SUMMARY
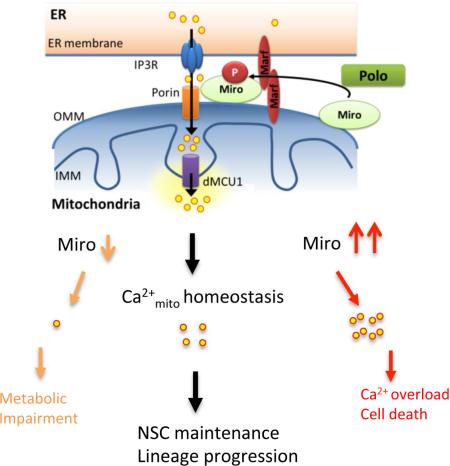
Mitochondria play central roles in buffering intracellular Ca2+ transients. While basal mitochondrial Ca2+ (Ca2+mito) is needed to maintain organellar physiology, Ca2+mito overload can lead to cell death. How Ca2+mito homeostasis is regulated is not well understood. Here we show that Miro, a known component of the mitochondrial transport machinery, regulates Drosophila neural stem cell (NSC) development through Ca2+mito homeostasis control independent of its role in mitochondrial transport. Miro interacts with Ca2+ transporters at the ER-mitochondria contact site (ERMCS). Its inactivation causes Ca2+mito depletion and metabolic impairment, whereas its overexpression results in Ca2+mito overload, mitochondrial morphology change, and apoptotic response. Both conditions impaired NSC lineage progression. Ca2+mito homeostasis is influenced by Polo-mediated phosphorylation of a conserved residue in Miro, which positively regulates Miro localization to, and the integrity of, ERMCS. Our results elucidate a regulatory mechanism underlying Ca2+mito homeostasis and how its dysregulation may impact NSC metabolism/development and contribute to disease.
Graphical Abstract
INTRODUCTION
Mitochondria are important for many aspects of cellular function, from ATP production and intermediary metabolism to apoptosis. Mitochondrial dysfunction has been broadly linked to the pathogenesis of a multitude of neurological disorders (Chan, 2006; Schon and Przedborski, 2011; Tait and Green, 2012; Wallace, 2005), emphasizing the particular importance of this organelle to neuronal function and maintenance. Mitochondrial function and Ca2+ signaling have been intimately linked. On one hand, Ca2+mito uptake helps buffer cytosolic Ca2+ signals and oscillations such as those generated by neuronal activation, thus amplifying and sustaining signals arising from neuronal activity while protecting neurons from the potentially damaging Ca2+ spikes (MacAskill et al., 2010; Sheng and Cai, 2012; Vos et al., 2010). On the other hand, Ca2+mito can positively regulate the activities of TCA cycle enzymes and electron transport chain components, stimulating ATP production (Cardenas et al., 2010; Jouaville et al., 1999; McCormack et al., 1990). Ca2+mito uptake primarily occurs at ERMCS where local Ca2+ can reach high levels (Rizzuto et al., 1993; Rizzuto et al., 1998). Proteins localized to ERMCS include those that mediate Ca2+ transfer, such as inositol 1,4,5-trisphosphate receptor (IP3R) at the ER, voltage-dependent anion-selective channel (VDAC) at the mitochondria outer membrane (MOM), mitochondrial Ca2+ uniporter (MCU) at the mitochondrial inner membrane (MIM) (Giorgi et al., 2015; Hajnoczky et al., 2002; Rowland and Voeltz, 2012), and mitofusin-2 (de Brito and Scorrano, 2008). In addition to transferring Ca2+, ERMCS also transfer lipids (Hayashi et al., 2009). Despite the importance of ERMCS to fundamental cellular functions and its potential link to disease conditions (Schon and Area-Gomez, 2013; Rizzuto et al., 2012), its roles during normal development remain mostly unexplored, and the signaling mechanisms regulating its integrity and function are largely unknown.
Mitochondrial rho GTPase (Miro) is a MOM protein containing two GTPase domains and two Ca2+-sensing EF hand domains (Fransson et al., 2003). Genetic studies in Drosophila (Guo et al., 2005) and mouse (Nguyen et al., 2014) revealed a key function of Miro in regulating the axonal transport of mitochondria, during which Miro forms a multi-protein complex with Milton and Kinesin heavy chain (KHC) to link mitochondria with motor molecules and the microtubule cytoskeleton (Glater et al., 2006). Through binding to the EF hands of Miro and altering protein-protein interactions within the transport machinery, cytosolic Ca2+ dynamically regulates the motility of mitochondria, offering one mechanism to match mitochondrial distribution with neuronal activity (Macaskill et al., 2009; Saotome et al., 2008; Wang and Schwarz, 2009). The PINK1/Parkin pathway, which is critically involved in maintaining mitochondrial function and mutations in which are associated with Parkinson's disease (PD), can regulate Miro stability and thus mitochondrial transport (Liu et al., 2012; Wang et al., 2011). Interestingly, despite the importance of the Miro/Milton/Khc complex in regulating mitochondrial transport in metazoans, Miro is the only component conserved in all eukaryotes, suggesting that it may perform some ancestral function that is independent of the transport complex. Indeed, functions of Miro not directly related to transport are emerging in yeast (Lee and Lu, 2014), where yeast Miro (Gem1) is localized to ERMCS (Kornmann et al., 2011) and regulates ER-associated mitochondrial division (Murley et al., 2013). The prominent roles of Miro in mitochondrial regulation and the importance of mitochondria to neuronal function and survival in humans promoted us to examine the physiological role of Miro in the nervous system of a multicellular organism.
RESULTS
Miro Regulates Drosophila NSC Maintenance in a Manner Independent of Mitochondrial Transport
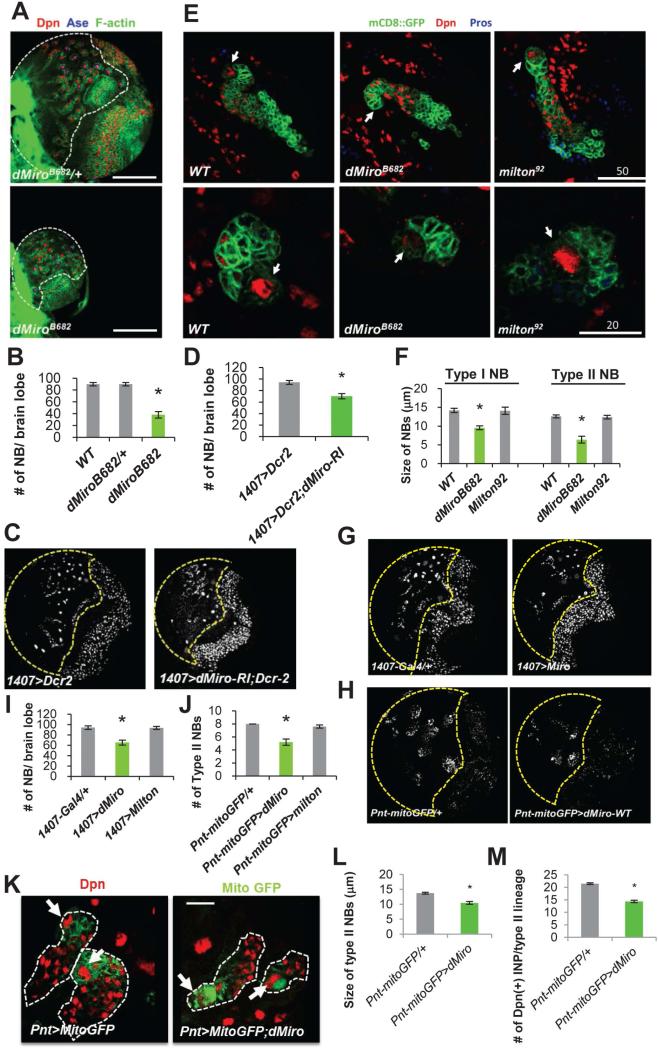
To investigate the physiological function of Miro in a developmental setting, we used Drosophila central brain NSCs called NBs. We examined the type I and type II NBs in the larval brains of animals carrying the dMiroB682 mutant allele, presumably a protein null (Figure S1A, S1B) with disrupted mitochondrial distribution in neuronal and muscle tissues and late-larvae or early-pupae lethality (Guo et al., 2005). The brain size and number of Deadpan (Dpn)-positive NBs were dramatically reduced in dMiroB682 homozygous mutants (Figure 1A, 1B). Loss of NB was also observed when dMiro was specifically knocked down in the NB lineages by RNAi (Figure 1C, 1D). To examine the effect of dMiro mutation on NB lineage architecture, we performed clonal analysis with MARCM (Lee and Luo, 2001). Type I and type II NB lineages of control animals each invariably contained one Dpn-positive primary NB, whereas the primary NBs were frequently lost in dMiroB682 mutant NB clones (Figure 1E). In cases where the primary NBs remained in the mutant clones, their sizes were noticeably smaller than control NBs (Figure 1E, 1F). These results indicate a critical and cell-autonomous role of Miro in the development and/or maintenance of larval brain NBs.
