Abstract
We have previously shown that Arcobacter butzleri induces intestinal, extra-intestinal, and systemic immune responses in perorally infected gnotobiotic IL-10–/– mice in a strain-dependent fashion. Here, we present a comprehensive survey of small and large intestinal expression profiles of inflammatory and regulatory mediators as well as of the matrix-degrading gelatinases MMP-2 and MMP-9 following murine A. butzleri infection. Gnotobiotic IL-10–/– mice were infected with A. butzleri strains CCUG 30485 or C1 of human and chicken origin, respectively. At day 6 following A. butzleri infection, mucin-2 mRNA, an integral part of the intestinal mucus layer, was downregulated in the colon, whereas TNF and IL-23p19 mRNA were upregulated in the ileum. Furthermore, IFN-γ, IL-17A, IL-1β, and IL-22 mRNA were upregulated in both colonic and ileal ex vivo biopsies at day 6 post strain CCUG 30485 infection. These changes were accompanied by downregulated colonic MMP-9 levels, whereas both MMP-2 and MMP-9 mRNA were upregulated in the ileum. In conclusion, these data indicate that A. butzleri infection induces changes in the expression of genes involved in pro-inflammatory and regulatory immune responses as well as in tissue degradation.
Keywords: Arcobacter butzleri, Campylobacterales, IL-23/Th17 axis, IL-22/IL-18 axis, pro-inflammatory immune responses, intestinal tract, gnotobiotic IL-10–/– mouse infection model, gelatinases, host defence, mucosal immunology
Introduction
Motile, spiral-shaped gram-negative bacteria of the genus Arcobacter represent a taxonomical branch of the Campylobacteraceae family of the cladus Campylobacterales. In the intestinal tract of animals, Arcobacter species are considered commensals [1], whereas, in humans, Arcobacter butzleri and Arcobacter cryaerophilus have been both classified as potential hazards by the International Commission on Microbiological Specifications for Food [2]. Given that routine diagnostic measures usually fail to reliably detect and identify Arcobacter, robust epidemiological data in humans are scarce [1]. In retrospective studies, however, Arcobacter spp. have been graded as the fourth most common species within the Campylobacterales clade isolated from diarrheal subjects. Both sporadic cases and outbreaks have been reported in humans [3, 4]. Patients acquire Arcobacter spp. predominantly via the peroral route upon ingestion of contaminated food or water and present with acute, but self-limiting, gastroenteritis or prolonged watery diarrhea that can even persist for several weeks [3, 4]. So far, however, information regarding Arcobacter–host interaction has been limited due to scarcity of appropriate animal infection models. In order to explore mechanisms underlying A. butzleri infection in vivo, we have recently applied gnotobiotic (i.e., secondary abiotic) IL-10–/– mice generated by broad-spectrum antibiotic treatment. Initially, this infection model had been established for investigating Campylobacter jejuni infection in vivo [5]. Given the taxonomic relationship between A. butzleri with the Campylobacteraceae family, we infected gnotobiotic IL-10–/– mice with A. butzleri strains CCUG 30485 or C1, that had initially been isolated from a diseased patient and fresh chicken meat, respectively [6, 7]. Within 1 week, infected mice displayed small and large intestinal as well as extra-intestinal and systemic immune responses but did not exhibit clinical or macroscopic disease [8, 9]. In the present study, we dissected the contribution of defined molecules to the observed local (i.e., intestinal) immune responses in more detail. We therefore surveyed the expression of genes encoding inflammatory and regulatory mediators as well as matrix-degrading metalloproteinases (MMP) such as gelatinases and mucin-2 within the colon and ileum during A. butzleri infection.
Materials and methods
Gnotobiotic mice
IL-10–/– mice (in C57BL/10 background, B10) were bred and maintained in the facilities of the “Forschungseinrichtungen für Experimentelle Medizin” (FEM, Charité – Universitätsmedizin, Berlin, Germany) under specific pathogen-free (SPF) conditions. To overcome physiological colonization resistance and assure stable colonization, gnotobiotic (i.e., secondary abiotic) IL-10–/– mice with a deprived gastrointestinal microbiota were generated following broad-spectrum antibiotic treatment as described earlier [10, 11]. In brief, mice were transferred to sterile cages and treated by adding ampicillin–sulbactam (1 g/l; Pfizer, Berlin, Germany), vancomycin (500 mg/l; Hexal, Holzkirchen, Germany), ciprofloxacin (200 mg/l; Hexal), imipenem (250 mg/l; Fresenius Kabi, Graz, Austria), and metronidazole (1 g/l; Braun, Melsungen, Germany) to the drinking water ad libitum starting at 3 weeks of age immediately after weaning and continued for approximately 3 months before the infection experiment [12]. Three days prior infection, the antibiotic cocktail was replaced by sterile tap water (ad libitum). Abiotic status of gnotobiotic mice was confirmed as described earlier [5, 10].
Arcobacter butzleri infection of mice
Four-month old female gnotobiotic IL-10–/– mice were perorally infected with approximately 109 viable colony forming units (CFU) of two different A. butzleri strains either (CCUG 30485 or C1 strain, respectively) by gavage in a total volume of 0.3 ml phosphate buffered saline (PBS) on two consecutive days (day 0 and day 1) as described previously [13, 14]. Naive age-matched gnotobiotic IL-10–/– mice served as uninfected controls. The A. butzleri reference strain CCUG 30485 was initially isolated from a fecal sample derived from a diarrheal patient [6], whereas the C1 strain was isolated from fresh chicken meat [7]. Both A. butzleri strains were grown on Kar maliagar (Oxoid, Wesel, Germany) for 2 days at 37 °C under microaerobic conditions using CampyGen gas packs (Oxoid) as described earlier [5, 8, 9].
