Abstract
OBJECTIVE
Controlling cardiovascular disease (CVD) risk factors in diabetes mellitus (DM) reduces the number of CVD events, but the effects of multifactorial risk factor control are not well quantified. We examined whether being at targets for blood pressure (BP), LDL cholesterol (LDL-C), and glycated hemoglobin (HbA1c) together are associated with lower risks for CVD events in U.S. adults with DM.
RESEARCH DESIGN AND METHODS
We studied 2,018 adults, 28–86 years of age with DM but without known CVD, from the Atherosclerosis Risk in Communities (ARIC) study, Multi-Ethnic Study of Atherosclerosis (MESA), and Jackson Heart Study (JHS). Cox regression examined coronary heart disease (CHD) and CVD events over a mean 11-year follow-up in those individuals at BP, LDL-C, and HbA1c target levels, and by the number of controlled risk factors.
RESULTS
Of 2,018 DM subjects (43% male, 55% African American), 41.8%, 32.1%, and 41.9% were at target levels for BP, LDL-C, and HbA1c, respectively; 41.1%, 26.5%, and 7.2% were at target levels for any one, two, or all three factors, respectively. Being at BP, LDL-C, or HbA1c target levels related to 17%, 33%, and 37% lower CVD risks and 17%, 41%, and 36% lower CHD risks, respectively (P < 0.05 to P < 0.0001, except for BP in CHD risk); those subjects with one, two, or all three risk factors at target levels (vs. none) had incrementally lower adjusted risks of CVD events of 36%, 52%, and 62%, respectively, and incrementally lower adjusted risks of CHD events of 41%, 56%, and 60%, respectively (P < 0.001 to P < 0.0001). Propensity score adjustment showed similar findings.
CONCLUSIONS
Optimal levels of BP, LDL-C, and HbA1c occurring together in individuals with DM are uncommon, but are associated with substantially lower risk of CHD and CVD.
Introduction
Cardiovascular diseases (CVD), including coronary heart disease (CHD), stroke, and heart failure (HF), are predominant causes of morbidity and mortality among persons with diabetes mellitus (DM) (1). An important focus of recent guidelines for the management of DM has been the control of modifiable risk factors for the primary prevention of CVD (1,2). Guidelines for over 15 years (3) have recommended tight control of glycated hemoglobin (HbA1c), blood pressure (BP), and LDL cholesterol (LDL-C). Despite evidence of benefit from tighter glycemic control on microvascular complications from the UK Prospective Diabetes Study (4), its effect on reducing the number of CVD events remains questionable in the light of recent clinical trials (5–7); yet the control of BP (8) and dyslipidemia (9,10) are well demonstrated to reduce CVD risks. While guidelines over the past 20 years have encouraged tighter control of blood glucose, BP, and LDL-C levels, studies in U.S. cohorts have shown poor CVD risk factor control in persons with DM (11–13); one recent study (14) showed only 25% to be at target levels for HbA1c, BP, and LDL-C simultaneously. The Steno-2 Trial (15) has shown that composite risk factor control in DM can reduce CVD events by >50%. However, data from community-dwelling individuals with DM on the relation of composite risk factor control with CVD risk are limited, especially from ethnically diverse U.S. population-based prospective studies. Understanding the extent to which CVD risk can be reduced from multiple risk factor control can be helpful in providing the evidence-based rationale for composite risk factor control efforts in DM management.
Given the importance of primary prevention of CVD in individuals with DM, in this report we examine, among three major multiethnic U.S. prospective studies of CVD, the association of individual and composite risk factor target attainment for BP, LDL-C, and HbA1c levels with future risk of CHD and CVD events over an average 11-year follow-up period in adults in whom DM had been diagnosed.