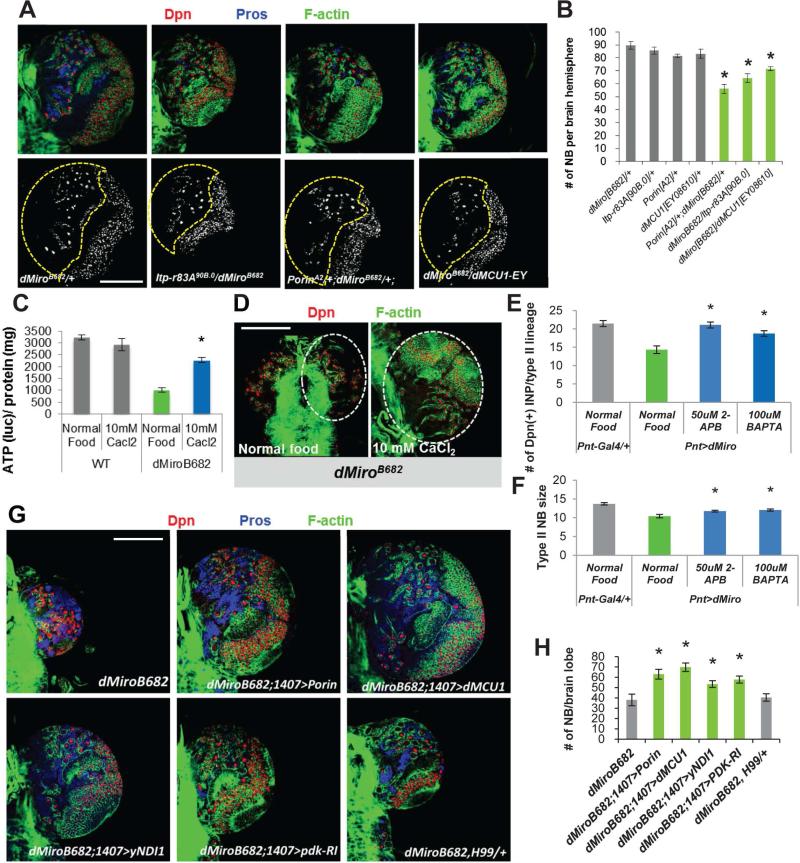
Figure 1. Regulation of NB Behavior by Miro.
(A, B) Reduction of NB number and brain size in dMiroB682 null mutant. Larval brains of dMiroB682 heterozygous and homozygous animals were immunostained for pan-NB marker Dpn, type I NB marker Ase, and actin. The central brain area is outlined with white dashed line.
(C, D) Reduction of NB number by NB-specific knockdown of dMiro. Control (1407>Dcr2) and dMiro RNAi (1407>Dcr2,dMiro-RI) larval brains were immunostained for Dpn.
(E) MARCM analysis of NB lineages in the dMiroB682 and milton92 backgrounds. Brains were stained for Dpn and Pros, a differentiation marker. NB clones are marked with GFP. Upper: Type II NBs, Lower: Type I NBs. Primary NBs are indicated with arrows. Note that the primary NB in the dMiroB682 type II NB clone is losing Dpn expression.
(F) Quantification of the size of NBs from E.
(G, H) Effects on NB number by pan-NB (1407-Gal4) or type II NB-specific (Pnt-Gal4) dMiro OE. Brains were stained for Dpn. The central brain area is outlined with yellow dashed line.
(I, J) Quantification of total NB (I) or type II NB (J) number from G and H, respectively.
(K-M) Effects of dMiro OE on type II NB size (L) and IP number (M). The type II NB lineages are outlined with white dashed line. Primary NBs are marked with arrows.
Error bar: SEM; *, p<0.05 versus control in Student's t-tests. n = 5. Scale bars, 100 μm (A, C, G); 20 μm (K).
See also Figure S1.
Type II NBs undergo self-renewing asymmetric divisions to generate immature intermediate progenitors (IPs), which progress into transit-amplifying mature IPs that also undergo self-renewing asymmetric divisions to generate ganglion mother cells (GMCs) and differentiated neuronal or glial progenies (Bello et al., 2006; Boone and Doe, 2008; Bowman et al., 2008). IPs sequentially express the transcription factors Dichaete (D), Grainy head (Grh), and Eyeless (Ey) during their transition from young to old IPs (Bayraktar and Doe, 2013). Thus, early-born (old) IPs express Ey, whereas late-born (young) IPs express D. In dMiroB682 mutant clones, young IPs and old IPs were both significantly reduced in number compared to control WT clones (Figure S1C, S1D). Loss-of-function (LOF) of dMiro thus reduces the number of transit-amplifying IPs, presumably as a result of stem cell loss during lineage progression.
Next, we examined Miro gain-of-function (GOF) effect using 1407-Gal4- or Pnt-Gal4-directed pan-NB or type II NB-specific Miro overexpression (OE), respectively. Miro OE led to a reduction of the number of total NBs in 1407-Gal4>dMiro-OE (Figure 1G, 1I) and occasional loss of type II NBs in Pnt-Gal4>dMiro-OE conditions (Figure 1H, 1J). A reduction of NB size was also seen in dMiro-OE animals (Figure 1K, 1L). In the Pnt-Gal4>dMiro-OE condition, the number of IPs, specifically the D+ young IPs in type II NB lineages, was reduced (Figure 1M). These results indicate that NB maintenance and lineage progression are sensitive to Miro dosage.
Miro is a key component of a conserved protein complex that transports mitochondria along microtubule tracks (Brickley et al., 2005; Fransson et al., 2006; Guo et al., 2005; Hirokawa, 1998; Pilling et al., 2006; Stowers et al., 2002). To test whether the function of Miro in mitochondrial trafficking is important for NB maintenance, we examined mitochondrial distribution in dMiro mutant clones by staining for ATPsyn5α. Mitochondrial distribution or morphology was not obviously affected by dMiro mutation in NB lineages (Figure S1E). To further assess the role of mitochondrial transport in NB development, we analyzed the LOF and GOF of Milton, another key component of the transport complex (Glater et al., 2006). Neither the number nor size of primary NBs was affected by NB-specific knockdown of Milton (Figure S1F), in milton92 mutant MARCM clones (Figure 1E, 1F), or when Milton was overexpressed (Figure 1I, 1J). The Milton RNAi line used here was known to be effective in altering neuronal mitochondrial transport or function (Liu et al., 2012; Fang et al., 2012; Iijima-Ando et al., 2009). Intriguingly, Miro-OE led to mitochondrial distribution defects in NBs, with mitochondria accumulating asymmetrically to one side of the perinuclear area, and this defect was partially rescued by Milton-RNAi (Figure S1G). However, Miro-OE effect on NB lineage development was not affected by Milton-RNAi (data not shown), suggesting that a transport-independent function of Miro is important for NB maintenance and lineage development.
Miro Regulates Ca2+mito Homeostasis
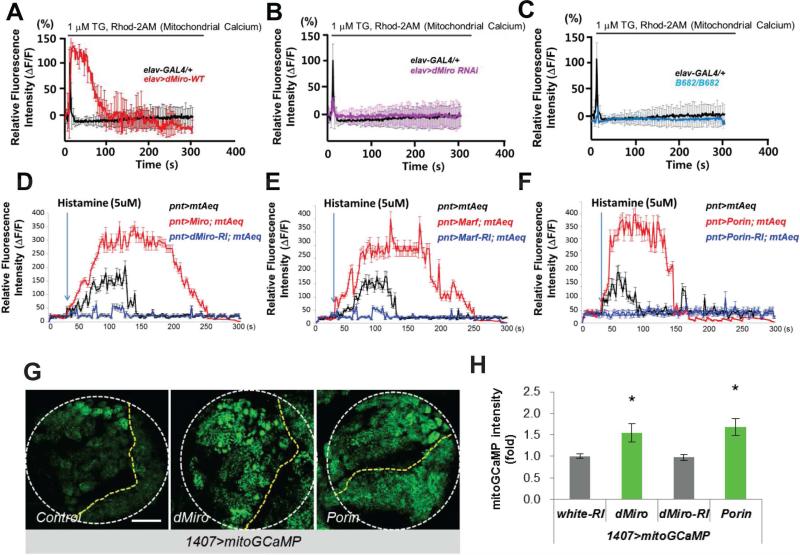
Miro contains GTPase domains and Ca2+-binding EF hand motifs, and its function in transporting neuronal mitochondria is regulated by activity-induced Ca2+ influx (Macaskill et al., 2009; Saotome et al., 2008; Wang and Schwarz, 2009). Yeast Gem1 and mammalian Miro1 (Rhot1) are localized to ERMCS (Kornmann et al., 2011), a structure that directs Ca2+ transfer from ER to mitochondria (Hayashi et al., 2009). To test whether Miro regulates Ca2+mito homeostasis in NSC lineage, we first measured Ca2+mito levels under dMiro LOF and GOF, using the Ca2+-sensitive fluorescent dye Rhod-2 AM. Consistent with a previous study (Guo et al., 2005), Rhod-2 AM preferentially monitored Ca2+mito in Drosophila neurons (Figure S2A). We loaded primary cultured neurons with Rhod-2 AM. Blocking ER Ca2+ uptake with thapsigagin (TG) increased cytosolic Ca2+ and induced a spike of Ca2+mito as reported (Perocchi et al., 2010), which quickly dissipated in control cells. Notably, in Miro-OE neurons the TG-induced Rhod-2 AM spike was stronger and persisted longer (Figure 2A), whereas TG induced a much weaker spike in dMiroB682 mutant or Miro-RNAi neurons than controls (Figure 2B, 2C). We also measured cytosolic Ca2+ response using Fluo-3AM. Overexpression of wild type but not a mutant form of Miro led to a decrease in the magnitude of cytosolic Ca2+ spikes induced by TG (Figure S2B).
Figure 2. Regulation of Ca2+mito by Miro.
(A-C) Effects of pan-neuronal elav-Gal4 driven dMiro OE (A) or dMiro RNAi (B), or dMiroB682 null mutation (C), on TG-stimulated Rhod-2 AM fluorescence in primary cultured fly neurons. The traces show mean response of cells present in the microscope field and are representative of more than 3 experiments.
(D-F) Effects of OE or RNAi of Miro (D), Marf (E), or Porin (F) on mitochondrial Ca2+ in type II NB lineages as monitored using mito-AEQ. The traces show mean response of 10 dissected larval brains present in the microtiter plate and are representative of more than 3 tests.
(G, H) Imaging of basal Ca2+mito using mito-GCaMP in larval brains without (control) or with Miro or Porin OE. White circle outline larval brain, yellow lines separate central brain (left) from the rest of brain. Images are representative of more than 5 samples. H, quantification of mito-GCaMP intensity in NBs from G.
Error bar: SEM; *, p<0.05 versus control in Student's t-tests. Scale bars, 100 μm (G).
See also Figure S2.
