Abstract
Myelin is a critical component of the nervous system facilitating efficient propagation of electrical signals and thus communication between the central and peripheral nervous systems and organ systems they innervate throughout the body. In instances of neurotrauma and neurodegenerative disease, injury to myelin is a prominent pathological feature responsible for conduction deficits and leaves axons vulnerable to damage from noxious compounds. Although the pathological mechanisms underlying myelin loss have yet to be fully characterized, oxidative stress appears to play a prominent role. Specifically, acrolein, a neurotoxic aldehyde that is both a product and instigator of oxidative stress, has been observed in studies to elicit demyelination through calcium-independent and -dependent mechanisms and also by affecting glutamate uptake and promoting excitotoxicity. Furthermore, pharmacological scavenging of acrolein has demonstrated a neuroprotective effect in animal disease models by conserving myelin structural integrity and alleviating functional deficits. This evidence is indicative that acrolein may be a key culprit of myelin damage while acrolein scavenging could potentially be a promising therapeutic approach for patients suffering from nervous system trauma and disease.
Keywords: demyelination, spinal cord injury, multiple sclerosis, neurotrauma, hydralazine
Introduction
Myelin damage is a significant pathogenic feature common to both trauma and disease that continues to pose a challenge in the treatment of neuropathologies. To establish novel effective therapeutics, a strong understanding of the molecular mechanisms governing myelin damage is warranted. This review will focus on evidence implicating acrolein as a causative agent of myelin damage and a compound with great potential to be targeted therapeutically to mitigate myelin damage and consequent functional deficits.
Pathological Significance of Myelin Damage in CNS Trauma and Disease
The structural integrity of myelin is essential for normal function in the central nervous system (CNS) and its destruction is a key characteristic observed in many neuropathologies, including multiple sclerosis (MS) and spinal cord injury (SCI) [1–5]. The CNS myelin sheath originates from oligodendrocytes and is composed of many compact layers tightly wrapped around axons, establishing a physical connection between myelin and axons at the paranodal region [1]. It has long been established that myelin insulates the internodal, juxtaparanodal and paranodal regions while leaving the nodes of Ranvier exposed. Given that this structure is vital for maintaining neuronal function, morphological studies have demonstrated significant demyelination in animal models of both SCI [4, 5] and MS [6–8], implicating myelin injury as a common feature of CNS trauma and disease. For example, compression and stretch SCI have shown immediate myelin damage such as elongation of the nodes of Ranvier and separation of the myelin and axolemma [9–11]. Such demyelination has been shown to progress with graded lengthening of the nodes two and five hours post stretch SCI in rats [11]. Furthermore, strikingly similar damage was also observed upon morphological analysis of excised spinal cords of experimental autoimmune encephalomyelitis (EAE) mice, a well-established model of MS, in which nodal lengthening and myelin retraction were also observed (unpublished observation, Shi). Thus the presence of structural myelin damage is an important component of CNS trauma and disease.
The functional capabilities of most axons in the CNS are dependent on the structural integrity of the myelin sheath. In non-pathological conditions axonal transmission of electrical depolarizing pulses is facilitated through the high expression of voltage-gated Na+ channels present in the nodes of Ranvier enabling saltatory conduction, in which pulses jump from one node to another [1]. Myelin enhances axonal conduction by both inhibiting ionic exchange across the membrane and increasing transverse resistance at the internodal region [12]. Accordingly, compromise of the structural integrity of myelin elicits significant conduction loss in spinal cord tissue partly due to the exposure and consequential activation of voltage-gated K+ channels typically residing in the juxtaparanodal region [1, 10, 11, 13–15]. Ex vivo studies of spinal cord tissue have demonstrated that myelin damage and subsequent exposure of K+ channels impaired signal propagation, indicated by a reduction of compound action potential (CAP) amplitude [10, 11, 14]. In fact, diminished CAP amplitudes were detected at the precise moment of physical damage indicating that a number of axons were not capable of signal transduction, likely due to immediate myelin injury [9, 10]. Interestingly, alleviation of functional deficits and partial recovery of CAP amplitude were observed following the application of a K+ channel blocker such as 4-aminopyridine (4-AP) and 4-aminopyridine-3-methanol (4-AP-3-MeOH), validating the role of K+ channel dysfunction in conduction loss [9, 11, 14–16]. Reinstated function of injured axons following the application of potassium channel blockers such as 4-AP has been observed in various models such as rat spinal nerve roots, ex vivo rat sciatic nerves [17, 18] and in cats with conduction and motor deficit due to chronic spinal cord injuries [19–21]. While both morphological and electrophysiological evidence have demonstrated functional consequences in diseases such as MS and SCI, up until recently knowledge regarding the mechanisms mediating myelin damage was limited. New evidence has emerged indicating that a variety of mechanisms could contribute to demyelination characteristic of CNS pathology.
Mechanisms of Myelin Damage
Demyelination is generally attributed to damage by both physical and chemical processes. In the case of trauma, primary injury mediated by physical forces triggers subsequent pathological molecular mechanisms, deemed secondary injury [22, 23]. Separation of these two components was postulated decades ago and has been supported by many subsequent studies [13, 22, 23]. Recently, this phenomenon was demonstrated in an ex vivo acute spinal cord injury model where a mechanical stretch injury resulted in immediate myelin damage indicated by lengthening of the nodal region and retraction of myelin from the paranodal region following the physical insult [11]. The detachment and degradation of myelin prompted immediate exposure of underlying juxtaparanodal K+ channels and simultaneous conduction failure. Interestingly, computational modeling studies have revealed that the paranodal region is subjected to a significant amount of stress under an external load such as compression, supporting the observation that physical force can cause myelin damage, especially in the paranodal region eliciting K+ channel dysregulation and conduction failure [10, 24]. The role of juxtaparanodal K+ channel exposure in mediating conduction loss is further supported by the observation that application of the K+ channel blocker 4-AP restored conduction in demyelinated axons, but was ineffective in myelin intact axons [9, 16, 25, 26]. Taken together, physical trauma can clearly produce myelin damage which could lead to functional loss.
