Abstract
Diabetes is among the biggest of the 21st-century global health challenges. In the U.S. and other high-income countries, thanks to investments in science, dedication to implementing these findings, and measurement of quality of care, there have been improvements in diabetes management and declines in rate of diabetes complications and mortality. This good news, however, is overshadowed by the ever-increasing absolute numbers of people with diabetes and its complications and the unprecedented growth of diabetes in low- and middle-income countries of the world. To comprehensively win the war against diabetes requires 1) concerted attention to prevention and 2) expansion of global research to better inform population-level policies to curb diabetes but also to better understand individual- and population-level variations in pathophysiology and phenotypes globally so that prevention and treatment can be tailored. For example, preliminary data show that thin people in low- and middle-income countries such as India commonly experience type 2 diabetes. Global studies comparing these thin Asian Indians with other high-risk groups such as Pima Indians, a population with a high mean BMI, suggest that type 2 diabetes may not be a single pathophysiological entity. Pima Indians may represent the well-studied phenotype of poor insulin action (type 2A), whereas Asian Indians represent the grossly understudied phenotype of poor insulin secretion (type 2B). This has major implications for diagnosis, prevention, and treatment and highlights the mismatch between where diabetes burdens occur (i.e., low- and middle-income countries) and where research happens (i.e., high-income countries). Correcting this imbalance will advance our knowledge and arsenal to win the global war against diabetes.
Introduction
Type 2 diabetes is a prototypical disease at the cross-section of contemporary globalization and health. In the U.S. and other high-income countries, some successes are evident in preventing or postponing complications of the disease by better implementation of quality of care. Yet, this “winning of a battle” hides a larger worrying issue of “losing the war,” stemming from the persistently high incidence of diabetes itself at home and from the expanding epidemic worldwide (Fig. 1). The war will not be won without viewing type 2 diabetes in its global context as the world becomes rapidly more interconnected in the midst of major demographic, economic, and environmental transitions. Although the majority of the disease burden resides in low- and middle-income countries, research into diabetes remains concentrated in a few high-income countries. This discrepancy between where the preponderance of the disease burden resides and where the research is conducted hampers our ability to better understand the differences in the pathophysiology, or phenotypes, of the disease. For example, studies in populations, such as Asian Indians, who have been exposed to generations of undernutrition suggest that type 2 diabetes may be highly prevalent even in thin people and may be driven not only by propensity to fat storage and insulin resistance but also primarily through innate and early problems with adequate insulin secretion. Furthermore, the etiology, clinical presentation, diagnosis, treatment, and prevention may differ by phenotypes. Studies in global settings, allowing for comparisons across populations (e.g., Asian Indians vs. Pima Indians), can better inform differences in phenotypes. It is therefore time to consider the pathophysiology of type 2 diabetes across the spectrum, from those dominantly affected by insulin resistance (type 2A) to those dominantly affected by insulin secretion (type 2B). With an increasingly interconnected world, investment in global collaboration in research and policy will be needed to win the war against diabetes.
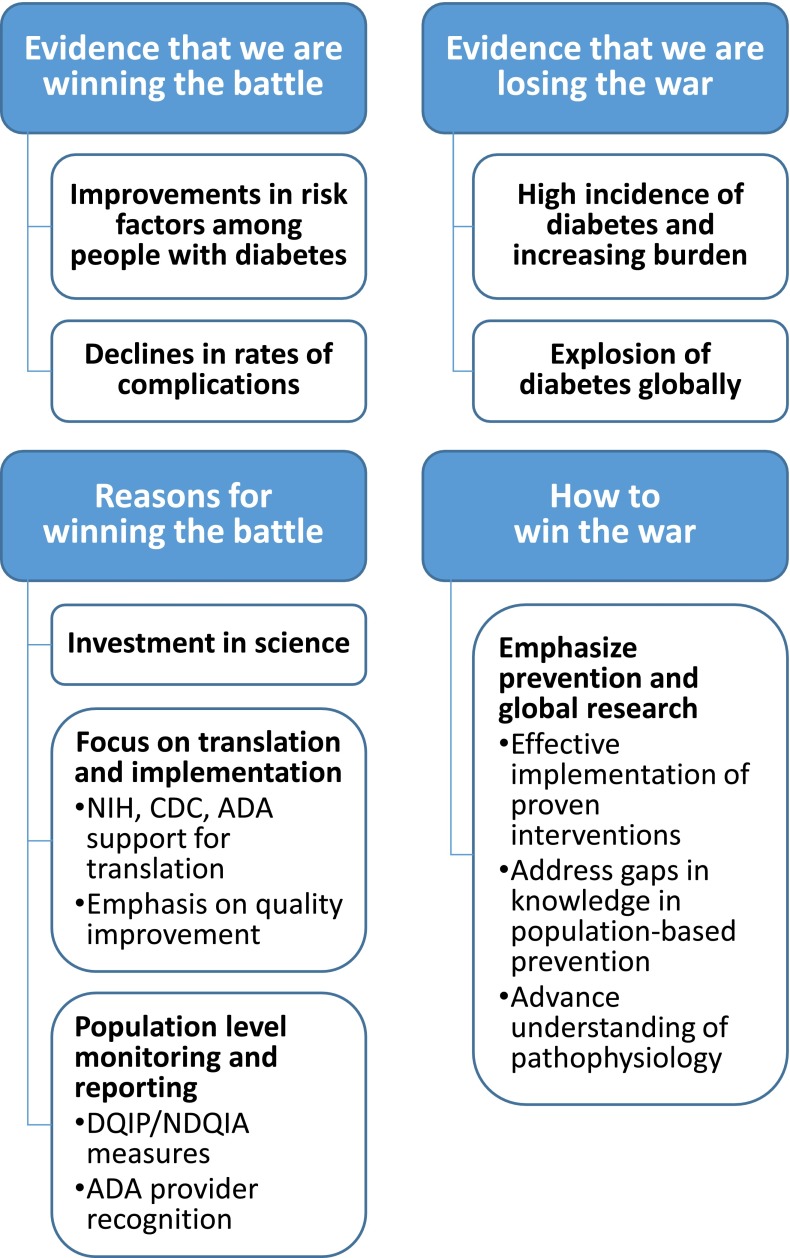
Figure 1.
Why we are winning the battle but losing the war? NIH, National Institutes of Health.
Winning the Battle
Declines in Rate of Complications Among People With Diabetes
Although diabetes (90–95% of which is type 2) remains a daunting public health problem, affecting 29.1 million individuals and costing $245 billion in the U.S. (1), there have been impressive improvements in outcomes among people with diabetes in the country over the past two decades (2) (Fig. 1). Mortality rates among both men and women with diabetes in the U.S. have declined substantially between 1997 and 2006 (3). Furthermore, rates of several diabetes complications have also declined between 1990 and 2010, including the incidence of acute myocardial infarction by 67.8%, death from hyperglycemic crisis by 64.4%, stroke by 52.7%, amputation by 51.4%, and end-stage renal disease by 28.3% (2). Such improvements are not limited to the U.S., as improvements in outcomes among people with diabetes have also been observed in other high-income countries (4,5).
