Abstract
OBJECTIVE
Early initiation of intensive diabetes therapy aimed at achieving near-normal glycemia reduces the early development of vascular complications in type 1 diabetes. We now assess whether intensive therapy compared with conventional therapy during the Diabetes Control and Complications Trial (DCCT) affected the incidence of cardiovascular disease over 30 years of follow-up.
RESEARCH DESIGN AND METHODS
The DCCT randomly assigned 1,441 patients with type 1 diabetes to intensive versus conventional therapy for a mean of 6.5 years, after which 93% were subsequently monitored during the observational Epidemiology of Diabetes Interventions and Complications (EDIC) study. Cardiovascular disease (nonfatal myocardial infarction and stroke, cardiovascular death, confirmed angina, congestive heart failure, and coronary artery revascularization) was adjudicated using standardized measures.
RESULTS
During 30 years of follow-up in DCCT and EDIC, 149 cardiovascular disease events occurred in 82 former intensive treatment group subjects versus 217 events in 102 former conventional treatment group subjects. Intensive therapy reduced the incidence of any cardiovascular disease by 30% (95% CI 7, 48; P = 0.016), and the incidence of major cardiovascular events (nonfatal myocardial infarction, stroke, or cardiovascular death) by 32% (95% CI −3, 56; P = 0.07). The lower HbA1c levels during the DCCT/EDIC statistically account for all of the observed treatment effect on cardiovascular disease risk. Increased albuminuria was also independently associated with cardiovascular disease risk.
CONCLUSIONS
Intensive diabetes therapy during the DCCT (6.5 years) has long-term beneficial effects on the incidence of cardiovascular disease in type 1 diabetes that persist for up to 30 years.
Introduction
Cardiovascular disease (CVD), the leading cause of death for men and women, is accelerated in type 1 diabetes (1–3). The effect of hyperglycemia on microvascular and macrovascular complications is well documented in longitudinal studies of patients with type 1 and type 2 diabetes (4–7). Early interventions designed to optimize glycemic control have been shown to reduce the development and progression of these complications (6,8,9). Participants in the Diabetes Control and Complications Trial (DCCT) with further follow-up in the Epidemiology of Diabetes Interventions and Complications (EDIC) study (DCCT/EDIC) demonstrated that intensive diabetes therapy aimed at near-normal blood glucose levels compared with conventional therapy during the DCCT reduced the subsequent development and progression of retinopathy, nephropathy, and neuropathy during EDIC (6,8,10). These beneficial effects from a period of intensive glycemic control persisted beyond the completion of the intervention trial and despite similar levels of glycemic control in the two former treatment groups during EDIC. We referred to this phenomenon as metabolic memory (11,12). In 2005, ∼10 years after the completion of the DCCT, we first reported that the period of initial intensive diabetes therapy also reduced the risk of developing CVD (i.e., nonfatal myocardial infarction and stroke, cardiovascular death, confirmed angina, and coronary artery revascularization) in EDIC, although the number of CVD events was relatively low (13).
In the long-term follow-up of the DCCT/EDIC study, with more than 2 decades of diabetes management that was no longer prescribed by study protocol, we have noted that the treatment group specific effects on microvascular complications, although declining, remain clinically significant (3,6,10). We now report the 30-year risk of CVD in the DCCT/EDIC cohort and evaluate treatment group differences and the association of HbA1c and albuminuria measures with this life-threatening complication of type 1 diabetes. We also examine the suggestion in the literature that HbA1c may relate more strongly to fatal rather than nonfatal CVD events (14–17).
Research Design and Methods
The methods of the DCCT and EDIC follow-up study have been described in detail (11,18–20). The DCCT (1983 and 1993) was designed to compare the effects of a randomly assigned intensive versus conventional diabetes treatment regimen on the development of microvascular complications.
Subjects
The DCCT randomized 1,441 volunteers with type 1 diabetes, age 13 to 39 years, to intensive (n = 711) versus conventional (n = 730) therapy. Participants with diabetes duration of 1 to 5 years without evidence of microvascular complications comprised the primary prevention cohort, and those with diabetes duration of 1 year up to 15 years with minimal retinopathy or nephropathy comprised the secondary cohort. Of these, 1,423 participants completed the study after a mean follow-up of 6.5 years. Subjects with a history of CVD or with hypertension (blood pressure ≥140/90 mmHg) or hypercholesterolemia (fasting serum cholesterol ≥3 SDs above age- and gender-specific means) were not eligible to participate (18). After the end of the DCCT, 1,394 of the surviving cohort (96% of the original DCCT cohort) joined the long-term EDIC follow-up study starting in 1994. Participants previously randomized to conventional therapy were taught intensive therapy, and ongoing care was returned to their usual health care team.
The current report includes follow-up through 31 December 2013, with 85.8% of the original cohort (93.2% of 1,327 surviving participants) remaining in the study (Supplementary Fig. 1). Apart from those who died or developed CVD, the remaining subjects participated in 96% of the possible follow-up period. DCCT/EDIC was approved by the institutional review boards of all participating centers, and all volunteers provided written informed consent.