It has been repeatedly shown that myelin damage and conduction loss directly resulting from physical trauma can be exacerbated even after mechanical forces are removed, an effect attributed to myelin damage mediated by the pathophysiological molecular mechanisms of secondary injury [4, 11, 27]. For example, using a well-controlled ex vivo spinal cord white matter preparation, Sun and colleagues have shown that immediate myelin damage resulting from physical trauma can further deteriorate through chemical reactions without concomitant physical loading [11]. Interestingly, it was also noted that while myelin damage resulting from primary injury does not depend on extracellular calcium, some components of secondary injury do [11, 28], further demonstrating the existence of two independent mechanisms.
It is worth noting that while myelin damage mediated by molecular signaling is clearly secondary to the physical impact in neurotrauma, chemical injury predominates in the case of neurodegenerative diseases where primary physical trauma is largely absent. The etiology of chemically-mediated injury in neurodegenerative disease is mostly unknown. It is generally believed that such pathology could be attributed to several known biochemical process such as autoimmune responses, particularly in MS, where myelin-reactive T-lymphocytes perceive myelin as foreign to the body and amount an immune response employing endogenous defense mechanisms to mediate its destruction [8, 29]. Although distinct processes initiate the molecular mechanisms underlying demyelination in trauma and neurodegenerative disease, downstream chemically-mediated myelin damage appears to be similar in both neurotrauma and chronic disease [10, 13, 14, 30]. Such scientific convergence facilitates the cross talk of these mechanisms and greatly enhances understanding of myelin damage in various pathological conditions as well as the development of effective treatments.
Based on decades of research in both the field of trauma and degenerative diseases, it is generally believed that biochemically induced myelin damage could result from multiple mechanisms including inflammation, oxidative stress, ischemia and glutamate toxicity [31]. Many studies indicate it is clear that biochemical mechanisms alone are capable of eliciting myelin damage similar to that elicited by physical force, however, over a prolonged time course. In particular, oxidative stress has emerged as a crucial factor in instigating myelin damage, and a key factor of oxidative stress, acrolein, has been shown to cause significant myelin damage in the absence of physical trauma [6, 30, 31]. Due to the fact that acrolein appears to be a novel mechanism of myelin damage, this review will focus on discussing evidence attributing acrolein as a key factor of myelin damage and also a novel effective therapeutic target to deter myelin damage and functional loss.
Acrolein
Acrolein (2-propenal) is produced endogenously through various mechanisms such as intracellular enzymatic oxidation of polyamine metabolites [32–36] and more importantly lipid peroxidation (LPO) [32, 37, 38]. As a highly electrophilic α, β-unsaturated aldehyde, acrolein is extremely reactive with nucleophilic DNA, the sulfhydryl groups of cysteine, histidine, lysine and arginine residues in proteins as well as phospholipids, and can also generate free radicals [32, 36, 39]. Acrolein interacts with proteins via the Michael addition reaction and is rapidly incorporated into proteins producing carbonyl derivatives [32, 40, 41]. In fact, oxidative stress is associated with the generation of many unsaturated aldehyde electrophiles such as 4-hydroxynonenal (HNE), 4-oxy-2-nonenal (ONE), Malondialdehyde (MDA), 4-hydroxy-2E-hexenal (HHE), and crotonaldehyde. However, of the aforementioned LPO aldehyde byproducts, acrolein is at least 100 times more reactive and is produced at 40 times its concentration [32, 36, 42]. In addition to its high reactivity, acrolein is relatively stable in aqueous conditions and has a half-life that is several orders of magnitude longer that of better known reactive oxygen species (ROS), such as hydroxyl radicals that decay within nanoseconds [42–45], suggesting that acrolein could incite long-lasting damage. Given that ROS scavengers did not prove to be clinically successful in mitigating neurological injuries [8, 31, 46, 47], this further promotes acrolein as a key factor to perpetuate oxidative stress and as a novel therapeutic target to mitigate such injury.
A unique characteristic of acrolein is its ability to exist as both a product and catalyst of LPO, indicative of a key role in perpetuating oxidative stress [6, 48–51]. After its production via LPO, it can react with xanthine oxidase to be converted back to superoxide, which can then react to promote LPO and consequently produce more acrolein [52–54]. The significance of this finding is only enhanced when taken into account with other studies that demonstrate the diffusive nature of acrolein. Hamann et al. incubated injured spinal cords, endogenously producing acrolein at an elevated concentration and rate, with healthy segments and demonstrated superoxide production and acrolein-mediated damage in both injured and uninjured segments [55]. Natural physiological antioxidants typically reduce this damage and prevent the elevation and diffusion of ROS and acrolein, in particular glutathione (GSH). As the most powerful endogenous antioxidant, GSH continuously scavenges acrolein in normal and disease states through 1, 4-addition reaction, forming a stable product that is non-toxic. However, GSH can be rapidly depleted during extreme oxidative stress [48, 50, 56]. Studies have shown that upon GSH depletion, pharmacological scavenging of acrolein in MS can suffice as a method for mitigating demyelination and subsequent functional deficits [57]. Together these studies demonstrate the key role of acrolein in enhancing oxidative stress, and thus it is likely responsible in part for perpetuating and propagating oxidative stress.
Acrolein-mediated Myelin Damage
In addition to its critical role in perpetuating oxidative stress, acrolein has also been implicated as a factor capable of directly exacerbating myelin damage. Oxidative stress in the CNS is especially detrimental to myelin given its main constituents are proteins and lipids—biopolymers vulnerable to progressively elevated acrolein levels due to the high potential for reactivity, rendering acrolein highly toxic to nervous tissue [53, 54, 58–61]. The presence of acrolein has been implicated in cases of enhanced oxidative stress, particularly where elevated tissue and systemic levels of acrolein have been shown in both SCI [55, 56, 61–64] and MS [57]. In fact, acrolein can increase up to 300% at 24 hours post-SCI and remain elevated for at least two weeks in a clinically relevant rat spinal cord contusion injury model [63]. Given that demyelination is a hallmark of both MS and SCI, this evidence points toward acrolein-mediated myelin damage as a key factor underlying symptoms of CNS trauma and disease. Furthermore, removal of acrolein by application of the scavenger hydralazine in EAE mice demonstrated a marked reduction in myelin damage and a parallel improvement of motor function [57]. In light of the aforementioned evidence, it seems likely that acrolein is in part mediating demyelination.