To detect Ca2+mito levels in vivo in NBs, we expressed genetically-encoded, mitochondria-targeted Ca2+mito reporters mito-Apoeaquorin (mtAEQ) (Terhzaz et al., 2006) or mito-GCaMP3 (Lutas et al., 2012) in type II NBs. With the mtAEQ reporter, dissected brains from transgenic (Tg) animals were treated with histamine, an IP3-generating agonist, to induce Ca2+ release from the ER and increase Ca2+mito uptake (Perocchi et al., 2010), and luminescence signals were immediately measured with an automated luminometer. Under this condition, Ca2+mito was significantly elevated when known components of ERMCS such as Marf and Porin/VDAC were overexpressed, and Ca2+mito was attenuated when they were inhibited (Figure 2E, 2F), validating the utility of the reporters in monitoring the effect of ERMCS on Ca2+mito homeostasis. We found that Ca2+mito was significantly increased by Miro-OE, but decreased by Miro-RNAi (Figure 2D). In contrast, Milton LOF or GOF had no obvious effect on Ca2+mito uptake under similar conditions (Figure S2C). With the mito-GCaMP3 reporter, we observed that basal fluorescence signal was significantly increased by Miro-OE or Porin-OE (Figure 2G, 2H). The expression level of mito-GCaMP protein was not altered by Miro-OE (Figure S2D). These results, and the observation that cytosolic Ca2+ level as measured with a cytosolic GCaMP reporter (Figure S2E-F), and mitochondrial membrane potential (Figure S2G, S2H) - the driving force of Ca2+mito entry, were not altered by Miro-OE, support that Miro specifically promotes Ca2+mito uptake. Miro therefore regulates Ca2+ transfer from ER to mitochondria, and this occurs in a manner seemingly independent of mitochondrial transport.
Miro-Mediated Ca2+mito Homeostasis Regulates Mitochondrial Activity
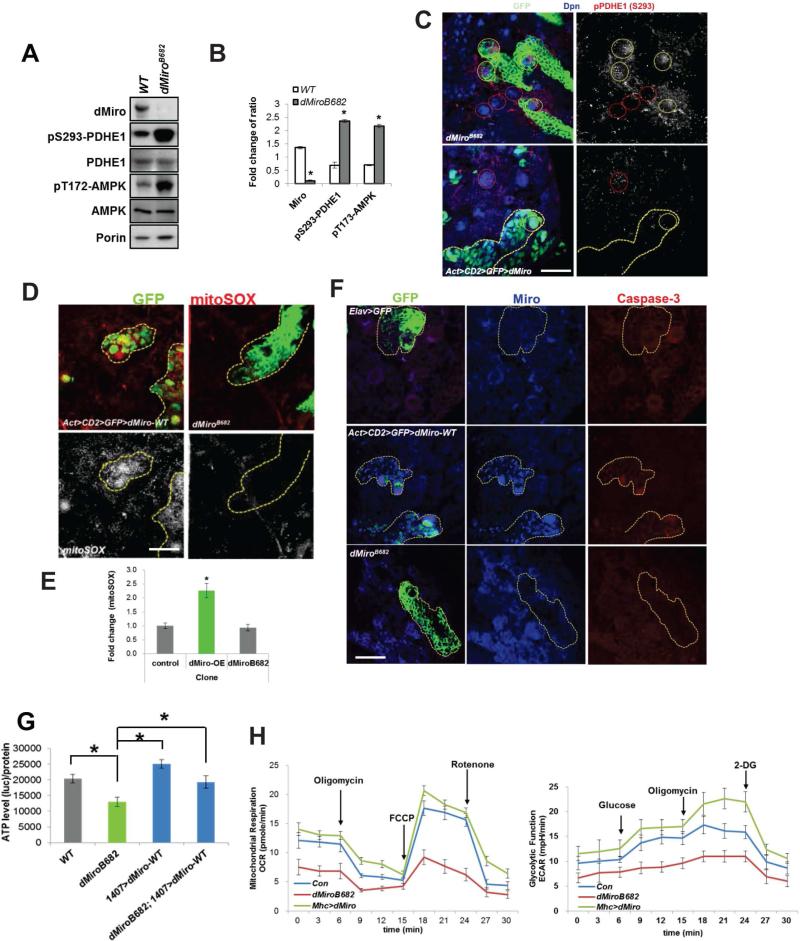
Ca2+mito uptake critically regulates many cellular functions, ranging from ATP production to cell death (Rizzuto et al., 2012). Sufficient Ca2+ uptake into the mitochondrial matrix is needed for ATP production by enhancing the activities of key metabolic enzymes. For example, the key TCA cycle enzyme pyruvate dehydrogenase (PDH) is inhibited by phosphorylation by PDH kinase (PDK). Ca2+mito activates PDH phosphatase to remove an inhibitory phosphate from PDHE1 subunit (Cardenas et al., 2010). Thus, Ca2+mito negatively influences p-PDHE1 level. Normalized p-S293-PDHE1 level was markedly increased in dMiro mutant but decreased in Miro-OE brain extracts (Figure 3A, 3B, S3A, S3B). Consistently, the intensity of p-PDHE1 immunosignal was increased in dMiro mutant but decreased in Miro-OE NB clones, compared to control NBs located outside of the clones in the same animals (Figure 3C). ATP production in larval brain was decreased in dMiroB682 mutant, which was rescued by NB-specific Miro-OE (Figure 3G). Measurement of oxygen consumption rate (OCR) confirmed alteration of mitochondrial metabolism under Miro LOF and GOF conditions (Figure 3H). In response to ATP depletion, AMP-activated protein kinase (AMPK) is activated by phosphorylation as a compensatory response to restore energy homeostasis (Hardie, 2007). AMPK was hyperphosphorylated in dMiro mutant (Figure 3A, 3B). These results suggest that reduced Ca2+mito uptake impairs mitochondrial metabolism and ATP production in Miro LOF condition.
Figure 3. Miro-Mediated Ca2+mito Homeostasis Regulates Mitochondrial Activity.
(A, B) Western blot (WB) analysis showing increased p-PDHE1 and p-AMPK in dMiro mutant. PDHE1, Porin, and actin serve as controls. Normalized protein levels are shown as the ratio of dMiro/Porin, pS293-PDHE1/PDHE1 and pT713-AMPK/AMPK from A.
(C-F) Immunostaining of p-PDHE1 (C), mito-SOX (D, E), or activated caspase 3 (F) in dMiro mutant MARCM clones (yellow-outlined in upper panels) or dMiro-OE Flip-out clones (yellow-outlined in lower panels). NBs within clones are marked with white circles and control NBs outside of the clones are marked with red circles in C.
(G) Effect of dMiro-WT OE on ATP level in dMiro mutant larval brain.
(H) Real-time changes in OCR and Glycolytic rate (ECAR) were measured in control, dMiro-WT-OE (Mhc>dMiro-WT) and dMiro mutant animals using the Seahorse Bioscience XF Analyzer.
Error bar: SEM; *, p<0.05 versus control in Student's t-tests. Scale bars, 50 μm (C), 20 μm (D, E).
See also Figure S3.
While mitochondrial metabolism requires sufficient levels of Ca2+mito, prolonged Ca2+mito uptake can lead to opening of the permeability transition pore and release of pro-apoptotic components such as Cytochrome (Cyt) c, a critical event in mitochondria-mediated cell death (Rizzuto et al., 2012). To test whether the detrimental effect of Miro-OE on NB maintenance is related to Ca2+mito overload-induced cytotoxicity, we analyzed mitochondrial redox status using MitoSOX Red, a mitochondrial superoxide indicator. MitoSOX signal was higher in Miro-OE than control clones. No obvious change in MitoSOX signal was observed in dMiro mutant clones (Figure 3D, 3E). Miro-OE but not dMiro mutant clones also showed increased staining of activated caspase-3 (Figure 3F), and release of Cyt c into the cytosol (Figure S3C, S3D), which are markers of apoptotic processes.
Under normal physiological conditions, the bulk of cellular Ca2+ resides within the ER lumen. ER Ca2+ depletion can cause ER stress and activate an unfolded protein response (Malhotra and Kaufman, 2011). We tested whether, by enhancing Ca2+mito uptake, Miro-OE might lower ER luminal Ca2+ level and cause ER stress. The ER stress marker p-eIF2α was significantly elevated in Miro-OE but not dMiro mutant NB clones (Figure S3E), suggesting that elevated Ca2+mito uptake may cause Ca2+mito overload, Ca2+ depletion from the ER, and ER stress, all of which may contribute to apoptotic responses in Miro-OE condition. To test this hypothesis, we first examined the effect of ER stress inducers on NB development. Acute treatment of 3rd instar larvae with 5mM DTT + 1μM TG resulted in loss of IPs (Figure S3G), indicating that ER stress can recapitulate Miro-OE effect in NB development. Importantly, two independent RNAi lines against the ER stress kinase PERK both effectively rescued dMiro-OE induced NB lineage defects (Figure S3F). Next, we estimated ER Ca2+ content by measuring ionomycin-releasable Ca2+ levels (Tu et al., 2006). Ionomycin is an ionophore that causes more complete emptying of ER Ca2+ stores than TG. In the absence of extracellular Ca2+, ionomycin elevates intracellular (cytosolic) Ca2+ by emptying intracellular stores, and the resulting transient Ca2+ peak gives a reasonable approximation of steady state ER Ca2+ content. With this method, we found that HeLa cells transfected with hMiro1 have less ER Ca2+ content than control cell transfected with empty vector (Figure S3H, I). Together, these results indicate that Miro-regulated Ca2+ homeostasis within the ER and mitochondria are both important for NB lineage development.