These improvements in diabetes complications are likely due to several factors; however, there are three that deserve special mention: investments in science, institutional orientation toward translation and implementation, and quality-of-care benchmarking efforts. First, investments in science leading to the development of new knowledge about the disease, better diagnostics, and a widening array of treatment options are all paying off. Namely, large clinical trials such as the Diabetes Control and Complications Trial (DCCT), the UK Prospective Diabetes Study (UKPDS), the Steno-2 study, and their successor mega-trials have helped to shape our understanding of diabetes management, treatment intensity and targets, and clinical practice. Second, there has been an increased emphasis on the implementation of proven interventions into clinical and public health practice and policy. Specifically, attention has been given to translational research to facilitate the implementation of proven interventions by the Centers for Disease Control and Prevention (CDC), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and the American Diabetes Association (ADA) (6–8). Large multicenter translational studies, such as the Translating Research Into Action for Diabetes (TRIAD), funded by the CDC, NIDDK, and the Department of Veterans Affairs, have generated valuable information around key factors to improve quality of care (9). Factors at the level of the health systems (i.e., financing, electronic record systems), disease management strategies (i.e., care coordination, diabetes teams, physician–patient communication), physician reimbursement (i.e., incentivizing quality as opposed to volume of services), and the patient (i.e., reducing out-of-pocket expenses, patient education and empowerment) each positively impact quality of care (10–14). Third, the measurement of quality of care, led by the national Diabetes Quality Improvement Project (DQIP), working through a coalition of influential private and public national organizations, and later to become the National Diabetes Quality Improvement Alliance (NDQIA), has helped to focus attention on implementation. The NDQIA develops, maintains, and promotes the use of an updated standardized measurement set (the NDQIA measures) for quality of diabetes care. Monitoring and reporting of quality of care among people with diabetes nationally by the CDC and NIDDK have focused attention on gaps and documented significant improvements in diabetes processes and intermediate outcomes (15–17). For example, such documentation has revealed that control of vascular risk factors, HbA1c, blood pressure, and LDL cholesterol among people with diabetes have all improved in the period 1999–2000 (17).
Losing the War
Although the declines in the rates of diabetes-related complications during the past two decades are noteworthy, we are still confronted by daunting challenges; namely, 1) the persistently high prevalence and incidence of diabetes in the U.S. (Fig. 2) and 2) the explosion of type 2 diabetes globally.
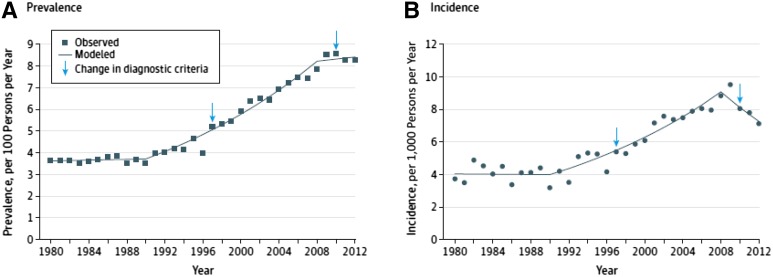
Figure 2.
Trends in age-adjusted diagnosed diabetes prevalence and incidence among adults aged 20–79 years, 1980–2012. Data from the National Health Interview Survey. Reproduced with permission from Geiss et al. (18).
Persistently High Prevalence and Incidence of Diabetes in the U.S.
The prevalence of diabetes in the U.S. has been rising at least since 1980, while the rising incidence of diabetes seems to have recently plateaued (18). Furthermore, the lifetime risk of diabetes in U.S. has been increasing over time: from 20.7% and 27.5% for males and females, respectively, born between 1985 and 1989, to 32.8% and 38.5% for those born in 2000 (19), to 40.2% and 39.5% for those born between 2000 and 2011 (20). The resulting consequences are that overall years spent with diabetes have increased by 156% for males and 70% for females (20) in the past 30 years. These increases in lifetime risk of diabetes are driven by two factors: 1) improved survival among those with diabetes, thanks to better implementation of proven interventions to prevent complications and delay mortality, and 2) continued high diabetes incidence. For a chronic disease such as type 2 diabetes, even small increases in incidence can have a dominant impact on lifetime risk and on population prevalence. As an illustration, the small increase in diabetes incidence in the U.S. between 2000 and 2004 resulted in 12 million more projected numbers of people with diabetes by 2050 (21). Furthermore, an estimated 86 million people in the U.S. have some form of prediabetes (impaired fasting glucose [IFG] and/or impaired glucose tolerance [IGT]) (1), and the annual rate of diabetes conversion in this group is severalfold than in people with normoglycemia (22). Thus, the impending growth of individuals with diabetes should be of great concern.
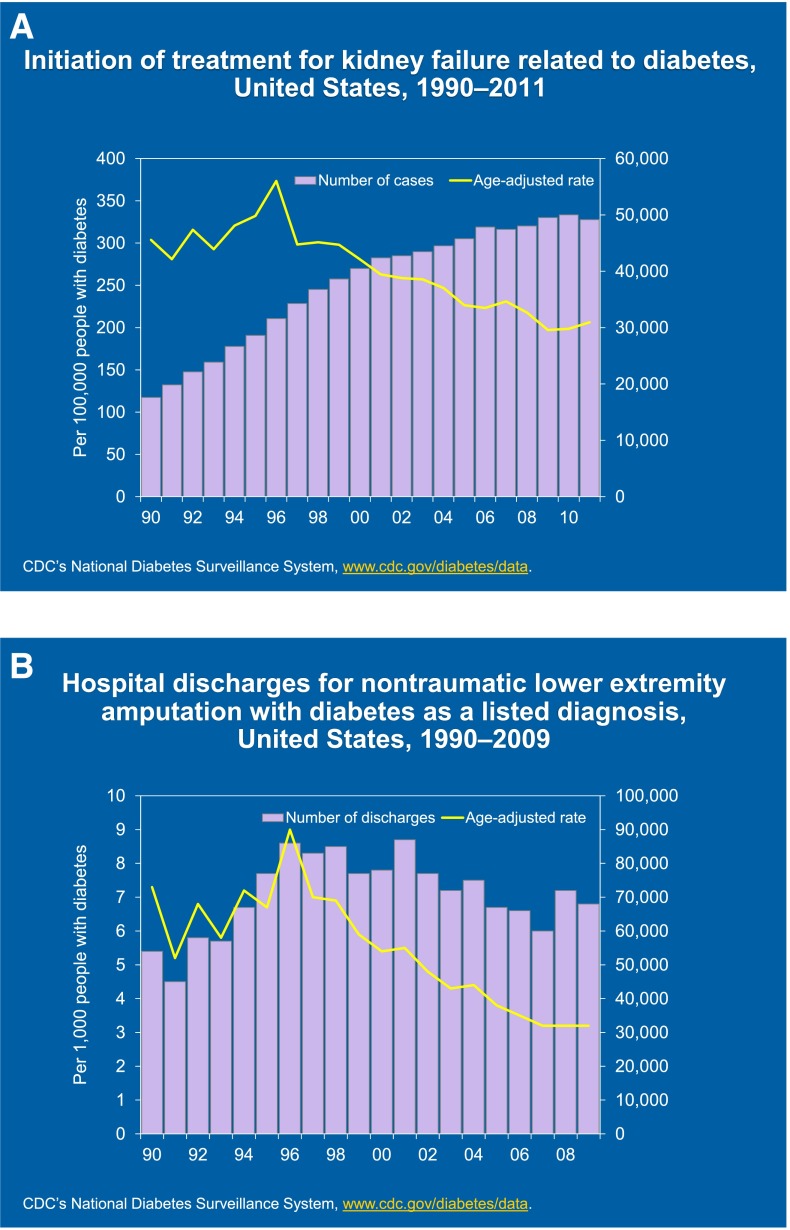
As a consequence of the increasing trend in numbers of people with diabetes in the U.S., even if the rate of complications is decreasing, the absolute numbers of people in the country with complications will continue to rise (Fig. 3). This will have enormous implications for health care systems and payers, as diabetes is already a costly disease (23) and was the leading contributor for inflation-adjusted health care spending among Medicare beneficiaries in 1997–2006 in the U.S. (24) and there will be ever-increasing numbers of people with diabetes and its complications to be managed.