While elevated acrolein levels and concomitant demyelination are observed under conditions of high oxidative stress, the direct role of acrolein-mediated myelin damage was only addressed in recent ex vivo studies. Since it is extremely difficult to evaluate the effect of endogenous acrolein on myelin in vivo, studies using ex vivo spinal cords were advantageous for providing physiological relevant tissue that could be monitored in a well-controlled environment. Perhaps the most powerful and direct study demonstrating acrolein-mediated myelin damage was the application of acrolein to isolated spinal cord tissue which resulted in demyelination, nodal lengthening and exposure of K+ channels visualized through the simultaneous use of label-free Coherent Anti-Stokes Raman Scattering (CARS) microscopy and immunohistochemistry, respectively [30]. The use of CARS is particularly unique given that it’s a label-free method for visualization of myelin and thus reduces the chance for further molecular perturbation [65]. Moreover, acrolein-mediated structural damage caused an immediate reduction in electrophysiological recordings of the CAP amplitude [30, 59, 64]. While application of K+ channels could restore the amplitude to some extent, acrolein scavenging with hydralazine significantly mitigated the progressive demyelination and conduction loss demonstrating the key role of acrolein in mediating myelin damage. Thus the combination of morphological and electrophysiological evidence clearly supports acrolein-mediated myelin damage in CNS trauma and disease by way of mechanisms outlined in Figure 1, which will be further delineated in the following section.
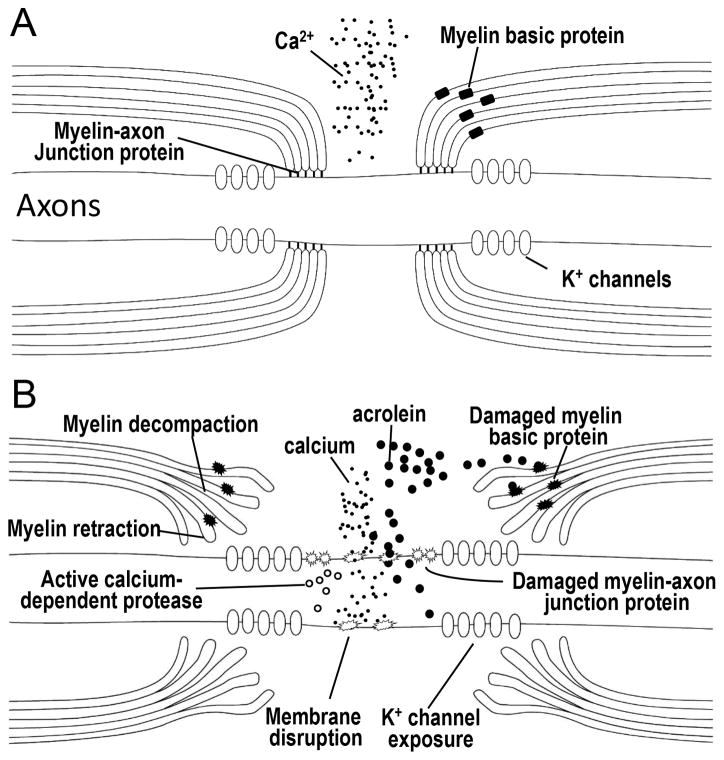
Figure 1.
Diagram representing the steps in acrolein-mediated myelin damage. For healthy nerves (A), proteins such as Caspr, contactin, Nfac, and spectrin comprise axoglial septate junctions that secure the myelin to the axon at the periphery of the nodes of Ranvier. These act to confine voltage gated fast K+ channels to the internode region. Myelin basic protein acts to hold the multi-layer of myelin together, forming a dense and compact structure of myelin. Intact axonal membranes at the nodal region maintain an ionic balance with low concentrations of intracellular Ca2+, keeping intracellular proteases inactive. Exposure to acrolein results in damage to myelin, myelin basic protein, the myelin–axon junction, and the axonal membranes (B). Influx of calcium activates proteases, which may cause further damage to the paranodal axoglial junction which result in detachment of myelin from axon and the retraction of myelin. Damage to the myelin and myelin basic protein lead to myelin decompaction. Ultimately, myelin decompaction and retraction cause exposure of K+ channels and prevent saltatory conduction and consequently result in axonal conduction block and disruption of neuronal function.
Molecular Mechanisms of Acrolein-mediated Myelin Damage
Acrolein can induce direct damage to the myelin sheath through splitting or decompaction in a Ca2+ independent manner. A physical connection among multiple layers of myelin lamellae is ensured by myelin basic protein (MBP) [66, 67] (Fig. 1). Contact between axons and myelin is established through various proteins that form the axo-glia complex including Caspr [68], NF 155 [69], contactin [70], protein 4.1 [71, 72] spectrin and actin [73, 74]. Given the existence of amino acid residues that are vulnerable to acrolein attack in MBP [75–77], acrolein likely reacts readily with the protein to form protein-adducts, resulting in the disruption of protein structure and function. Such damage likely culminates in decompaction or splitting of the myelin lamellae. In fact, direct treatment of spinal cord tracts with acrolein resulted in degraded MBP and concomitant myelin splitting in the paranodal region, visualized by CARS and immunohistochemistry [30]. Damage to MBP mediated by acrolein was shown to be Ca2+ independent given that incubation with the Ca2+ chelator, ethylene glycol tetraacetic acid (EGTA), did not alleviate paranodal myelin splitting [30]. These data are consistent with the hypothesis that acrolein attacks MBP directly, likely forming acrolein-MBP adducts, which does not require calcium.