Miro Interacts with Ca2+ Transporters at the ERMCS to Regulate Ca2+mito Homeostasis and NB Maintenance
Transfer of Ca2+ from the ER to mitochondria is a major function of the ERMCS carried out by the Ca2+ transporters IP3R, VDAC, and MCU localized at the ER, MOM, and MIM respectively. We found that similar to Miro case, knocking down fly IP3R (Venkatesh and Hasan, 1997), Porin (VDAC) (Messina et al., 1996), or MCU resulted in reduced NB number (Figures S4A-S4D), supporting the importance of ER-mitochondria Ca2+ transfer to NB maintenance. In addition, overexpressing these Ca2+ transporters also modestly reduced NB number (Figure S4E, S4F). We further tested the genetic interaction between Miro and the Ca2+ transporters. Although the heterozygous conditions for dMiro or each of the Ca2+ transporter genes alone had no obvious effect on NB development, double heterozygous combinations between dMiro and the Ca2+ transporters resulted in reduced NB number (Figure 4A, 4B), supporting that Miro functionally interacts with Ca2+ transporters at the ERMCS to control Ca2+mito homeostasis, mitochondrial function, and NB maintenance. Consistently, partial LOF of IP3R or Porin reduced Ca2+mito to basal levels in Miro-OE condition and blocked the Miro-OE effect on NB maintenance (Figure S4G, S4H).
Figure 4. Functional Interaction between Miro and Ca2+ Transporter at the ERMCS.
(A, B) Transheterozygotes between dMiro and IP3R (Itp-r83A), Porin, or dMCU mutants resulted in reduction of NB number. Larval brains were immunostained for Dpn, Pros, and F-actin (cell cortex). Central brain area is outlined with dashed line.
(C, D) Effects of CaCl2 feeding in rescuing brain ATP level (C) and brain size (D) of dMiro mutant. Larval brains were immunostained for Dpn and F-actin. Brain lobes are outlined.
(E, F) Quantification of the effects of 2-APB and BAPTA feeding in rescuing the reduced IP number (E) and NB size (F) caused by dMiro OE.
(G, H) Genetic interactions showing rescue of NB number in dMiro mutant by the OE of Porin, dMCU, yNDI1, or PDK-RNAi, but not the H99 heterozygous background. Larval brains were immunostained for Dpn, Pros, and F-actin. H, quantification of number of NBs shown in G.
Error bar: SEM; *, p<0.05 in Student's t-tests. Scale bars, 100 μm.
See also Figure S4.
We next took a pharmacological approach to test the role of Ca2+mito homeostasis in mediating Miro function in NSCs. Previous studies showed that feeding flies with CaCl2 could increase intracellular Ca2+ levels, especially at the larval stage (Dube et al., 2000), and that exogenous Ca2+ could overcome genetic deficits in intracellular Ca2+ signaling (Howard, 1984). We found that Ca2+mito was increased upon CaCl2 feeding (Figure S4I, S4J), and that feeding dMiroB682 mutant larvae with CaCl2 partially restored ATP production (Figure 4C) and rescued the brain size reduction and NB loss (Figure 4D). Conversely, Ca2+mito overload caused by Miro-OE was attenuated (Figure S4I, S4J), and the reduction of NB size and loss of IPs in Pnt>Miro-OE condition was rescued by reducing Ca2+mito level via Ca2+ chleation with bis (2-aminophenoxy) ethane tetra-acetic acid (BAPTA), or IP3R inhibition with 2-aminoethoxydiphenyl borate (2-APB) (Figure 4E, 4F).
We also tested the interaction between Miro and the ER-mitochondria Ca2+ transporters using available GOF alleles. NB-specific OE of Porin or dMCU significantly rescued the NB-loss phenotype seen in dMiroB682 mutant (Figure 4G, 4H). Consistent with mitochondrial metabolic impairment due to reduced Ca2+mito uptake being responsible for the NB phenotype in dMiroB682 mutant, overexpression of yeast NDI1 (yNDI1), which encodes a single-peptide alternative complex-I and is functional in boosting fly mitochondrial complex-I activity (Bahadorani et al., 2010; Sanz et al., 2010), partially restored NB number in dMiroB682 mutant. Enhancing PDH activity by PDK RNAi had similar effect (Figure 4G, 4H). This contrasts with the lack of effect of overexpressing the apoptosis inhibitor p35 (data not shown), or the compound removal of three cell death executers in flies (Reaper, Grim, and Hid) using the H99 deficiency (Foley and Cooley, 1998) (Figures 4G, 4H). These results support the notion that the Miro-LOF effect on NB maintenance is due to Ca2+mito depletion and metabolic impairment as apposed to Ca2+mito overload and apoptotic activation in Miro-GOF condition.
Regulation of Miro Function by Polo-Mediated Phosphorylation at Serine 66
Despite the importance of ER-mitochondria Ca2+ signaling to cellular physiology, little is known how this process is regulated. Implication of Miro as a key player in this process offers the opportunity to investigate the regulatory mechanisms. By aligning the sequence of Miro homologs from fly to humans, we identified a conserved, putative phosphorylation site for Polo-like kinases (PLKs) in the N-terminal GTPase domain of Miro (Figure 5A, S5A). Of the two GTPase domains of Miro, the N-terminal one is particularly important for ERMCS localization (Kornmann et al., 2011), mitochondrial morphology and transport, and cell survival (Babic et al., 2015). Polo is also of particular interest as previous studies identified it as a regulator of NB self-renewal in Drosophila (Wang et al., 2007). Our immunohistochemical and biochemical analyses indicated that a fraction of Polo protein is localized to mitochondria (Figure S5B-E). To test for a molecular link between Miro and Polo in NB maintenance, we first asked whether Miro and Polo physically interact in vivo. In co-IP experiments using elav>Polo-GFP fly head extracts, we detected Polo-GFP in complex with endogenous dMiro (Figure 5B). Co-expression with Polo resulted in increased dMiro phosphorylation at Ser but not Thr residue(s) (Figure 5C, 5D). To test whether Polo directly phosphorylates Miro, we performed in vitro kinase assays using PLK1 as kinase and GST-dMiro fusion proteins as substrates. PLK1 strongly phosphorylated Miro-FL (aa 1-634) and Miro-N (aa 1–213), but not Miro-M (aa 210-393) or Miro-C (aa 390-634) (Figure 5E). None of the GST-Miro fusion proteins was phosphorylated by a control kinase PKC-zeta (Figure S5F, G). We next searched for the target site of Polo in Miro-N by focusing on conserved Ser residues, including Ser66 that matches the consensus site for PLKs (Figure 5A, S5A). We note that the corresponding sites in mammalian Miro1 and Miro2 may be better targets for PLK2 or 3, which prefers an acidic residue at +1 position (Rozeboom and Pak, 2012). A S66A mutation led to a dramatic reduction of Miro-N phosphorylation by PLK1 compared to mutating other conserved Ser residues (Figure 5F). S66 in dMiro N-terminal GTPase domain thus represents a major phosphorylation site for PLK1 in vitro.
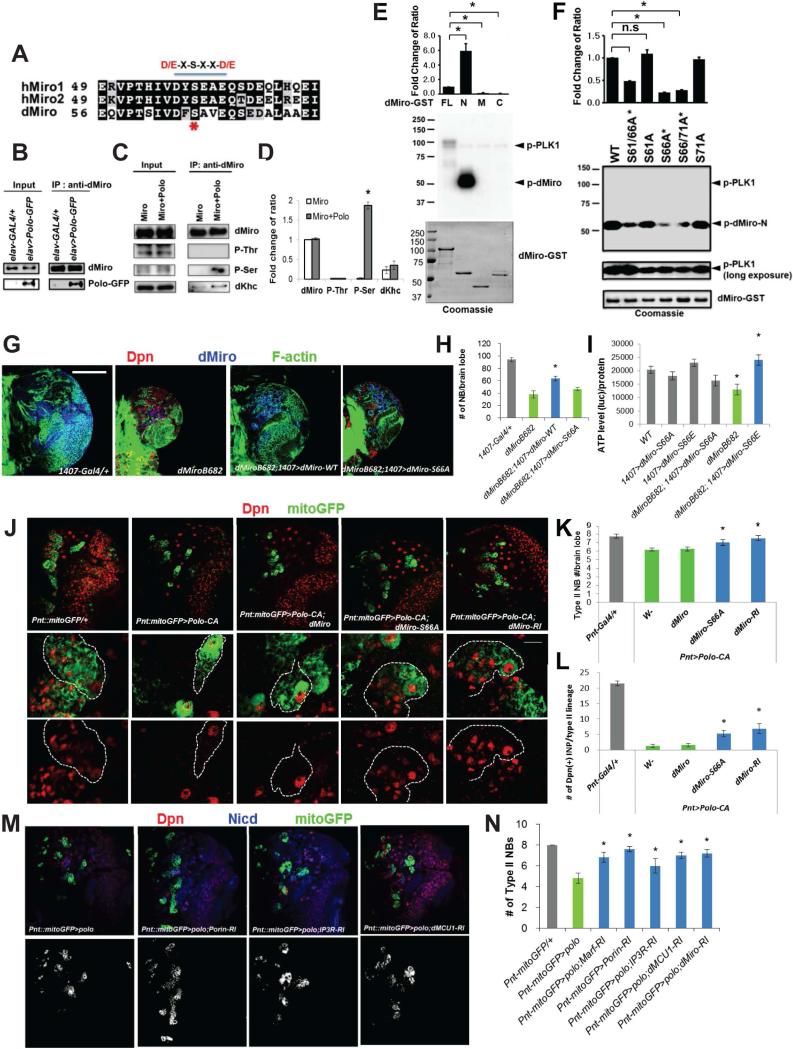
Figure 5. Regulation of Miro Function by Polo-Mediated Phosphorylation at Serine 66.
(A) Comparison of candidate Polo/PLK phosphorylation motifs at the N-terminus of Miro. The consensus D/E-X-S-X-X-D/E motif is underlined, and S66 indicated by asterisk.
(B) Polo-GFP is present in dMiro IP prepared from fly brain extracts.
(C, D) Increased dMiro phosphorylation at Ser but not Thr residue(s) after Polo co-expression in fly brain. D, quantification of normalized phospho-Ser intensity from C.