Sampling procedures
Mice were sacrificed by isofluran treatment (Abbott, Greifswald, Germany) on day 6 or 16 p.i. Tissue samples from ileum and colon were removed under sterile conditions.
Real-time PCR
Total RNA was isolated from snap frozen colonic and ileal ex vivo biopsies, reverse transcribed, and analyzed as described previously [15]. Briefly, murine mRNAs coding for mucin-2, IFN-γ, TNF, IL-17A, IL-1β, IL-23p19, IL-22, IL-18, MMP-2, MMP-9, TIMP-1, and TIMP-3 were detected by real-time polymerase chain reaction (PCR) with specific primers and quantified by analysis with the Light Cycler Data Analysis Software (Roche). The mRNA of the housekeeping gene for hypoxanthine-phosphoribosyltransferase (HPRT) was used as reference; the mRNA expression levels of the individual genes were normalized to the lowest measured value and expressed as fold expression (arbitrary units).
Statistical analysis
Medians and levels of significance were determined using Mann–Whitney U test (GraphPad Prism v6.05, La Jolla, CA, USA). Two-sided probability (P) values of ≤0.05 were considered significant. Experiments were reproduced twice.
Ethics statement
All animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, registration number G0184/12). Animal welfare was monitored twice daily by assessment of clinical conditions.
Results
Peroral A. butzleri infection of gnotobiotic IL-10–/– mice
In the present study, we performed for the first time a comprehensive survey of intestinal expression profiles of inflammatory mediators including T helper cell (Th) 17 cytokines important for host antimicrobial immunity and matrix-degrading gelatinases during A. butzleri infection. To address this, we infected gnotobiotic IL-10–/– mice [11] with the A. butzleri strains CCUG 30485 or C1 (109 CFU each) perorally by gavage [8, 9]. Mice were stably colonized by either strain with median intestinal loads of 108 CFU per gram feces until the end of the experiment at day 16 p.i. [8, 9]. Interestingly, even though mice did not display any overt clinical signs of enteric disease including wasting, diarrhea, or occurence of blood in fecal samples at day 6 or day 16 p.i., distinct pro-inflammatory immune responses could be observed in small and large intestinal as well as extra-intestinal and systemic compartments [8, 9].
Large intestinal gene expression of mucin-2, pro-inflammatory mediators, and gelatinases in A. butzleri-infected gnotobiotic IL-10–/– mice
Within the mucus layer of the small and large intestinal tract, mucin-2 is expressed and contributes significantly to combating bacterial infection and, subsequently, maintenance of intestinal epithelial barrier function [16, 17]. We therefore investigated whether A. butzleri infection affects mucin-2 expression in the colon of gnotobiotic IL-10–/– mice. At either time point of C1 strain infection and at day 6 following CCUG 30485 strain challenge, colonic mucin-2 expression was downregulated (p < 0.05–0.01; Fig. 1). Next, we determined expression of pro-inflammatory mediators in large intestines of A. butzleri-infected mice. Whereas colonic TNF mRNA levels were rather comparable between infected and naive mice (Fig. 2B), IFN-γ, IL-17A, and IL-1β mRNA expression were upregulated as early as 6 days p.i. (p < 0.01–0.001; Fig. 2A, C, and D) and remained elevated until day 16 p.i. in case of IL-17A (p < 0.05; Fig 2C), but not of the remaining ones after infection with A. butzleri strain CCUG 30485 (Fig. 2A and D). In addition, colonic IL-17A mRNA levels increased at day 16 following C1 strain infection (p < 0.05; Fig. 2C). Moreover, large intestinal expression of IL-23p19, a master regulator of mucosal immunity during intestinal infection and inflammation [18], did not change upon A. butzleri infection (Fig. 3A), whereas colonic IL-22 was upregulated at day 6 following CCUG 30485 strain infection (p < 0.001; Fig. 3B), as observed for IFN-γ, IL-17A, and IL-1β. Colonic IL-18 mRNA levels, however, were increased at day 16, but not day 6, following CCUG 30485 strain infection, and upregulated in C1 strain-infected mice at either time point (p < 0.05–0.01; Fig. 3C). Given that matrix-degrading gelatinases are upregulated during intestinal inflammation upon infection with Toxoplasma gondii [15] and C. jejuni [19–21], we analyzed expression of the genes encoding MMP-2 and -9 following A. butzleri infection. Moreover, we determined large intestinal expression of the tissue inhibitors of metalloproteinases (TIMP) -1 and -3, that counteract gelatinase expression and function [22, 23]. Whereas colonic MMP-2 expression was virtually unchanged in A. butzleri-infected mice (Fig. 4A), MMP-9 was downregulated at day 6 following CCUG 30485 and C1 strain infection (p < 0.01 and p < 0.05, respectively; Fig. 4B). Of the analyzed TIMPs, only TIMP-1 mRNA was upregulated in colonic ex vivo biopsies at day 16 following A. butzleri C1 strain infection (p < 0.05; Fig. 5). Hence, large intestinal immune responses upon A. butzleri infection shown previously [8, 13] are associated with a strain-dependent expression pattern of mucus constituents, pro-inflammatory mediators including Th17 cytokines and their regulatory molecules as well as of matrix-degrading gelatinases.