Study Procedures
During the DCCT, HbA1c levels were measured quarterly, and fasting lipids, serum creatinine, albumin excretion rate (AER), and other CVD risk factors were measured annually in a central laboratory (18,20). Annual electrocardiograms were centrally read while masked to treatment assignment. DCCT methods were continued during EDIC follow-up; however, the frequency of assessment differed. HbA1c was measured annually in EDIC, and fasting lipids and renal function were measured in alternate years in EDIC (yearly in DCCT). The time-weighted mean HbA1c represented the total glycemic exposure during DCCT/EDIC with weights of 0.25 and 1 for quarterly DCCT and annual EDIC values, respectively, up to the measure immediately preceding the event or censoring for those without an event.
To be consistent with contemporary guidelines, sustained microalbuminuria was defined as an AER of at least 30 on two consecutive occasions and albuminuria as ≥300 mg/24 h, and each was counted as present if end-stage renal disease (ESRD) occurred. ESRD included kidney transplantation or dialysis (18). Kidney failure was defined as ESRD or the development of an estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration equation.
Diabetes Therapy
During the DCCT, intensive therapy consisted of three or more daily insulin injections or use of an external insulin infusion pump, with dose adjustments based on at least four self-monitored glucose measurements per day. Daily glucose goals were 70–120 mg/dL before meals and <180 mg/dL peak levels after meals. The HbA1c goal was a value of <6.05% (42.6 mmol/mol), 2 SDs above the nondiabetic mean. Conventional therapy used one or two daily injections of insulin and had no glucose goals beyond prevention of hyper- and hypoglycemia.
Outcomes
The primary outcome was the time to the first of any of the following types of cardiovascular events: nonfatal myocardial infarction or stroke; death judged to be secondary to CVD; subclinical (“silent”) myocardial infarction detected on an annual electrocardiogram (21); angina confirmed by ischemic changes with exercise tolerance testing or by clinically significant obstruction on coronary angiography; congestive heart failure (CHF) with paroxysmal nocturnal dyspnea, orthopnea, or marked limitation of physical activity caused by heart disease; or revascularization with angioplasty and/or coronary artery bypass (22).
The occurrence of any CVD event was routinely documented during annual participant study visits, with additional medical records sought to verify all self-reported events. To ensure complete capture of all known CVD events before year end, staff efforts were escalated in early 2013 to ensure full reporting of all known CVD events irrespective of completion of the annual visit. Medical records describing cardiovascular events, including electrocardiograms and cardiac enzymes, were obtained and centrally adjudicated by a Mortality and Morbidity Review Committee masked to DCCT treatment assignment, HbA1c, and glucose levels. Only events adjudicated as definite cardiovascular events were included in these analyses (21).
Statistical Analysis
Previous analyses of CVD events through year 11 of EDIC were conducted after 50 DCCT conventional treatment group subjects had experienced a cardiovascular event (13). The research group also prespecified that analyses of risk factors for CVD events would be embargoed until 100 such conventional cases were observed. That landmark was reached near the end of 2013. In this report we present updated analyses of the incidence of CVD through 31 December 2013, the differences between former DCCT treatment groups, and the association of CVD events with HbA1c and renal outcomes. Results nominally significant at P < 0.05 (two-sided) are cited.
Clinical characteristics were compared using Wilcoxon rank sum tests for quantitative variables and χ2 tests for categorical variables (23). The Kaplan-Meier method estimated the cumulative incidence of the first cardiovascular event (24). The Cox proportional hazards model estimated covariate effects (hazard ratio [HR] and 95% CI) on CVD risk that were tested using the Wald test (25). The corresponding risk reduction was calculated as 100 (1 − HR). The Kaplan-Meier estimate of the underlying incidence (hazard) function within groups was computed, and a smooth estimate was obtained using natural cubic splines with 2 degrees of freedom (26). Event rates, including all (i.e., multiple) events in all subjects, are presented as the number per 100 patient-years, and the difference was tested using robust Poisson regression analysis (24). Cox models also assessed the effects of time-dependent covariates on CVD risk such as the updated mean HbA1c.
Results
Cohort characteristics at DCCT baseline, DCCT close-out, year 10–11 of EDIC, and year 19–20 are described in Supplementary Table 1. Owing to exclusion criteria, no participants had hypertension or hypercholesterolemia at DCCT baseline, and only 5% had microalbuminuria. The only significant group difference was a minimally higher systolic blood pressure in the conventional treatment group. At DCCT study end, the conventional and intensive treatment groups had diverged with regard to several established and putative CVD risk factors (e.g., BMI and triglycerides) and differed in the levels of microvascular outcomes. By EDIC year 11, the prevalence of microalbuminuria and albuminuria remained higher in the former conventional treatment group. However, there were small differences between groups in other traditional CVD risk factors and a negligible difference in HbA1c. Additional differences emerged by year 20, albeit small (e.g., a lower LDL cholesterol in the conventional therapy group). The prevalence of cardioprotective medication use increased by year 11 and increased further by year 20, with no major differences in medication use or type between groups.