(E, F) In vitro kinase assays showing phosphorylation of dMiro-N by PLK1 (E), and the strong effect of S66A mutation in blocking PLK1 effect (F). Bar graph shows quantification of normalized phospho-dMiro signal (top). GST-dMiro proteins were visualized by autoradiography (middle panels) or Coomassie blue staining (bottom panels).
(G, H) Rescue of brain size (G) and NB number (H) in dMiro mutant by dMiro-WT but not -S66A. Larval brains were immunostained for Dpn, Pros, and F-actin.
(I) Rescue of reduced brain ATP level in dMiro mutant by dMiro-S66E, but not -S66A.
(J-L) Rescue of type II NB number (J, K) or IP number (J, L) in Polo-CA OE animals by dMiro-S66A or dMiro-RNAi. Larval brains were immunostained for Dpn and GFP. Dashed lines mark type II NB lineages.
(M, N) Rescue of type II NB number in Polo OE animals by RNAi of ERMCS components. Larval brains were immunostained for Dpn, GFP and NICD (cell membrane). N, quantification of number of NBs in M.
Error bar: SEM; *, p<0.05 in Student's t-tests. Scale bars, 100 μm (G, upper panel of J, M ), 20 μm (lower panel of J).
See also Figure S5.
To study the physiological effects of Polo-mediated phosphorylation of Miro in vivo, we generated Tgs expressing wild type dMiro (dMiro-WT) or dMiro-S66A. To circumvent overexpression artifacts, Tgs that express dMiro-WT and dMiro-S66A at comparable, but less than endogenous dMiro expression levels (Figure 6F, S3A), were chosen for further analyses. The NB-loss phenotype seen in dMiro mutant was significantly rescued by 1407-Gal4-driven expression of dMiro-WT but not dMiro-S66A (Figure 5G, 5H). This effect is likely related to the function of Miro in modulating Ca2+mito homeostasis, as dMiro-S66A exhibited compromised ability to induce Ca2+mito uptake compared to dMiro-WT (Figure S5H, S2B). Similarly, reduced ATP production in dMiro mutant larval brain was rescued by 1407-Gal4 driven dMiro-WT, but not -S66A (Figure 5I). Unlike in dMiro-WT case, NB clones overexpressing dMiro-S66A did not show increased mitochondrial ROS (Figure S5J). Phosphorylation of S66 in dMiro thus positively influences its function in regulating mitochondrial function and NB maintenance under basal conditions.
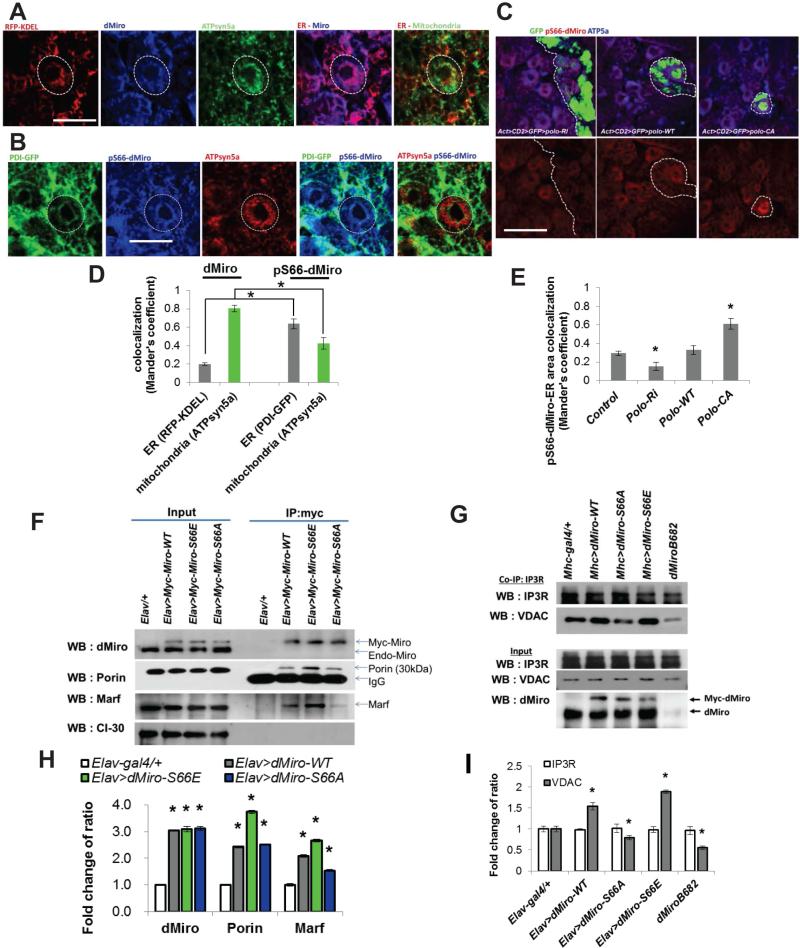
Figure 6. Phosphorylation of Miro Regulates the Integrity of ERMCS.
(A, B) Immunostaining showing localization of p-S66-Miro to ERMCS of larval brain NBs. Circles mark the NBs of interest. Larval brains were stained for the indicated antibodies.
(C) Effects of Polo on p-S66-Miro immunosignal in Flip-out NB clones with Polo overexpressed or knocked down by RNAi. Clones are marked with GFP.
(D, E) Quantification of dMiro and p-dMiro colocalization with ER and mitochondria shown in A and B (D) or p-dMiro colocalization with ER shown in C (E), using Mander's coefficient.
(F, H) Co-IP assays showing the effects of S66A and S66E mutations on Miro interaction with Porin and Marf. H, quantification of proteins pulled down by co-IP in F.
(G, I) Co-IP assays showing the effects of dMiro mutation or OE of Miro variants on IP3R-VDAC interaction. I, quantification of proteins pulled down by co-IP in G.
Error bar: SEM; *, p<0.05 in Student's t-tests. Scale bars, 20 μm.
See also Figure S6.
Consistent with Polo playing a role in regulating Ca2+mito homeostasis, Ca2+mito level was increased by Polo-OE but moderately decreased by Polo-RNAi (Figure S5I). Moreover, Polo-OE caused significant loss of type II NBs and IPs, which was rescued by dMiro RNAi or dMiro-S66A co-expression (Figure 5J-L), or by RNAi of genes mediating ER-mitochondria Ca2+ transfer (Figure 5M, 5N). Importantly, the NB and IP loss caused by Polo-OE was also partially rescued by pharmacological interventions that reduce Ca2+mito (Figure S5K, L). This effect was specific, as NB and IP losses caused by Numb-OE (Ouyang et al., 2011) was not rescued by similar treatments (Figure S5M). The Polo-Miro axis thus plays a physiological role in regulating NB maintenance via Ca2+mito homeostasis.
Phosphorylation of Miro by Polo Regulates the Integrity of ERMCS
We next sought to understand the mechanism by which Polo phosphorylation of Miro affects Ca2+mito homeostasis. We first tested whether phosphorylation affects the localization of Miro. For this purpose, we generated an antibody against dMiro phosphorylated at S66 (p-S66-Miro), the specificity of which was demonstrated by the absence of signal in dMiro mutant (Figure S6A-C), its sensitivity to phosphatase treatment (Figure S6D), and its response to altered Polo activity (see later). To specifically examine the localization of Miro or p-S66-Miro with respect to mitochondria and ER, we used red fluorescent protein (RFP) tagged with the ER-retention sequence KDEL, or ER resident protein PDI tagged with GFP as ER markers, and ATPsyn5α as mitochondrial marker. Compared to the rather uniform mitochondrial localization of Miro protein in general, p-S66-Miro exhibited a granular localization pattern that colocalized with ATPsyn5α and overlapped extensively with ER markers in the perinuclear area (Figure 6A, B, D). The abundance of p-S66-Miro in the perinuclear area was reduced by Polo RNAi but increased by Polo-WT or constitutively active Polo (Polo-CA) OE (Figure 6C, E). Phosphorylation of Miro by Polo at S66 thus promotes its localization to the ERMCS.
To test whether Miro interacts with proteins localized to ERMCS and whether this interaction is affected by its phosphorylation status, we performed co-IP assays using fly brain tissues expressing dMiro-WT, dMiro-S66A, or phospho-mimetic dMiro-S66E, which functioned similarly as dMiro-WT in NBs when overexpressed (Figure 6F, 5I). Tg expression was driven by the pan-neuronal elav-Gal4 driver. Miro-WT exhibited physical association with VDAC/Porin and Mitofusin/Marf, two key components of the ERMCS (de Brito and Scorrano, 2008; Rowland and Voeltz, 2012). Importantly, the Miro-Porin and Miro-Marf interactions were significantly enhanced by S66E, whereas the Miro-Marf interaction was attenuated by S66A mutation (Figure 6F, H), suggesting that S66 phosphorylation positively influences the interaction of Miro with proteins at the ERMCS.
We next examined the effect of Miro phosphorylation on ERMCS integrity. To this end, we analyzed the IP3R-VDAC interaction in brain tissues of dMiro mutant or Tg flies expressing Miro variants. Although the expression level of IP3R or VDAC was unaltered in all genotypes tested, their physical association was reduced in dMiro mutant or Miro-S66A Tg tissues, but increased in Miro-WT or Miro-S66E Tg tissues (Figure 6G, I). Since S66 is located within the first GTPase domain of Miro, we examined the effect of S66 phosphorylation status on GTPase activity. In vitro GTPase assays showed time-dependent GTP hydrolysis by the N-terminal GTPase domain of Miro-WT and Miro-S66D or Miro-S66E, with phosphomimetic Miro exhibiting greater GTPase activity than Miro-WT (Figure S6E). Moreover, prior phosphorylation by Polo significantly enhanced the GTPase activity of Miro-WT, but not Miro-S66A (Figure S6F). We conclude that Polo-mediated phosphorylation positively regulates the GTPase activity of Miro and the recruitment of Miro to ERMCS, where p-S66-Miro enhances the integrity of this ER-mitochondria tethering complex.