The existing evidence suggests that myelin damage can also be initiated through acrolein-mediated enzymatic damage by Ca2+ dependent mechanisms. In contrast with myelin splitting, acrolein-mediated retraction of myelin from the paranodal region only occurs in the presence of extracellular Ca2+ since application of EGTA blocked acrolein-mediated myelin retraction [30]. Additionally, calcium is a major co-enzyme of proteases such as calcium-activated neutral proteinase (calpain) [78]. Given that calpain has directly access to interact with and damage proteins critical for the function of the axo-glial complex, specifically spectrin and protein 4.1, activation of calpain could lead to the disruption of these structures and the consequential instability of axo-glial junction, disassociation of myelin from axon and subsequently the retraction of myelin at the paranodal region [78, 79]. This was demonstrated when acrolein application for 12 hours significantly altered the structure of the axo-glial complex as seen by Caspr dissociation from the paranodal region [30]. Axonal neurofascin 186 (NF186) and myelinic NF155 were also shown to separate into three noticeable components indicative of NF186 remaining in the nodal region while NF155 receded with the retracting myelin, further document the disassociation and destruction of paranodal myelin strcuture [30]. Furthermore, abnormal distribution of Caspr is also observed in cases of chronic MS and associated with myelin damage [80]. Together, this data indicates that acrolein-mediated damage directly affects myelin sheath and the paranodal protein complex in both a Ca2+-independent (direct) and Ca2+-dependent (indirect) manner leading to myelin splitting and retraction.
Glutamate excitotoxicity is a recognized mechanism of CNS myelin damage [81–83]. It is known that glutamate can encourage calcium influx through the activation of NMDA receptors [84], where calcium activates calpain, which could lead to myelin damage [78, 85, 86]. Recent evidence points towards the critical role of acrolein in mediating demyelination through inhibition of glutamate uptake transporters and enhancement of excitotoxic damage. Elevated levels of glutamate have been observed in the CSF of MS patients where chronic oxidative stress exists [87]. The significance of this finding is given by studies demonstrating that direct application of 1.0 and 0.1 mM glutamate to ex vivo spinal cord tissue resulted in splitting and retraction of myelin from the paranodal region as well as axo-glial complex disruption [83]. This demyelination was also observed with exposure and redistribution of K+ channels and a consistent decrease in conduction which could be recovered following application of 100 μM 4-AP [83]. These characteristics parallel what is observed when acrolein is applied [30]. The link between acrolein and glutamate-mediated demyelination is further highlighted by the demonstrated ability of HNE, an aldehyde produced through LPO similar to acrolein, but less reactive, to directly bind to the glutamate uptake transporter, GLT-1 (EAAT2), to inhibit its activity and elevate extracellular glutamate levels [32, 88–90]. Given the presence of cysteine residues within the active region of GLT-1 and acrolein’s high reactivity to cysteines [91], it is likely that acrolein will also bind to this region and inhibit uptake activity. This is further strengthened by the fact that blockage of iGluRs, overactivated by excessive extracellular glutamate, can reduce demyelination, axonal damage and motor deficits in MS [92]. Therefore, it is likely that acrolein-mediated inhibition of glutamate transporters, subsequent increase of extracellular glutamate and excitotoxicity may also play key roles in causing acrolein-induced demyelination in both SCI and MS, where glutamate toxicity is a well-established pathological mechanism.
Mitigating Acrolein-mediated Myelin Damage and Improving Functional Recovery
Neuroprotective strategies targeting acrolein removal have been investigated given its significant detrimental role in promoting myelin damage. Recent studies have demonstrated that the FDA approved antihypertensive hydralazine can scavenge and neutralize both acrolein and acrolein-protein adducts [48, 55, 64, 93–96]. Binding of acrolein to hydralazine is initiated through a Michael addition reaction between the carbonyl and hydrazine functional groups, respectively [97]. Hydralazine application has demonstrated significant benefit by exhibiting therapeutic concentrations in CNS only 2 hours after application [63]. Specifically, 20 μmol/L of hydralazine was detected in spinal cord tissue 2 hours following an intraperitoneal (IP) injection, which is comparable with a previous established therapeutic concentration [98]. Furthermore, low doses of hydralazine (1–5 mg/kg), comparable to a safe concentration used to treat pediatric patients (7.5 mg/kg), are still capable of significantly scavenging acrolein in rats and mice [57, 62, 63]. As no significant adverse side effects were observed with application of lower dosages [56, 57], hydralazine has great potential for neuroprotection given its nature to actively scavenge acrolein in the CNS at low concentrations.
Hydralazine’s therapeutic benefit has been shown in vitro and in vivo in SCI and MS. Ex vivo SCI studies demonstrated that acrolein-mediated neuronal damage was alleviated with the presence of hydralazine [48, 55, 64]. Approximately 50 to 70 percent of acrolein levels in the spinal cord are sequestered following application of hydralazine [63]. In vivo studies of rat contusive SCI showed that neutralizing acrolein following hydralazine exposure resulted in significant reduction of tissue damage and motor and sensory deficits [62, 63]. Similar effects are also observed in a mouse model of MS [57]. EAE mice exhibiting elevated levels of acrolein and compromised motor function were treated with hydralazine, which inhibited further myelin damage and behavioral deficits and reversed the progressive symptoms of EAE [57]. Morphological and functional improvements elicited by anti-acrolein treatment further validate the causal role of acrolein in myelin damage. Therefore, there is great potential for hydralazine treatment in cases of MS and SCI where chronic oxidative stress and acrolein are present.
While the blood pressure-lowering properties of hydralazine may contraindicate its use as a carbonyl scavenger in some disease contexts, especially in acute stages when adequate blood perfusion of vital organs is critical, in the context of SCI these pharmacological properties may be desirable. Chronic SCI patients often experience acute hypertension due to autonomic dysreflexia which could lead to life-threatening consequences [99–101], the systemic vasodilatory effects of hydralazine might be beneficial. Indeed, it has been shown that SCI patients receiving antihypertensive therapies were less likely to have elevated blood pressure and a consequential autonomic dysreflexia attack [100]. Therefore, in addition to neutralizing acrolein, hydralazine may also offer therapeutic benefit by curtailing post-SCI hypertension, effectively reducing the risk of autonomic dysreflexia in SCI patients.