Research Design and Methods
Study Population
We included subjects ≥18 years of age with diagnosed DM who were free of known CVD at baseline from three National Institutes of Health–sponsored prospective studies of persons with follow-up for the following CVD events: 1) the Atherosclerosis Risk in Communities (ARIC) study (16), 2) the Multi-Ethnic Study of Atherosclerosis (MESA) (17), and 3) the Jackson Heart Study (JHS) (18). MESA included Caucasians, African Americans, Hispanics, and Chinese; the ARIC study was composed of Caucasians and African Americans; and the JHS included African Americans exclusively. For our study, we identified those persons with diagnosed DM, as defined by a self-reported physician diagnosis or whether they took medication to lower their blood glucose levels at the time of the identified baseline visit, where HbA1c and other risk factor information were available (1990–1992 in the ARIC study, 2000–2002 in the JHS, and 2003–2004 for visit 2 in the MESA when baseline HbA1c measures were available). ARIC study clinical centers included Washington County, MD; Forsyth County, NC; Jackson, MS; and Minneapolis, MN; MESA included clinical sites at the University of Minnesota (Minneapolis, MN), Northwestern University (Chicago, IL), Johns Hopkins University (Baltimore, MD), Columbia University (New York, NY), Wake Forest University (Winston-Salem, NC), and the University of California, Los Angeles (Los Angeles, CA); the JHS has a single site in Jackson, MS. Recruitment was based on probability sampling of four communities from lists of driver licenses, voter registration cards, or identification cards for the ARIC study; lists of residents, dwellings, telephone exchanges, Medicare beneficiaries, and referrals by participants for the MESA; and the Accudata volunteer list and eligible ARIC study participants for the JHS. The mean (maximum) follow-up time, in years, for the subjects included was 14.9 (20.9) for the ARIC study, 7.9 (9.9) for the JHS, and 8.0 (10.5) for the MESA. JHS subjects who were also part of the ARIC study were excluded from our JHS data set and included only in the ARIC study data set used for our analysis. From the original cohort, sample sizes of 15,792 for the ARIC study, 6,814 for the MESA, and 5,302 for the JHS, we identified those individuals with diagnosed DM based on Examination 2 from the ARIC study and the MESA; and, after excluding those with known prior CVD and missing covariate information, 825, 740, and 453 subjects, respectively, were included in the ARIC study, MESA, and JHS data sets, for a total sample size of 2,018 subjects (Supplementary Fig. 1). We excluded prevalent CVD on the basis of information on prior myocardial infarction (MI) and stroke, as well as data on prior bypass surgery, angioplasty, and HF. Information on HF was missing in 8 ARIC study subjects, and in the JHS, 18 subjects did not have information on HF medication. In the ARIC study, 2 participants had missing stroke data and 91 had information missing on bypass surgery and/or coronary angioplasty; in the JHS, 3 subjects had information missing on bypass surgery and/or coronary angioplasty. These subjects (n = 121) were all assumed not to have prior CVD. There were also 77, 37, and 43 persons with DM in the ARIC study, MESA, and JHS, respectively, who were not included because of missing key risk factor information. These persons tended to be 1.8 years younger than those included in our sample. They had a higher HbA1c (9.0% vs. 7.8%) and a lower HDL cholesterol (HDL-C) (37.9 vs. 46.6 mg/dL), but other demographic and risk factors were comparable.
Measurements
Goal or recommended levels of risk factors for this study were based on levels recommended by the American Diabetes Association during 2000–2002, which corresponds to the enrollment periods in the JHS and the MESA (19). Detailed specimen and data collection for the MESA, ARIC study, and JHS have been previously published (16–18). For BP, the average of two sitting BP readings was used. LDL-C was calculated from the Friedwald formula using measurements of total cholesterol, HDL-C, and triglycerides from standardized assays (Roche). HbA1c level was determined by high-performance liquid chromatography (Tosoh Bioscience). Study subjects were considered to be at their goal level if their specific laboratory value was at or below the following cut points, as recommended by guidelines that were in effect during the conduct of the examinations (19): LDL-C level <2.6 mmol/L (100 mg/dL), HbA1c level <53.0 mmol/mol (7%), and BP level <130/80 mmHg. The impact of a more recently defined cut point for BP control of <140/80 mmHg (20) was examined in a secondary analysis to examine the impact of this more modest, but evidence-based, goal. We also examined the number of factors controlled as none, any one, any two, or a composite goal of all three factors: BP <130/80 mmHg, LDL-C <2.6 mmol/L (100 mg/dL), and HbA1c <53.0 mmol/mol (7%). A BMI ≥30 kg/m2 was used to define obesity. Information on medications for the lowering of lipid levels, BP, and DM (insulin or oral DM medication) was obtained from questionnaires and participants bringing in pill containers at the study visit with the medication recorded.
