intestinal inflammation in this experimental colitis model.
Fig. 3.
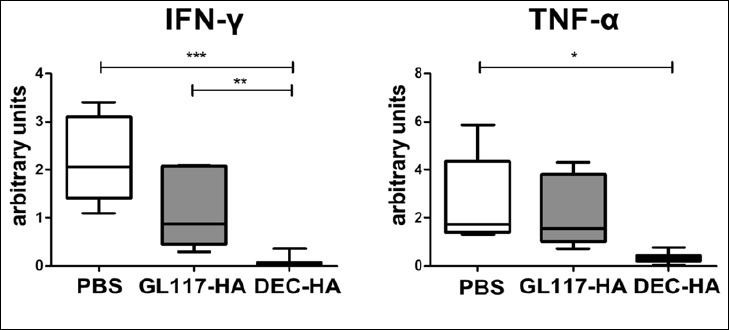
IFN-γ and TNF-α expression in the colon of VILLIN-HA transgenic mice is significantly reduced after DEC-HA treatment. HA-specific CD4+Foxp3– T cells were adoptively transferred into DEC-HA-, GL117-HA- or PBS-treated VILLIN-HA transgenic mice. On day 6 post cell transfer, quantitative real-time PCR analysis for IFN-γ and TNF-α was performed of colon biopsies and normalized to the expression of the house keeping gene RPS-9. Data from three independent experiments are shown as means ± SEM. Statistical analysis was performed using one-way ANOVA (*p < 0.05; **p < 0.01, ***p < 0.001)
Protection against intestinal inflammation is mediated by the induction of Foxp3+ Treg
In previous studies, we and others have shown that antigen-targeting to DEC-205 in vivo leads to the de novo generation and expansion of antigen-specific Foxp3+ Treg [14, 15]. To evaluate the protective mechanism of DEC-HA treatment, cells from the colonic lamina propria, the MLN, and spleens were analyzed for the presence of HA-specific CD4+Foxp3+ T cells. In GL117-HA- and PBS-treated mice, between 2% and 12% of the transferred HA-specific CD4+Foxp3– T cells were converted into Foxp3+ Treg (Fig. 4). Remarkably, DEC-HA treatment led to conversion of up to 40% of the transferred HA-specific T cells into Foxp3+ Treg in all analyzed organs. Interestingly, Foxp3+ Treg were not only detectable in high numbers in the spleen but more importantly in the colonic lamina propria and the MLN. Hence, these results show that antigen-targeting to DEC-205 provides protection from severe colitis via the efficient conversion of naive HA-specific CD4+Foxp3 T cells into HA-specific Foxp3+ Treg in the gut.
Fig. 4.
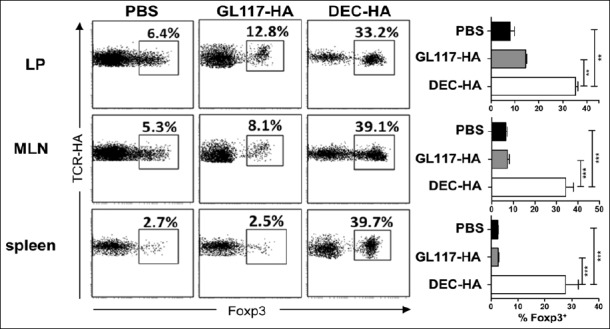
DEC-HA treatment leads to the de novo generation of Foxp3+ Treg in VILLIN-HA transgenic mice. HA-specific CD4+Foxp3– T cells were adoptively transferred into DEC-HA-, GL117-HA-, or PBS-treated VILLIN-HA transgenic mice. On day 6 posttransfer, spleens, MLN, and cells of the colonic lamina propria (LP) were analyzed by flow cytometry for the expression of the transgenic TCR and intracellular Foxp3. Data from three independent experiments are shown as means ± SEM. Statistical analysis was performed using one-way ANOVA (**p < 0.01; ***p < 0.001)
Discussion
The loss of immunological tolerance towards self or harmless antigens is an initial event for the manifestation of inflammatory disorders, i.e., autoimmune diseases [21]. With the discovery of Treg, defined by the expression of the transcription factor Foxp3, it has been demonstrated in different studies over the past years that this cell subset is able to maintain homeostasis of the immune system and thereby to prevent the onset of autoimmune responses [22, 23]. One promising approach for the in vivo generation of Treg is to target antigens to immature DCs expressing the surface receptor DEC-205 [10, 24]. However, in the context of mature DCs, antigen-targeting to DEC-205 leads to the induction of efficient immune responses, presumably