Abstract
The identification of druggable molecular alterations represents one of the greatest advances in cancer treatment. Such progress is particularly evident for lung cancer, which now has numerous molecularly defined subsets such as epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements. However, understanding of the significance of these genomic alterations is largely limited to incurable, metastatic lung cancer. ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial) is a National Cancer Institute–sponsored initiative to address these questions in earlier-stage disease.
Molecular profiling has become standard of care for advanced (metastatic) lung cancer. For non-squamous non-small cell lung cancer (NSCLC), which accounts for more than half of all lung cancer cases, routine testing for epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements is recommended. In cases with identified EGFR (approximately 15% of NSCLC) or ALK alterations (approximately 5% of NSCLC), molecularly targeted therapy with EGFR- or ALK-targeting drugs is now the preferred initial approach to treatment. These drugs have impressive efficacy (radiographic response rates and progression-free survival (PFS) approximately twice that of conventional cytotoxic chemotherapy) (see Figure 1) and are generally well tolerated. The common toxicities of EGFR inhibitors reflect the normal distribution of the EGFR molecule in epidermal and epithelial tissues, with acneiform rash and diarrhea the most common side effects. Approved and investigational ALK inhibitors generally cause gastrointestinal toxicities (nausea, vomiting, diarrhea, transaminitis). Additionally, the first-generation ALK inhibitor crizotinib may cause transient visual changes (such as “flashing lights” and “shadows” that do not affect visual acuity), edema, and renal insufficiency. Both classes of drugs, and indeed the majority of molecularly targeted therapies, are orally bioavailable, making treatment highly convenient for patients.
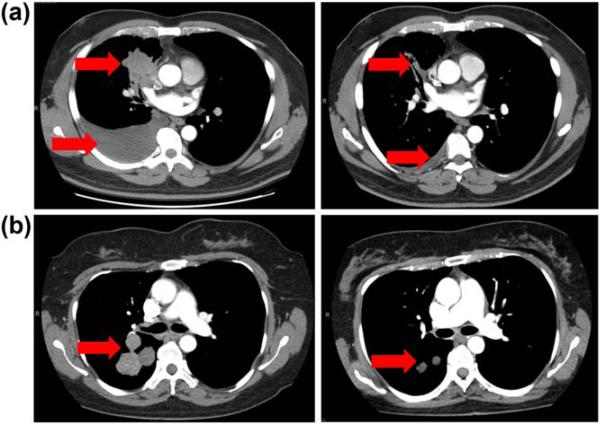
Figure 1.
Dramatic therapeutic effects of molecularly targeted therapies demonstrated in serial axial chest computed tomography images. (a) Radiographic response to epidermal growth factor receptor (EGFR) inhibition in patient with EGFR mutant non-small cell lung cancer (NSCLC) after 2 mo of treatment. (b) Ongoing radiographic response to anaplastic lymphoma kinase (ALK) inhibition in patient with ALK rearranged NSCLC after 6 mo of treatment.
CURRENT STATE OF EGFR- AND ALK-DIRECTED THERAPIES
In recent years, we have seen further advances in our understanding of EGFR and ALK biology, including molecular etiologies of secondary resistance and the development of second- and third-generation EGFR and ALK inhibitors to overcome these events. EGFR mutant NSCLC generally refers to cases with sensitizing mutations in the EGFR kinase domain (exon 19 deletions or exon 21 L858R substitutions). These activating mutations result in constitutive activity of the EGFR kinase domain, generating survival and proliferative signals through the PI3K-Akt-mTOR and Ras-Raf-MEK pathways. In these cases, EGFR inhibitors such as erlotinib, gefitinib, and afatinib in the first-line setting yield response rates in excess of 75%, median PFS of approximately 12 mo, and overall survival (OS) exceeding 2 y. To place these results in context, in NSCLC without actionable molecular alterations treated with conventional chemotherapy, response rates are approximately 30%, median PFS 6 mo, and median OS 12 mo. The increasingly common practice of performing repeat tumor biopsies for molecular profiling at the time of disease progression after treatment with EGFR inhibitors has provided insight into mechanisms of resistance. These include secondary Exon 20 T790M mutations (approximately 50% of cases, in which substitution of a bulky methionine residue for a threonine residue results in greater affinity for the ATP substrate and reduced binding of the EGFR inhibitor), MET amplification, PIK3CA mutations, epithelial-to-mesenchymal transition, and even histologic transformation to small cell lung cancer.1 EGFR inhibitors in clinical use and undergoing development are characterized as first-generation (reversible binding to the EGFR molecule), second-generation (irreversible covalent binding to EGFR molecule), and third-generation (mutation specific binding to the EGFR molecule). Third-generation EGFR inhibitors in particular have demonstrated promising activity against the resistant T790M mutation.