Fig. 1.
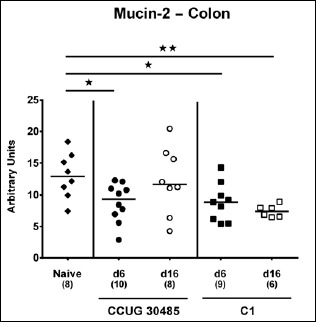
Changes in colonic mucin-2 mRNA levels in gnotobiotic IL-10–/– mice following A. butzleri infection. Gnotobiotic IL-10–/– mice were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 (circles) or strain C1 (squares). Uninfected mice served as negative control (naive; closed diamonds). Mucin-2 mRNA levels were determined in ex vivo colonic biopsies taken at days (d) 6 (closed symbols) or 16 (open symbols) postinfection. Medians (black bars), numbers of analyzed animals, and significance levels as determined by the Mann–Whitney U test are indicated (*p < 0.05; **p < 0.01). Data shown were pooled from three independent experiments
Fig. 2.
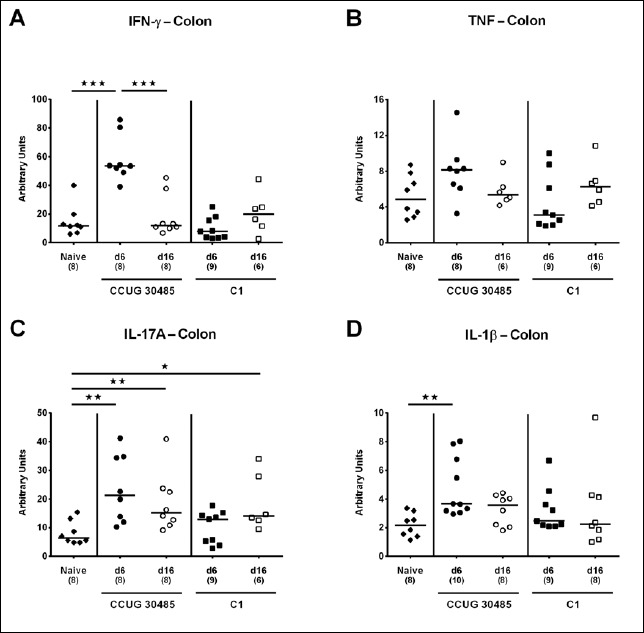
Colonic expression of genes coding for pro-inflammatory cytokines in gnotobiotic IL-10–/– mice following A. butzleri infection. Gnotobiotic IL-10–/– mice were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 (circles) or strain C1 (squares). Uninfected mice served as negative control (naive; closed diamonds). A) IFN-γ, B) TNF, C) IL-17A, and D) IL-1β mRNA levels were determined in ex vivo colonic biopsies taken at days (d) 6 (closed symbols) or 16 (open symbols) postinfection. Medians (black bars), numbers of analyzed animals, and significance levels as determined by the Mann-Whitney U test are indicated (*p < 0.05; **p < 0.01; ***p < 0.001). Data shown were pooled from three independent experiments
Fig. 3.
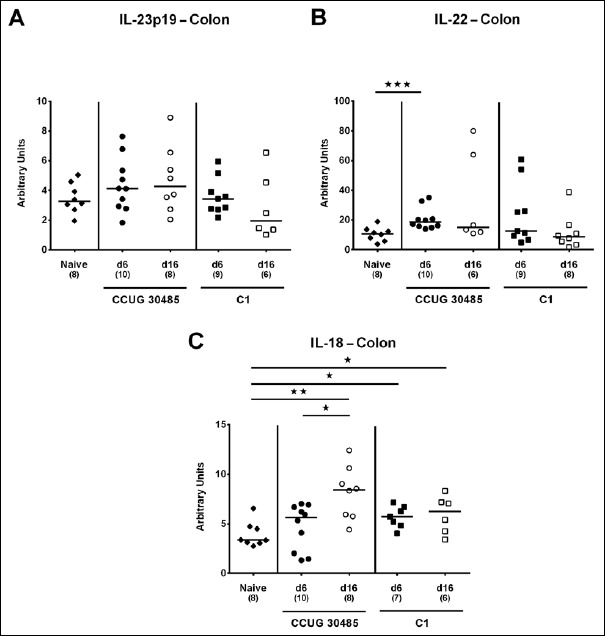
Colonic expression of genes coding for IL-23p19, IL-22, and IL-18 in gnotobiotic IL-10–/– mice following A. butzleri infection. Gnotobiotic IL-10–/– mice were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 (circles) or strain C1 (squares). Uninfected mice served as negative control (naive; closed diamonds). A) IL-23p19, B) IL-22, and C) IL-18 mRNA levels were determined in ex vivo colonic biopsies taken at days (d) 6 (closed symbols) or 16 (open symbols) postinfection. Medians (black bars), numbers of analyzed animals, and significance levels as determined by the Mann–Whitney U test are indicated (*p < 0.05; **p < 0.01; ***p < 0.001). Data shown were pooled from three independent experiments
Fig. 4.