Figure 3.
Initiation of treatment for kidney failure related to diabetes (A) and hospital discharges for nontraumatic lower-extremity amputation with diabetes as a listed diagnosis (B) in the U.S., 1990–2011. Lines represent age-adjusted rates, and bars represent number of cases. Data from the CDC National Surveillance System (94) and personal communication (N.R. Burrows).
Explosion of Type 2 Diabetes Globally
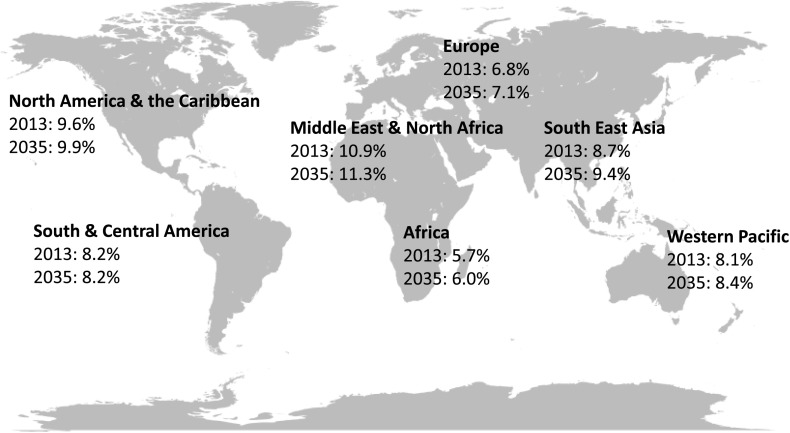
A larger worrying story, which the U.S., with its large immigrant population and global interconnectedness, would ignore at its own peril, is that diabetes has emerged as a major public health problem worldwide, with pandemic growth driven by changing demographic, socioeconomic, and lifestyle patterns across the globe. The International Diabetes Federation (IDF) estimates that there were 415 million people with diabetes in 2015 and projects that the absolute number will reach 642 million by 2040, affecting all regions of the world (25) (Fig. 4). Current estimates suggest that three-quarters of those affected by diabetes live in low- and middle-income countries, where the disease disproportionately affects younger people at the prime of their economic productivity. The overall increase in diabetes prevalence has also been steeper in low- and middle-income countries than in affluent high-income countries (26). The IDF also estimates that globally at least 316 million people (6.9% of the world’s adult population) had IGT in 2013 (27) and that low- and middle-income countries will experience a 50% increase in IGT prevalence by 2035, compared with 41% increase in high-income countries (27).
Figure 4.
Comparative prevalence of diabetes in people aged 20–79 years by world regions. Data from IDF Diabetes Atlas (27).
How to Win the War?
Emphasize Prevention and Global Research
Given the ever-growing and worldwide burden of diabetes, driven by high incidence and high numbers of people with complications, emphasis on primary prevention is critical (Fig. 1). The emphasis on the prevention of type 2 diabetes requires two sets of strategies: 1) effective implementation of proven preventive interventions in people at high risk (i.e., IGT) in the immediate term and 2) aggressive long-term global collaboration and research to a) address gaps in knowledge in population-based primary prevention for improving diet and activity at the societal level and b) advance the understanding of differences in pathophysiology, especially in hitherto understudied but very large populations in the rapidly developing economies of the world.
Effective Implementation of Proven Preventive Interventions in People at High Risk
Strong evidence from a number of countries, highlighting the global nature of science today, demonstrates that lifestyle intervention and also metformin use in people with IGT can prevent or slow progression to diabetes (28–30). Evidence also exists that interventions applied to people with IGT are cost-effective and can reduce diabetes complications, such as cardiovascular mortality and retinopathy, and can improve quality of life (31).
Several barriers need to be overcome to effectively implement these preventive interventions for people with prediabetes at scale (22,32). First, despite the high prevalence of prediabetes (i.e., one of every three adults) in the U.S., almost 90% of people with prediabetes remain undetected (33). Improved detection of prediabetes followed by implementation of proven interventions for prevention in high-risk groups will slow the expansion of diabetes. Over the years, however, there has been considerable debate around the evidence for the benefits and costs of screening for prediabetes and diabetes (34,35). Screening policies have varied from the liberal position of the ADA (36) to a more restrictive policy of the United States Preventive Services Task Force (USPSTF) (37). Much new evidence has accumulated over the past decade, and current evidence tips the scale toward screening for hyperglycemia (31), which can also be viewed as a gateway to diabetes prevention and management (38). The USPSTF has recently issued a broader set of criteria for screening, which, while moving in the right direction, still does not address the issue of nonobese people with prediabetes (39).
Screening for prediabetes, however, is only the first step and needs to be coupled with strengthening of infrastructure and financing mechanisms to sustainably and effectively deliver interventions (22). A recent systematic review indicates that translation of diabetes prevention in real-life settings is feasible (40), and a variety of successful initiatives already exist to implement diabetes prevention nationally by using existing community or social resources and networks (41,42). Expanding such initiatives across the country is needed, and some international examples may also serve as useful models (43). Although lifestyle interventions should be the primary strategy for the prevention of diabetes among people with prediabetes, there are data on effectiveness and cost-effectiveness that also support the use of metformin in people at high risk (22,44). Yet, indication for metformin use in people with prediabetes to prevent diabetes incidence is still not approved, and use of metformin among those at high risk of diabetes is exceedingly low (45).
Although unequivocal evidence exists for prevention of diabetes in high-risk groups (i.e., IGT) and should be aggressively pursued in the immediate term, data suggest that the impact on diabetes prevalence from this approach alone would be rather modest and the U.S. diabetes prevalence would still be likely to increase dramatically over the next 20 years (46). Therefore, although focusing on implementing currently proven interventions in people with IGT is important in the short term, a longer-term strategy for effective prevention will need to go beyond this narrow high-risk group and address the major gaps in knowledge concerning improving diet and physical activity in the population at large. Broader diabetes prevention efforts will also need research to better understand the pathophysiology of diabetes, so that more tailored measures of prevention can be evolved for various subpopulations.
Aggressive Long-term Global Collaboration and Research
In today’s increasingly globalized world, driven by unprecedented movement of trade, capital, goods, people, ideas, and information, every challenge confronting comprehensive type 2 diabetes prevention is shared across the world in common, and solutions are also likely to require global collaboration. Clues from global health research can help our shared understanding of pathophysiology and cross-border sharing of best practices in prevention, management, and policy for diabetes.
Address Gaps in Knowledge in Population-Based Primary Prevention.
Population-based approaches aimed at improving diet and physical activity of the entire society to primarily prevent diabetes are clearly very appealing. However, few data are available regarding interventions to prevent diabetes in people with normoglycemia (22). Furthermore, multipronged community-based strategies that have been tested at the population level for preventing diabetes have thus far not demonstrated encouraging results (47). There are major impediments to strategies that can improve healthy lifestyles at the societal level. For example, despite knowing that adequate fruit and vegetable intake is recommended, we cannot implement targets because the global supply does not meet the demand, and analysis of data indicates that globally there is, on average, a 22% shortage in the supply of fruits and vegetables to meet this demand based on a recommended intake of at least five portions each day. Furthermore, this shortage is as high as 58% in low-income countries (48). We therefore need more research in order to know what to do to improve the supply of healthy foods and to make it affordable.