Mean HbA1c during the average 6.5 years of DCCT intensive therapy was ∼2% (20 mmol/mol) lower than that during conventional therapy (7.2 vs. 9.1% [55.6 vs. 75.9 mmol/mol], P < 0.001). Subsequently during EDIC, HbA1c differences between the treatment groups dissipated. At year 11 of EDIC follow-up and most recently at 19–20 years of EDIC follow-up, there was only a trivial difference between the original intensive and conventional treatment groups in the mean level of HbA1c (Supplementary Table 1). However, after a mean of 26 years of follow-up (maximum 30 years), differences in weighted mean HbA1c during the DCCT/EDIC follow-up remained significant between the former intensive and conventional treatment groups (weighted mean HbA1c of 7.8 ± 0.9 vs. 8.2 ± 0.9% [61.3 ± 10.0 vs. 66.3 ± 10.1 mmol/mol], respectively, P < 0.0001).
By year end 2013, 366 adjudicated cardiovascular events occurred in 184 subjects, 149 among 82 former intensive treatment group subjects and 217 among 102 former conventional treatment group subjects (Table 1). The event rates in the intensive and conventional treatment groups were 0.81 and 1.18 per 100 patient-years, respectively (P = 0.06). The rates of specific clinical events were also consistently lower in the original intensive treatment group, but not significantly owing to the smaller numbers of events.
Table 1.
Cardiovascular events in each original treatment group of the DCCT
| Event | Intensive-treatment group |
Conventional-treatment group |
||||||
|---|---|---|---|---|---|---|---|---|
| Patients* | Events† | Initial events‡ | Secondary events§ | Patients* | Events† | Initial events‡ | Secondary events§ | |
| n (%) | n | n | n | n (%) | n | n | n | |
| Any CVD events | 82 (11.5) | 149 | 82 | 67 | 102 (14.0) | 217 | 102 | 115 |
| 1. Nonfatal acute MI | 24 (3.4) | 26 | 19 | 7 | 29 (4.0) | 35 | 23 | 12 |
| 2. Nonfatal cerebrovascular event | 8 (1.1) | 9 | 7 | 2 | 12 (1.6) | 13 | 11 | 2 |
| 3. Death from CVD | 9 (1.3) | 9 | 5 | 4 | 16 (2.2) | 16 | 8 | 8 |
| 4. Silent MI | 20 (2.8) | 21 | 19 | 2 | 33 (4.5) | 36 | 24 | 12 |
| 5. Confirmed angina | 20 (2.8) | 20 | 11 | 9 | 22 (3.0) | 33 | 11 | 22 |
| 6. Revascularization | 40 (5.6) | 62 | 20 | 42 | 48 (6.6) | 71 | 22 | 49 |
| 7. CHF | 2 (0.3) | 2 | 1 | 1 | 10 (1.4) | 13 | 3 | 10 |
| MACE | 39 (5.5) | 44 | 39 | 5 | 49 (6.7) | 64 | 49 | 15 |
| 1. Nonfatal acute MI | 24 (3.4) | 26 | 24 | 2 | 29 (4.0) | 35 | 28 | 7 |
| 2. Nonfatal cerebrovascular event | 8 (1.1) | 9 | 8 | 1 | 12 (1.6) | 13 | 11 | 2 |
| 3. Death from CVD | 9 (1.3) | 9 | 7 | 2 | 16 (2.2) | 16 | 10 | 6 |
MI, myocardial infarction.
*Number of patients with each type of event, regardless of whether it is the initial event for that subject.
†The total number of events of each type in all subjects.
‡If a patient had multiple initial events on the same day, then only the most severe initial event is tabulated according to the following order of severity: nonfatal acute MI, CHF, revascularization, and confirmed angina.
§The number of additional events of other types experienced by the subjects with a specific event. For example, 24 subjects experienced a total of 26 acute nonfatal MIs, of which it was the initial event for 19 subjects and a subsequent (secondary) event for 7 subjects.
Overall, 115 of the 715 subjects (16.1%) in the secondary intervention cohort experienced 223 CVD events vs. 69 of 726 (9.5%) in the primary prevention cohort who experienced 143 events (HR 1.45; 95% CI 1.08, 1.96; P = 0.0143), and 96 of 761 males (12.6%) had 184 CVD events compared with 88 of 680 females (12.9%) who experienced 182 CVD events (HR 0.99; 95% CI 0.74, 1.32; P = 0.9337).