Conclusion
Acrolein is likely a critical component of myelin damage in CNS trauma and disease. Elevated tissue and systemic levels have been determined in both SCI and MS. Given the composition of the myelin sheath, it is likely that acrolein can easily attack the lipid membrane and proteins critical for maintaining the axo-myelin junction and axolemma space. Furthermore, acrolein can lead to heightened calpain activation and excitotoxic damage. The mechanisms by which acrolein can damage myelin can be mitigated through acrolein scavenging strategies. Ex vivo and in vivo hydralazine application have exhibited a neuroprotective effect, reducing myelin damage in spinal cord tissue and improving conduction, indicative of its potential as an effective therapeutic intervention. Given that, like acrolein, many unsaturated aldehyde electrophiles have been linked to various pathological conditions and likely share similar mechanisms of cellular destruction with acrolein, the knowledge gained in this line of research can facilitate the understanding of other various unsaturated aldehyde electrophiles. In addition, some known acrolein scavengers are also capable of scavenging other unsaturated aldehyde such as HNE and MDA, further strengthening the broader application of such knowledge in combating these unsaturated aldehyde toxicants [98]. Among many possible future studies that could advance our understanding of unsaturated aldehyde toxicants in the pathological processes of neuronal trauma and disease, the confirmation and characterization of protein damage by various unsaturated aldehyde electrophiles has great potential to further advance this line of work. This will require the use of a more chemically definitive method or more contemporary approaches, such as biotin-tagging approach, to quantify the augmented protein adduction by LPO products-unsaturated aldehyde electrophiles, in relevant SCI and MS models [102]. Acquisition of additional data characterizing interactions between the discussed aldehydes and a variety of proteins will not only facilitate the utility of acrolein-protein adducts as a biomarkers correlated with pathological changes, but also as an evaluation index of the effectiveness of anti-acrolein or anti-aldehyde treatment.
Abbreviations
- 4-AP
4-aminopyridine
- 4-AP-3-MeOH
4-aminopyridine-3-methanol
- CAP
compound action potential
- CARS
Coherent Anti-Stokes Raman Scattering microscopy
- Caspr
contactin associate protein
- CNS
central nervous system
- GLT-1 (EAAT2)
glia glutamate transporter-1
- iGluRs
ionotropic glutamate receptors
- IP
intraperitoneal injection
- EAE
experimental autoimmune encephalomyelitis
- EGTA
ethylene glycol tetraacetic acid
- GSH
glutathione
- HNE
4-hydroxynonenal
- LPO
lipid peroxidation
- MBP
myelin basic protein
- MS
multiple sclerosis
- NF155
neurofascin 155
- NF186
neurofascin 186
- OS
oxidative stress
- ROS
reactive oxygen species
- SCI
spinal cord injury
Footnotes
Declaration of interests
This work was supported by the Indiana State Department of Health (Grant # 204200), National Institutes of Health (Grant # NS073636), and Indiana CTSI Collaboration in Biomedical Translational Research (CBR/CTR) Pilot Program Grant (Grant # RR025761).
References
- 1.Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–80. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- 2.McDonald JW, Belegu V. Demyelination and remyelination after spinal cord injury. J Neurotrauma. 2006;23:345–59. doi: 10.1089/neu.2006.23.345. [DOI] [PubMed] [Google Scholar]
- 3.Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280–91. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- 4.Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Central Nervous System Trauma. 1985;2:299–315. doi: 10.1089/cns.1985.2.299. [DOI] [PubMed] [Google Scholar]
- 5.Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373–83. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- 6.Tully M, Shi R. New Insights in the Pathogenesis of Multiple Sclerosis—Role of Acrolein in Neuronal and Myelin Damage. International journal of molecular sciences. 2013;14:20037–20047. doi: 10.3390/ijms141020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudick RA, Trapp BD. Gray-matter injury in multiple sclerosis. N Engl J Med. 2009;361:1505–6. doi: 10.1056/NEJMcibr0905482. [DOI] [PubMed] [Google Scholar]
- 8.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 9.Jensen JM, Shi R. Effects of 4-aminopyridine on stretched mammalian spinal cord: the role of potassium channels in axonal conduction. J Neurophysiol. 2003;90:2334–40. doi: 10.1152/jn.00868.2002. [DOI] [PubMed] [Google Scholar]
- 10.Ouyang H, Sun W, Fu Y, Li J, Cheng JX, Nauman E, et al. Compression induces acute demyelination and potassium channel exposure in spinal cord. J Neurotrauma. 2010;27:1109–20. doi: 10.1089/neu.2010.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun W, Fu Y, Shi Y, Cheng JX, Cao P, Shi R. Paranodal myelin damage after acute stretch in Guinea pig spinal cord. J Neurotrauma. 2012;29:611–9. doi: 10.1089/neu.2011.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. 4. McGraw-hill; 2000. [Google Scholar]
- 13.Shi R, Sun W. Potassium channel blockers as an effective treatment to restore impulse conduction in injured axons. Neurosci Bull. 2011;27:36–44. doi: 10.1007/s12264-011-1048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun W, Smith D, Fu Y, Cheng JX, Bryn S, Borgens R, et al. Novel potassium channel blocker, 4-AP-3-MeOH, inhibits fast potassium channels and restores axonal conduction in injured guinea pig spinal cord white matter. J Neurophysiol. 2010;103:469–78. doi: 10.1152/jn.00154.2009. [DOI] [PubMed] [Google Scholar]
- 15.Leung G, Sun W, Brookes S, Smith D, Shi R. Potassium channel blocker, 4-Aminopyridine-3-Methanol, restores axonal conduction in spinal cord of an animal model of multiple sclerosis. Exp Neurol. 2011;227:232–235. doi: 10.1016/j.expneurol.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi R, Blight AR. Compression injury of mammalian spinal cord in vitro and the dynamics of action potential conduction failure. J Neurophysiol. 1996;76:1572–1580. doi: 10.1152/jn.1996.76.3.1572. [DOI] [PubMed] [Google Scholar]