Miro Plays Conserved Roles in Regulating Ca2+mito Homeostasis in Mammalian Cells
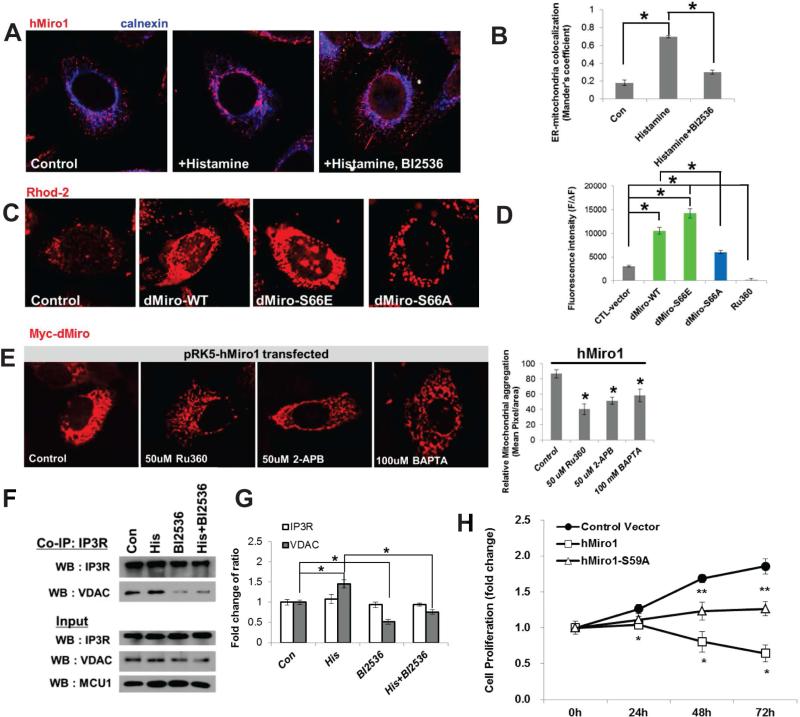
To test the conservation of the mechanisms of Miro function and regulation, we turned to the human cervical cancer cell line (HeLa) and mouse NSCs. Addition of IP3R agonist histamine to HeLa cells enhanced the recruitment of hMiro1 to the perinuclear ER area, and this effect was ameliorated by the co-treatment with BI2536, a specific chemical inhibitor of PLK1 (Figure 7A, B, S7B). Monitoring Ca2+mito with Rhod-2 AM showed that Ca2+mito was significantly increased in cells transfected with dMiro-WT or -S66E, but less so with dMiro-S66A (Figure 7C, 7D). Previous studies showed that hMiro1 (Fransson et al., 2003) or dMiro (Liu et al., 2012) caused aberrant mitochondrial morphology when overexpressed. We found that hMiro1-induced mitochondrial aggregation in HeLa cells was attenuated by treatment with 2-APB or Ru360, cell-permeant pharmacological inhibitor of IP3R or MCU, respectively (Tang et al., 2005; Garcia-Rivas Gde et al., 2006), or by chelating Ca2+ with BAPTA-AM (Figure 7E), suggesting that Miro-regulated Ca2+mito homeostasis underlies its effect on mitochondrial morphology. Moreover, Myc-tagged dMiro-WT, -S66E, or -S66D exhibited more pronounced perinuclear and aggregated localization than Miro-S66A (Figure S7C). These results suggest that as in fly tissues, phosphorylation of Miro at S66 site promotes Miro localization to ERMCS in HeLa cells. We also examined the effect of Polo-Miro signaling on the integrity of the ER-mitochondria tethering complexes. The interaction between VDAC and IP3R was weakened by treatment with BI2536 (Figure 7F, 7G). Immunostaining with ER and mitochondrial markers reveal that ER-mitochondria interaction was increased in cell transfected with hMiro1-WT, but not with hMiro1-SA (Figure S7E, S7F), supporting a critical role of PLK signaling in ER-mitochondria interaction and Ca2+mito uptake. Finally, we examined the effect of Miro on the proliferation of mouse NSCs. Transfection of NSCs with hMiro1-WT attenuated NSC proliferation, whereas hMiro1-S59A (equivalent of dMiro-S66A) was less effective in this aspect (Figure 7H). Mitochondrial metabolism as monitored by OCR was increased by hMiro1-WT but decreased by hMiro1-SA in mouse NSCs (Figure S7G). Supporting the importance of ER-mitochondria Ca2+ transfer in NSC regulation, pharmacological inhibition of Ca2+mito uptake by Ru360, and to a lesser extent by 2-APB, attenuated NSC proliferation (Figure S7D).
Figure 7. Regulation of Ca2+mito by Miro in Mammalian Cells.
(A, B) Immunostaining showing that the PLK inhibitor BI2536 blocked histamine-induced hMiro1 recruitment to perinuclear ER area in HeLa cells. B, quantification of Miro-Calnexin colocalization using Mander's coefficient.
(C, D) Effects of dMiro variants on Rhod-2 AM fluorescence in HeLa cells. D, quantification of Rhod-2 signals. RU360 treatment serves as control.
(E) Effects of pharmacological intervention of ER-mitochondrial Ca2+ transfer (RU360, 2-APB) or Ca2+ availability (BAPTA) on hMiro1-induced mitochondrial morphology change visualized with mitochondria-localized hMiro. Bar graph shows quantification of mitochondrial aggregation.
(F, G) Co-IP assay showing effect of PLK inhibition on IP3R-VDAC interaction in HeLa cells. G, quantification of signal intensity change shown in F.
(H) Effects of hMiro1-WT and hMiro1-S59A transfection on mammalian NSC (ReNcell CX) proliferation monitored over a 72h time course.
Error bar: SEM; *, p<0.05 in Student's t-tests. Scale bars, 100 μm.
See also Figure S7.
DISCUSSION
Mitochondrial and Ca2+ signaling pathways are intimately connected in many aspects of cellular physiology, from metabolism and ATP production to cell death. Previous studies have emphasized the key role of Miro in regulating mitochondrial transport by sensing local elevation of cytosolic Ca2+ concentration elicited by neuronal activity. This mechanism links mitochondrial distribution to the subcellular need for Ca2+ buffering. In this study we demonstrate a conserved role of Miro in regulating Ca2+mito homeostasis in an apparently transport-independent manner. Miro localizes to ERMCS and interacts with its protein components. We further show that this function of Miro is regulated by Polo-mediated phosphorylation at a conserved site in the N-terminal GTPase domain. The identification of new regulators of Ca2+mito homeostasis offers the opportunity to elucidate how Ca2+mito uptake is controlled in different cell types of multicellular organisms, and how defects in this process may impact development and disease.
Our results identify the Polo/Miro signaling axis as an important regulator of Ca2+mito uptake. ER is a major source of Ca2+mito, which is transported to mitochondrial matrix through Ca2+ transporters located at the ERMCS. Despite detailed biophysical studies of Ca2+mito uptake, little is known how this process is regulated to meet the developmental and physiological needs of distinct cell types in a multicellular organism. We show that Miro is localized to ERMCS, where it interacts with components of the ERMCS to modulate the integrity of this quasi-synaptic structure and the inter-organelle Ca2+ transfer. Our genetic and biochemical studies further identify Polo kinase as a key regulator of Miro-controlled Ca2+mito homeostasis. Polo-induced phosphorylation of Miro promotes the localization of Miro to ERMCS, enhancing the interaction between Miro and components of the ER-mitochondria tethering complex and the integrity of the tethering complex. Our identification of S66 in the N-terminal GTPase domain as a key regulatory site of Miro is consistent with the known importance of this GTPase domain to Miro function (Kornmann et al., 2011; Babic et al., 2015). Our results also uncover a new mechanism of action for Polo in NSC self-renewal and differentiation. Dysregulation of PLKs has been implicated in cancer (Craig et al., 2014) and neurodegenerative diseases such as PD (Mbefo et al., 2010). Given that Miro OE-induced cell death involves Ca2+mito overload, mitochondrial dysfunction, oxidative stress, ER stress, and caspase 3 activation, key features broadly implicated in neurodegenerative diseases, it would be interesting to test in various disease conditions whether disease-causing genes or signaling pathways converge on Miro, and whether pharmacological inhibition of PLK-Miro signaling may offer broad therapeutic benefits.
Our analyses of the effect of Miro LOF and GOF in the fly NB lineages reveal a critical role of Ca2+mito homeostasis in cell fate determination. Miro LOF and GOF both impairs NB maintenance and lineage development, but through different mechanisms. Miro LOF causes Ca2+mito depletion and reduced ATP production, impairing cell growth, a key requirement of NB maintenance (Song and Lu, 2011), and causing premature differentiation and loss of NB stemness through a non-apoptotic mechanism. In contrast, Miro GOF causes Ca2+mito overload, oxidative stress, and activation of the apoptotic cascade, leading to failed maintenance of NB and IPs. These results reveal hypersensitivity of NSC maintenance and lineage progression to Ca2+mito levels. A role for Ca2+ signaling in progenitor maintenance was previously shown in the fly hematopoietic system (Shim et al., 2013). However, distinct from the importance of Ca2+mito to NB maintenance reported here, the maintenance of hematopoietic progenitors involves cytosolic Ca2+, which is mediated by CaMKII signaling and is sensitive to SERCA activity (Shim et al., 2013), whereas NSC maintenance by Miro-mediated Ca2+mito homeostasis is insensitive to SERCA manipulation (data not shown). Together with previous studies implicating mitochondrial regulators or structural proteins in stem cell differentiation (Kasahara et al., 2013; Mitra et al., 2012; Lee et al., 2013; Teixeira et al., 2015), our result broadens the impact of mitochondria on developmental processes. Given the emerging connection between mitochondrial metabolism and epigenetic modifications (Wallace, 2010; Castegna et al., 2015), we speculate that there exist a mechanistic link between Miro-controlled Ca2+mito homeostasis and epigenetic regulation in the nucleus. Future studies will establish, at the molecular level, how developmental pathways dictating stem cell self-renewal vs. differentiation decision are connected with signaling pathways regulating mitochondrial function.