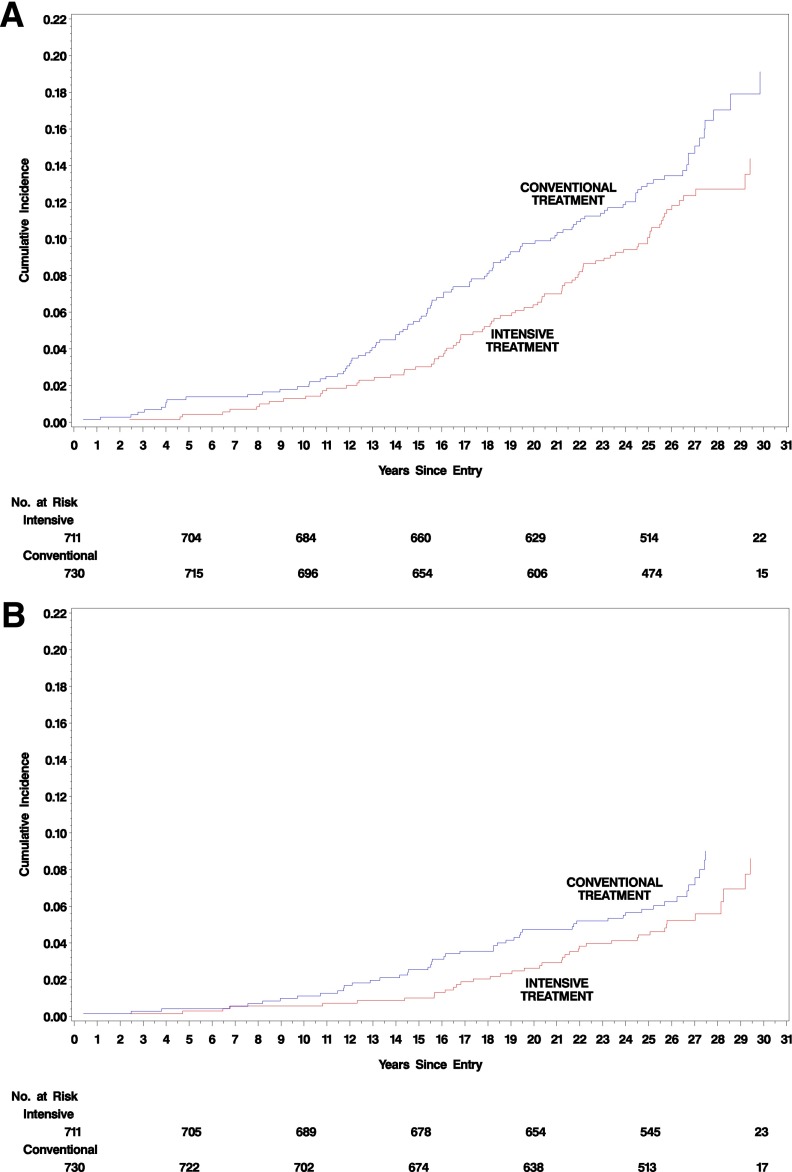
Figure 1A shows the Kaplan-Meier estimated cumulative incidence of the first occurrence of any cardiovascular event within each DCCT treatment group. The incidence within the original DCCT intensive group is 30% lower than that of the conventional group (95% CI 7, 48; P = 0.016). The incidence of the first occurrence of a nonfatal myocardial infarction, stroke, or cardiovascular death (major adverse cardiac events [MACE]) was reduced 32% with intensive compared with conventional therapy (95% CI −3, 56; P = 0.07) (Fig. 1B).
Figure 1.
Cumulative incidence of cardiovascular outcomes in the conventional treatment and intensive treatment groups during up to 30 years of DCCT/EDIC treatment and follow-up. A: The first of any of the predefined CVD outcomes. The risk reduction with intensive therapy was 30% (95% CI 7, 48; P = 0.016). B: The first occurrence of MACE. The risk reduction with intensive therapy was 32% (95% CI −3, 56; P = 0.07).
The effects on cardiovascular events (i.e., excluding stroke) were similar, with a 31% risk reduction in the former intensive group compared with the conventional group (95% CI 6, 49; P = 0.020). Furthermore, there is the suggestion of a greater reduction in the risk of less frequent fatal events with intensive versus conventional therapy (6 vs. 16, HR 0.33) than nonfatal events (71 vs. 85, HR 0.73).