It is now widely recognized that type 2 diabetes is occurring with worrying frequency at younger ages, especially affecting large numbers of people in low- and middle-income countries. First noted in the late 1990s as an emerging public health problem (49,50), data from investigations, such as the SEARCH for Diabetes in Youth (SEARCH) study, have now carefully documented the prevalence and incidence of youth-onset type 2 diabetes and highlighted risk factors for it, notably, ethnicity (e.g., Native Americans, Asian Pacific Islanders) (51) and childhood obesity (52,53). A study of a nationally representative sample of over 7,000 schoolchildren in the U.S., followed from ages 5 to 14 with serial objective measures of anthropometry, showed that by age 5, 14.9% of children were already overweight and 12.5% were obese (54). Importantly, although prevalence of obesity increased with age, incidence decreased with age and was highest at younger ages. Furthermore, incidence of obesity from ages 5 to 14 was four times higher in children who were overweight at age 5 compared with those who were normal weight at that age. In addition, a large randomized controlled trial of a comprehensive school-based program found no difference in risk of obesity between intervention and control schools (55). These data taken together suggest that the antecedents of childhood obesity and thus of young-onset type 2 diabetes are likely established well before school age and that the window of opportunity for intervention is preschool or sooner, perhaps even in utero (54). However, we need a better and fuller understanding of the early-life factors that predispose individuals and subpopulations to obesity and type 2 diabetes, and global studies are likely to be of great value, as childhood obesity and young-onset type 2 diabetes are happening at quickening pace, especially in economically transforming low- and middle-income countries (56).
Advance the Understanding of Differences in Pathophysiology.
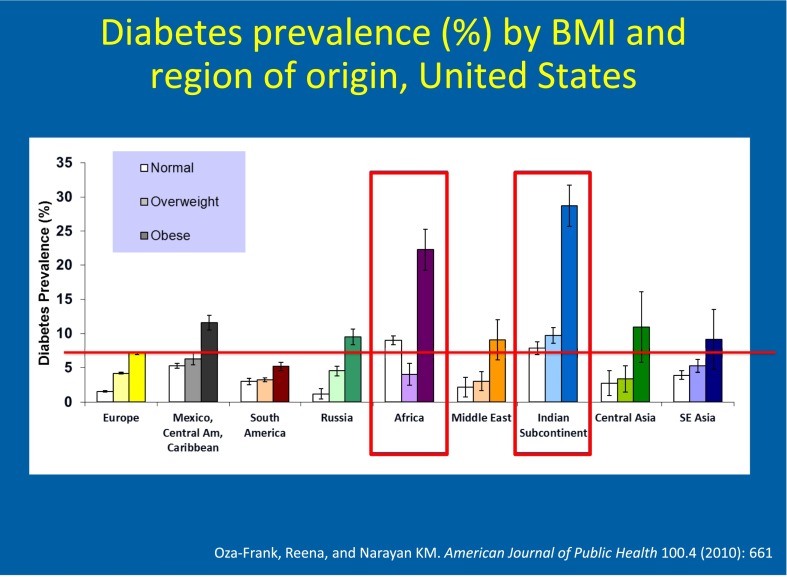
Viewing type 2 diabetes in its global context offers exciting and productive opportunities for a better understanding its complex pathophysiology. For example, people of South Asian descent, a population of nearly 1.7 billion people worldwide, are at extremely high risk for type 2 diabetes and have unique susceptibilities to the disease (57,58). The prevalence of diabetes in India has continued to rise dramatically over recent decades (59), and data now indicate a higher prevalence in Indians in urban India than in Indian migrants in the U.S. (60). Furthermore, among foreign-born people in the U.S., a fast-growing population of over 36 million, people from the Indian subcontinent have the highest diabetes prevalence among all immigrants to the country (61) (Fig. 5). South Asians have the highest diabetes prevalence among all ethnic groups in the U.S., other than Native Americans (62,63), and develop the disease at younger ages and at lower levels of BMI (61,64–66).
Figure 5.
Diabetes prevalence, by region of birth and BMI category: National Health Interview Survey. Reproduced with permission from Oza-Frank and Narayan (61).
South Asians also exhibit other unique features in terms of diabetes development. For example, South Asians were five to nine times more likely than other racial/ethnic groups to exhibit dysglycemia and dyslipidemia (characterized by low HDL cholesterol and high triglycerides), even at a healthy BMI (67). The South Asian population may offer unique opportunities to investigate the causes of type 2 diabetes and cardiometabolic metabolic diseases in people who are relatively thin, a phenomenon being reported in other populations either living in or whose ancestry is from parts of the world undergoing recent economic development (68,69). This concept of a unique South Asian phenotype has been described by others (70,71) and goes back several centuries. Diabetes was described in ancient times in the preinsulin era by the Egyptians, Arabs, Greeks, and Indians. A sixth-century Indian Ayurvedic textbook, Charaka Samhita, even identified two forms of diabetes: “There are two forms of diabetes (Madhu Meha or honey urine): one associated with emaciation, dehydration, polyuria, and lassitude, and the other with stout build, gluttony, obesity, and sleepiness” (72).
An important area for future investigation of phenotypes relevant to type 2 diabetes prevention and treatment is the role of innate or early problems with insulin secretion. In fact, some authors have recently called for a reclassification of diabetes with a greater β-cell–centric approach (73). Genetic studies add to this discourse and reveal two insights: 1) that there are only a few overlaps between genes identified for type 2 diabetes and those for obesity, thereby indicating that pathophysiological processes other than obesity and insulin resistance may be important in the development of diabetes (74), and 2) that the majority of type 2 diabetes genes seem to be related to the β-cell rather than to insulin resistance. South Asians may be a good population group to investigate the role of β-cell dysfunction in pathogenic progression of type 2 diabetes.
Population-based data comparing U.S. Pima Indians and Asian Indians from Chennai, India, have revealed several intriguing findings, notably, the relatively poorer insulin secretion at baseline and 30 and 120 min after an oral glucose tolerance test and the relatively higher proportion of isolated IFG (Table 1). As isolated IFG is primarily the result of hepatic insulin resistance, with early-phase, stationary impairment in β-cell function (75), and is diagnosed as a fasting plasma glucose ≥5.6 mmol/L and <7.0 mmol/L (76), these comparisons indicate that these two high prevalence groups may have very different pathways to developing diabetes. The Asian Indian type 2 diabetes phenotype may involve greater innate insulin secretion problems, both in the fasting state and in response to glucose challenges. This has implications for prevention and treatment. In a trial of a stepwise strategy of lifestyle intervention and metformin, when required, among South Asians with prediabetes, there was an overall relative risk reduction of 32% of progression to diabetes. Importantly, in the group with isolated IFG, the relative risk reduction was a meager 12% (77). Similarly, a study from Japan has also shown a null effect of lifestyle intervention among Japanese people with isolated IFG (78). The results of these studies indicate that measures to improve insulin action may have limited effect in people whose primary problem may be with insulin secretion and that other types of interventions may be needed in these populations.
Table 1.
Comparison of diabetes in Pima Indians and Asian Indians
| Pima Indians | Asian Indians (Chennai, India) | |
|---|---|---|
| Prevalence of diabetes* | Very high (50% by age 55 years) | Very high (50% by age 55 years) |
| Obesity profile | Very obese (mean BMI 33.7 kg/m2, mean waist circumference 108.6 cm) | Relatively thinner (mean BMI 25.7 kg/m2, mean waist circumference 83.1 cm) |
| Glucose profile | Higher mean 2-h plasma glucose | Higher mean fasting plasma glucose |
| Prediabetes distribution | Nearly two-thirds of prediabetes is isolated IGT | Over two-thirds of prediabetes is isolated IFG |
| Insulin resistance vs. secretion | 2–4.5 times more insulin resistant than Asian Indians across BMI and glucose strata | One-half to one-third of insulin secretion in Pima Indians across BMI and glucose strata |
Data from Gila River Indian community in Arizona cohort (96), data from the National Health and Nutrition Examination Survey (NHANES) and Centre for Cardiometabolic Risk Reduction in South Asia (CARRS) surveillance study (U. Gujral, personal communication), and comparisons between the Gila River Indian community in Arizona and the CARRS surveillance study (97).
