Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease with unknown origins. Neurodegeneration in ALS mouse models occurs together with signs of disrupted blood–spinal cord barrier (BSCB) and regressed capillary network, but the molecular pathways contributing to these vascular pathologies remain unknown. We show that motor neurons of human sporadic ALS patients (n = 12) have increased gene expression of PDGFC and its activator PLAT and presymptomatic activation of the PDGF-CC pathway in SOD1G93A mice leads to BSCB dysfunction. Decrease of Pdgfc expression in SOD1G93A mice restored vascular barrier properties, reduced motor neuron loss and delayed symptom onset by up to 3 weeks. Similarly, lower expression levels of PDGFC or PLAT in motor neurons of sporadic ALS patients were correlated with older age at disease onset. PDGF-CC inhibition and restoration of BSCB integrity did not prevent capillary regression at disease end stage. Lower vessel density was found in spinal cords of sporadic ALS patients and the degree of regression in SOD1G93A mice correlated with more aggressive progression after onset regardless of BSCB status. We conclude that PDGF-CC-induced BSCB dysfunction can contribute to timing of ALS onset, allow insight into disease origins and development of targeted novel therapies.
Keywords: ALS, Blood-brain barrier, Small vessel disease, Cerebral blood flow, Neuroprotection
Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly progressing disease where motor neurons in spinal cord and brain degenerate resulting in paralysis and death [6]. Most ALS cases appear sporadic (sALS), while 10 % are familial (fALS). A proportion of fALS cases is caused by specific mutations in genes including superoxide dismutase 1 (SOD1) [33] and their transgenic overexpression is used to model ALS-like disease in rodents [17]. The factors causing sALS are a poorly understood interplay between age, genetic background or environmental factors [3, 18] and can modulate the latency to onset even within fALS cases carrying the same mutation [31].
Elevated immunoglobulin (IgG) and albumin levels were detected in the cerebrospinal fluid of sALS patients [21], which suggests dysfunction of the blood–brain barrier (BBB) and its spinal cord counterpart (BSCB). Recently several SOD1 ALS mouse models were found to have dysfunctional BSCB already at a presymptomatic stage of the disease [26, 44]. IgG extravasation was observed in spinal cords of SOD1G93A mice before detectable inflammation or motor neuron loss, suggesting that BSCB dysfunction could promote neuronal injury [26, 44]. It is still unknown, which molecular signalling pathways or cell types promote this BSCB dysfunction. The maintenance of vascular barrier properties is the result of a dynamic interplay between the endothelial cells (EC) lining the vessel wall, contractile vascular mural cells regulating the vessel diameter and perivascular astrocytes connecting the vessel with neuronal synapses [25]. These cells coordinate blood flow dynamics, selective molecular uptake, and are commonly referred to as the neurovascular unit [27].
We have previously shown that platelet-derived growth factor C (PDGF-CC) pathway is activated and contributes to BBB dysfunction and oedema after ischemic brain injury [36] and other neuropathologies. Since the inhibition of PDGF-CC signalling by imatinib was beneficial in stroke [36], traumatic spinal cord injury response [1], and other neuropathologies [2, 13, 37] we here utilised genetic and pharmacological methods to inhibit the PDGF-CC pathway in SOD1G93A fALS mice.
Materials and methods
Mice strains, housing, and ALS evaluation
The SOD1G93A (B6SJL-Tg(SOD1*G93A)1Gur/J) and SOD1G85R [B6.Cg-Tg(SOD1*G85R)148Dwc/J] strains were a kind gift from Prof. Stefan Marklund at Umeå University, Sweden. Generation of Pdgfc−/− mice was described previously [9]. Strains were backcrossed to the C57BL/6CR background for at least 10 generations. B6SJL-Tg(SOD1)2Gur/J mice overexpressing wild-type SOD1 were purchased from the Jackson Laboratory. Mice were housed in individually ventilated cages in a specific pathogen-free facility and given free access to food and water. Symptomatic mice were given solid drink (95-23-100) from Nova SCB. Transgene-bearing mice were identified by PCR genotyping as described previously [32]. SOD1 copy number was determined with qPCR using fluorescent probes for hSOD1 and mApoL and was carried out as described in guidelines by the Jackson Laboratory [20]. Mice with more than a 0.5 ΔCt differences from the SOD1G93A reference DNA (Jackson Laboratories) were discarded from the colony. Body weight was measured in a blinded fashion three times a week from the age of 8 weeks. Onset was defined retrospectively as the day of maximum body weight. Progression was defined as time from onset to loss of more than 10 % of the body weight. From the time when mice lost support in two limbs they were monitored daily and survival (critical endpoint) was determined as mouse inability to right itself within 30 s from being placed on either side [7, 40].
Patient description and bioinformatics
Human spinal cord tissues from sALS patients and non-demented controls used for histological analysis were retrieved from the Netherland's Brain Bank (NBB, http://www.brainbank.nl/). Information about the patients and controls used in the mRNA expression analysis of laser-captured motor neurons were published previously and described within [30], as was information on the patients used for the RNA sequencing of frontal cortex tissue [29]. All patients classified as sALS cases were assessed and found negative for C9Orf72 mutations. Expression data sets GSE67196 (cortex) [29] and GSE18920 (laser-captured motoneurons) [30] were downloaded from NCBI Gene Expression omnibus (http://www.ncbi.nlm.nih.gov/geo/). For GSE18920, the raw.cel files were reanalysed using Affymetrix Expression Console to obtain summarised expression values for transcripts presented as normalised probe intensity units. Samples from patient 55 and 65 of GSE18920 were excluded from further analysis, since they turned out as outliers in a hierarchical clustering analysis.
Imatinib administration and cohort design
Imatinib mesylate tablets (Glivec® Novartis) were pulverised and dissolved in PBS. Animals were fed 100 μl of the solution by gavage twice a day to a total dose of 100 or 150 mg/kg/day for survival and tracer experiments, respectively. Mice in survival cohorts were treated from the age of 8 weeks until the critical endpoint. Mice involved in the fluorescent tracer studies were treated for 7 days from the onset age at 14 weeks. For the imatinib cohort SOD1G93A female littermates were randomly split to control and treatment groups. For the Pdgfc deficiency experiment mice were organised in gender-balanced cohorts. Mice in treatment and gene ablation groups, which displayed severe non-ALS-related health issues, were removed from further cohort analysis.
Histology and immunostainings
Immunohistochemistry and beta galactosidase detection
Paraffin-embedded tissues were cut at 6 (mice) or 10 μm (human) thickness, rehydrated, retrieved in EDTA or citrate buffer at pH 6.0, blocked with TNB solution (NEN Life Sciences) and incubated with one of the following antibodies overnight: a custom rabbit anti-PDGF-C-core domain, ChAT (144P Millipore), GFAP (Z0334 DAKO), CD3 (1477 Serotec), Iba1 (019-19741 Wako), APP (MAB348 Millipore), Mac3 (553322 DB Pharmingen) or B220 (550539 BD Pharmingen). Following PBS washes, sections were incubated with biotinylated anti-rabbit antibody solution (656140 Life Technologies), developed with ABC Elite (PK-6100) and DAB substrate (SK-4100) from Vector Laboratories according to manufacturers instructions. Spinal cord tissues from Pdgfc−/− mice stained simultaneously were used as negative control. Beta galactosidase activity was detected using a standard protocol as described previously [36].