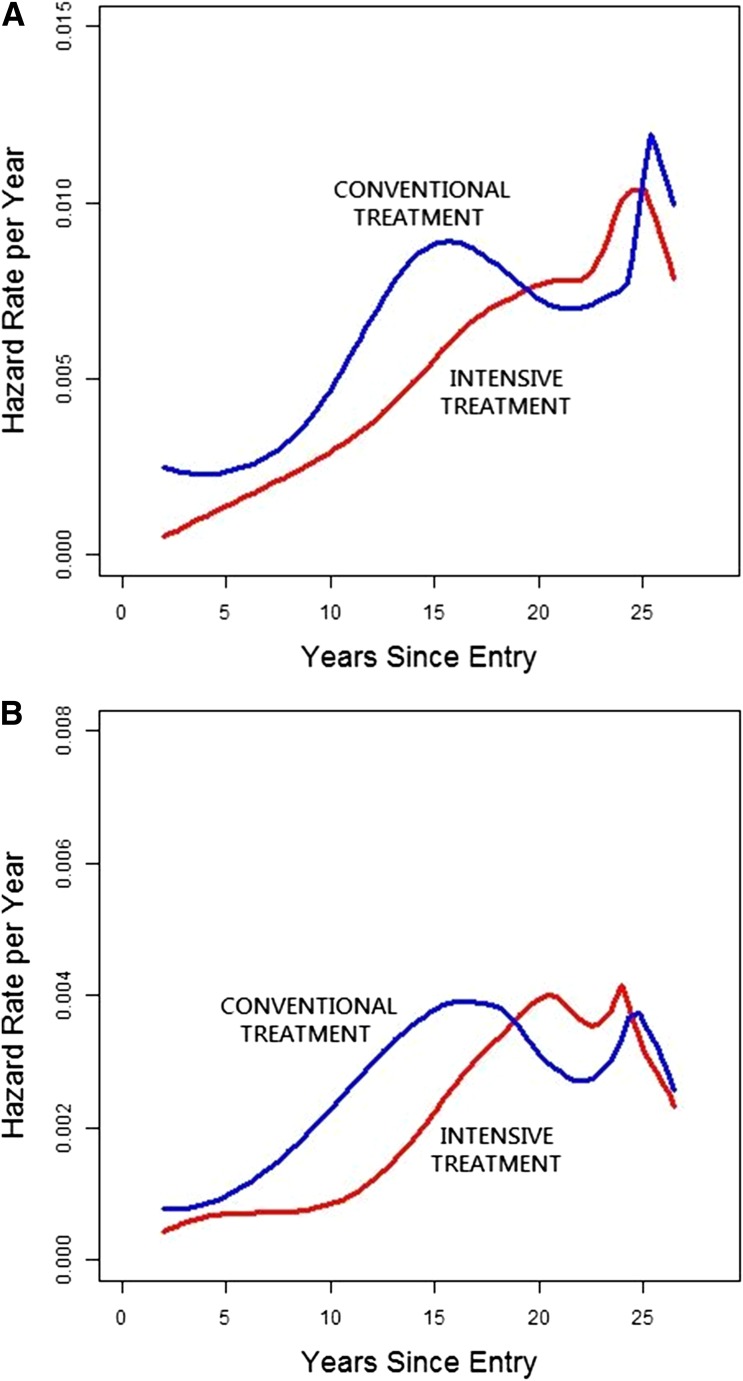
Figure 2 shows the underlying instantaneous (day-to-day) incidence (HR) functions. For any CVD and for MACE, the incidence function in the former conventional group was higher than that in the intensive group over the first ∼17 years of follow-up, but the curves became equal in later years. The estimates beyond 25 years, however, are imprecise because the number at risk declines due to the staggered entry of subjects into the DCCT during 1983–1989.
Figure 2.
Underlying HR function representing the risk of a new event among those remaining at risk at every point in time. Note that the number of subjects at risk declines beyond year 24 owing to staggered entry of subjects during 1983–1989. A: Any CVD. B: MACE.
Proportional hazards models, adjusted for selected baseline factors, also assessed the association of selected time-dependent covariates with CVD risk and the effect of DCCT treatment group before and after adjustment for each of these limited factors (Table 2). The HR of intensive versus conventional treatment, adjusted only for baseline factors, was 0.70 (P = 0.015). The five subjects with a history of kidney failure had a 4.8-fold risk of CVD than did those without antecedent kidney failure, but adjustment for kidney failure did not materially alter the treatment group effect, perhaps because of the small number of affected subjects. A history of sustained microalbuminuria (71 subjects) or albuminuria (27 subjects) was each significantly associated with about a twofold increased risk of CVD versus those without and explained a sufficient part of the treatment group effect to render it no longer significant (P = 0.10 and P = 0.06, respectively).
Table 2.
Proportional hazards models of the effect of time-dependent covariates on the risk of CVD and of the effect of the treatment group after adjustment for the time-dependent covariate
| Effect of time-dependent covariate* |
Treatment group adjusted for specific time-dependent covariate* |
|||
|---|---|---|---|---|
| Time-dependent covariate | HR (95% CI) | P value | HR (95% CI) | P value |
| None | — | — | 0.70 (0.52, 0.93) | 0.0151 |
| Sustained microalbuminuria (yes vs. no)‡ | 2.28 (1.67, 3.11) | <0.0001 | 0.78 (0.58, 1.05) | 0.1038 |
| Macroalbuminuria (yes vs. no)§ | 2.22 (1.46, 3.38) | 0.0002 | 0.75 (0.56, 1.01) | 0.0594 |
| Kidney failure (yes vs. no)¶ | 4.84 (1.93, 12.11) | 0.0008 | 0.71 (0.53, 0.95) | 0.0199 |
| Mean HbA1c during DCCT/EDIC# | ||||
| Per 10% increase | 1.35 (1.21, 1.51) | <0.0001 | 1.00 (0.72, 1.39) | 0.9843 |
| Per 10% decrease | 0.72 (0.63, 0.81) | |||
*Each of the above five models was also adjusted for the following DCCT baseline characteristics: HbA1c value, age, cholesterol level, and smoking status.