EXPERIMENTAL PROCEDURES
Fly Genetics
Fly culture and crosses were performed according to standard procedures and raised at indicated temperatures. To generate UAS-dMiro-S66A and UAS-dMiro-S66E Tg flies, wild-type dMiro cDNA was modified using the QuikChange Multi kit (Stratagene) to introduce the S66A and S66E mutations. The insert of UAS-dMiro-WT-Myc was replaced with dMiro-S66A and dMiro-S66E cDNAs to generate UAS-dMiro-S66A-Myc and UAS-dMiro-S66E-Myc, respectively. The resulting pUAST-Miro constructs were injected into w– embryos to obtain Tg lines according to established procedures (Bestgene, Inc.).
MARCM and Flip-Out Clonal Analysis
To generate NB MARCM clones and overexpression clones, larvae at 24h after-larvae-hatching (ALH) were heat-shocked at 37°C for 90 min and further aged for 72 hrs at 25°C before dissection. MARCM analyses were performed essentially as described (Song and Lu, 2011). For making overexpression clones, w, hsFLP; Actin 5c>CD2>Gal4, UAS-GFP-NLS was crossed with the indicated UAS lines and 24h ALH larvae were heat-shocked at 37°C for 90 min and further aged for 72 hrs at 25°C before dissection.
Pharmacological Treatment
2-APB (Sigma), Ru360 (Calbiochem), EDTA/EGTA (Sigma), CaCl2 (Sigma), BAPTA (Life Technologies), Histamine (Sigma), Thapsigargin (Calbiochem), or BI2536 (Selleckchem) dissolved in 0.5% DMSO was mixed in instant Drosophila media or cell culture at the indicated final concentrations. 0.5% DMSO alone was used as vehicle control. For treatment of flies, embryos were collected on drug-containing food or control food for 6 hrs at 25°C and allowed to develop further to 120 hr ALH before larval brain dissection and immunostaining.
Immunohistochemistry
To generate the p-S66 Miro antibody, a synthetic peptide with the sequence SIVDFpSAVEQS was used to elicit immune response in rabbits. Purification of the phospho-specific antibody was performed by AbFrontier (Seoul, Korea). Purified p-S66 antibody was diluted at 1:200 for immunostaining. For larval brain immunostaining, larvae were dissected in Schneider's medium (Invitrogen) and fixed with 4% formaldehyde in PEM buffer (100 mM PIPES at pH 6.9, 1 mM EGTA, 1 mM MgCl2) for 23 min at room temperature. For ER-mitochondria interaction analysis, confocal image stacks were automatically thresholded using ImageJ. Colocalization between organelles was quantified using the Manders’ algorithm (Manders et al., 1993).
Co-Immunoprecipitation and Western Blot Analyses
Approximately 25 adult fly thoraces were collected and homogenized in HBS buffer (210 mM Mannitol, 70 mM Sucrose, 5 mM HEPES pH 7.4, 1 mM EGTA) containing protease inhibitor (Sigma). The lysate was centrifuged at 1,300g for 10 min, and the supernatant was collected and centrifuged again at 13,000g for 15 min. For co-IP, the cytosolic and the mitochondrial fractions were incubated with anti-GFP or anti-Myc conjugated protein G-agarose beads (Upstate) at 4 °C for 2 hours. Beads were washed 3x with PBS for 5 min each. Proteins were eluted and analyzed by WB as described (Wu et al., 2013).
In Vitro Kinase and GTPase Assays
The conditions of the in vitro kinase assays were essentially as described (Lee et al., 2010). GST-Miro fusion protein production was done as described (Liu et al., 2012). Briefly, cDNAs encoding fragments of dMiro were cloned into a glutathione S-transferase (GST)-encoding vector, pGEX-6P-1 (GE Healthcare), and GST fusion production was carried out in E. coli strain BL21(DE3) cells according to Manufacturer's instructions. PLK1 and PKC-zeta were purchased from ProQinase. The GTPase assay for dMiro was performed according to a published procedure (Koshiba et al., 2011).
ATP Measurement
The level of ATP in Drosophila larval brain was measured essentially as previously described (Wu et al., 2013), using a luciferase-based bioluminescence assay kit (ATP Bioluminescence Assay Kit HS II, Roche Applied Science).
Calcium Imaging
For calcium imaging in primary neural cells, ~50 third instar Tg larval brains expressing Mito-GFP, Mito-GFP+UAS-dMiro-WT, Mito-GFP+UAS-dMiro-S66A, or Mito-GFP+dMiro RNAi driven by elav-GAL4 were collected. Primary neural cells were prepared according to published procedures (Egger et al., 2013). To monitor mitochondrial or cytosolic Ca2+ levels, primary neural cells were loaded with 1 μM Rhod-2 AM or Fluo-3 AM (Molecular Probes), respectively, in physiological salt solution [pH7.4] (150 mM NaCl, 4 mM KCl, 1 mM MgCl2, 5.6 mM glucose, 5 mM HEPES) for 30 min at 37 °C. Primary neural cells were treated with 1 μM Thapsigargin (Calbiochem) for intracellular Ca2+ perturbation. Calcium imaging was carried out using an inverted confocal microscope (LSM 510 META and LIVE 5, Carl Zeiss) with a 40× objective.
For measuring mitochondrial Ca2+ in mammalian cells, HeLa cells were transfected with control pCDNA3.0 vector or Myc-hMiro1, Myc-hMiro2, Myc-dMiro-WT, Myc-dMiro-S66E, or Myc-dMiro-S66A plasmids cloned in pCDNA3.0. After transfection, HeLa cells were loaded with Rhod-2 AM (10 μM in DMEM media, Molecular Probe) for 30 min at room temperature. Cells were then washed with DMEM for 30 min. Single cell fluorescence was excited at 545 nm and images of the emitted fluorescence obtained on a Leica TCS SP5 AOBS confocal microscope were processed with LAS AF (Leica).
Statistical Analysis
Statistical significance of all data were evaluated by two-tailed paired or unpaired Student's t-tests. For studies employing multiple testing, we used a one-way ANOVA followed by Bonferroni post hoc test. All data were presented as mean±s.e.m.
Supplementary Material
Highlights.
Mitochondrial Rho GTPase Miro regulates NSC maintenance and lineage maturation
Miro controls mitochondrial Ca2+ homeostasis independent of its role in transport
Miro interacts with Ca2+ transporters at the ER-mitochondria contact site (ERMCS)
Miro activity is positively regulated by Polo kinase through phosphorylation
ACKNOWLEDGEMENTS
We are grateful to Drs. K Zinsmaier, DM Glover, J Lipsick, WM Saxton, D Smith, JK Chung, I Bezprozvanny, F Kawasaki, A. Whitworth, YN Jan, L Luo, M Guo, D Walker, S Davies, G Hasan, J Nambu, and the VDRC and Bloomington Stock Center for fly stocks and reagents. We thank members of the Lu laboratory for discussions and help. Supported by the NIH (R01NS083417 and R01MH080378 to BL), NRF (2014M3A908034462 to KL and 2015R1D1A1A01059079 to SL) and the Brainpool program of KOFST (to SL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
S.Lee, K-S.L and S.H. designed the study, performed the experiments, analyzed data, wrote the manuscript, and contributed equally. S.Liu., D-Y.L, and S.H.H performed some of the experiments and contributed data. K.Y. participated in the supervision of the study and provided key reagents. B.L. conceived and supervised the study and wrote the manuscript.
See Extended Experimental Procedures for additional details.