The pace of discovery is even more impressive for ALK-positive lung cancer. In 2007, fusions of the echinoderm microtubule-associated protein-like 4 (EML4) gene with the ALK gene were discovered in lung cancer. Analogous to EGFR mutations, EML4-ALK fusions result in constitutive tyrosine kinase activity, dependence of the cancer cell on activated downstream mitogenic pathways, and exquisite sensitivity to ALK inhibition. By 2011, the ALK inhibitor crizotinib was FDA approved for these cases. With crizotinib, response rates may exceed 70% and median PFS may exceed 10 mo. Resistance appears due to several possible molecular events, including secondary ALK mutations, ALK copy number gain, and alterations in other oncogenes such as EGFR and KRAS. In 2013, the second-generation ALK inhibitor ceritinib, which has considerable activity after crizotinib failure, was FDA approved.
EMERGING TARGETED THERAPIES FOR LUNG CANCER
Due to multiplex genomic analysis, such as that conducted by the Lung Cancer Mutation Consortium, and the emergence of clinically available Next Generation sequencing, understanding of the molecular underpinnings and vulnerabilities of lung cancer has evolved well beyond EGFR and ALK.2 ROS1 rearrangements occur in 1%–2% of NSCLC. ROS1 has a high degree of homology with ALK (approximately 50% within the kinase domain and 75% within the ATP-binding site), and the majority of cases respond to the first-generation ALK inhibitor crizotinib; however, certain other ALK inhibitors such as alectinib do not appear to have activity against ROS1-positive cases. BRAF mutations occur in 1%–3% of NSCLC. Of these, approximately 50% are V600 and respond to BRAF inhibitors such as vermurafenib and dabrafenib, both currently approved for V600 BRAF mutant melanoma. HER2 mutations (in contrast to amplification, which is the pathogenic event in breast and gastro-esophageal cancer) occur in 2%–4% of NSCLC. Dual EGFR/HER2 inhibitors such as afatinib and lapatinib, as well as the anti-HER2 antibody trastuzumab, have activity against these cases. Gene fusions involving the rearranged during transfection (RET) gene occur in approximately 1% of NSCLC. Several commercially available multitargeted kinase inhibitors have RET activity (e.g., vandetanib, sorafenib, sunitinib, cabozantinib); cabozantinib has led to radiographic responses.