‡Sustained microalbuminuria was defined by a history of microalbuminuria (AER ≥30 mg/24 h) on at least two consecutive visits, or ESRD (dialysis or transplant).
§Macroalbuminuria was defined by a history of albuminuria (AER ≥300 mg/24 h) or ESRD.
¶Kidney failure was defined by a history of eGFR <15 mL/min/1.73 m2 or ESRD.
#The weighted mean HbA1c up to the time of each CVD event was calculated as the weighted mean of the quarterly values during DCCT plus the annual values during EDIC, weighted respectively by ¼ and 1 to reflect the interval between values during the DCCT and EDIC. The log mean HbA1c value was used so that the HR per c-fold change in risk is c3.17155, where 3.17155 is the estimated regression coefficient; a c of 1.1 corresponds to a 10% increase in the mean glycosylated hemoglobin value, and a c of 0.9 to a 10% decrease.
A 10% lower updated mean HbA1c during DCCT/EDIC (e.g., 8 vs. 7.2%) was associated with an HR of 0.72, representing a 28% reduction in the risk of a cardiovascular event (95% CI 19, 37; P < 0.0001). The updated log mean DCCT/EDIC HbA1c explained all of the treatment group effect on risk of CVD, the treatment group HR being exactly 1 and no longer significant (P = 0.99) after adjustment for HbA1c.
Conclusions
We have previously demonstrated the beneficial effects of an average of 6.5 years of intensive versus conventional diabetes therapy on the risk of any CVD and also on the standardized MACE outcome (13). This was based on analysis of events when at least 50 conventional subjects had experienced a CVD event (mean of 17 years of follow-up). The current analysis (mean of 26 years of follow-up) was performed after at least 100 cases (184 total events) in the former conventional group had occurred. The cumulative incidence curves for any CVD and MACE within the former intensive and conventional groups continue to demonstrate a treatment effect with a continuing difference in cumulative incidence over time. However, the risk reduction of any CVD with intensive therapy through 2013 is now less than that reported previously through 2004 (30% [P = 0.016] vs. 47% [P = 0.005]), and likewise, the risk reduction per 10% lower mean HbA1c through 2013 was also somewhat lower than previously reported but still highly statistically significant (17% [P = 0.0001] vs. 20% [P = 0.001]).
The cumulative incidence curves (Fig. 1A and B) are a function of past differences between former treatment groups that are perpetuated into the future and of differences in the day-to-day incidences (current risks) over time. The latter may be a more direct measure of the long-term metabolic memory effects. Plots of these incidence functions (Fig. 2A and B) for any CVD and MACE show that the incidences (risk of an event at each day) in both former treatment groups were increasing with time, that in the conventional group higher than intensive, and that beyond ∼17 years of follow-up, the incidences in the two groups were similar. As noted, the incidence estimates beyond 25 years are less precise owing to the declining numbers at risk. These suggest that the beneficial metabolic memory effect on the underlying incidence of any CVD and MACE may be diminishing with longer follow-up.
The treatment group effect for any CVD in the 2005 report through 20 years of follow-up was 42%, whereas through 30 years, it has declined to 30% (P = 0.016) (13). The actual decline may be greater because the latter estimate includes CHF, whereas the former does not, and there is now a fivefold difference in the few cases of CHF between treatment groups (2 intensive vs. 10 conventional cases). However, it is unlikely there were many heart failure events at the 2005 follow-up. Likewise, the risk reduction for MACE has declined from 57 to 32%, the latter no longer being statistically significant (P = 0.07). The difference in CHF between groups is of interest, but the numbers are too small to permit any definitive statement or analysis of additional, potentially confounding, factors.
Adjusting for the effects of microalbuminuria and albuminuria individually, both reduce the HR for the treatment group effect on any CVD such that it is no longer statistically significant (P = 0.10 and P = 0.06, respectively). A greater effect is apparent, however, when adjustment is made for the updated mean HbA1c from DCCT and EDIC combined, such that the HR becomes 1.00 (P = 0.98). It is thus likely that the better glycemia experienced during DCCT with intensive therapy has CVD benefits beyond the subsequent development of microalbuminuria.