due to their increased expression of costimulatory molecules and pro-inflammatory cytokines [13, 25]. Due to the large number of exogenous antigens and the presence of a tremendous amount of commensal gut bacteria, a state of “physiological inflammation” prevails in the gut [16]. These circumstances potentially impede the successful induction of tolerance through antigen-targeting to DEC-205. However, data from different studies suggest that especially intestinal DCs show lower expression of costimulatory and MHC II molecules as well as lower pro-inflammatory (i.e., IL-12) but higher expression of anti-inflammatory (i.e., TGF-β, IL-10) cytokines than DCs from other organs [26, 27]. These ambivalent conditions in the gut raise the question whether DEC-205-mediated antigen-targeting is sufficient to induce tolerogenic rather than immunogenic responses in mucosal tissues. Antigen-targeting to DEC-205 is known to confer tolerance mainly via three mechanisms: the deletion and anergy of T cells and the conversion of naive CD4+ T cells into Foxp3+ Treg [24, 28, 29]. In this study, we identified that DEC-HA treatment converted naive CD4+ Foxp3– T cells into Foxp3+ Treg, unperturbed by the mucosal environment and thereby mediating protection from intestinal inflammation. These data again highlight the potential of Treg for the suppression/prevention of inflammatory disorders similar to previous studies [4, 5]. Interestingly, the induction was detectable in the spleen, the gut-draining mesenteric lymph nodes, and in the lamina propria of the colon, which suggests that the approach of DEC-205-targeted delivery of antigens in general systemically induces Foxp3+ Treg.
This approach of tolerance induction is dependent on an antigen specifically linked to the disease [30, 31]. CBir1 is a bacterial flagellin and the first antigen shown to have a direct pathogenic effect in the development of T-cell-mediated colitis in mice and humans. Cong and colleagues demonstrated that adoptive transfer of CD4+ T cells from C3H/HeJBir mice into immune-deficient SCID mice leads to the onset of intestinal inflammation [32]. Monocytes isolated from CD patients produce significantly higher amounts of IL-6 after stimulation with CBir1 flagellin than do monocytes from healthy controls. In addition, lamina propria mononuclear cells isolated from CD patients produce increased IFN-γ upon CBir1 flagellin stimulation compared to lamina propria mononuclear cells from healthy controls, supporting a pathogenic role for CBir1-specific responses in CD [33]. These data suggest that CBir1 could be a promising antigen for therapeutic intervention in IBD.
Taken together, our results clearly show that antigen-targeting to DEC-205 – despite of a physiologic state of inflammation in the gut – is sufficient to convert antigen-specific CD4+ T cells into Foxp3+ Treg leading to the amelioration of intestinal inflammation and making this approach a potential candidate for therapeutic intervention in human IBD.
Acknowledgements
We thank Witold Bartosik, Mechthild Hemmler-Roloff, and Patrick Juszczak for excellent technical assistance.