THE CURRENT STATE OF EARLY-STAGE NSCLC
In contrast to advanced stage NSCLC, early-stage NSCLC has seen few medical advances in recent years. There have been substantial improvements in surgical techniques, including robotically assisted minimally invasive procedures, which are associated with faster healing times, shorter hospital stays, and lower complication rates. Stereotactic radiation therapy techniques, in which high-dose radiation is delivered to tumor with minimal exposure to surrounding normal structures, yields disease control rates far superior to that of conventional fractionated radiation and comparable to that achieved with surgical resection. However, medical therapies for early-stage disease have not moved beyond the incorporation of cisplatin chemotherapy-based adjuvant (post-operative) therapy, which provides an 11% relative and 5% absolute reduction in the risk of death, albeit with substantial toxicity.3 Despite all of these advances, 5-y survival remains 50%–60% for stage 1, 30%–40% for stage 2, and 15%–20% for stage 3 lung cancer. Perhaps surprisingly, in contrast to breast cancer and even relatively rare gastrointestinal stromal tumors, the role of targeted therapies as adjuvant treatment for lung cancer remains unknown. Several clinical trials have investigated the role of molecularly targeted therapies for surgically resected, early-stage NSCLC. However, to date none has provided a definitive answer. Two phase III trials (NCI-C BR19 and RADIANT) evaluated post-operative EGFR inhibitor therapy, but neither trial restricted enrollment to cases with EGFR mutations. The SELECT trial enrolled only EGFR mutant resected NSCLC, but was not randomized. Even less is known about early-stage ALK-positive NSCLC. No clinical trials of adjuvant ALK inhibitor therapy have been performed. It is not even clear if ALK rearrangements convey a beneficial or detrimental effect on outcomes in early-stage NSCLC.4,5
ALCHEMIST: MOLECULAR DIAGNOSTICS AND TARGETED THERAPIES FOR EARLY-STAGE NSCLC
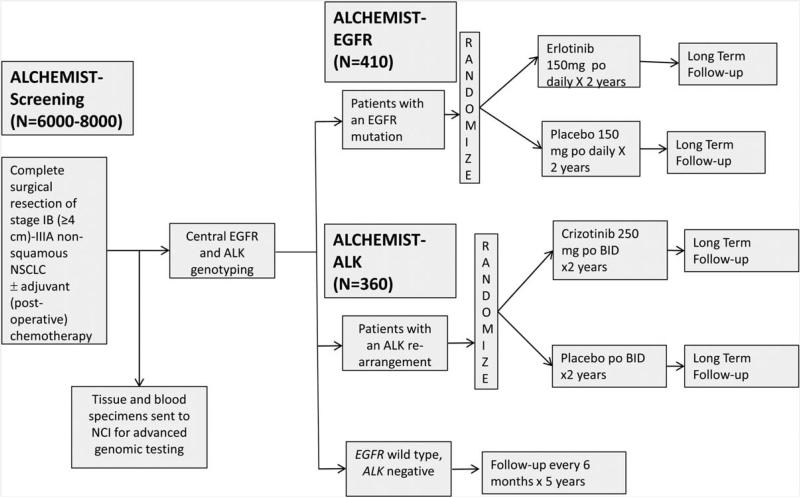
Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial (ALCHEMIST) is a National Cancer Institute (NCI)–sponsored National Clinical Trials Network (NCTN) initiative to address the role of genomic profiling and molecular targeted therapies for early-stage NSCLC. ALCHEMIST is a clinical trial platform that currently consists of three integrated protocols: ALCHEMIST-Screening (A151216; NCT02194738), ALCHEMIST-EGFR (A081105; NCT02193282), and ALCHEMIST-ALK (E4512; NCT02201992). ALCHEMIST-Screening and ALCHEMIST-EGFR are being coordinated by the Alliance for Clinical Trials in Oncology. ALCHEMIST-ALK is being coordinated by the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network (ECOG-ACRIN) Cancer Research Group. All trials are open throughout the NCTN of the NCI. In ALCHEMIST-Screening, up to 8,000 patients with pathologically confirmed stage 1–3 nonsquamous NSCLC will be enrolled either before or after surgical resection (see Figure 2). Tumors will be centrally genotyped for EGFR mutations and ALK rearrangements. Blood and tumor samples will also be collected for advanced genomic analysis at the NCI. Patients with EGFR mutations or ALK rearrangements will be referred to ALCHEMIST-EGFR and -ALK treatment trials, respectively. All other patients will be followed for relapse and survival. Available biopsies at recurrence will be collected to characterize clonal evolution. ALCHEMIST-Screening has been designed with broad eligibility criteria and a flexible enrollment process to facilitate accrual and generalizability (see Table 1). Patients may be enrolled before surgery (preregistration), after surgery but before adjuvant therapy, or after completion of any adjuvant therapy. For the ALCHEMIST-EGFR and ALCHEMIST-ALK treatment trials, patients must meet eligibility for ALCHEMIST-Screening and have an identified activating EGFR mutation or ALK rearrangement.