Although the pathogenesis of coronary artery disease (CAD) in type 1 diabetes is still poorly understood and there are morphologic and risk factor differences, as reviewed elsewhere, atherosclerosis is clearly a slowly progressive state that starts in childhood and is likely to be accelerated in the presence of diabetes (16). This acceleration is likely to result from many pathways, including renal disease and lipid and blood pressure changes (16). We previously showed a lower incidence of hypertension in the former intensive therapy group (27) as well as decreased glucose-based atherosclerosis mechanisms, including glyco-oxidative stress (5), inflammation (28), and advanced glycosylated end-product formation (29). Thus this acceleration of atherosclerosis that is apparent in type 1 diabetes appears to have been reduced by a 6-year period of DCCT intensive therapy, a benefit that has continued to be evident many years later. This is consistent with the chronicity of the atherosclerotic process; as such, individuals will be subsequently progressing from a lower degree of glycemic damage. Thus, seeing a strong continuing—although somewhat reduced—treatment effect many years after the randomized treatment phase stopped (and after which both groups had similar glucose control) is consistent with the natural history of atherosclerosis itself and with the DCCT metabolic memory observations with all other complications (3,19).
The extent to which metabolic memory reflects delayed progression and the carrying forward of a reduced degree of glycemic damage or a long-term alteration of future risk remains unclear. Mechanistically, this may reflect a complex balance of alterations in tissue-destructive and potential protective factors. Another possible explanation for the equilibration of the current risks beyond year 17, principally due to a stabilization of the risk in the conventional group, is the possible “exhaustion of susceptible” subjects in the conventional group, more so than the intensive group, whereby subjects who are predisposed (genetically or otherwise) experience a CVD event early in follow-up, and those remaining at risk beyond some point in time are protected. This could be assessed if and when a suitable risk function based on genetic or other factors is established, which could be the subject of future reports. The current data support the benefit of early intensive therapy intervention in young adults with recent-onset type 1 diabetes, when the atherosclerotic process can be attenuated in its relatively early stage, but does not necessarily apply to subjects with older-onset type 2 diabetes with more advanced CVD. For instance, in the extended follow-up of the Veterans Affairs Diabetes Trial (VADT), although the time to the first major CVD event increased with intensive glycemic control, there was no significant difference in cardiovascular mortality (30). Similar to the inclusion criteria and study follow-up of the DCCT/EDIC study, the UK Prospective Diabetes Study (UKPDS), with its inclusion of younger patients with newly diagnosed diabetes and an absence of recent CVD events, demonstrated significant reductions in myocardial infarction and mortality in the former intensive treatment patient cohort, unlike the results of other studies of type 2 diabetes, including the VADT, involving older individuals with longer duration of diabetes and a greater incidence of preexisting CVD and/or CVD risk factors at baseline (7,30–32).
An interesting observation in DCCT/EDIC is the potentially stronger effect of intensive therapy on protecting against fatal (n = 25) rather than nonfatal CAD. This preliminary observation will require further confirmation when we have a larger number of fatal CAD events in the future. This was examined because of a suggestion in the literature that HbA1c may be a stronger predictor of fatal than nonfatal CAD events (15,33). Taken together these data may explain some of the inconsistencies of the glycemia-CAD relationship in past studies and point toward mechanisms that may be particularly related to glycemia per se, for example, mechanisms underlying plaque erosion, thrombosis, and renal disease, all of which have been associated with a more fatal presentation of CAD (34,35).
The current analyses have strengths and weaknesses. The DCCT/EDIC includes a well-characterized cohort of individuals with type 1 diabetes monitored for up to 30 years, with entry criteria designed to address the development and progression of the complications of type 1 diabetes. The CVD adjudication process ensures capture of valid and verifiable CVD events. Over time, the occurrence of CVD events has increased, reflective of the increasing duration of type 1 diabetes and advancing age of the cohort. However, the mean age (∼56 years) of the cohort remains relatively young when considering CVD events. In addition, the exclusion of individuals with preexisting hypertension, hyperlipidemia, or CVD, coupled with the continued relatively small number of CVD events, may limit applicability of these results to the population with type 1 diabetes as a whole.
In summary, intensive diabetes therapy during the DCCT (6.5 years) has long-term, clinically beneficial effects on the incidence of CVD in this cohort with type 1 diabetes. Efforts to make intensive diabetes management attainable at a young age must continue so as to reduce the rates of life-threatening CVD over the life span for patients with diabetes.
Supplementary Material
Article Information
Funding. The DCCT/EDIC has been supported by cooperative agreement grants (1982-1993, 2012-2017) and contracts (1982-2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (current grant numbers U01-DK-094176 and U01-DK-094157), and by the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program (1993-2007), and the Clinical and Translational Science Center Program (2006-present), Bethesda, MD.