REFERENCE
- Babic M, Russo GJ, Wellington AJ, Sangston RM, Gonzalez M, Zinsmaier KE. Miro's N-Terminal GTPase Domain Is Required for Transport of Mitochondria into Axons and Dendrites. J Neurosci. 2015;35:5754–5771. doi: 10.1523/JNEUROSCI.1035-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadorani S, Cho J, Lo T, Contreras H, Lawal HO, Krantz DE, Bradley TJ, Walker DW. Neuronal expression of a single-subunit yeast NADH-ubiquinone oxidoreductase (Ndi1) extends Drosophila lifespan. Aging Cell. 2010;9:191–202. doi: 10.1111/j.1474-9726.2010.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar OA, Doe CQ. Combinatorial temporal patterning in progenitors expands neural diversity. Nature. 2013;498:449–455. doi: 10.1038/nature12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello B, Reichert H, Hirth F. The brain tumor gene negatively regulates neural progenitor cell proliferation in the larval central brain of Drosophila. Development. 2006;133:2639–2648. doi: 10.1242/dev.02429. [DOI] [PubMed] [Google Scholar]
- Boone JQ, Doe CQ. Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev Neurobiol. 2008;68:1185–1195. doi: 10.1002/dneu.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SK, Rolland V, Betschinger J, Kinsey KA, Emery G, Knoblich JA. The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev Cell. 2008;14:535–546. doi: 10.1016/j.devcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley K, Smith MJ, Beck M, Stephenson FA. GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: association in vivo and in vitro with kinesin. J Biol Chem. 2005;280:14723–14732. doi: 10.1074/jbc.M409095200. [DOI] [PubMed] [Google Scholar]
- Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castegna A, Iacobazzi V, Infantino V. The mitochondrial side of epigenetics. Physiol Genomic. 2015;47:299–307. doi: 10.1152/physiolgenomics.00096.2014. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Craig SN, Wyatt MD, McInnes C. Current assessment of polo-like kinases as anti-tumor drug targets. Expt Opin Drug Disc. 2014;9:773–789. doi: 10.1517/17460441.2014.918100. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- Dube KA, McDonald DG, O'Donnell MJ. Calcium homeostasis in larval and adult Drosophila melanogaster. Arch Insect Biochem Physiol. 2000;44:27–39. doi: 10.1002/(SICI)1520-6327(200005)44:1<27::AID-ARCH4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Egger B, van Giesen L, Moraru M, Sprecher SG. In vitro imaging of primary neural cell culture from Drosophila. Nat Protocol. 2013;8:958–965. doi: 10.1038/nprot.2013.052. [DOI] [PubMed] [Google Scholar]
- Fang Y, Soares L, Teng X, Geary M, Bonini NM. A novel Drosophila model of nerve injury reveals an essential role of Nmnat in maintaining axonal integrity. Curr Biol. 2012;22:590–595. doi: 10.1016/j.cub.2012.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley K, Cooley L. Apoptosis in late stage Drosophila nurse cells does not require genes within the H99 deficiency. Development. 1998;125:1075–1082. doi: 10.1242/dev.125.6.1075. [DOI] [PubMed] [Google Scholar]
- Fransson A, Ruusala A, Aspenstrom P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- Fransson S, Ruusala A, Aspenstrom P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochemi Biophysi Res Commun. 2006;344:500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- Garcia-Rivas Gde J, Carvajal K, Correa F, Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br J Pharmacol. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Missiroli S, Patergnani S, Duszynski J, Wieckowski MR, Pinton P. Mitochondria-associated membranes: composition, molecular mechanisms, and physiopathological implications. Antioxid Redox Signal. 2015;22:995–1019. doi: 10.1089/ars.2014.6223. [DOI] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G, Yi M. Old players in a new role: mitochondria-associated membranes, VDAC, and ryanodine receptors as contributors to calcium signal propagation from endoplasmic reticulum to the mitochondria. Cell Calcium. 2002;32:363–377. doi: 10.1016/s0143416002001872. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trend Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Howard J. Calcium enables photoreceptor pigment migration in a mutant fly. J Exp Biol. 1984;113:471–475. [Google Scholar]
- Iijima-Ando K, Hearn SA, Shenton C, Gatt A, Zhao L, Iijima K. Mitochondrial mislocalization underlies Abeta42-induced neuronal dysfunction in a Drosophila model of Alzheimer's disease. PloS one. 2009;4:e8310. doi: 10.1371/journal.pone.0008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara A, Cipolat S, Chen Y, Dorn GW, 2nd, Scorrano L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science. 2013;342:734–737. doi: 10.1126/science.1241359. [DOI] [PubMed] [Google Scholar]
- Kornmann B, Osman C, Walter P. The conserved GTPase Gem1 regulates endoplasmic reticulum-mitochondria connections. Proc Natl Acad Sci USA. 2011;108:14151–14156. doi: 10.1073/pnas.1111314108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Holman HA, Kubara K, Yasukawa K, Kawabata S, Okamoto K, MacFarlane J, Shaw JM. Structure-function analysis of the yeast mitochondrial Rho GTPase, Gem1p: implications for mitochondrial inheritance. J Biol Chem. 2011;286:354–362. doi: 10.1074/jbc.M110.180034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Lu B. The myriad roles of Miro in the nervous system: axonal transport of mitochondria and beyond. Front Cell Neurosci. 2014;8:330. doi: 10.3389/fncel.2014.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Wu Z, Song Y, Mitra SS, Feroze AH, Cheshier SH, Lu B. Roles of PINK1, mTORC2, and mitochondria in preserving brain tumor-forming stem cells in a noncanonical Notch signaling pathway. Genes Dev. 2013;27:2642–2647. doi: 10.1101/gad.225169.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Liu HP, Lin WY, Guo H, Lu B. LRRK2 kinase regulates synaptic morphology through distinct substrates at the presynaptic and postsynaptic compartments of the Drosophila neuromuscular junction. J Neuro Sci. 2010;30:16959–16969. doi: 10.1523/JNEUROSCI.1807-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Liu S, Sawada T, Lee S, Yu W, Silverio G, Alapatt P, Millan I, Shen A, Saxton W, Kanao T, et al. Parkinson's Disease-Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria. PLoS Genet. 2012;8:e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutas A, Wahlmark CJ, Acharjee S, Kawasaki F. Genetic analysis in Drosophila reveals a role for the mitochondrial protein p32 in synaptic transmission. G3. 2012;2:59–69. doi: 10.1534/g3.111.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill AF, Atkin TA, Kittler JT. Mitochondrial trafficking and the provision of energy and calcium buffering at excitatory synapses. Eur J Neurosci. 2010;32:231–240. doi: 10.1111/j.1460-9568.2010.07345.x. [DOI] [PubMed] [Google Scholar]
- Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb Perspect Biol. 2011;3:a004424. doi: 10.1101/cshperspect.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manders EMM, Verbeek FJ, Aten JA. Measurement of Colocalization of Objects in Dual-Color Confocal Images. J Microsc. 1993;169:375–382. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- Mbefo MK, Paleologou KE, Boucharaba A, Oueslati A, Schell H, Fournier M, Olschewski D, Yin G, Zweckstetter M, Masliah E, et al. Phosphorylation of synucleins by members of the Polo-like kinase family. J Biol Chem. 2010;285:2807–2822. doi: 10.1074/jbc.M109.081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- Messina A, Neri M, Perosa F, Caggese C, Marino M, Caizzi R, De Pinto V. Cloning and chromosomal localization of a cDNA encoding a mitochondrial porin from Drosophila melanogaster. FEBS Lett. 1996;384:9–13. doi: 10.1016/0014-5793(96)00268-2. [DOI] [PubMed] [Google Scholar]
- Mitra K, Rikhy R, Lilly M, Lippincott-Schwartz J. DRP1-dependent mitochondrial fission initiates follicle cell differentiation during Drosophila oogenesis. J Cell Biol. 2012;197:487–497. doi: 10.1083/jcb.201110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murley A, Lackner LL, Osman C, West M, Voeltz GK, Walter P, Nunnari J. ER-associated mitochondrial division links the distribution of mitochondria and mitochondrial DNA in yeast. eLife. 2013;2:e00422. doi: 10.7554/eLife.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Oh SS, Weaver D, Lewandowska A, Maxfield D, Schuler MH, Smith NK, Macfarlane J, Saunders G, Palmer CA, et al. Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease. Proc Natl Acad Sci USA. 2014;111:E3631–3640. doi: 10.1073/pnas.1402449111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Petritsch C, Wen H, Jan L, Jan YN, Lu B. Dronc caspase exerts a non-apoptotic function to restrain phospho-Numb-induced ectopic neuroblast formation in Drosophila. Development. 2011;138:2185–2196. doi: 10.1242/dev.058347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozeboom AM, Pak DT. Identification and functional characterization of polo-like kinase 2 autoregulatory sites. Neurosci. 2012;202:147–157. doi: 10.1016/j.neuroscience.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A, Soikkeli M, Portero-Otin M, Wilson A, Kemppainen E, McIlroy G, Ellila S, Kemppainen KK, Tuomela T, Lakanmaa M, et al. Expression of the yeast NADH dehydrogenase Ndi1 in Drosophila confers increased lifespan independently of dietary restriction. Proc Natl Acad Sci USA. 2010;107:9105–9110. doi: 10.1073/pnas.0911539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnoczky G. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci USA. 2008;105:20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon EA, Area-Gomez E. Mitochondria-associated ER membranes in Alzheimer disease. Mol Cell Neurosci. 2013;55:26–36. doi: 10.1016/j.mcn.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Schon EA, Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng ZH, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012;13:77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Mukherjee T, Mondal BC, Liu T, Young GC, Wijewarnasuriya DP, Banerjee U. Olfactory control of blood progenitor maintenance. Cell. 2013;155:1141–1153. doi: 10.1016/j.cell.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Lu B. Regulation of cell growth by Notch signaling and its differential requirement in normal vs. tumor-forming stem cells in Drosophila. Genes Dev. 2011;25:2644–2658. doi: 10.1101/gad.171959.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers RS, Megeath LJ, Gorska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Tait SW, Green DR. Mitochondria and cell signalling. J Cell Sci. 2012;125:807–815. doi: 10.1242/jcs.099234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TS, Slow E, Lupu V, Stavrovskaya IG, Sugimori M, Llinas R, Kristal BS, Hayden MR, Bezprozvanny I. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington's disease. Proc Natl Acad Sci USA. 2005;102:2602–2607. doi: 10.1073/pnas.0409402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira FK, Sanchez CG, Hurd TR, Seifert JR, Czech B, Preall JB, Hannon GJ, Lehmann R. ATP synthase promotes germ cell differentiation independent of oxidative phosphorylation. Nat Cell Biol. 2015;17:689–696. doi: 10.1038/ncb3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhzaz S, Southall TD, Lilley KS, Kean L, Allan AK, Davies SA, Dow JA. Differential gel electrophoresis and transgenic mitochondrial calcium reporters demonstrate spatiotemporal filtering in calcium control of mitochondria. J Biol Chem. 2006;281:18849–18858. doi: 10.1074/jbc.M603002200. [DOI] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh K, Hasan G. Disruption of the IP3 receptor gene of Drosophila affects larval metamorphosis and ecdysone release. Curr Biol. 1997;7:500–509. doi: 10.1016/s0960-9822(06)00221-1. [DOI] [PubMed] [Google Scholar]
- Vos M, Lauwers E, Verstreken P. Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front Synaptic Neurosci. 2010;2:139. doi: 10.3389/fnsyn.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. The epigenome and the mitochondrion: bioenergetics and the environment [corrected]. Genes Dev. 2010;24:1571–1573. doi: 10.1101/gad.1960210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ouyang Y, Somers WG, Chia W, Lu B. Polo inhibits progenitor self-renewal and regulates Numb asymmetry by phosphorylating Pon. Nature. 2007;449:96–100. doi: 10.1038/nature06056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, Lavoie MJ, Schwarz TL. PINK1 and Parkin Target Miro for Phosphorylation and Degradation to Arrest Mitochondrial Motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Sawada T, Shiba K, Liu S, Kanao T, Takahashi R, Hattori N, Imai Y, Lu B. Tricornered/NDR kinase signaling mediates PINK1-directed mitochondrial quality control and tissue maintenance. Genes Dev. 2013;27:157–162. doi: 10.1101/gad.203406.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.