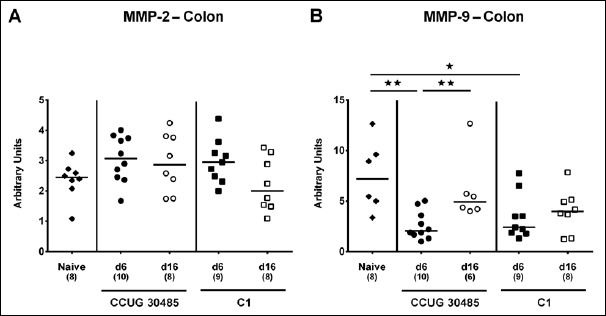
Colonic expression of genes coding for MMP-2 and MMP-9 in gnotobiotic IL-10–/– mice following A. butzleri infection. Gnotobiotic IL-10–/– mice were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 (circles) or strain C1 (squares). Uninfected mice served as negative control (naive; closed diamonds). A) MMP-2 and B) MMP-9 mRNA levels were determined in ex vivo colonic biopsies taken at days (d) 6 (closed symbols) or 16 (open symbols) postinfection. Medians (black bars), numbers of analyzed animals, and significance levels as determined by the Mann–Whitney U test are indicated (*p < 0.05; **p < 0.01). Data shown were pooled from three independent experiments
Fig. 5.
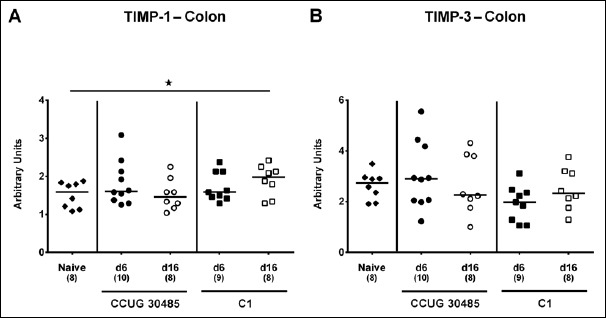
Colonic expression of genes coding for TIMP-1 and TIMP-3 in gnotobiotic IL-10–/– mice following A. butzleri infection. Gnotobiotic IL-10–/– mice were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 (circles) or strain C1 (squares). Uninfected mice served as negative control (naive; closed diamonds). A) TIMP-1 and B) TIMP-3 mRNA levels were determined in ex vivo colonic biopsies taken at days (d) 6 (closed symbols) or 16 (open symbols) postinfection. Medians (black bars), numbers of analyzed animals, and significance levels as determined by the Mann–Whitney U test are indicated (*p < 0.05). Data shown were pooled from three independent experiments
Small intestinal gene expression of mucin-2, pro-inflammatory mediators, and gelatinases in A. butzleri-infected gnotobiotic IL-10–/– mice
We next surveyed respective mRNA levels within a different compartment of the gastrointestinal tract, namely, the distal small intestines of A. butzleri-infected gnotobiotic IL-10–/– mice. Ileal mucin-2 expression was rather unaffected by A. butzleri infection. At day 16 following C1 strain infection, however, mice displayed lower mucin-2 levels than at day 6 p.i. (p < 0.05; Fig. 6). At day 6 following infection with either A. butzleri strain, ileal IFN-γ mRNA expression was upregulated (p < 0.01–0.001; Fig. 7A), whereas TNF mRNA levels were higher at either time point of CCUG 30485 strain infection and additionally at day 16 following C1 strain infection (p < 0.05–0.01; Fig. 7B). Ileal IL-17A and IL-1β were both upregulated at day 6 and the former additionally at day 16 following CCUG 30485 strain infection (p < 0.01–0.001; Fig. 7C and D). Increased IL-23p19 and IL-22 mRNA levels could be detected in small intestines of A. butzleri CCUG 30485 strain-infected mice at day 6 and day 16 p.i. (p < 0.05–0.001; Fig. 8A and B) and the latter additionally at day 6 following C1 strain infection (p < 0.01; Fig. 8B), whereas ileal IL-18 mRNA expression remained virtually unchanged during infection with either strain (Fig. 8C). A. butzleri CCUG 30485 strain-induced upregulation of pro-inflammtory cytokines at day 6 p.i. was accompanied by elevated ileal MMP-2 and MMP-9 mRNA expression (p < 0.05–0.01; Fig. 9). As opposed to the large intestines, MMP-9 was upregulated in the distal small intestinal tract at day 6 following C1 strain infection but returned to basal levels thereafter (p < 0.01 and p < 0.05, respectively; Fig. 9B). Interestingly, ileal expression levels of TIMP-1 and TIMP-3 were rather comparable between infected and naive mice (Fig. 10). Only TIMP-1 mRNA levels of CCUG 30485 strain infected mice were higher at day 6 versus day 16 p.i. (p < 0.01; Fig. 10A). These data clearly indicate not only strain- and kinetic- but also tissue-dependent differences in expression patterns of mucus constituents, pro-inflammatory mediators including Th17 cytokines and their regulatory molecules as well as of matrix-degrading gelatinases.
Fig. 6.
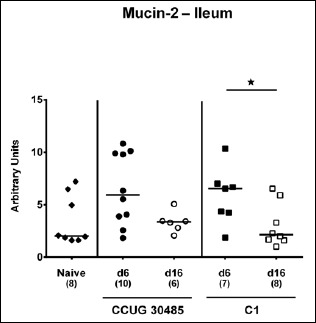
Changes in ileal mucin-2 mRNA levels in gnotobiotic IL-10–/– mice following A. butzleri infection. Gnotobiotic IL-10–/– mice were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 (circles) or strain C1 (squares). Uninfected mice served as negative control (naive; closed diamonds). Mucin-2 mRNA levels were determined in ex vivo ileal biopsies taken at days (d) 6 (closed symbols) or 16 (open symbols) postinfection. Medians (black bars), numbers of analyzed animals, and significance levels as determined by the Mann–Whitney U test are indicated (*p < 0.05). Data shown were pooled from three independent experiments
Fig. 7.