Immunofluorescence
Following PBS and PFA perfusion, tissues were incubated with 30 % sucrose solution overnight, embedded in frozen section media and snap frozen on dry ice. 30 and 16 μm sections were cut on a Micron cryostat for BSCB tracer quantification and histology staining. The following antibodies were used for immunostainings: a-Podocalyxin (AF1556 R&D), a-Collagen IV (2150-1470 Serotec), Claudin-5 (352588 Thermo Fisher) and a-PDGFRα (3164 Cell Signaling). Secondary goat antibodies conjugated to Alexa Fluor 488, 555, 594 or 647 were purchased from Life Technologies. Images were acquired using Zeiss LSM 700 confocal microscope and Zen software.
Nissl staining for motoneuron count
Motoneurons in the ventral horns of SOD1G93A or non-transgenic mice on Pdgfc+/+, +/− and −/− background, and of SOD1G93A mice treated with imatinib mesylate or PBS were counted at the onset or at end-stage disease. Since neuromuscular junction denervation occurs early in ALS [10] and results in loss of expression of choline acetyltransferase (ChAT) [39], we preferred to count viable motor neurons as Nissl-positive cells with a diameter greater than 20 μm and with clearly visible nucleolus to ChAT immunostaining to avoid false-negative counts. Coronal sections from frozen lumbar spinal cords were rehydrated for 40 min in PBS and thereafter permeabilised for 10 min in PBS/0.1 % Triton X-100 before labelling with a green fluorescent Nissl probe (1:300 in PBS) for 20 min at RT (NeuroTrace® 500/525 green fluorescent Nissl stain, Invitrogen, OR, USA). Excess tracer was washed away with PBS/0.1 % Triton X-100 followed by a 2 h wash in PBS before mounting in Prolong Gold antifade reagent with DAPI (Invitrogen). Viable alpha motor neurons (αMNs), defined as polygonal Nissl+ cells were counted in both ventral horns by an investigator blinded to the sample information. Individual observations are based on an average of five lumbar spinal cord sections per mouse and the data expressed as the percent of difference between mean αMN number ± s.e.m. from the number of mice stated in the respective figure legends. Statistical analysis was performed using the non-paired Student's t test and statistical significance was defined as p < 0.05.
BSCB integrity with IgG and tracer quantification
Nonadjacent 30 μm sections separated by at least 240 μm with a mean of 8.2 ± 0.2 slides per animal were used for leakage quantification. Sections were photographed at 10× objective magnification with the central canal aligned in the middle. Region of interest (ROI) was defined as combined grey and white matter area of the spinal cord excluding the meninges, ventral artery and ventral median fissure. Images were quantified using the ImageJ software by setting a common pixel intensity threshold and calculating the pixel-positive area. Two-tailed Mann–Whitney test was chosen for the analysis of IgG and tracer leakage and statistical significance was defined as p < 0.05.
Exogenous BSCB tracers
Cadaverine-Alexa-Fluor-555 (A30677), BSA-Alexa-Fluor-555 (A34786) and 70 kDa dextran-tetramethylrhodamine (TMR) (D1818) were purchased from Life Technologies, resuspended in PBS to a concentration of 0.5, 2.5 and 2.5 mg/ml, respectively, and injected at 100 μl per 20 g of body weight via tail vein. Tracers were allowed to circulate for 2 h (cadaverine-A555) or 16 h (BSA-A555 and Dextran-TMR) followed by anaesthesia with Hypnorm and midazolam, cardiac perfusion with PBS and perfusion-fixation with 4 % PFA.
Vascular length measurements
An average of 4 tissue sections per animal were cut at 30 μm thickness and were stained with antibody against Podocalyxin to visualise vasculature. For human tissue vessels in 3 sections were stained with anti-CD34 antibody (M7165 Dako). Images fitting the ventral horn grey matter were analysed with ImageJ software by threshold selection and skeletonisation function. Each n replicate data is an average of 8 (mouse) or 6 (human) ventral horns. Spinal cord tissues in 3 out of 42 mice were underexposed and needed adjustment in gain until vasculature was visible. The increase in signal intensity was normalised using a standard sample analysed at both levels of gain. The total length of fragments per frame was presented as mean ± s.e.m. Statistical analysis was performed using the Student's t test and statistical significance was defined as p < 0.05.
mRNA, protein expression and proteolytic tPA activity
mRNA expression
Lumbar spinal cords were dissected, frozen in RNALater solution (Qiagen 76106) and kept at −80 °C. Whole tissue RNA was isolated with TRIzol reagent (Invitrogen 15596-026) and RNeasy (Qiagen 74106) according to the manufacturers' instructions. Total RNA (1 μg) was reverse transcribed with iScript cDNA synthesis kit (Bio-Rad 170-8891). qPCR was performed using KAPA SYBR Fast polymerase (KAPA Biosystems KK4601) and 10 ng cDNA per reaction with duplicate repeats. Average ΔCt values from three independent runs were used as a conclusive result for each mouse. Expression levels were normalised to the expression of ribosomal protein L19 (Rpl19). The following primers were used for qPCR analysis: Pdgfc (F) 5′-TTTGGGCTGGAAGATCCAGA-3′, (R) 5′-CTGTCCTCTTTAGCTCTTCC-3′, Plat (tPA) (F) 5′-GCTGAGTGCATCAACTGGAA-3′, (R) 5′-GCCACGGTAAGTCACACCTT-3′, Pdgfa (F) 5′-GCTGCGGATACCTCGCCCAT-3′, (R) 5′- AGTCGCTGGAGGTCCCGGAT-3′, Rpl19 (F) 5′-GGTGACCTGGATGAGAAGGA-3′, (R) 5′-TTCAGCTTGTGGATGTGCTC-3′.