CVD and CHD Event Definitions and Ascertainment
For the ARIC study, MESA, and JHS, incident CVD was defined as a MI, CHD death, cardiac procedure (percutaneous coronary interventions, bypass surgery, or coronary revascularization), stroke, or HF; incident CHD was defined as a MI, CHD death, or cardiac procedure. The adjudication process for events involved a panel to review hospitalization and death data per study protocols previously published (16–18). All events were adjudicated from medical records and death certificates for end-point classification and assignment of incidence dates by the morbidity and mortality classification/review committee in three studies.
Statistical Methods
Using the risk factor and CVD event variables from each study, we created new variables with consistent definitions and then pooled the subjects with DM among cohorts. All continuous variables used in our analyses were normally distributed and, thus, were compared between those at versus those not at target levels for HbA1c, BP, and LDL-C using the Student t test. The χ2 test for proportions was used to compare categorical variables, with a test of trend used to examine cumulative CVD event incidence by the number of risk factors under control. CVD/CHD event rates were calculated as per 1,000 person-years. Cox proportional hazards regression models (providing hazard ratios [HRs] and 95% CIs) examined the relation of being at individual and composite risk factor targets with the risk of incident CHD and CVD events, unadjusted and then adjusted for age, sex, ethnicity, smoking status, HDL-C level, BMI, family history of premature CVD, lipid-lowering medication, hypertension medication, and antidiabetes medication (insulin or oral hypoglycemic therapy). To eliminate the potential bias of confounding by indication in those at target levels versus those not at target levels for individual or composite risk factors, we recalculated the HRs in Cox regression adjusted for propensity score (21). Propensity scores were calculated using logistic regression adjusted by age, sex, ethnicity, systolic and diastolic BP (for LDL-C and HbA1c propensity score), HbA1c (for LDL-C and BP propensity score), LDL-C (for BP and HbA1c propensity score), HDL-C, BMI, smoking status, family history of premature CVD, lipid-lowering medication, hypertension medication, and antidiabetes medication. We included subgroup analyses and interaction terms for sex (male vs. female), ethnicity (African American vs. other races), and cohort (ARIC study, MESA, JHS) with each risk factor variable (and the number of risk factors) to examine the heterogeneity of effects across these strata. We also examined effects stratified by DM duration in a subgroup of participants with this information available. A two-tailed α value of < 0.05 (and P < 0.1 for interaction test) was considered statistically significant. Data analyses used SAS (version 9.4; SAS Institute, Cary, NC).
Results
For the pooled cohort, 2,018 persons with diagnosed DM were included, with 43% male and a mean ± SD age of 60.1 ± 9.7 years (age range 28–86 years); ethnicity included 30% Caucasian, 55% African American, 11% Hispanic, and 4% Asian/Pacific Islander. The JHS had fewer participants who were at the BP target, whereas the ARIC study had fewer participants at LDL-C and HbA1c targets; the MESA had the highest percentage of subjects at composite control (Table 1). The mean duration of DM was 9.9 years and mean follow-up time in our analysis was 10.8 years (range 0.2–20.9 years). Although 41.8%, 32.1%, and 41.9% of subjects were at target individually for BP, LDL-C, and HbA1c, respectively, only 7.2% of subjects were at target for all three factors.
Table 1.