- 17.Bostock H, Sherratt RM, Sears TA. Overcoming conduction failure in demyelinated nerve fibres by prolonging action potentials. Nature. 1978;274:385–7. doi: 10.1038/274385a0. [DOI] [PubMed] [Google Scholar]
- 18.Targ EF, Kocsis JD. 4-Aminopyridine leads to restoration of conduction in demyelinated rat sciatic nerve. Brain Research. 1985;328:358–61. doi: 10.1016/0006-8993(85)91049-2. [DOI] [PubMed] [Google Scholar]
- 19.Blight AR. Effect of 4-aminopyridine on axonal conduction-block in chronic spinal cord injury. Brain Research Bulletin. 1989;22:47–52. doi: 10.1016/0361-9230(89)90126-3. [DOI] [PubMed] [Google Scholar]
- 20.Blight AR, DeCrescito V. Morphometric analysis of experimental spinal cord injury in the cat: the relation of injury intensity to survival of myelinated axons. Neuroscience. 1986;19:321–341. doi: 10.1016/0306-4522(86)90025-4. [DOI] [PubMed] [Google Scholar]
- 21.Blight AR, Gruner JA. Augmentation by 4-aminopyridine of vestibulospinal free fall responses in chronic spinal-injured cats. Journal of the Neurological Sciences. 1987;82:145–159. doi: 10.1016/0022-510x(87)90014-1. [DOI] [PubMed] [Google Scholar]
- 22.Young W. Secondary injury mechanisms in acute spinal cord injury. J Emergency Med. 1993;11:13–22. [PubMed] [Google Scholar]
- 23.Blight AR. Effects of silica on the outcome from experimental spinal cord injury: Implication of macrophages in secondary tissue damage. Neuroscience. 1994;60:263–273. doi: 10.1016/0306-4522(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 24.Babbs CF, Shi R. Subtle paranodal injury slows impulse conduction in a mathematical model of myelinated axons. PLoS One. 2013;8:e67767. doi: 10.1371/journal.pone.0067767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi R, Blight AR. Differential effects of low and high concentrations of 4-aminopyridine on axonal conduction in normal and injured spinal cord. Neuroscience. 1997;77:553–562. doi: 10.1016/s0306-4522(96)00477-0. [DOI] [PubMed] [Google Scholar]
- 26.Shi R, Kelly TM, Blight AR. Conduction block in acute and chronic spinal cord injury: Different dose-response characteristics for reversal by 4-Aminopyridine. Exp Neurology. 1997;148:495–501. doi: 10.1006/exnr.1997.6706. [DOI] [PubMed] [Google Scholar]
- 27.Blight AR. Morphometric analysis of a model of spinal cord injury in guinea pigs, with behavioral evidence of delayed secondary pathology. J Neurol Sci. 1991;103:156–171. doi: 10.1016/0022-510x(91)90159-5. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y, Kim S, Huff TB, Borgens RB, Park K, Shi R, et al. Effective repair of traumatically injured spinal cord by nanoscale block copolymer micelles. Nat Nanotechnol. 2010;5:80–7. doi: 10.1038/nnano.2009.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waxman SG. Membranes, myelin, and the pathophysiology of multiple sclerosis. N Engl J Med. 1982;306:1529–33. doi: 10.1056/NEJM198206243062505. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Sun W, McBride JJ, Cheng JX, Shi R. Acrolein induces myelin damage in mammalian spinal cord. J Neurochem. 2011;117:554–64. doi: 10.1111/j.1471-4159.2011.07226.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4- hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology & Medicine. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 33.Seiler N. Oxidation of polyamines and brain injury. Neurochem Res. 2000;25:471–90. doi: 10.1023/a:1007508008731. [DOI] [PubMed] [Google Scholar]
- 34.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faroon O, Roney N, Taylor J, Ashizawa A, Lumpkin MH, Plewak DJ. Acrolein health effects. Toxicol Ind Health. 2008;24:447–90. doi: 10.1177/0748233708094188. [DOI] [PubMed] [Google Scholar]
- 36.Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicological Sciences. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 37.Uchida K. Current status of acrolein as a lipid peroxidation product. Trends in Cardiovascular Medicine. 1999;9:109–13. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–62. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- 39.Lambert C, Li J, Jonscher K, Yang TC, Reigan P, Quintana M, et al. Acrolein inhibits cytokine gene expression by alkylating cysteine and arginine residues in the NF-kappaB1 DNA binding domain. J Biol Chem. 2007;282:19666–75. doi: 10.1074/jbc.M611527200. [DOI] [PubMed] [Google Scholar]
- 40.Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998;273:16058–66. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 41.Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, et al. Protein- bound acrolein: potential markers for oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4882–7. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. Rev Environ Contam Toxicol. 1995;144:95–146. doi: 10.1007/978-1-4612-2550-8_2. [DOI] [PubMed] [Google Scholar]
- 43.Dreosti. Trace elements, micronuteitions, and free radicals. Vol. 149. Human Press; Clifton, NJ: 1991. [Google Scholar]
- 44.Roots R, Okada S. Estimation of life times and diffusion distances of radicals involved in x-ray-induced DNA strand breaks of killing of mammalian cells. Radiat Res. 1975;64:306–20. [PubMed] [Google Scholar]
- 45.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford: Oxford University Press; 1999. [Google Scholar]
- 46.Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–71. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 48.Hamann K, Shi R. Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury. J Neurochem. 2009;111:1348–56. doi: 10.1111/j.1471-4159.2009.06395.x. [DOI] [PubMed] [Google Scholar]
- 49.Shi R, Luo L. The role of acrolein in spinal cord injury. Applied Neurology. 2006;2:22–27. [Google Scholar]