Western blotting
Frozen lumbar spinal cords were lysed in 50 mM Tris–HCl pH 7.5, 100 mM NaCl, 0.5 % SDS, 1 % Triton X-100, 0.5 % deoxycholate supplemented with protease inhibitors (Complete Protease Inhibitor Cocktail Tablets and PhosphoStop, both from Roche), using a Precellys24 homogenizer (Bertin). Protein content was determined using Pierce BCA Protein Assay Reagent (Thermo Scientific) according to manufacturer's instructions. 10 μg of total protein was separated on SDS-PAGE (4–12 % gradient gel, Invitrogen) and proteins where transferred on an Immobilon-P PVDF membrane (Millipore). Membrane was then probed with custom rabbit anti-PDGF-C-core domain antibody and rabbit anti-actin antibody (cat. #4967, Cell Signalling) as a loading control. Horseradish peroxidase conjugated secondary goat anti-rabbit antibody was detected using ECL substrate (both from Roche). For immunoprecipitation, lysates described above were adjusted to 750 μg total protein and cleared with protein G Sepharose (GE Healthcare, Uppsala, Sweden) for 1 h at 4 °C. Thereafter, custom rabbit antiserum to PDGFRα (kind gift from Carl-Henrik Heldin, Ludwig Institute for Cancer Research Ltd. Uppsala, Sweden) was added to the samples and incubated for 16 h at 4 °C. The antiserum was then precipitated with protein G Sepharose for 1 h at 4 °C. Sepharose beads were washed with ice cold TBS and boiled in Laemmli buffer. Precipitated proteins were separated on SDS-PAGE (4–12 % gradient gel) and then transferred on a nitrocellulose membrane (Invitrogen). Membrane was then sequentially probed with mouse anti-phosphorylated tyrosine antibody (clone 4G10, Millipore) and rabbit anti-PDGFRα antibody (#3164, Cell Signaling) as loading control. Horseradish peroxidase conjugated secondary antibodies anti-mouse and anti-rabbit, as well as ECL prime detection kit were from GE Healthcare. Pictures were obtained by the imaging system FluroChem Q (AlphaInnotec). Quantification was performed using the ImageJ software on the unmodified picture files. The intensity of the phosphorylated tyrosine band over the PDGFRα band intensity was applied as the ratio readout. The p value was calculated using one-tailed, Student's t test.
Proteolytic activity of tPA
Frozen lumbar spinal cords were homogenised in 0.4 M HEPES; 0.1 M NaCl; pH 7.4. Avidin-coated plates (Molecular Innovations, AVI-PLATE) were incubated with biotinylated PAI-1 protein (Molecular Innovations, NTBIOC-PAI) followed by incubation with tissue lysates or defined amounts of recombinant mouse tPA protein (Molecular Innovations, MTPA) to generate the standard curve. The amount of bound tPA was detected using rabbit anti-mouse tPA antibody (Molecular Innovation, ASMTPA-GF-HT) followed by incubation with donkey anti-rabbit-HRP antibody (Jackson Immuno Research, 711-036-152). Peroxidase activity was detected with tetramethylbenzidine (Molecular Innovations, TMB) chromogenic substrate and measured at 450 nm using the SoftMax Pro suite. The amount of tPA activity was related to the total protein content as measured by the BCA kit (Pierce) and presented as ng of active tPA/mg of total protein.
Statistical analysis
Data are expressed as mean ± SEM. Statistical comparisons were made with GraphPad Prism software using Student's t test (2-tailed unless otherwise indicated; for 2 groups meeting normal distribution criteria: mRNA expression, tPA activity, densitometry and vessel density) or Mann–Whitney test (for 2 groups not meeting normal distribution criteria: BSCB integrity degree in histology sections). Pearson's regression was used for correlation analysis. Kaplan–Meier survival curves and probability estimates were calculated using Log rank (Mantel–Cox) and Gehan– Breslow–Wilcoxon tests. For all tests, a p value of 0.05 or less was considered significant.
Ethical approval
All the work involving animal or human subjects/tissues has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and with national legislation as well as our institutional guidelines. Animal experiments were approved and performed according to the guidelines of the North Stockholm Animal Ethics Committee. Ethical approval for the use of the human samples analysed in this publication was obtained from the regional ethical review board in Stockholm, Sweden (Regionala Etikprövningsnämnden, Stockholm, EPN, http://www.epn.se/sv/stockholm/omnaemnden/), approval number 2012/111-31/1(Eva Hedlund). Experiments were performed in compliance with the ARRIVE guidelines.
Experimental design
For tissue section analysis (e.g. PDGFC expression staining or motor neuron count) blinding was performed by third party concealment of treatments or genotypes and assignment of numeric codes to each group. For treatment cohort randomisation each even and odd mouse in a litter was separated to control and treatment groups. For mouse cohort evaluation analysis blinding was performed by a third party concealment of the mouse tag. Sample size for SOD1 ALS mice cohorts was chosen in concordance with “Guidelines for preclinical in vivo evaluation of pharmacological active drugs for ALS/MND” [24]. Recommended cohort size contained a minimum of 12 gender-matched mice per control and treatment group.
Results
The PDGF-CC signalling pathway is activated before the onset of ALS symptoms
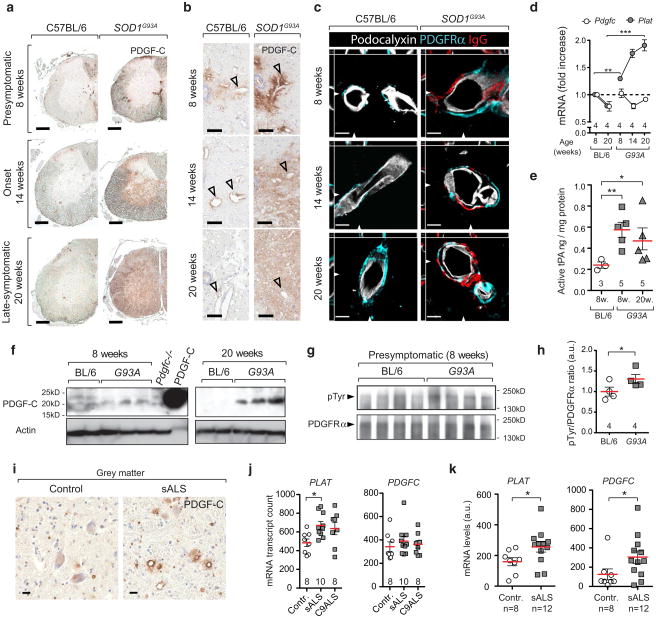