Figure 2.
ALCHEMIST schema.
Table 1.
ALCHEMIST pre-registration/registration eligibility criteria
| Pre-registration eligibility criteria |
| • Clinical stage IB (≥4 cm), II, or IIIA non-squamous NSCLC |
| • ECOG Performance Status 0-1 |
| • No neoadjuvant (pre-operative) therapy (chemotherapy or radiation therapy) |
| • Age ≥18 years |
| • No prior or concurrent malignancy within 5 years, except non-melanoma skin carcinoma or in situ carcinomas |
| • No prior treatment with agents targeting EGFR mutation or ALK rearrangement |
| • No patients known to be pregnant or lactating |
| Eligibility criteria |
| • Completely resected non-squamous NSCLC |
| • Pathologically staged IB (≥4 cm)-IIIA disease |
| • Adequate FFPE tissue available for central EGFR and ALK genotyping for all patients, including those already identified to carry eligible EGFR or ALK alterations |
ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; FFPE, formalin fixed paraffin embedded; NSCLC, non-small cell lung cancer.
In the treatment trials, patients will be randomized to placebo vs. erlotinib or crizotinib after completion of standard adjuvant therapy. The timing of randomization varies according to administration of other therapies: if no adjuvant therapy, within 90 days of surgery; if adjuvant chemotherapy, within 180 days of surgery; if adjuvant chemotherapy and postoperative radiation therapy, within 270 days of surgery. Study treatment is given for up to 2 y. This duration of therapy was selected because it represents the period of greatest risk of disease relapse after surgical resection of lung cancer. Both trials are double blind studies with a primary endpoint of overall survival (OS). ALCHEMIST-EGFR will enroll 410 patients, which provides 85% power with one-sided type I error rate of 0.05 to demonstrate an OS hazard ratio (HR) of 0.67 favoring erlotinib. Erlotinib will be administered at the standard dose and schedule of 150 mg orally daily. ALCHEMIST-ALK will enroll 378 patients to provide 80% power and one-sided type I error of 0.05 to demonstrate an OS HR of 0.67 favoring crizotinib. Crizotinib will be administered at the standard dose and schedule of 250 mg orally twice daily. In both treatment trials, patients will undergo radiographic follow-up with chest computed tomography (CT) scans every 6 mo for 2 y, then every 12 mo. In the future, additional treatment trials for specific molecular subsets may be added to the ALCHEMIST platform.
As of January 2015, ALCHEMIST has been activated at over 500 centers nationwide. Similar to other ongoing NCI platforms such as the MATCH and Lung-MAP trials, the successful completion of ALCHEMIST will require broad endorsement and participation from academic and community sites alike. The advent of widespread radiographic screening for lung cancer may increase numbers of early-stage lung cancer, but at the same time, it will heighten the need to improve disease understanding and treatment options. Only with a comprehensive national effort such as ALCHEMIST can these goals be realized.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerber DE, Gandhi L, Costa DB. Management and future directions in non-small cell lung cancer with known activating mutations. Am. Soc. Clin. Oncol. Educ. Book. 2014:e353–e365. doi: 10.14694/EdBook_AM.2014.34.e353. [DOI] [PubMed] [Google Scholar]
- 3.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 4.Blackhall FH, Peters S, Bubendorf L, et al. Prevalence and clinical outcomes for patients with ALK-positive resected stage I to III adenocarcinoma: results from the European Thoracic Oncology Platform Lungscape Project. J. Clin. Oncol. 2014;32:780–787. doi: 10.1200/JCO.2013.54.5921. [DOI] [PubMed] [Google Scholar]
- 5.Yang P, Kulig K, Boland JM, et al. Worse disease-free survival in never-smokers with ALK 1 lung adenocarcinoma. J. Thorac. Oncol. 2012;7:90–97. doi: 10.1097/JTO.0b013e31823c5c32. [DOI] [PMC free article] [PubMed] [Google Scholar]