- 50.Shi R, Rickett T, Sun W. Acrolein-mediated injury in nervous system trauma and diseases. Mol Nutr Food Res. 2011;55:1320–31. doi: 10.1002/mnfr.201100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tully M, Zheng L, Shi R. Acrolein detection: potential theranostic utility in multiple sclerosis and spinal cord injury. Expert Rev Neurother. 2014:1–7. doi: 10.1586/14737175.2014.918849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adams JD, Jr, Klaidman LK. Acrolein-induced oxygen radical formation. Free Radical Biology & Medicine. 1993;15:187–93. doi: 10.1016/0891-5849(93)90058-3. [DOI] [PubMed] [Google Scholar]
- 53.Luo J, Shi R. Acrolein induces axolemmal disruption, oxidative stress, and mitochondrial impairment in spinal cord tissue. Neurochem Int. 2004;44:475–86. doi: 10.1016/j.neuint.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Luo J, Shi R. Acrolein induces oxidative stress in brain mitochondria. Neurochem Int. 2005;46:243–52. doi: 10.1016/j.neuint.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Hamann K, Durkes A, Ouyang H, Uchida K, Pond A, Shi R. Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J Neurochem. 2008;107:712–21. doi: 10.1111/j.1471-4159.2008.05622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng L, Park J, Walls M, Tully M, Jannasch A, Cooper B, et al. Determination of Urine 3-HPMA, a Stable Acrolein Metabolite in a Rat Model of Spinal Cord Injury. J Neurotrauma. 2013;30:1334–41. doi: 10.1089/neu.2013.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leung G, Sun W, Zheng L, Brookes S, Tully M, Shi R. Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune enchephalomyelitis mouse. Neuroscience. 2011;173:150–5. doi: 10.1016/j.neuroscience.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Picklo MJ, Montine TJ. Acrolein inhibits respiration in isolated brain mitochondria. Biochim Biophys Acta. 2001;1535:145–52. doi: 10.1016/s0925-4439(00)00093-4. [DOI] [PubMed] [Google Scholar]
- 59.Shi R, Luo J, Peasley MA. Acrolein inflicts axonal membrane disruption and conduction loss in isolated guinea pig spinal cord. Neuroscience. 2002;115:337–340. doi: 10.1016/s0306-4522(02)00457-8. [DOI] [PubMed] [Google Scholar]
- 60.Liu-Snyder P, McNally H, Shi R, Borgens RB. Acrolein-mediated mechanisms of neuronal death. J Neurosci Res. 2006;84:209–18. doi: 10.1002/jnr.20863. [DOI] [PubMed] [Google Scholar]
- 61.Luo J, Uchida K, Shi R. Accumulation of acrolein-protein adducts after traumatic spinal cord injury. Neurochem Res. 2005;30:291–5. doi: 10.1007/s11064-005-2602-7. [DOI] [PubMed] [Google Scholar]
- 62.Due MR, Park J, Zheng L, Walls M, Allette YM, White FA, et al. Acrolein involvement in sensory and behavioral hypersensitivity following spinal cord injury in the rat. J Neurochem. 2014;128:776–86. doi: 10.1111/jnc.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park J, Zheng L, Marquis A, Walls M, Duerstock B, Pond A, et al. Neuroprotective role of hydralazine in rat spinal cord injury-attenuation of acrolein-mediated damage. J Neurochem. 2014;129:339–49. doi: 10.1111/jnc.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamann K, Nehrt G, Ouyang H, Duerstock B, Shi R. Hydralazine inhibits compression and acrolein-mediated injuries in ex vivo spinal cord. J Neurochem. 2008;104:708–18. doi: 10.1111/j.1471-4159.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Fu Y, Zickmund P, Shi R, Cheng JX. Coherent anti-stokes Raman scattering imaging of axonal myelin in live spinal tissues. Biophys J. 2005;89:581–91. doi: 10.1529/biophysj.105.061911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci. 2006;63:1945–61. doi: 10.1007/s00018-006-6094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Readhead C, Takasashi N, Shine HD, Saavedra R, Sidman R, Hood L. Role of myelin basic protein in the formation of central nervous system myelin. Ann N Y Acad Sci. 1990;605:280–5. doi: 10.1111/j.1749-6632.1990.tb42401.x. [DOI] [PubMed] [Google Scholar]
- 68.Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, et al. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol. 1997;139:1495–506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tao-Cheng JH, Rosenbluth J. Axolemmal differentiation in myelinated fibers of rat peripheral nerves. Brain Res. 1983;285:251–63. doi: 10.1016/0165-3806(83)90023-8. [DOI] [PubMed] [Google Scholar]
- 70.Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J, et al. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci. 2000;20:8354–64. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menegoz M, Gaspar P, Le Bert M, Galvez T, Burgaya F, Palfrey C, et al. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 1997;19:319–31. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- 72.Denisenko-Nehrbass N, Oguievetskaia K, Goutebroze L, Galvez T, Yamakawa H, Ohara O, et al. Protein 4. 1B associates with both Caspr/paranodin and Caspr2 at paranodes and juxtaparanodes of myelinated fibres. Eur J Neurosci. 2003;17:411–6. doi: 10.1046/j.1460-9568.2003.02441.x. [DOI] [PubMed] [Google Scholar]
- 73.Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, et al. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J Neurosci. 2006;26:5230–9. doi: 10.1523/JNEUROSCI.0425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kontrogianni-Konstantopoulos A, Frye CS, Benz EJ, Jr, Huang SC. The prototypical 4.1R-10-kDa domain and the 4. 1g-10-kDa paralog mediate fodrin-actin complex formation. J Biol Chem. 2001;276:20679–87. doi: 10.1074/jbc.M010581200. [DOI] [PubMed] [Google Scholar]
- 75.Fraser PE, Deber CM. Surface accessibility of 13C-labeled lysine residues in membrane-bound myelin basic protein. J Biol Chem. 1984;259:8689–92. [PubMed] [Google Scholar]
- 76.Yokoyama A, Igarashi K, Sato T, Takagi K, Otsuka IM, Shishido Y, et al. Identification of myelin transcription factor 1 (MyT1) as a subunit of the neural cell type-specific lysine-specific demethylase 1 (LSD1) complex. J Biol Chem. 2014;289:18152–62. doi: 10.1074/jbc.M114.566448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gibson BW, Gilliom RD, Whitaker JN, Biemann K. Amino acid sequence of human myelin basic protein peptide 45–89 as determined by mass spectrometry. J Biol Chem. 1984;259:5028–31. [PubMed] [Google Scholar]