Other investigations also lend support to the possibility that β-cell function may be declining very early in the natural history among South Asians. For example, in a cross-sectional study of South Asian Indians with different glycemic status, β-cell function was reduced in those with even mild dysglycemia (e.g., fasting glucose 100–126 mg/dL and/or 2-h glucose 140–199 mg/dL) regardless of age, adiposity, insulin sensitivity, or family history (79). β-Cell function was also more strongly associated with prediabetes and type 2 diabetes than with insulin resistance in young adults in Chennai, India, and in a cohort of South Asian Indian migrants in the U.S. (80,81). Comparisons between South Asians and other racial/ethnic groups have also been informative. For example, an analysis from the Whitehall II cohort study in the U.K. suggested that South Asians may have a poorer β-cell reserve relative to Europeans (82), and a study in the U.S. showed South Asians had poorer β-cell function compared with whites, blacks, Hispanics, and Chinese Americans (83). A study in the Netherlands suggested that family members of South Asians with type 2 diabetes may have poorer β-cell adaptation than similar Dutch individuals (84). Although informative, most of the published data suggesting that South Asian populations may have susceptibility for poor β-cell function are from cross-sectional studies, and investments in longitudinal multiethnic studies are needed to establish causality and mechanism.
The role of early β-cell dysfunction in the pathophysiology of diabetes may be especially important in populations or subgroups where prevalence of type 2 diabetes is high at low BMIs, a phenomenon that is quite common in populations of emerging economies, where nutrition transitions are overlapping with centuries of undernutrition (68,69). For example, in a population-based study in Chennai, India, approximately 20% of people with BMI <18.5 kg/m2 had type 2 diabetes (U. Gujral, personal communication) and overall diabetes prevalence was high, even though more than two-thirds of men and half of women had BMI <25 kg/m2. Intriguingly, historical data suggest that the South Asian population may have had good nutrition status in the Mesolithic period, as indicated by their tallness (85), and that the population may have been growing undernourished for generations. This may point to the role of maternal and/or early childhood nutrition, as the window of maximal opportunity for height, a marker of maternal and early nutrition and possibly of metabolic capacity, may be in the first 3 years of life (86,87). A number of studies also support the role of transgenerational metabolic pathways linking early malnourishment with diabetes risk (88,89).
Time for New Classification of Type 2 Diabetes?
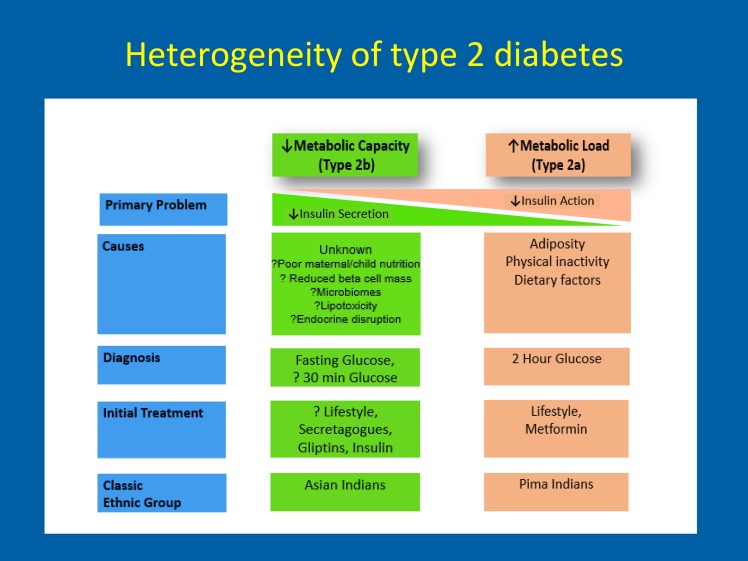
Although both insulin resistance and impaired insulin secretion are important in the pathophysiology of type 2 diabetes, studies in populations, such as South Asians, especially in comparison with groups such as the Pima Indians, raise the question of whether it is time to seriously consider heterogeneity within type 2 diabetes development and diagnosis. In particular, could there be different phenotypic presentations of type 2 diabetes described by the relative roles of insulin resistance and β-cell function? Is it time to consider the pathophysiology of type 2 diabetes across the spectrum, from those dominantly affected by insulin resistance (type 2A) to those dominantly affected by insulin secretion (type 2B)? For example, the traditional and well-studied phenotype of type 2 diabetes, mainly a manifestation of increased metabolic load, defined largely by impaired insulin action, and driven by adiposity, physical inactivity, and adult dietary factors, may be a distinct type (Fig. 6), whereas the far lesser studied phenotype, characterized by reduced metabolic capacity and impaired β-cell function and driven by hitherto incompletely understood factors, such as maternal and child nutrition, reduced β-cell mass, microbiomes, and endocrine disruptors, may be another. Studies across global populations may shed further insights into type 2 diabetes phenotypes and may point to differences in etiology, pathophysiology, diagnosis, prevention, and treatment by diabetes subtypes.
Figure 6.
Heterogeneity of type 2 diabetes: a hypothesis.