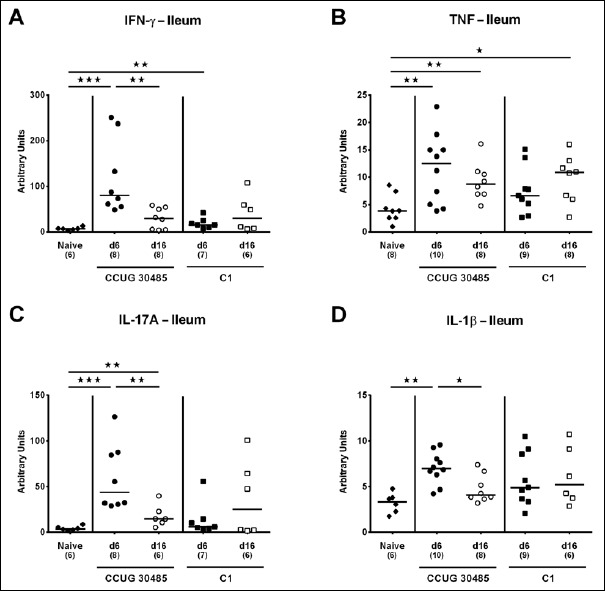
Ileal expression of genes coding for pro-inflammatory cytokines in gnotobiotic IL-10–/– mice following A. butzleri infection. Gnotobiotic IL-10–/– mice were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 (circles) or strain C1 (squares). Uninfected mice served as negative control (naive; closed diamonds). A) IFN-γ, B) TNF, C) IL-17A, and D) IL-1β mRNA levels were determined in ex vivo ileal biopsies taken at days (d) 6 (closed symbols) or 16 (open symbols) postinfection. Medians (black bars), numbers of analyzed animals, and significance levels as determined by the Mann-Whitney U test are indicated (*p < 0.05; **p < 0.01; ***p < 0.001). Data shown were pooled from three independent experiments
Fig. 8.
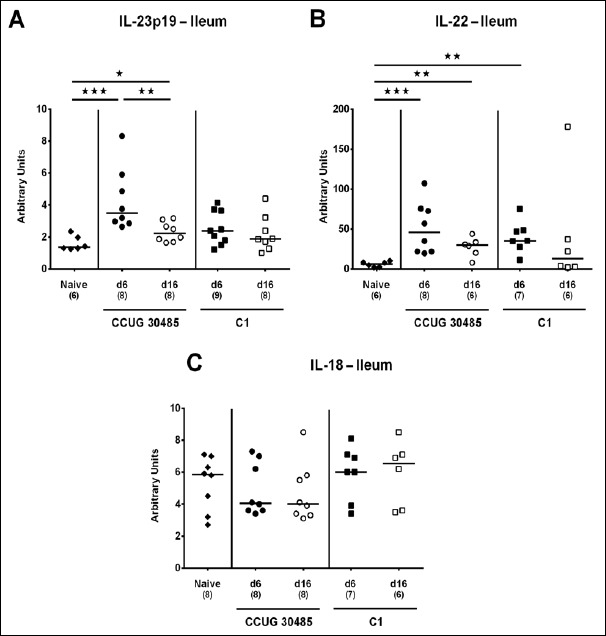
Ileal expression of genes coding for IL-23p19, IL-22, and IL-18 in gnotobiotic IL-10–/– mice following A. butzleri infection. Gnotobiotic IL-10–/– mice were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 (circles) or strain C1 (squares). Uninfected mice served as negative control (naive; closed diamonds). A) IL-23p19, B) IL-22, and C) IL-18 mRNA levels were determined in ex vivo ileal biopsies taken at days (d) 6 (closed symbols) or 16 (open symbols) postinfection. Medians (black bars), numbers of analyzed animals, and significance levels as determined by the Mann–Whitney U test are indicated (*p < 0.05; **p < 0.01; ***p < 0.001). Data shown were pooled from three independent experiments
Fig. 9.
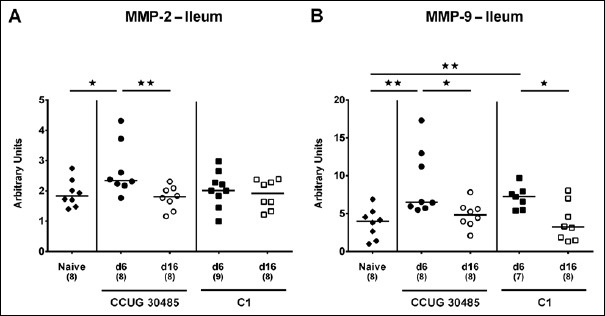
Ileal expression of genes coding for MMP-2 and MMP-9 in gnotobiotic IL-10–/– mice following A. butzleri infection. Gnotobiotic IL-10–/– mice were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 (circles) or strain C1 (squares). Uninfected mice served as negative control (naive; closed diamonds). A) MMP-2 and B) MMP-9 mRNA levels were determined in ex vivo ileal biopsies taken at days (d) 6 (closed symbols) or 16 (open symbols) postinfection. Medians (black bars), numbers of analyzed animals, and significance levels as determined by the Mann–Whitney U test are indicated (*p < 0.05; **p < 0.01). Data shown were pooled from three independent experiments
Fig. 10.