References
- 1.Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P: Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 130, 323–333; quiz 591 (2006) [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein GR, Thomsen OØ, Schreiber S, Lawrance IC, Hanauer SB, Hanauer SB, Bloomfield R, Sandborn WJ; Precise 3 Study Investigators: Continuous therapy with certolizumab pegol maintains remission of patients with Crohn’s disease for up to 18 months. Clin Gastroenterol Hepatol 8, 600–609 (2010) [DOI] [PubMed] [Google Scholar]
- 3.Shevach EM: Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30, 636–645 (2009) [DOI] [PubMed] [Google Scholar]
- 4.Mottet C, Uhlig HH, Powrie F: Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 170, 3939–3943 (2003) [DOI] [PubMed] [Google Scholar]
- 5.Powrie F: T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity 3, 171–174 (1995) [DOI] [PubMed] [Google Scholar]
- 6.McMurchy AN, Bushell A, Levings MK, Wood KJ: Moving to tolerance: clinical application of T regulatory cells. Semin Immunol 23, 304–313 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, Nussenzweig M.: Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci 987, 15–25 (2003) [DOI] [PubMed] [Google Scholar]
- 8.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y: Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204, 1775–1785 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F: A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204, 1757–1764 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, Steinman RM: The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol 151, 673–684 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM: Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8(+) T cell tolerance. J Exp Med 196, 1627–1638 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahnke K, Qian YJ, Knop J, Enk AH: Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood 101, 4862–4869 (2003) [DOI] [PubMed] [Google Scholar]
- 13.Idoyaga J, Lubkin A, Fiorese C, Lahoud MH, Caminschi I, Huang Y, Rodriguez A, Clausen BE, Park CG, Trumpfheller C, Steinman RM: Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc Natl Acad Sci U S A 108, 2384–2389 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian Y, von Boehmer H, Mahnke K, Buer J: On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes 54, 3395–3401 (2005) [DOI] [PubMed] [Google Scholar]
- 15.Stern JN, Keskin DB, Kato Z, Waldner H, Schallenberg S, Anderson A, von Boehmer H, Kretschmer K, Strominger JL: Promoting tolerance to proteolipid protein-induced experimental autoimmune encephalomyelitis through targeting dendritic cells. Proc Natl Acad Sci U S A 107, 17280–17285 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiocchi C: Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am J Physiol-Gastrointest Liver Physiol 273, G769-G775 (1997) [DOI] [PubMed] [Google Scholar]
- 17.Westendorf AM, Templin M, Geffers R, Deppenmeier S, Gruber AD, Probst-Kepper M, Hansen W, Liblau RS, Gunzer F, Bruder D, Buer J: CD4+ T cell mediated intestinal immunity: chronic inflammation versus immune regulation. Gut 54, 60–69 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H: Thymic Selection of CD8+ Single Positive Cells with a Class-Ii Major Histocompatibility Complex-Restricted Receptor. J Exp Med 180, 25–34 (1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westendorf AM, Fleissner D, Deppenmeier S, Gruber AD, Bruder D, Hansen W, Liblau R, Buer J: Autoimmune-mediated intestinal inflammation-impact and regulation of antigen-specific CD8+ T cells. Gastroenterology 131, 510–524 (2006) [DOI] [PubMed] [Google Scholar]
- 20.Westendorf AM, Fleissner D, Groebe L, Jung S, Gruber AD, Hansen W, Buer J: CD4+Foxp3+ regulatory T cell expansion induced by antigen-driven interaction with intestinal epithelial cells independent of local dendritic cells. Gut 58, 211–219 (2009) [DOI] [PubMed] [Google Scholar]
- 21.Atkinson TP: Immune deficiency and autoimmunity. Curr Opin Rheumatol 24, 515–521 (2012) [DOI] [PubMed] [Google Scholar]
- 22.Fontenot JD, Gavin MA, Rudensky AY: Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4, 330–336 (2003) [DOI] [PubMed] [Google Scholar]
- 23.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY: Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22, 329–341 (2005) [DOI] [PubMed] [Google Scholar]
- 24.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC: Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 194, 769–779 (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM: A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med 204, 1095–1106 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell SJ, Rigby R, English N, Mann SD, Knight SC, Kamm MA, Stagg AJ: Migration and maturation of human colonic dendritic cells. J Immunol 166, 4958–4967 (2001) [DOI] [PubMed] [Google Scholar]
- 27.Sato A, Hashiguchi M, Toda E, Iwasaki A, Hachimura S, Kaminogawa S: CD11b+ Peyer’s patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J Immunol 171, 3684–3690 (2003) [DOI] [PubMed] [Google Scholar]
- 28.Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC: Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dentritic cells in vivo. Immunity 20, 695–705 (2004) [DOI] [PubMed] [Google Scholar]
- 29.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H: Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol 6, 1219–1227 (2005) [DOI] [PubMed] [Google Scholar]
- 30.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM: Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest 113, 1296–1306 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elson CO: Commensal bacteria as targets in Crohn’s disease. Gastroenterology 119, 254–257 (2000) [DOI] [PubMed] [Google Scholar]
- 32.Cong Y, Brandwein SL, McCabe RP, Lazenby A, Birkenmeier EH, Sundberg JP, Elson CO: CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med 187, 855–864 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen C, Landers CJ, Derkowski C, Elson CO, Targan SR: Enhanced CBir1-specific innate and adaptive immune responses in Crohn’s disease. Inflamm Bowel Dis 14, 1641–1651 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]