Conclusions
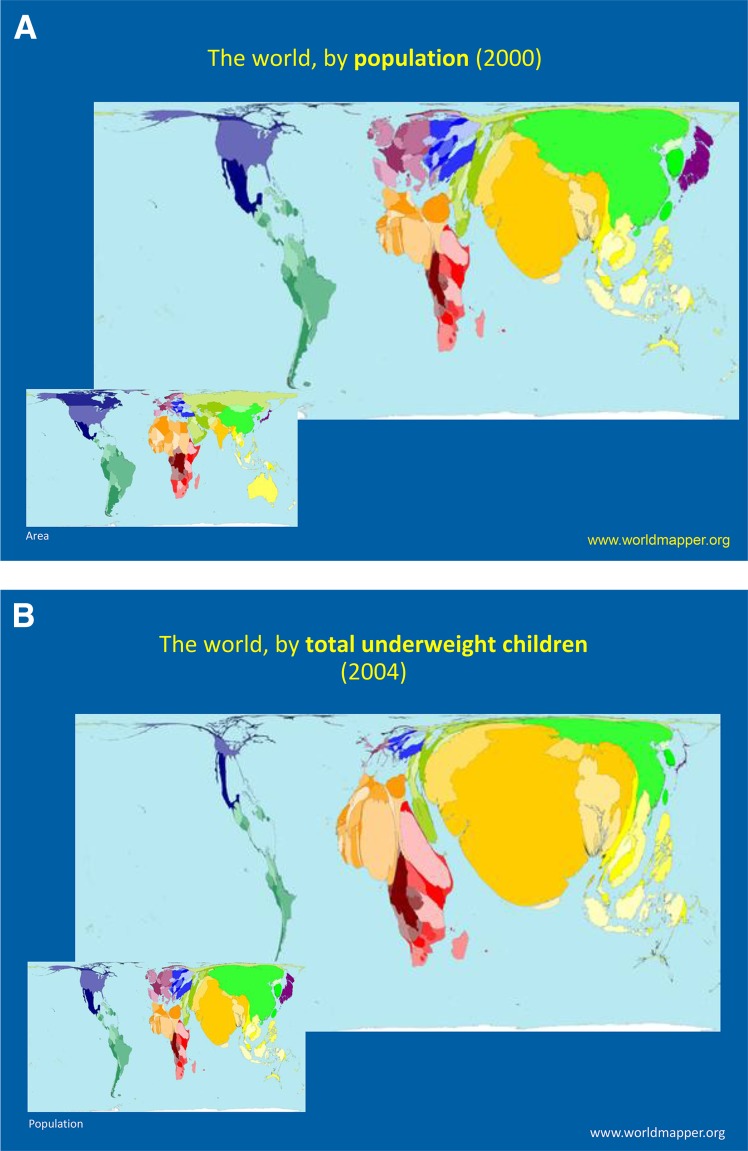
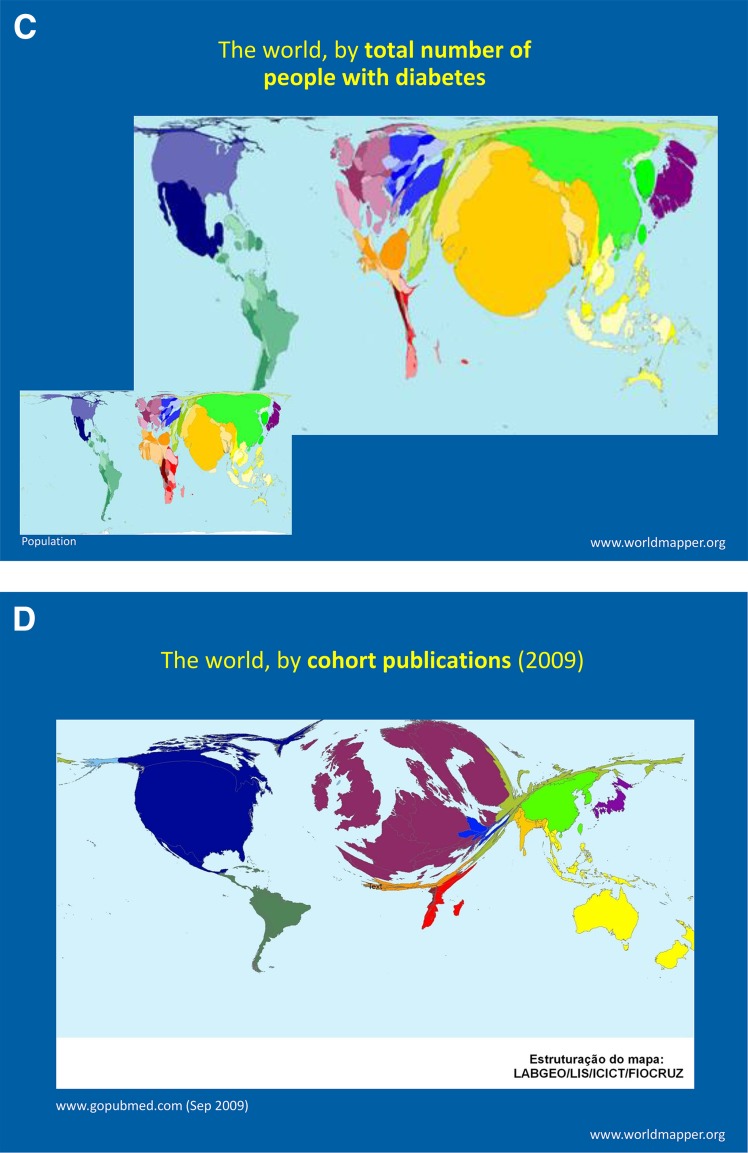
The reductions in complication rates among people with type 2 diabetes in the U.S. and in high-income countries are encouraging, but the war against diabetes needs a new era of informed prevention through expanded global collaboration in research and policy. Although the global burden of type 2 diabetes points to the need for aggressive global collaboration in science to advance prevention and treatment, there is currently a huge mismatch between where the diabetes burdens reside globally and where research is concentrated (Fig. 7). Correcting this imbalance will be important, and research into type 2 diabetes and related noncommunicable diseases in low- and middle-income countries offers huge scientific opportunities (90). Perhaps there are lessons to be learned for type 2 diabetes and other noncommunicable diseases from how global collaborations in science and policy have helped with the fight against HIV and AIDS (91,92). We are now in a highly interconnected global world and all problems—whether it is climate change, water security, HIV, or Ebola—cut across national borders in ways unimaginable, and efficient and effective solutions need expanded global engagement in science (93). The war against type 2 diabetes is no exception and cannot be comprehensively won without the investment in collaborative global diabetes research.
Figure 7.
World map sized to relative proportion of country population (A), prevalence of underweight in children (B), diabetes prevalence (C), and birth cohorts (D) available for research (95).
Article Information
Acknowledgments. K.M.V.N. especially thanks Dr. Peter Bennett for inspiring him into diabetes epidemiology and Dr. William Knowler for teaching him the rigors of science and is grateful to the innumerable colleagues at the Phoenix Epidemiology and Clinical Research Branch at the NIDDK, at the Division of Diabetes Translation at the CDC, in the several national and international multicenter studies he has had the privilege to be involved with, and at the Emory Global Diabetes Research Center. K.M.V.N. also thanks Mohammed Ali, Lisa Staimez, Shivani Patel, Mary Beth Weber, Unjali Gujral, Samrawit Yisahak, and Mark Hutcheson for their valuable comments on the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1.Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2014 [Internet]. Available from http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed 23 January 2016
- 2.Gregg EW, Li Y, Wang J, et al. . Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–1523 [DOI] [PubMed] [Google Scholar]
- 3.Gregg EW, Cheng YJ, Saydah S, et al. . Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey. Diabetes Care 2012;35:1252–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennon B, Leese GP, Cochrane L, et al. . Reduced incidence of lower-extremity amputations in people with diabetes in Scotland: a nationwide study. Diabetes Care 2012;35:2588–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikonen TS, Sund R, Venermo M, Winell K. Fewer major amputations among individuals with diabetes in Finland in 1997-2007: a population-based study. Diabetes Care 2010;33:2598–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narayan KM, Benjamin E, Gregg EW, Norris SL, Engelgau MM. Diabetes translation research: where are we and where do we want to be? Ann Intern Med 2004;140:958–963 [DOI] [PubMed] [Google Scholar]
- 7.Narayan KM, Gregg EW, Engelgau MM, et al. . Translation research for chronic disease: the case of diabetes. Diabetes Care 2000;23:1794–1798 [DOI] [PubMed] [Google Scholar]
- 8.Garfield SA, Malozowski S, Chin MH, et al.; Diabetes Mellitus Interagency Coordinating Committee (DIMCC) Translation Conference Working Group . Considerations for diabetes translational research in real-world settings. Diabetes Care 2003;26:2670–2674 [DOI] [PubMed] [Google Scholar]
- 9.TRIAD Study Group Health systems, patients factors, and quality of care for diabetes: a synthesis of findings from the TRIAD study. Diabetes Care 2010;33:940–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr EA, Gerzoff RB, Krein SL, et al. . Diabetes care quality in the Veterans Affairs Health Care System and commercial managed care: the TRIAD study. Ann Intern Med 2004;141:272–281 [DOI] [PubMed] [Google Scholar]
- 11.Mangione CM, Gerzoff RB, Williamson DF, et al.; TRIAD Study Group . The association between quality of care and the intensity of diabetes disease management programs. Ann Intern Med 2006;145:107–116 [DOI] [PubMed] [Google Scholar]
- 12.Schmittdiel JA, Uratsu CS, Karter AJ, et al. . Why don’t diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med 2008;23:588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ettner SL, Thompson TJ, Stevens MR, et al.; TRIAD Study Group . Are physician reimbursement strategies associated with processes of care and patient satisfaction for patients with diabetes in managed care? Health Serv Res 2006;41:1221–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karter AJ, Stevens MR, Herman WH, et al.; Translating Research Into Action for Diabetes Study Group . Out-of-pocket costs and diabetes preventive services: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care 2003;26:2294–2299 [DOI] [PubMed] [Google Scholar]
- 15.Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med 2002;136:565–574 [DOI] [PubMed] [Google Scholar]
- 16.Saaddine JB, Cadwell B, Gregg EW, et al. . Improvements in diabetes processes of care and intermediate outcomes: United States, 1988-2002. Ann Intern Med 2006;144:465–474 [DOI] [PubMed] [Google Scholar]
- 17.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–1624 [DOI] [PubMed] [Google Scholar]
- 18.Geiss LS, Wang J, Cheng YJ, et al. . Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA 2014;312:1218–1226 [DOI] [PubMed] [Google Scholar]
- 19.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–1890 [DOI] [PubMed] [Google Scholar]
- 20.Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KM, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985-2011: a modelling study. Lancet Diabetes Endocrinol 2014;2:867–874 [DOI] [PubMed] [Google Scholar]
- 21.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005-2050. Diabetes Care 2006;29:2114–2116 [DOI] [PubMed] [Google Scholar]
- 22.Narayan KM, Williamson DF. Prevention of type 2 diabetes: risk status, clinic, and community. J Gen Intern Med 2010;25:154–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Diabetes Association Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:596–615 [DOI] [PubMed] [Google Scholar]