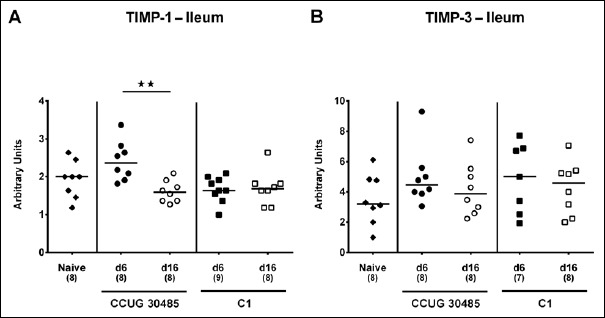
Ileal expression of genes coding for TIMP-1 and TIMP-3 in gnotobiotic IL-10–/– mice following A. butzleri infection. Gnotobiotic IL-10–/– mice were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 (circles) or strain C1 (squares). Uninfected mice served as negative control (naive; closed diamonds). A) TIMP-1 and B) TIMP-3 mRNA levels were determined in ex vivo ileal biopsies taken at days (d) 6 (closed symbols) or 16 (open symbols) postinfection. Medians (black bars), numbers of analyzed animals, and significance levels as determined by the Mann–Whitney U test are indicated (**p < 0.01). Data shown were pooled from three independent experiments
Discussion
In the present study, we performed a survey of small and large intestinal gene expression levels of molecules that are involved in mediating host resistance against bacterial infections during murine A. butzleri infection. Upon peroral infection, pathogens are confronted with the mucus layer protecting the underlying mucosal epithelial tissue from damage and preserving epithelial barrier function [24]. Mucins including mucin-2 are secreted complex glycoproteins that give mucus its viscous consistency and act as first line defence against pathogens [24]. To date, however, no information regarding Arcobacter–mucin interactions is available. In our study, colonic mucin-2 mRNA was downregulated in mice infected with either strain at day 6 p.i. and additionally at day 16 following A. butzleri C1 strain infection. Mucins have further been shown to act as major chemoattractants for bacterial pathogens such as C. jejuni [25]. Upon binding, C. jejuni–mucin-2 interaction results in reduced pathogenic growth and, besides other transcriptomic changes, in enhanced transcription of mucin-degrading enzymes in C. jejuni [26]. We could recently show that C. jejuni infection of conventionally colonized IL-10–/– mice was accompanied by a decrease in intestinal mucin-2 mRNA expression [27], which was also true for A. butzleri-infected gnotobiotic IL-10–/– animals as shown here. Notably, isolator raised germfree mice exhibited an extremely thin colonic mucus layer that upon exposure with bacterial products such as peptidoglycan or lipopolysaccharide could be quickly restored to levels observed in conventionally colonized mice [28]. Nevertheless, our data presented here need to be interpretated with caution given that we analyzed mRNA expression of mucin-2 but neither the translated protein nor the complex mucus layer.
Following stable intestinal colonization, A. butzleri-infected mice did not exhibit macroscopic disease but distinct pro-inflammatory sequelae that were not restricted to the small and large intestines, given that pronounced immune responses could be observed in extra-intestinal including systemic compartments [8, 9, 13, 14]. Both innate and adaptive immune responses upon C. jejuni infection were characterized by elevated expression of genes encoding Th17 cytokines in human intestinal ex vivo biopsies [29]. Accordingly, our results indicate that IFN-γ, IL-22, and IL-17A were upregulated in both murine colon and ileum after A. butzleri infection. This is well in line with our previous studies revealing colonic increases in respective mediators upon murine C. jejuni infection [19]. In C. jejuni-induced colitis, both T lymphocytes and innate lymphoid cells (ILCs) were sources for IFN-γ, IL-22, and IL-17A secretion in a time- and organ-specific fashion [30], whereas IFN-γ played a critical role particularly during the early phase of acute C. jejuni infection [29]. The triad IFN-γ, IL-22, and IL-17A exert potential bactericidal properties including enhanced β-defensin production, which has been shown an effective antimicrobial mechanism directed against C. jejuni [31] and presumably also against A. butzleri. Elevated expression of the gene for IL-17A is further associated with early neutrophil recruitment to sites of infection [32], and enhanced IL-17A and IFN-γ secretion with tissue damage upon C. jejuni infection [30]. Given that IL-23 is a known master regulator of mucosal immune responses upon intestinal infection and inflammation [18], we assessed intestinal IL-23p19 expression in A. butzleri-infected mice. Following A. butzleri CCUG 30485, but not C1 infection, ileal, but not colonic IL-23p19 mRNA levels, were upregulated. Hence, it is tempting to speculate that IL-23 might also act as a central regulator of immune responses during Arcobacter infections in a strain- and organ-specific fashion. The exact underlying mechanisms, however, remain to be elucitated.