Current understanding of the mechanisms leading to BSCB dysfunction in ALS is limited and has not been attributed to specific signalling pathways. We observed increased PDGF-C immunostaining (Fig. 1a) associated with blood vessels at the presymptomatic age of 8 weeks (Fig. 1b) and accumulated in parenchyma during onset and symptomatic stages (14 and 20 weeks) in spinal cords of SOD1G93A mice. Moreover, vessels displaying BSCB dysfunction were surrounded by perivascular astrocytes expressing the receptor for PDGF-CC—PDGFRα (Fig. 1c). We found that in the spinal cord tissue the cells with the strongest expression of the Pdgfc mRNA transcript belonged to a subpopulation of ChAT+ neuronal cells as shown by location of β-galactosidase activity in Pdgfc+/lacZ mice (Online Resource 1a, b, c, d). Latent PDGF-CC protein is secreted to the extracellular matrix and needs to be proteolytically cleaved by the tissue plasminogen activator (tPA) to become an active ligand [11]. Although Pdgfc mRNA expression level in SOD1G93A spinal cords was stable, expression of the tPA gene (Plat) was increased already in the presymptomatic stage (Fig. 1d). Since tPA function can be modified by protein inhibitors we also examined the levels of proteolytically active tPA in spinal cords of SOD1G93A mice and observed presymptomatic increase in tPA activity (Fig. 1e), together with accumulation of cleaved 22-kDa PDGF-C protein (Fig. 1f; Online Resource 2a, b, c). Accordingly, we found an indication of ligand-dependent activation with increase of PDGFRα tyrosine-phosphorylation in lumbar spinal cord tissue of presymptomatic SOD1G93A mice (Fig. 1g, h; Online Resource 2d). PDGF-CC-induced BSCB dysfunction is unlikely caused by SOD1 biochemical activity since it was also present in mice overexpressing enzymatically inactive SOD1G85R mutation (Online Resource 3a, b) but not in mice overexpressing non-mutated SOD1 (SOD1WT) (Online Resource 4a, b). Since signalling pathways activated in SOD1 fALS models can differ from sporadic disease, we examined sALS cases and found elevated IHC staining for PDGF-C protein in sALS patients (Fig. 1i). This finding was confirmed by increased transcription levels of PLAT in sALS frontal cortex (Fig. 1j) [29] and in laser-captured spinal cord motor neurons of sALS patients (Fig. 1k) [30]. Spinal cord motor neurons showed increased expression of both PLAT and PDGFC, which was not observed in remaining anterior horn tissue or cerebellum (Online Resource 5 a, b). These findings indicate that increase in PDGF-CC signalling is common in both fALS mouse models and human sALS tissue. PDGFRα can be also activated by PDGF-AA, which does not require extracellular activation and is constitutively secreted by cells expressing the Pdgfa gene [14]. Its expression was not changed during the course of the disease in SOD1G93A mice, or in sALS samples (Online Resource 6a, b), which suggests that PDGF-CC is responsible for the increased receptor phosphorylation. Vascular barrier dysfunction in SOD1G93A mice was not visibly associated with general changes in endothelial cell polarisation (Online Resource 7), a well-known characteristic for BBB disruption resulting from impaired Wnt/β-catenin signalling or CCM1 deficiency [19, 22].
Fig. 1.
The PDGF-CC signalling pathway is activated before symptom onset of ALS. a, b Expression of PDGF-C in SOD1G93A mice as detected by immunohistochemistry shows PDGF-C-positive areas around vessels (arrowheads) in 8-week-old mice and in the parenchyma of 14- or 20-week-old mice. Staining target is indicated in the upper right corner. Bar 200 μm (a), 20 μm (b). c Vessels outlined with podocalyxin staining (white) show BSCB dysfunction (IgG— red) and presence of perivascular astrocytes expressing PDGFRα (turquoise) in 8, 14 and 20-week-old SOD1G93A mice. Z stack cross-section location is shown with arrowheads. Bar 10 μm. d Quantification of mRNA expression for Pdgfc and Plat (tPA) in SOD1G93A mice (n = 4). Mean ± s.e.m, two-tailed t test **p = 0.008, ***p = 0.0002. e Quantification of proteolytically active tPA in spinal cords of C57BL/6 (n = 3) and SOD1G93A mice at age of 8 weeks (n = 5) and 20 weeks (n = 5). Mean ± s.e.m, two-tailed t test **p = 0.003, *p = 0.019. f Western blotting against the PDGF-C protein displaying accumulation of the 22-kDa active core domain. Tissue lysate from Pdgfc−/− mouse spinal cord and recombinant PDGF-C core protein were used as controls. g Western blotting against pTyr and PDGFRα in spinal cord tissue lysates. h Calculated density ratio of pTyr and PDGFRα bands. One-tailed t test *p = 0.048. i Increased expression of PDGF-C in medulla oblongata of sALS cases and controls as detected by immunohistochemistry (n = 4). Staining target is indicated in the upper right corner. Bar 20 μm. j mRNA expression of PDGFC and PLAT in the frontal cortex of control (n = 8), sporadic (n = 10) and C9orf72 (n = 8) ALS patients. Mean ± s.e.m, two-tailed t test *p = 0.006. k mRNA expression of PDGFC and PLAT (tPA) in laser-captured motoneurons of sALS patients (n = 12) and controls (n = 8). Mean ± s.e.m, two-tailed t test *p = 0.026 (PLAT), *p = 0.033 (PDGFC)
Decreased PDGF-CC activity restores BSCB integrity and delays ALS onset
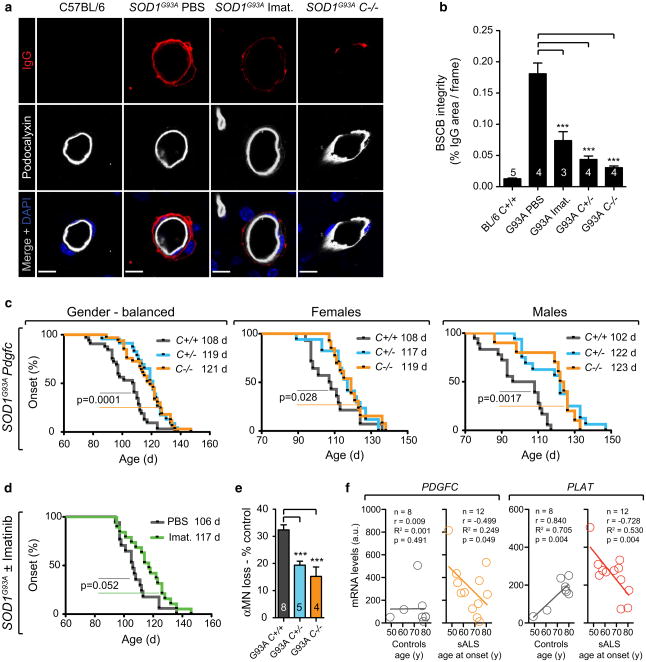
To evaluate the effect of increased PDGF-CC activity on the BSCB dysfunction the SOD1G93A strain was crossed with Pdgfc−/− mice (SOD1G93APdgfc−/−) or treated with PDGFRα tyrosine kinase inhibitor imatinib for 7 days from the age of 14 weeks. BSCB function was restored in SOD-1G93APdgfc−/−, SOD1G93APdgfc+/− and imatinib-treated SOD1G93A mice with a decreased area of endogenous IgG found abluminally from the endothelium membrane. This would point to an effective IgG clearance after barrier restoration (Fig. 2a, b). We used fluorophore-conjugated tracers to describe the characteristics of BSCB deficiency and found that cadaverine (0.9 kDa) Alexa Fluor-555 (A555), bovine serum albumin (BSA)-A555 (69 kDa), as well as 70 kDa dextran tetramethylrhodamine (TMR) were found abluminally or within endothelium of SOD1G93A mice after 2 or 16 h post-injection (Online Resource 8a, b, c, g, h). The uptake of all three tracers was reduced in mice pretreated with imatinib for 7 days (Online Resource 8d, e, f). Endothelial tight junctions were not visibly impaired in leaky vessels (Online Resource 9 and Online Video 1), which together with the endothelial tracer location would suggest that transcellular uptake might be increased in BSCB dysfunction in ALS. To assess the role of PDGF-CC-induced BSCB dysfunction on ALS onset, we followed SOD1G93APdgfc−/− mice and SOD1G93A mice treated with imatinib from the presymptomatic age of 8 weeks. In SOD1G93APdgfc−/− mice the onset of symptoms, defined as the age of maximum body weight, was delayed by 13.5 days (12 %) compared to SOD1G93APdgfc+/+ (median 121 vs. 107.5 days) (Fig. 2c; Online Resource 10). The latency to onset was gender-related with longer delay in males (21 days, 19.6 %) than females (11 days, 10.2 %) (Fig. 2c; Online Resource 10). A similar effect of delayed onset was also observed in haplodeficient SOD1G93APdgfc+/− mice (Fig. 2c; Online Resource 10). SOD1G93A mice treated with imatinib also showed a delay in onset by 11 days (10.4 %) as compared with vehicle-treated controls (117 vs. 106 days) (Fig. 2d; Online Resource 10). Since muscle wasting is driven by motor neuron loss we also evaluated the effect of the PDGF-CC pathway on motor neuron counts. We found that in control SOD1G93APdgfc+/+ mice 32 % of motor neurons were lost at 14 weeks, whereas only about 15 % were lost in SOD1G93APdgfc−/− mice as referred to genotype controls without SOD1G93A (Fig. 2e). To verify if increased PDGF-CC signalling could have an effect on the onset of sporadic forms of ALS, we correlated mRNA expression levels of PDGFC and PLAT in neurons with patient age at disease onset. We found that similarly to SOD1G93A mice with reduced PDGF-CC activity, decreased expression of PGDFC or PLAT in neuronal cells was significantly correlated with increased latency to sALS onset (Fig. 2f). Expression of PDGFC and PLAT was not simply decreasing with age in control subjects (Fig. 2f) or correlated in the anterior horn fraction (Online Resource 11) with the exception of neuronal PLAT, which increased with age in neurons of elderly control subjects (Fig. 2f). Taken together our results indicate that the degree of PDGF-CC activity can affect age of onset in both a fALS model and sporadic human disease.
Fig. 2.
Decreased activity of the PDGF-CC pathway restores BSCB integrity and delays ALS onset. a BSCB dysfunction in lumbar spinal cords of 14-week-old mice. IgG (red) location in reference to the endothelial cell membrane outlined by podocalyxin staining (white). SOD1G93A mice were given PBS or 150 mg/kg/day imatinib for 7 days or were bred onto the Pdgfc−/− background. Bar 10 μm. b Quantification of BSCB dysfunction as shown by IgG area immunofluorescence from corresponding lumbar region sections. PBS-treated SOD1G93A mice (n = 4), imatinib-treated SOD1G93A (n = 3, p = 0.0008), SOD1G93APdgfc−/− (n = 4, p = 0.0002), SOD1G93APdgfc+/− (n = 4, p = 0.0002). Mean ± s.e.m, two-tailed Mann-Whitney test. c Event estimates of disease onset in gender-balanced SOD1G93APdgfc+/+ mice (black, n = 32) compared to SOD1G93APdgfc−/− (orange, n = 23) and SOD1G93APdgfc+/− (turquoise, n = 33) together with gender specific values for males and female cohorts. p values for Gehan–Breslow–Wilcoxon tests are indicated and median onset values are shown in legends. d Event estimates of disease onset in SOD1G93A female mice treated with PBS (black, n = 17) or imatinib (100 mg/kg/day) (green, n = 19) from the age of 8 weeks. e Quantification of motor neuron loss in SOD1G93APdgfc+/+ (n = 8) vs. SOD1G93APdgfc−/− (n = 4, ***p = 0.0008) or SOD1G93APdgfc+/− (n = 5, ***p = 0.0005) mice at ALS onset (14 weeks) as referred to % of genotype control without SOD1G93A. Mean ± s.e.m, two-tailed t test. f Correlated values for neuronal mRNA expression of PDGFC or PLAT and age of sALS onset. r Pearson correlation, R2 coefficient of determination, p one-tailed t test p value
Vascular regression occurs despite BSCB restoration and correlates with disease severity
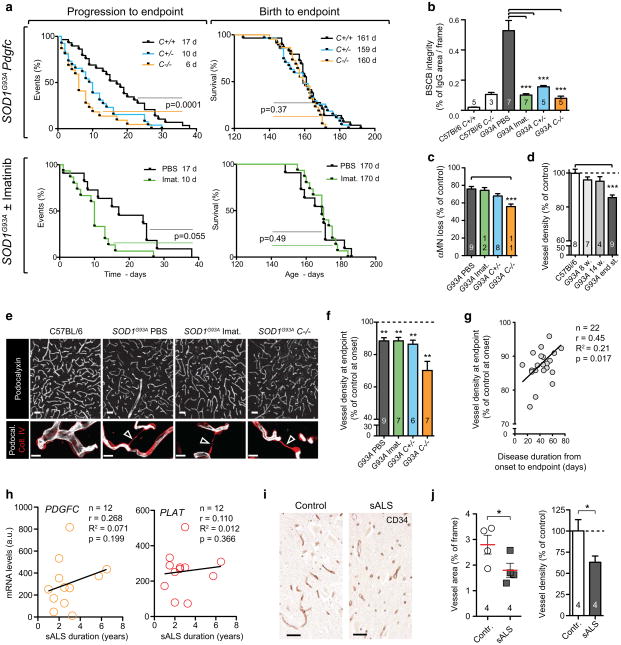
To assess the role of PDGF-CC activity after onset, we followed SOD1G93A mice with ablated Pdgfc expression or PDGFRα inhibition until the critical endpoint. The progression phase, defined as time between onset and loss of more than 10 % of maximum body weight, was not affected in imatinib-treated SOD1G93A mice and was shorter in SOD1G93APdgfc−/− mice compared to SOD1G93APdgfc+/+ controls (median 31 vs. 42.5 days) (Online Resource 12a, b). Intriguingly, PDGF-CC inhibition was associated with shortened duration of the progression to endpoint phase to 6 days for the SOD1G93APdgfc−/− a APdgfc+/− or imatinib-treated SOD1G93A cohorts as compared with 17 days for the SOD1G93APdgfc+/+ control group (Fig. 3a; Online Resource 12c). As a consequence, there was no observable difference in survival, defined as time from birth to critical endpoint (Fig. 3a; Online Resource 12c). Since end-stage disease acceleration occurred despite restored BSCB properties (Fig. 3b), noticeable sparing of alpha motor neurons in the SOD1G93APdgfc−/− mice (Fig. 3c) and without noticeable differences in neuroinflammation (Online Resource 13a, b), it suggested that other mechanisms can contribute to disease duration after onset. The Pdgfc null mice were reported to have developmental cerebrovascular phenotypes [12], which could contribute to disease acceleration after onset, yet the acceleration was also present to a lesser extent in SOD1G93APdgfc+/− strain and imatinib-treated mice (Fig. 3a; Online Resource Figure 12c). We examined if disease-induced capillary regression after onset (Fig. 3d) [26] was connected with BSCB status and observed that vessels continued to regress despite restored BSCB (Fig. 3e, f). Vascular regression was associated with presence of collagen sleeves, which are remnants of basement membrane after endothelial cell degeneration [8] (Fig. 3e). Moreover, the degree of vessel regression at end stage was positively correlated with more aggressive disease after onset regardless of BSCB restoration (Fig. 3g). Similarly to our observations in fALS mice, duration of sALS was not associated with expression levels of PDGFC or PLAT (Fig. 3h) and could be modified by capillary regression since it was also present in the grey matter of sALS spinal cord tissue (Fig. 3i, j). In summary, our observations suggest that the vascular phenotype of PDGF-CC-induced BSCB dysfunction and disease-induced capillary regression are present in a fALS mouse model as well as in sALS; however, these phenotypes are not directly consequential and they can independently affect specific disease stages.