- 24.Thorpe KE, Florence CS, Howard DH, Joski P. The impact of obesity on rising medical spending. Health Aff (Millwood) 2004;23(Suppl.):W4-480–4-486 [DOI] [PubMed] [Google Scholar]
- 25.International Diabetes Federation IDF Diabetes Atlas. 7th ed Brussels, Belgium: International Diabetes Federation, 2015 [Google Scholar]
- 26.Hwang CK, Han PV, Zabetian A, Ali MK, Narayan KM. Rural diabetes prevalence quintuples over twenty-five years in low- and middle-income countries: a systematic review and meta-analysis. Diabetes Res Clin Pract 2012;96:271–285 [DOI] [PubMed] [Google Scholar]
- 27.International Diabetes Federation IDF Diabetes Atlas. 6th ed. Brussels, Belgium, International Diabetes Federation, 2013 [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuomilehto J, Lindström J, Eriksson JG, et al.; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 30.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V; Indian Diabetes Prevention Programme (IDPP) . The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 31.Narayan KM, Gujral UP. Evidence tips the scale toward screening for hyperglycemia. Diabetes Care 2015;38:1399–1401 [DOI] [PubMed] [Google Scholar]
- 32.Fradkin JE, Roberts BT, Rodgers GP. What’s preventing us from preventing type 2 diabetes? N Engl J Med 2012;367:1177–1179 [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention (CDC) Awareness of prediabetes--United States, 2005-2010. MMWR Morb Mortal Wkly Rep 2013;62:209–212 [PMC free article] [PubMed] [Google Scholar]
- 34.Engelgau MM, Narayan KM, Herman WH. Screening for type 2 diabetes. Diabetes Care 2000;23:1563–1580 [DOI] [PubMed] [Google Scholar]
- 35.Echouffo-Tcheugui JB, Ali MK, Griffin SJ, Narayan KM. Screening for type 2 diabetes and dysglycemia. Epidemiol Rev 2011;33:63–87 [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Preventive Services Task Force Screening for type 2 diabetes mellitus in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2008;148:846–854 [DOI] [PubMed] [Google Scholar]
- 38.Narayan KM, Weber MB. Screening for hyperglycemia: the gateway to diabetes prevention and management for all Americans. Ann Intern Med 2015;162:795–796 [DOI] [PubMed] [Google Scholar]
- 39.Siu AL; U.S. Preventive Services Task Force . Screening for abnormal blood glucose and type 2 diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2015;163:861–868 [DOI] [PubMed] [Google Scholar]
- 40.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31:67–75 [DOI] [PubMed] [Google Scholar]
- 41.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med 2008;35:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sattin RW, Williams LB, Dias J, et al. . Community trial of a faith-based lifestyle intervention to prevent diabetes among African-Americans. J Community Health 2016;41:87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saaristo T, Moilanen L, Korpi-Hyövälti E, et al. . Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010;33:2146–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatterjee R, Narayan KM, Lipscomb J, Phillips LS. Screening adults for pre-diabetes and diabetes may be cost-saving. Diabetes Care 2010;33:1484–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moin T, Li J, Duru OK, et al. . Metformin prescription for insured adults with prediabetes from 2010 to 2012: a retrospective cohort study. Ann Intern Med 2015;162:542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gregg EW, Boyle JP, Thompson TJ, Barker LE, Albright AL, Williamson DF. Modeling the impact of prevention policies on future diabetes prevalence in the United States: 2010-2030. Popul Health Metr 2013;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satterfield DW, Volansky M, Caspersen CJ, et al. . Community-based lifestyle interventions to prevent type 2 diabetes. Diabetes Care 2003;26:2643–2652 [DOI] [PubMed] [Google Scholar]
- 48.Siegel KR, Ali MK, Srinivasiah A, Nugent RA, Narayan KM. Do we produce enough fruits and vegetables to meet global health need? PLoS ONE 2014;9:e104059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. . Type 2 diabetes among North adolescents: an epidemiologic review and a public health perspective. J Pediatr 2000;136:664–672 [DOI] [PubMed] [Google Scholar]
- 50.Narayan KM, Fagot-Campagna A, Imperatore G. Type 2 diabetes in children: a problem lurking for India? Indian Pediatr 2001;38:701–704 [PubMed] [Google Scholar]
- 51.Dabelea D, Bell RA, D’Agostino RB Jr, et al.; Writing Group for the SEARCH for Diabetes in Youth Study Group . Incidence of diabetes in youth in the United States. JAMA 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 52.Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics 2005;116:473–480 [DOI] [PubMed] [Google Scholar]
- 53.Kaufman FR. Type 2 diabetes mellitus in children and youth: a new epidemic. J Pediatr Endocrinol Metab 2002;15(Suppl. 2):737–744 [DOI] [PubMed] [Google Scholar]
- 54.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med 2014;370:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foster GD, Linder B, Baranowski T, et al.; HEALTHY Study Group . A school-based intervention for diabetes risk reduction. N Engl J Med 2010;363:443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lobstein T, Jackson-Leach R, Moodie ML, et al. . Child and adolescent obesity: part of a bigger picture. Lancet 2015;385:2510–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gujral UP, Pradeepa R, Weber MB, Narayan KM, Mohan V. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci 2013;1281:51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sattar N, Gill JM. Type 2 diabetes in migrant south Asians: mechanisms, mitigation, and management. Lancet Diabetes Endocrinol 2015;3:1004–1016 [DOI] [PubMed] [Google Scholar]
- 59.Mohan V, Deepa M, Deepa R, et al. . Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban South India--the Chennai Urban Rural Epidemiology Study (CURES-17). Diabetologia 2006;49:1175–1178 [DOI] [PubMed] [Google Scholar]
- 60.Gujral UP, Narayan KM, Pradeepa RG, et al. . Comparing type 2 diabetes, prediabetes, and their associated risk factors in Asian Indians in India and in the U.S.: the CARRS and MASALA Studies. Diabetes Care 2015;38:1312–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oza-Frank R, Narayan KM. Overweight and diabetes prevalence among US immigrants. Am J Public Health 2010;100:661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venkataraman R, Nanda NC, Baweja G, Parikh N, Bhatia V. Prevalence of diabetes mellitus and related conditions in Asian Indians living in the United States. Am J Cardiol 2004;94:977–980 [DOI] [PubMed] [Google Scholar]