In the intestinal tract, IL-22, as member of the IL-10 family, exerts a dichotomous mode of action in a tissue-specific manner. Whereas pro-inflammatory properties of IL-22 have been shown in the small intestines [15], IL-22 induced anti-inflammatory responses and tissue repair in the colon [33, 34]. Recently, we showed that colonic apoptosis and pro-inflammatory immune responses were accompanied by increased IL-22 but also IL-18 mRNA levels in the large intestines of C. jejuni-infected mice [19, 21]. These data support our results obtained from A. butzleri-infected mice presented here. In the colon, but not ileum, IL-18 was upregulated upon A. butzleri infection with either strain during both the early and late phase of infection, whereas colonic IL-22 mRNA increased until day 6 following CCUG 30485 strain infection. We could recently show that IL-22 induced the expression of IL-18 mRNA in intestinal epithelial cells following Citrobacter rodentium and T. gondii infection [35]. Conversely, IL-18 was able to amplify IL-22 production from ILCs and Th1 mediated intestinal inflammation [35]. Future studies need to unravel whether such a mutual regulation of IL-22 and IL-18 were also true for Arcobacter infection. Given that matrix-degrading enzymes such as gelatinases are upregulated upon intestinal inflammation [15, 36, 37], we determined intestinal expression levels of MMP-2 and MMP-9 and their endogenous inhibitors including TIMP-1 and TIMP-3 during murine Arcobacter infection. As early as 6 days following A. butzleri strain CCUG 30485 infection, both ileal MMP-2 and MMP-9 mRNA were upregulated, whereas, unexpectedly, MMP-9 was even downregulated in the large intestines at day 6 following infection with either strain. In our previous C. jejuni infection studies, MMP-2, but not MMP-9 mRNA, was upregulated in large intestines of infected infant mice, whereas mRNA levels of genes for both TIMP-1 and TIMP-3 were virtually unchanged similar to A. butzleri infection investigated here.
Conclusion
In conclusion, our data indicate that murine A. butzleri infection induces changes in the intestinal expression levels of genes encoding distinct pro-inflammatory, regulatory, and matrix-degrading molecules essential for immunity and tissue turnover, respectively.
Acknowledgements
We thank Michaela Wattrodt, Ursula Rüschendorf, Ines Puschendorf, Alexandra Bittroff-Leben, Ulrike Hagen, Gernot Reifenberger, Uwe Lohmann, and the staff of the animal research facility for excellent technical assistance and animal breeding.
References
- 1.Ho HT, Lipman LJ, Gaastra W: Arcobacter, what is known and unknown about a potential foodborne zoonotic agent! Vet Microbiol 115(1–3), 1– 13 (2006) [DOI] [PubMed] [Google Scholar]
- 2.ICMSF ICoMSfF (2002): Microbiological Testing in Food Safety Management, 7, ed. Tompkin RB. Kluwer Academic/Plenum Publishers, New York, NY, p. 171 [Google Scholar]
- 3.Collado L, Figueras MJ: Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clin Microbiol Rev 24(1), 174–192 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lappi V, Archer JR, Cebelinski E, Leano F, Besser JM, Klos RF, Medus C, Smith KE, Fitzgerald C, Davis JP: An outbreak of foodborne illness among attendees of a wedding reception in Wisconsin likely caused by Arcobacter butzleri. Foodborne Pathog Dis 10(3), 250–255 (2013) [DOI] [PubMed] [Google Scholar]
- 5.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kuhl AA, Dasti JI, Zautner AE, Munoz M, Loddenkemper C, Gross U, Gobel UB, Heimesaat MM: Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PloS One 6(6), e20953 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandamme P, Pugina P, Benzi G, Van Etterijck R, Vlaes L, Kersters K, Butzler JP, Lior H, Lauwers S: Outbreak of recurrent abdominal cramps associated with Arcobacter butzleri in an Italian school. J Clin Microbiol 30(9), 2335–2337 (1992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karadas G, Sharbati S, Hanel I, Messelhausser U, Glocker E, Alter T, Gölz G: Presence of virulence genes, adhesion and invasion of Arcobacter butzleri. J Appl Microbiol 115(2), 583–590 (2013) [DOI] [PubMed] [Google Scholar]
- 8.Gölz G, Karadas G, Alutis ME, Fischer A, Kuhl AA, Breithaupt A, Gobel UB, Alter T, Bereswill S, Heimesaat MM: Arcobacter butzleri induce colonic, extra-intestinal and systemic inflammatory responses in gnotobiotic IL-10 deficient mice in a strain-dependent manner. PloS One 10(9), e0139402 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heimesaat MM, Karadas G, Alutis M, Fischer A, Kuhl AA, Breithaupt A, Gobel UB, Alter T, Bereswill S, Gölz G: Survey of small intestinal and systemic immune responses following murine Arcobacter butzleri infection. Gut Pathog 7, 28 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Gobel UB, Liesenfeld O: Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol 177(12), 8785–8795 (2006) [DOI] [PubMed] [Google Scholar]