Fig. 3.
Vascular regression occurs despite BSCB restoration and correlates with disease severity. a Event estimates of endpoint survival from birth and disease progression to endpoint phase. SOD1G93APdgfc+/+ (n = 32) or SOD1G93A PBS-treated mice (black, n = 17) are compared to SOD1G93APdgfc−/− (orange, n = 23), SOD1G93APdgfc+/− (turquoise, n = 33) and SOD1G93A treated with 100 mg/kg/day imatinib from the age of 8 weeks (green, n = 19). p values for Gehan–Breslow–Wilcoxon tests are indicated and median onset values are shown in legends. b BSCB integrity determined by IgG quantification in endpoint SOD1G93A mice treated with PBS (n = 7), imatinib (n = 7, ***p = 0.0001), SOD1G93APdgfc+/− (n = 5, ***p = 0.0001) and SOD1G93APdgfc−/− (n = 5, ***p = 0.0001). Mean ± s.e.m, two-tailed Mann–Whitney test. c Motor neuron loss in endpoint SOD1G93A mice treated with PBS (n = 9), imatinib (n = 12) and SOD1G93APdgfc−/− (n = 11, ***p = 0.0001), data are presented as % of genotype control without SOD1G93A. Mean ± s.e.m, two-tailed t test. d Vascular regression in the ventral horn of SOD1G93A calculated as skeletonised vessel length and referenced to C57Bl/6 (n = 8), SOD1G93A (n = 9, ***p = 0.0001). Mean ± s.e.m, two-tailed t test. e Vascular network in the ventral horn at disease endpoint visualised with podocalyxin staining (white, bar 50 μm) and basement membrane collagen IV (red) to visualise empty collagen sleeves (lower panel arrowheads), bar 10 μm. f Quantification of vascular density at disease endpoint in SOD1G93A mice treated with PBS (n = 9, **p = 0.007) as well as in mice with restored BSCB properties: imatinib (n = 7, **p = 0.009), SOD1G93APdgfc+/− (n = 6, **p = 0.004) or SOD1G93APdgfc−/− (n = 7, **p = 0.003). Values are related to respective SOD1G93APdgfc allele control at onset age of 14 weeks. Mean ± s.e.m, two-tailed t test. g Positive correlation between vascular regression from panel f and disease duration after onset. Results from SOD1G93A PBS-treated, SOD1G93A imatinib-treated and SOD1G93APdgfc+/− mice were grouped and correlation is indicated with a black line. h Lack of correlation between neuronal PDGFC or PLAT mRNA expression and duration of sALS. i Vascular network in spinal cord ventral horn in sALS patients stained with the CD34 antibody. Staining target is indicated in the upper right corner. Bar 50 μm. j Vascular density in sALS patients quantified as vessel area or skeletonised fragment length density (n = 4). Mean ± s.e.m, one-tailed t test
Discussion
The origins of ALS and factors affecting the onset of sporadic forms remain largely unknown. Vascular barrier dysfunction in sALS was proposed more than three decades ago [21] and studies in fALS rodent models imply that early BSCB dysfunction could exacerbate disease symptoms [15, 41, 44]. Exogenous administration of the anti-coagulant drug warfarin showed increased spinal cord haemorrhage and acceleration of disease onset in SOD1G93A model [41]; however, knowledge regarding endogenous molecular pathways leading to BSCB dysfunction is limited and as a consequence the therapeutic opportunities remain restricted.
We show that PDGF-C protein found in a neuronal subpopulation is increased in human sALS and in SOD1 mouse models from the presymptomatic stage as evidenced by increased activity of tPA, accumulation of cleaved PDGF-CC ligand and elevated phosphorylation of the PDGFRα. Using Pdgfc-deficient mice or pharmacological PDGFRα inhibition with imatinib, we were able to restore BSCB properties in the SOD1G93A model, which led to increased latency to disease onset. The onset delay observed in SOD1G93APdgfc−/− (21 days) or SOD1G93APdgfc+/− males (20 days) was the strongest genetic effect recorded for this strain and outperformed transgenic overexpression of parvalbumin [5] or glutamate transporter EAAT2 [16]. A similar correlation between decreased expression of PDGFC or PLAT and delayed onset age in sALS patients suggests that this mechanism is common between familial and sporadic disease and clinically relevant. In the light of recent evidence for increased PDGF-C protein concentration in the cerebral spinal fluid of traumatic injury patients [37] our findings could provide a mechanistic explanation for increased ALS risk after head trauma [35]. Therefore, the therapeutic potential for PDGF-C inhibition could be applied to delay onset in familial ALS cases and in immediate treatment of traumatic injury events to decrease acute consequences of BBB dysfunction and probability of sALS onset.
Our onset-specific observation is consistent with the studies of cell-specific ablation of mutant LoxSOD1G37R, which show that neuron stress is associated with disease onset [7, 43] but not later disease stages and can be augmented by dysfunctional BSCB. Although endothelial cells clearly contribute to the physical formation of the BSCB by means of tight junctions, the regulation of barrier activity is dependent on other cell types including, pericytes, astrocytes and neuronal activity [4, 28]. Our findings support this concept and point to a neuronal source of PDGF-C and the previously described astrocyte PDGFRα location [36] as a mechanism of BSCB dysfunction in ALS. tPA can be secreted by several cell types including interneuron synapses upon depolarisation [23] and prolonged increase in its activity can result in proteolytic activation of PDGF-C and stimulation of PDGFRα-positive perivascular astrocytes. As a consequence BSCB dysfunction and additional neuron insult can lead to acceleration of onset. Imatinib was designed to target the Abl kinase in chronic myelogenous leukaemia and apart from PDGFRα it can also inhibit PDGFRβ and c-Kit kinase activity. Long-term PDGFRβ inhibition can cause decreased pericyte coverage and BBB dysfunction [4], which was also reported in ALS tissue [42] and needs to be considered in long-term imatinib use. Since imatinib has broad range of tyrosine kinase inhibition, a future therapeutic approach should focus on development of specific anti-PDGF-C antibodies.