- 78.Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–6. doi: 10.1016/s0166-2236(99)01479-4. [DOI] [PubMed] [Google Scholar]
- 79.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 80.Wolswijk G, Balesar R. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain. 2003;126:1638–49. doi: 10.1093/brain/awg151. [DOI] [PubMed] [Google Scholar]
- 81.Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–92. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- 82.Lima RR, Guimaraes-Silva J, Oliveira JL, Costa AM, Souza-Rodrigues RD, Dos Santos CD, et al. Diffuse axonal damage, myelin impairment, astrocytosis and inflammatory response following microinjections of NMDA into the rat striatum. Inflammation. 2008;31:24–35. doi: 10.1007/s10753-007-9046-y. [DOI] [PubMed] [Google Scholar]
- 83.Fu Y, Sun W, Shi Y, Shi R, Cheng JX. Glutamate excitotoxicity inflicts paranodal myelin splitting and retraction. PLoS One. 2009;4:e6705. doi: 10.1371/journal.pone.0006705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nicole O, Ali C, Docagne F, Plawinski L, MacKenzie ET, Vivien D, et al. Neuroprotection mediated by glial cell line-derived neurotrophic factor: involvement of a reduction of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. J Neurosci. 2001;21:3024–33. doi: 10.1523/JNEUROSCI.21-09-03024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shields DC, Schaecher KE, Saido TC, Banik NL. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci U S A. 1999;96:11486–91. doi: 10.1073/pnas.96.20.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shields DC, Tyor WR, Deibler GE, Hogan EL, Banik NL. Increased calpain expression in activated glial and inflammatory cells in experimental allergic encephalomyelitis. Proc Natl Acad Sci U S A. 1998;95:5768–72. doi: 10.1073/pnas.95.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stover JF, Pleines UE, Morganti-Kossmann MC, Kossmann T, Lowitzsch K, Kempski OS. Neurotransmitters in cerebrospinal fluid reflect pathological activity. Eur J Clin Invest. 1997;27:1038–43. doi: 10.1046/j.1365-2362.1997.2250774.x. [DOI] [PubMed] [Google Scholar]
- 88.Blanc EM, Keller JN, Fernandez S, Mattson MP. 4-hydroxynonenal, a lipid peroxidation product, impairs glutamate transport in cortical astrocytes. Glia. 1998;22:149–60. doi: 10.1002/(sici)1098-1136(199802)22:2<149::aid-glia6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 89.Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, et al. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Abeta1–42. J Neurochem. 2001;78:413–6. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 90.Springer JE, Azbill RD, Mark RJ, Begley JG, Waeg G, Mattson MP. 4-hydroxynonenal, a lipid peroxidation product, rapidly accumulates following traumatic spinal cord injury and inhibits glutamate uptake. Journal of Neurochemistry. 1997;68:2469–76. doi: 10.1046/j.1471-4159.1997.68062469.x. [DOI] [PubMed] [Google Scholar]
- 91.Trotti D, Rizzini BL, Rossi D, Haugeto O, Racagni G, Danbolt NC, et al. Neuronal and glial glutamate transporters possess an SH-based redox regulatory mechanism. Eur J Neurosci. 1997;9:1236–43. doi: 10.1111/j.1460-9568.1997.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 92.Gonsette RE. Neurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicity. J Neurol Sci. 2008;274:48–53. doi: 10.1016/j.jns.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 93.Burcham PC, Kerr PG, Fontaine F. The antihypertensive hydralazine is an efficient scavenger of acrolein. Redox Rep. 2000;5:47–9. doi: 10.1179/rer.2000.5.1.47. [DOI] [PubMed] [Google Scholar]
- 94.Burcham PC, Kaminskas LM, Fontaine FR, Petersen DR, Pyke SM. Aldehyde-sequestering drugs: tools for studying protein damage by lipid peroxidation products. Toxicology. 2002;181–182:229–36. doi: 10.1016/s0300-483x(02)00287-1. [DOI] [PubMed] [Google Scholar]
- 95.Burcham PC, Fontaine FR, Kaminskas LM, Petersen DR, Pyke SM. Protein adduct-trapping by hydrazinophthalazine drugs: mechanisms of cytoprotection against acrolein-mediated toxicity. Mol Pharmacol. 2004;65:655–64. doi: 10.1124/mol.65.3.655. [DOI] [PubMed] [Google Scholar]
- 96.Burcham PC, Pyke SM. Hydralazine inhibits rapid acrolein-induced protein oligomerization: role of aldehyde scavenging and adduct trapping in cross-link blocking and cytoprotection. Mol Pharmacol. 2006;69:1056–65. doi: 10.1124/mol.105.018168. [DOI] [PubMed] [Google Scholar]
- 97.Kaminskas LM, Pyke SM, Burcham PC. Reactivity of hydrazinophthalazine drugs with the lipid peroxidation products acrolein and crotonaldehyde. Org Biomol Chem. 2004;2:2578–84. doi: 10.1039/B408796H. [DOI] [PubMed] [Google Scholar]
- 98.Liu-Snyder P, Borgens RB, Shi R. Hydralazine rescues PC12 cells from acrolein- mediated death. J Neurosci Res. 2006;84:219–27. doi: 10.1002/jnr.20862. [DOI] [PubMed] [Google Scholar]
- 99.Hubli M, Gee CM, Krassioukov AV. Refined Assessment of Blood Pressure Instability After Spinal Cord Injury. Am J Hypertens. 2014 doi: 10.1093/ajh/hpu122. [DOI] [PubMed] [Google Scholar]
- 100.Zhu C, Galea M, Livote E, Signor D, Wecht JM. A retrospective chart review of heart rate and blood pressure abnormalities in veterans with spinal cord injury. J Spinal Cord Med. 2013;36:463–75. doi: 10.1179/2045772313Y.0000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gunduz H, Binak DF. Autonomic dysreflexia: an important cardiovascular complication in spinal cord injury patients. Cardiol J. 2012;19:215–9. doi: 10.5603/cj.2012.0040. [DOI] [PubMed] [Google Scholar]
- 102.Galligan JJ, Smathers RL, Fritz KS, Epperson LE, Hunter LE, Petersen DR. Protein carbonylation in a murine model for early alcoholic liver disease. Chem Res Toxicol. 2012;25:1012–21. doi: 10.1021/tx300002q. [DOI] [PMC free article] [PubMed] [Google Scholar]