- 63.Lee JWR, Brancati FL, Yeh H-C. Trends in the prevalence of type 2 diabetes in Asians versus whites: results from the United States National Health Interview Survey, 1997-2008. Diabetes Care 2011;34:353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oza-Frank R, Ali MK, Vaccarino V, Narayan KM. Asian Americans: diabetes prevalence across U.S. and World Health Organization weight classifications. Diabetes Care 2009;32:1644–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mather HM, Keen H. The Southall Diabetes Survey: prevalence of known diabetes in Asians and Europeans. Br Med J (Clin Res Ed) 1985;291:1081–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Creatore MI, Moineddin R, Booth G, et al. . Age- and sex-related prevalence of diabetes mellitus among immigrants to Ontario, Canada. CMAJ 2010;182:781–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel S, Shivashankar R, Ali MK, et al. . Is the “South Asian phenotype” unique to South Asians? Comparing cardiometabolic risk factors in the CARRS and NHANES studies. Glob Heart. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maskarinec G, Grandinetti A, Matsuura G, et al. . Diabetes prevalence and body mass index differ by ethnicity: the Multiethnic Cohort. Ethn Dis 2009;19:49–55 [PMC free article] [PubMed] [Google Scholar]
- 69.Chan WB, Tong PCY, Chow CC, et al. . The associations of body mass index, C-peptide and metabolic status in Chinese type 2 diabetic patients. Diabet Med 2004;21:349–353 [DOI] [PubMed] [Google Scholar]
- 70.Unnikrishnan R, Anjana RM, Mohan V. Diabetes in South Asians: is the phenotype different? Diabetes 2014;63:53–55 [DOI] [PubMed] [Google Scholar]
- 71.Enas EA, Mohan V, Deepa M, Farooq S, Pazhoor S, Chennikkara H. The metabolic syndrome and dyslipidemia among Asian Indians: a population with high rates of diabetes and premature coronary artery disease. J Cardiometab Syndr 2007;2:267–275 [DOI] [PubMed] [Google Scholar]
- 72.MacFarlane I, Bliss M, Jackson J, Williams G. Diabetes in its historical and social context. In Textbook of Diabetes. 2nd ed. Hoboken, NJ, Blackwell Scientific Publications, 1997 [Google Scholar]
- 73.Schwartz SS, Epstein S, Corkey BE, Grant SFA, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes: rationale and implications of the β-cell-centric classification schema. Diabetes Care 2016;39:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med 2010;363:2339–2350 [DOI] [PubMed] [Google Scholar]
- 75.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29:1130–1139 [DOI] [PubMed] [Google Scholar]
- 76.Nathan DM, Davidson MB, DeFronzo RA, et al.; American Diabetes Association . Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753–759 [DOI] [PubMed] [Google Scholar]
- 77.Weber MB, Harish R, Staimez LR, et al. . Reduction in diabetes incidence differs by prediabetes type in a randomized translational trial of prevention [Abstract]. Diabetes 2015;64(Suppl. 1A):LB46 [Google Scholar]
- 78.Saito T, Watanabe M, Nishida J, et al.; Zensharen Study for Prevention of Lifestyle Diseases Group . Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352–1360 [DOI] [PubMed] [Google Scholar]
- 79.Staimez LR, Weber MB, Ranjani H, et al. . Evidence of reduced β-cell function in Asian Indians with mild dysglycemia. Diabetes Care 2013;36:2772–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohan V, Amutha A, Ranjani H, et al. . Associations of β-cell function and insulin resistance with youth-onset type 2 diabetes and prediabetes among Asian Indians. Diabetes Technol Ther 2013;15:315–322 [DOI] [PubMed] [Google Scholar]
- 81.Gujral UP, Narayan KM, Kahn SE, Kanaya AM. The relative associations of β-cell function and insulin sensitivity with glycemic status and incident glycemic progression in migrant Asian Indians in the United States: the MASALA study. J Diabetes Complications 2014;28:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ikehara S, Tabák AG, Akbaraly TN, et al. . Age trajectories of glycaemic traits in non-diabetic South Asian and white individuals: the Whitehall II cohort study. Diabetologia 2015;58:534–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kanaya AM, Herrington D, Vittinghoff E, et al. . Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care 2014;37:1621–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jainandunsing S, Özcan B, Rietveld T, et al. . Failing beta-cell adaptation in South Asian families with a high risk of type 2 diabetes. Acta Diabetol 2015;52:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lukacs JR, Pal JN. Skeletal variation among Mesolithic people of the Ganga Plains: new evidence of habitual activity and adaptation to climate. Asian Perspect 2003;42:329–351 [Google Scholar]
- 86.Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:e473–480 [DOI] [PubMed] [Google Scholar]
- 87.Stein AD, Wang M, Martorell R, et al.; Cohorts Group . Growth patterns in early childhood and final attained stature: data from five birth cohorts from low-and middle-income countries. Am J Hum Biol 2010;22:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanafi MY, Saleh MM, Saad MI, Abdelkhalek TM, Kamel MA. Transgenerational effects of obesity and malnourishment on diabetes risk in F2 generation. Mol Cell Biochem 2016;412:269–280 [DOI] [PubMed] [Google Scholar]
- 89.Hardikar AA, Satoor SN, Karandikar MS, et al. . Multigenerational undernutrition increases susceptibility to obesity and diabetes that is not reversed after dietary recuperation. Cell Metab 2015;22:312–319 [DOI] [PubMed] [Google Scholar]
- 90.Jaacks LM, Ali MK, Bartlett J, et al. . Global noncommunicable disease research: opportunities and challenges. Ann Intern Med 2015;163:712–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Narayan KM, Ali MK, Koplan JP. Global noncommunicable diseases--where worlds meet. N Engl J Med 2010;363:1196–1198 [DOI] [PubMed] [Google Scholar]
- 92.Narayan KM, Ali MK, del Rio C, Koplan JP, Curran J. Global noncommunicable diseases--lessons from the HIV-AIDS experience. N Engl J Med 2011;365:876–878 [DOI] [PubMed] [Google Scholar]
- 93.Richmond G. Global science engagement. Science 2016;351:427. [DOI] [PubMed] [Google Scholar]
- 94.Centers for Disease Control and Prevention Data and statistics, diabetes [Internet]. Available from http://www.cdc.gov/diabetes/data/. Accessed 16 February 2016
- 95.Worldmapper. The world as you’ve never seen it before [Internet]. Available from http://www.worldmapper.org/. Accessed 25 January 2016
- 96.Pavkov ME, Hanson RL, Knowler WC, Bennett PH, Krakoff J, Nelson RG. Changing patterns of type 2 diabetes incidence among Pima Indians. Diabetes Care 2007;30:1758–1763 [DOI] [PubMed] [Google Scholar]
- 97.Staimez LR, Deepa M, Ali MK, Mohan V, Narayan KM, Hanson RL. The tale of two Indians: a comparison of beta-cell function and insulin resistance between Pima Indians and Asian Indians [Abstract]. Diabetes 2014;63(Suppl. 1):A400 [Google Scholar]