- 11.Haag LM, Fischer A, Otto B, Plickert R, Kuhl AA, Gobel UB, Bereswill S, Heimesaat MM: Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10–/– mice via Toll-like-receptor-2 and -4 signaling. PloS One 7(7), e40761 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heimesaat MM, Lugert R, Fischer A, Alutis M, Kuhl AA, Zautner AE, Tareen AM, Gobel UB, Bereswill S: Impact of Campylobacter jejuni cj0268c knockout mutation on intestinal colonization, translocation, and induction of immunopathology in gnotobiotic IL-10 deficient mice. PloS One 9(2), e90148 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gölz G, Karadas G, Fischer A, Gobel UB, Alter T, Bereswill S, Heimesaat MM: Toll-like receptor-4 is essential for Arcobacter butzleri-induced colonic and systemic immune responses in gnotobiotic IL-10–/– mice. Eur J Microbiol Immunol 5(4), 321–332 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heimesaat MM, Karadas G, Fischer A, Gobel UB, Alter T, Bereswill S, Gölz G: Toll-lLike receptor-4 dependent small intestinal immune responses following murine Arcobacter butzleri infection. Eur J Microbiol Immunol 5(4), 333–342 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Holscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O: Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med 206(13), 3047–3059 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuckin MA, Linden SK, Sutton P, Florin TH: Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9(4), 265–278 (2011) [DOI] [PubMed] [Google Scholar]
- 17.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L: Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295(5560), 1726–1729 (2002) [DOI] [PubMed] [Google Scholar]
- 18.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F: Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464(7293), 1371–1375 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alutis ME, Grundmann U, Fischer A, Hagen U, Kuhl AA, Gobel UB, Bereswill S, Heimesaat MM: The role of gelatinases in Campylobacter jejuni infection of gnotobiotic mice. Eur J Microbiol Immunol 5(4), 256–267 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alutis ME, Grundmann U, Fischer A, Kuhl AA, Bereswill S, Heimesaat MM: Selective gelatinase inhibition reduces apoptosis and pro-inflammatory immune cell responses in Campylobacter jejuni-infected gnotobiotic IL-10 deficient mice. Eur J Microbiol Immunol 4(4), 213–222 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alutis ME, Grundmann U, Hagen U, Fischer A, Kuhl AA, Gobel UB, Bereswill S, Heimesaat MM: Matrix metalloproteinase-2 mediates intestinal immunopathogenesis in Campylobacter jejuni-infected infant mice. Eur J Microbiol Immunol 5(3), 188–198 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA: Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4(2), 197–250 (1993) [DOI] [PubMed] [Google Scholar]
- 23.Goetzl EJ, Banda MJ, Leppert D: Matrix metalloproteinases in immunity. J Immunol 156(1), 1–4 (1996) [PubMed] [Google Scholar]
- 24.Strugala V, Allen A, Dettmar PW, Pearson JP: Colonic mucin: methods of measuring mucus thickness. P Nutr Soc 62(1), 237–243 (2003) [DOI] [PubMed] [Google Scholar]
- 25.Hugdahl MB, Beery JT, Doyle MP: Chemotactic behavior of Campylobacter jejuni. Infect Immun 56(6), 1560–1566 (1988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu QV, McGuckin MA, Mendz GL: Campylobacter jejuni response to human mucin MUC2: modulation of colonization and pathogenicity determinants. J Med Microbiol 57(Pt 7), 795–802 (2008) [DOI] [PubMed] [Google Scholar]
- 27.Otto B, Haag LM, Fischer A, Plickert R, Kuhl AA, Gobel UB, Heimesaat MM, Bereswill S: Campylobacter jejuni induces extra-intestinal immune responses via Toll-like-receptor-4 signaling in conventional IL-10 deficient mice with chronic colitis. Eur J Microbiol Immunol 2(3), 210–219 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersson J, Schreiber O, Hansson GC, Gendler SJ, Velcich A, Lundberg JO, Roos S, Holm L, Phillipson M: Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol 300(2), G327–333 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards LA, Nistala K, Mills DC, Stephenson HN, Zilbauer M, Wren BW, Dorrell N, Lindley KJ, Wedderburn LR, Bajaj-Elliott M: Delineation of the innate and adaptive T-cell immune outcome in the human host in response to Campylobacter jejuni infection. PloS One 5(11), e15398 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik A, Sharma D, St Charles J, Dybas LA, Mansfield LS: Contrasting immune responses mediate Campylobacter jejuni-induced colitis and autoimmunity. Mucosal Immunol 7(4), 802–817 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zilbauer M, Dorrell N, Boughan PK, Harris A, Wren BW, Klein NJ, Bajaj-Elliott M: Intestinal innate immunity to Campylobacter jejuni results in induction of bactericidal human beta-defensins 2 and 3. Infect Immun 73(11), 7281–7289 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouyang W, Kolls JK, Zheng Y: The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28(4), 454–467 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA: Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29(6), 947–957 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W: Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14(3), 282–289 (2008) [DOI] [PubMed] [Google Scholar]
- 35.Munoz M, Eidenschenk C, Ota N, Wong K, Lohmann U, Kuhl AA, Wang X, Manzanillo P, Li Y, Rutz S, Zheng Y, Diehl L, Kayagaki N, van Lookeren-Campagne M, Liesenfeld O, Heimesaat M, Ouyang W: Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity 42(2), 321–331 (2015) [DOI] [PubMed] [Google Scholar]
- 36.Salmela MT, MacDonald TT, Black D, Irvine B, Zhuma T, Saarialho-Kere U, Pender SL: Upregulation of matrix metalloproteinases in a model of T cell mediated tissue injury in the gut: analysis by gene array and in situ hybridisation. Gut 51(4), 540–547 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heimesaat MM, Dunay IR, Fuchs D, Trautmann D, Fischer A, Kuhl AA, Loddenkemper C, Siegmund B, Batra A, Beres will S, Liesenfeld O: The distinct roles of MMP-2 and MMP-9 in acute DSS colitis. Eur J Microbiol Immunol 1(4), 302–310 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]