Apart from BSCB dysfunction, fALS mouse models display other vascular pathologies such as decreased cerebral blood flow (CBF) or capillary network regression [26, 44]. It is yet unclear to which extent these phenotypes contribute to specific disease stages. We have confirmed that capillary regression is strongest at the end stage in fALS mice and further show that regression occurs despite BSCB restoration, which implies that these two pathologies are not successively linked. Importantly, we show that capillary regression can also occur in sALS and the degree of vascular regression in fALS mice correlated with disease duration after onset, with more aggressive progression to end stage occurring in mice with stronger capillary degeneration. This inefficient structure of the capillary network is a plausible explanation for decreased grey matter perfusion found in human ALS patients using 18fuoro-deoxyglucose PET imaging [34, 38]. These human studies also point to a strong correlation between decreased CBF and increased disease severity, which together with our findings, indicate that vascular delivery of oxygen and nutrients can be a critical factor for disease progression and survival.
Taken together, our observations help to explain how vascular barrier and regression phenotypes in ALS contribute to specific disease stages. Early activation of the PDGF-CC pathway is a significant contributor to presymptomatic BSCB dysfunction and disease onset. However, efficient tissue perfusion through the capillary network can be an important factor controlling the duration of the disease after onset.
Supplementary Material
Acknowledgments
This work was supported by grants from the Thierry Latran Foundation (U. E.), the Leducq Foundation (U. E.), Swedish Research Council (U.E. 2011-3861), the Swedish Governmental Agency for Innovation Systems (VINNOVA, U. E., L. F. 2011-03503), the Ragnar Söderberg Foundation (E. H. M245/11), the Swedish Brain Foundation (U. E., E. H. FO2012-0055) and the Håll-sten Research Foundation (U. E.), the Birgit Backmark Donation (E. H.), the Åhlen's Foundation (E. H. mA9/11, mA5/h12 and mB8/h13), the Swedish Stroke Foundation (I. N.), the Swedish Research Council (I. N. 524-2008-785, L. F. 524-2008-777 and 521-2012-1853), the National Institutes of Health (D. A. L. R01 NS079639) and Karolinska Institutet. Human post mortem tissues were provided by the Netherland's Brain Bank (NBB). We would like to thank Sofia Wittgren and Mark Warnock for technical assistance. We would also like to thank R. K. Filipkowski for comments on the manuscript.
Footnotes
Electronic supplementary material: The online version of this article (doi:10.1007/s00401-015-1520-2) contains supplementary material, which is available to authorized users.
Supplementary information: Supplementary figures and video are present as Online Resource on the Acta Neuropathologica journal website.
Compliance with ethical standards
Conflict of interest: The authors declare that no conflict of interest exists.
Contributor Information
Sebastian A. Lewandowski, Email: sebastian.lewandowski@ki.se.
Ulf Eriksson, Email: ulf.pe.eriksson@ki.se.
References
- 1.Abrams MB, Nilsson I, Lewandowski SA, Kjell J, Codeluppi S, Olson L, Eriksson U. Imatinib enhances functional outcome after spinal cord injury. PLoS One. 2012;7:e38760. doi: 10.1371/journal.pone.0038760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adzemovic MV, Zeitelhofer M, Eriksson U, Olsson T, Nilsson I. Imatinib ameliorates neuroinflammation in a rat model of multiple sclerosis by enhancing blood-brain barrier integrity and by modulating the peripheral immune response. PLoS One. 2013;8:e56586. doi: 10.1371/journal.pone.0056586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9:617–628. doi: 10.1038/nrneurol.2013.203. [DOI] [PubMed] [Google Scholar]
- 4.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 5.Beers DR, Ho BK, Siklos L, Alexianu ME, Mosier DR, Mohamed AH, Otsuka Y, Kozovska ME, McAlhany RE, Smith RG, Appel SH. Parvalbumin overexpression alters immune-mediated increases in intracellular calcium, and delays disease onset in a transgenic model of familial amyotrophic lateral sclerosis. J Neurochem. 2001;79:499–509. doi: 10.1046/j.1471-4159.2001.00582.x. [DOI] [PubMed] [Google Scholar]
- 6.Bento-Abreu A, Van Damme P, Van Den Bosch L, Robberecht W. The neurobiology of amyotrophic lateral sclerosis. Eur J Neurosci. 2010;31:2247–2265. doi: 10.1111/j.1460-9568.2010.07260.x. [DOI] [PubMed] [Google Scholar]
- 7.Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 8.Brown WR. A review of string vessels or collapsed, empty basement membrane tubes. J Alzheimers Dis. 2010;21:725–739. doi: 10.3233/JAD-2010-100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding H, Wu X, Bostrom H, Kim I, Wong N, Tsoi B, O'Rourke M, Koh GY, Soriano P, Betsholtz C, Hart TC, Marazita ML, Field LL, Tam PP, Nagy A. A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat Genet. 2004;36:1111–1116. doi: 10.1038/ng1415. [DOI] [PubMed] [Google Scholar]
- 10.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Fredriksson L, Li H, Fieber C, Li X, Eriksson U. Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 2004;23:3793–3802. doi: 10.1038/sj.emboj.7600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredriksson L, Nilsson I, Su EJ, Andrae J, Ding H, Betsholtz C, Eriksson U, Lawrence DA. Platelet-derived growth factor C deficiency in C57BL/6 mice leads to abnormal cerebral vascularization, loss of neuroependymal integrity, and ventricular abnormalities. Am J Pathol. 2012;180:1136–1144. doi: 10.1016/j.ajpath.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredriksson L, Stevenson TK, Su EJ, Ragsdale M, Moore S, Craciun S, Schielke GP, Murphy GG, Lawrence DA. Identification of a neurovascular signaling pathway regulating seizures in mice. Ann Clin Transl Neurol. 2015;2:722–738. doi: 10.1002/acn3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fruttiger M, Calver AR, Richardson WD. Platelet-derived growth factor is constitutively secreted from neuronal cell bodies but not from axons. Curr Biol. 2000;10:1283–1286. doi: 10.1016/S0960-9822(00)00757-0. [DOI] [PubMed] [Google Scholar]
- 15.Garbuzova-Davis S, Haller E, Saporta S, Kolomey I, Nicosia SV, Sanberg PR. Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res. 2007;1157:126–137. doi: 10.1016/j.brainres.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Guo H, Lai L, Butchbach ME, Stockinger MP, Shan X, Bishop GA, Lin CL. Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum Mol Genet. 2003;12:2519–2532. doi: 10.1093/hmg/ddg267. [DOI] [PubMed] [Google Scholar]
- 17.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 18.Johnston CA, Stanton BR, Turner MR, Gray R, Blunt AH, Butt D, Ampong MA, Shaw CE, Leigh PN, Al-Chalabi A. Amyotrophic lateral sclerosis in an urban setting: a population based study of inner city London. J Neurol. 2006;253:1642–1643. doi: 10.1007/s00415-006-0195-y. [DOI] [PubMed] [Google Scholar]
- 19.Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, Dejana E. CCM1 regulates vascularlumen organization by inducing endothelial polarity. J Cell Sci. 2010;123:1073–1080. doi: 10.1242/jcs.059329. [DOI] [PubMed] [Google Scholar]
- 20.Leitner M, Menzies S, Lutz C. Working with ALS Mice. Guidelines for preclinical testing and colony management. Prize4Life and The Jackson Laboratory. 2010:1–21. http://www.researchals.org/uploaded_files/p4l_jax_sod1manual_20091202_29aPcx.pdf.