The following industry contributors provided free or discounted supplies or equipment to support participants’ adherence to the study but have had no role in the DCCT/EDIC study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), LifeScan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi (Bridgewater, NJ).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.A.G.-K. and J.M.L. obtained funding for the study and wrote the manuscript. R.A.G.-K., J.M.L., J.-Y.C.B., G.M.L., D.J.B., and T.J.O. wrote sections of the manuscript and reviewed and edited the manuscript. J.M.L. directed the statistical analyses. J.M.L. and J.-Y.C.B. contributed to the analysis plan specifications for the manuscript. J.-Y.C.B. conducted the statistical analyses and reviewed and edited the manuscript. J.M.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
Members of the DCCT/EDIC Writing Group: Rose A. Gubitosi-Klug (Case Western Reserve University, Cleveland, OH), John M. Lachin (George Washington University, Rockville, MD), Jye-Yu C. Backlund (George Washington University, Rockville, MD), Gayle M. Lorenzi (University of California, San Diego, La Jolla, CA), David J. Brillon (Weill Cornell Medical College, New York, NY), and Trevor J. Orchard (University of Pittsburgh, Pittsburgh, PA).
Footnotes
Clinical trial reg. nos. NCT00360815 and NCT00360893, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-1990/-/DC1.
A complete list of participants in the DCCT/EDIC Research Group is presented in the Supplementary Material published online for the article in N Engl J Med 2015;372:1722–1733. Members of the DCCT/EDIC Writing Committee are presented in the appendix.
References
- 1.Ford ES. Trends in predicted 10-year risk of coronary heart disease and cardiovascular disease among U.S. adults from 1999 to 2010. J Am Coll Cardiol 2013;61:2249–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding JL, Shaw JE, Peeters A, Guiver T, Davidson S, Magliano DJ. Mortality trends among people with type 1 and type 2 diabetes in Australia: 1997-2010. Diabetes Care 2014;37:2579–2586 [DOI] [PubMed] [Google Scholar]
- 3.Orchard TJ, Nathan DM, Zinman B, et al.; Writing Group for the DCCT/EDIC Research Group . Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan DM, Lachin J, Cleary P, et al.; Diabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research Group . Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003;348:2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol 1995;75:894–903 [DOI] [PubMed] [Google Scholar]
- 6.Aiello LP; DCCT/EDIC Research Group . Diabetic retinopathy and other ocular findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 8.Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA, Nathan DM; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 2015;64:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 10.Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group . Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirai FE, Moss SE, Klein BE, Klein R. Relationship of glycemic control, exogenous insulin, and C-peptide levels to ischemic heart disease mortality over a 16-year period in people with older-onset diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR). Diabetes Care 2008;31:493–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein BE, Klein R, McBride PE, et al. . Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Arch Intern Med 2004;164:1917–1924 [DOI] [PubMed] [Google Scholar]
- 16.Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care 2006;29:2528–2538 [DOI] [PubMed] [Google Scholar]
- 17.de Ferranti SD, de Boer IH, Fonseca V, et al. . Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 2014;37:2843–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The DCCT Research Group The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes 1986;35:530–545 [PubMed] [Google Scholar]
- 19.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 20.Epidemiology of Diabetes Interventions and Complications (EDIC) Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epidemiology of Diabetes Interventions and Complications Research Group Protocol [article online], 1996. Available from https://edic.bsc.gwu.edu/documents/26117/391689/EDIC+Protocol+Sept+2013. Accessed 30 September 2013
- 22.Backlund JY, Determan A, Cleary P. Validation of deaths and reported cardiovascular cerebrovascular events (Abstract). Control Clin Trials 2003;23:2S [Google Scholar]
- 23.Snedecor GW, Cochran WG. Statistical Methods. 7th ed. Ames, Iowa, Iowa State University Press, 1980 [Google Scholar]
- 24.Lachin JM. Biostatistical Methods: The Assessment of Relative Risks. 2nd ed. New York, John Wiley and Sons, 2011 [Google Scholar]
- 25.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed. New York, John Wiley and Sons, 2002 [Google Scholar]
- 26.Hastie TJ. Statistical models. In Generalized Additive Models. Chambers JM, Hastie TJ, Eds, Pacific Grove, Wadsworth and Brooks/Cole, 1992 [Google Scholar]
- 27.de Boer IH, Kestenbaum B, Rue TC, et al.; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group . Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med 2008;168:1867–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopes-Virella MF, Carter RE, Gilbert GE, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications Cohort Study Group . Risk factors related to inflammation and endothelial dysfunction in the DCCT/EDIC cohort and their relationship with nephropathy and macrovascular complications. Diabetes Care 2008;31:2006–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monnier VM, Bautista O, Kenny D, et al. . Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 1999;48:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 32.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 33.Hayward RA, Reaven PD, Wiitala WL, et al.; VADT Investigators . Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–2206 [DOI] [PubMed] [Google Scholar]
- 34.Conway B, Costacou T, Orchard T. Is glycaemia or insulin dose the stronger risk factor for coronary artery disease in type 1 diabetes? Diab Vasc Dis Res 2009;6:223–230 [DOI] [PMC free article] [PubMed]
- 35.Orchard TJ, Costacou T. When are type 1 diabetic patients at risk for cardiovascular disease? Curr Diab Rep 2010;10:48–54 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.