- 21.Leonardi A, Abbruzzese G, Arata L, Cocito L, Vische M. Cerebrospinal fluid (CSF) findings in amyotrophic lateral sclerosis. J Neurol. 1984;231:75–78. doi: 10.1007/BF00313720. [DOI] [PubMed] [Google Scholar]
- 22.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lochner JE, Honigman LS, Grant WF, Gessford SK, Hansen AB, Silverman MA, Scalettar BA. Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol. 2006;66:564–577. doi: 10.1002/neu.20250. [DOI] [PubMed] [Google Scholar]
- 24.Ludolph AC, Bendotti C, Blaugrund E, Hengerer B, Loffer JP, Martin J, Meininger V, Meyer T, Moussaoui S, Robberecht W, Scott S, Silani V, Van Den Berg LH. Guidelines for the preclinical in vivo evaluation of pharmacological active drugs for ALS/MND: report on the 142nd ENMC international workshop. Amyotroph Lateral Scler. 2007;8:217–223. doi: 10.1080/17482960701292837. [DOI] [PubMed] [Google Scholar]
- 25.Mae M, Armulik A, Betsholtz C. Getting to know the cast–cellular interactions and signaling at the neurovascular unit. Curr Pharm Des. 2011;17:2750–2754. doi: 10.2174/13816121179744011. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki K, Masamoto K, Morimoto N, Kurata T, Mimoto T, Obata T, Kanno I, Abe K. Early and progressive impairment of spinal blood flow–glucose metabolism coupling in motor neuron degeneration of ALS model mice. J Cereb Blood Flow Metab. 2011;32:456–467. doi: 10.1038/jcbfm.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuwelt EA. Mechanisms of disease: the blood–brain barrier. Neurosurgery. 2004;54:131–140. doi: 10.1227/01.NEU.0000097715.11966.8E. [DOI] [PubMed] [Google Scholar]
- 28.Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnar Z, O'Donnell ME, Povlishock JT, Saunders NR, Sharp F, Stanimirovic D, Watts RJ, Drewes LR. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prudencio M, Belzil VV, Batra R, Ross CA, Gendron TF, Pregent LJ, Murray ME, Overstreet KK, Piazza-Johnston AE, Desaro P, Bieniek KF, DeTure M, Lee WC, Biendarra SM, Davis MD, Baker MC, Perkerson RB, van Blitterswijk M, Stetler CT, Rademakers R, Link CD, Dickson DW, Boylan KB, Li H, Petrucelli L. Distinct brain transcriptome profiles in C9orf72-associated and sporadic ALS. Nat Neurosci. 2015;18:1175–1182. doi: 10.1038/nn.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabin SJ, Kim JM, Baughn M, Libby RT, Kim YJ, Fan Y, La Spada A, Stone B, Ravits J. Sporadic ALS has compartment-specific aberrant exon splicing and altered cell-matrix adhesion biology. Hum Mol Genet. 2010;19:313–328. doi: 10.1093/hmg/ddp498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regal L, Vanopdenbosch L, Tilkin P, Van den Bosch L, Thijs V, Sciot R, Robberecht W. The G93C mutation in superoxide dismutase 1: clinicopathologic phenotype and prognosis. Arch Neurol. 2006;63:262–267. doi: 10.1001/archneur.63.2.262. [DOI] [PubMed] [Google Scholar]
- 32.Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364:362. doi: 10.1038/364362c0. [DOI] [PubMed] [Google Scholar]
- 33.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 34.Rule RR, Schuff N, Miller RG, Weiner MW. Gray matter perfusion correlates with disease severity in ALS. Neurology. 2010;74:821–827. doi: 10.1212/WNL.0b013e3181d3e2dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seelen M, van Doormaal PT, Visser AE, Huisman MH, Roozekrans MH, de Jong SW, van der Kooi AJ, de Visser M, Voermans NC, Veldink JH, van den Berg LH. Prior medical conditions and the risk of amyotrophic lateral sclerosis. J Neurol. 2014;261:1949–1956. doi: 10.1007/s00415-014-7445-1. [DOI] [PubMed] [Google Scholar]
- 36.Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, Strickland DK, Betsholtz C, Eriksson U, Lawrence DA. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su EJ, Fredriksson L, Kanzawa M, Moore S, Folestad E, Stevenson TK, Nilsson I, Sashindranath M, Schielke GP, Warnock M, Ragsdale M, Mann K, Lawrence AE, Medcalf RL, Eriksson U, Murphy GG, Lawrence DA. Imatinib treatment reduces brain injury in a murine model of traumatic brain injury. Front Cell Neurosci. 2015;9:385. doi: 10.3389/fncel.2015.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Laere K, Vanhee A, Verschueren J, De Coster L, Driesen A, Dupont P, Robberecht W, Van Damme P. Value of 18fluorodeoxyglucose-positron-emission tomography in amyotrophic lateral sclerosis: a prospective study. JAMA Neurol. 2014 doi: 10.1001/jamaneurol.2014.62. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Salvaterra PM, Loera S, Chiu AY. Brain-derived neurotrophic factor spares choline acetyl-transferase mRNA following axotomy of motor neurons in vivo. J Neurosci Res. 1997;47:134–143. doi: 10.1002/(SICI)1097-4547(19970115)47:2<134:AID-JNR2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 40.Weydt P, Hong SY, Kliot M, Moller T. Assessing disease onset and progression in the SOD1 mouse model of ALS. NeuroReport. 2003;14:1051–1054. doi: 10.1097/01.wnr.0000073685.00308.89. [DOI] [PubMed] [Google Scholar]
- 41.Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma Q, Zuniga E, Wang Y, Zhong Z, Sullivan JS, Griffin JH, Cleveland DW, Zlokovic BV. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci USA. 2014 doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. 2012;125:111–120. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O'Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.