Characteristics of all participants with diagnosed DM and no CVD at baseline in the individual parent cohort studies and in the pooled sample
| ARIC study | MESA | JHS | All 3 studies pooled | |
|---|---|---|---|---|
| DM | 825 | 740 | 453 | 2,018 |
| Age (years) | 58.5 ± 5.7 | 66.2 ± 9.5 | 53.6 ± 10.7 | 60.1 ± 9.7 |
| Age range (years) | 47–69 | 46–86 | 28–82 | 28–86 |
| Male sex | 355 (43.0%) | 372 (50.3%) | 143 (31.6%) | 870 (43.1%) |
| White | 450 (54.6%) | 152 (20.5%) | N/A | 602 (29.8%) |
| African American | 373 (45.2%) | 278 (37.6%) | 453 (100%) | 1,104 (54.7%) |
| Hispanic | N/A | 228 (30.8%) | N/A | 228 (11.3%) |
| Asian/Pacific Islander | 2 (0.2%) | 72 (11.6%) | N/A | 84 (4.2%) |
| DM duration (years) | 10.0 ± 9.4 | 10.6 ± 8.4 | 8.6 ± 8.8 | 9.9 ± 9.0 |
| BP at target (<130/80 mmHg) | 431 (52.2%) | 376 (50.8%) | 37 (8.2%) | 844 (41.8%) |
| LDL-C at target (<2.6 mmol/L [100 mg/dL]) | 157 (19.0%) | 353 (47.7%) | 137 (30.2%) | 647 (32.1%) |
| HbA1c at target (<53.0 mmol/mol [7%]) | 266 (32.2%) | 378 (50.1%) | 201 (44.4%) | 845 (41.9%) |
| None (BP, LDL-C, HbA1c) at target | 228 (27.6%) | 104 (14.1%) | 176 (38.9%) | 508 (25.2%) |
| Any one (BP, LDL-C, HbA1c) at target | 374 (45.3%) | 267 (36.1%) | 189 (41.7%) | 830 (41.1%) |
| Any two (BP, LDL-C, HbA1c) at target | 189 (22.9%) | 267 (36.1%) | 78 (17.2%) | 534 (26.5%) |
| All three (BP, LDL-C, HbA1c) at target | 34 (4.1%) | 102 (13.8%) | 10 (2.2%) | 146 (7.2%) |
| Follow-up time (years) | 14.9 ± 5.6 | 7.9 ± 1.4 | 8.0 ± 1.4 | 10.8 ± 5.1 |
| Incident CVD (n/1,000 person-years) | 444 (45.4) | 105 (24.4) | 65 (19.1) | 614 (35.1) |
| Incident CHD (n/1,000 person-years) | 288 (26.9) | 66 (14.9) | 24 (6.8) | 378 (20.3) |
Continuous variables are presented as the mean ± SD; categorical variables are presented as frequencies (%); incident events were presented as the number of events (event rates). The JHS (n = 3,675) excludes those counted in the ARIC study cohort. Asian/Pacific Islanders are all Chinese American in the MESA and except for two unspecified Asian/Pacific Islanders from the ARIC study. DM duration data were available in 602 ARIC study participants, 457 MESA participants, and 348 JHS participants.
Table 2 shows LDL-C, HDL-C, BMI, DM duration, percentage African American, current smoker, and receiving hypertension and lipid-lowering medication to be significantly different between subjects at versus those not at BP targets. Age, systolic BP, diastolic BP, HbA1c, BMI, percentage male, percentage African American, and percentage receiving hypertension, DM, and lipid-lowering medication were significantly different between subjects at versus those not at LDL-C target level. Age, LDL-C level, HDL-C level, BMI, DM duration, percentage African American, current smokers, and proportion of subjects receiving antidiabetes medication were significantly different between those at versus not at the HbA1c target. Age, BMI, percentage male, African American, current smokers, and lipid-lowering medication use were significantly different between subjects at versus those not at composite risk factor targets.
Table 2.
Baseline characteristics by BP, LDL-C, HbA1c, and composite (BP, LDL-C, HbA1c) targets among subjects with DM from the pooled cohort
| BP |
LDL-C |
HbA1c |
Composite target |
|||||
|---|---|---|---|---|---|---|---|---|
| At target (<130/80 mmHg) (N = 844) | Not at target (≥130/80 mmHg) (N = 1,174) | At target (<2.6 mmol/L [100 mg/dL]) (N = 647) | Not at target (≥2.6 mmol/L [100 mg/dL]) (N = 1,371) | At target (<53.0 mmol/mol [7%]) (N = 845) | Not at target (≥53.0 mmol/mol [7%]) (N = 1,173) | At target (N = 146) | Not at target (N = 1,872) | |
| Age, years | 60.3 ± 8.4 | 59.9 ± 10.6 | 61.8 ± 10.4 | 59.3 ± 9.3§ | 61.3 ± 10.4 | 59.3 ± 9.2§ | 62.5 ± 9.4 | 59.9 ± 9.8† |
| Male | 386 (45.7%) | 484 (41.2%)* | 305 (47.1%) | 565 (41.2%)* | 383 (45.3%) | 487 (41.5%) | 79 (54.1%) | 791 (42.3%)† |
| African American | 344 (40.8%) | 760 (64.7%)§ | 330 (51.0%) | 774 (56.5%)* | 434 (51.4%) | 670 (57.1%)* | 49 (33.6%) | 1,055 (56.4%)§ |
| Current smoker | 157 (18.6%) | 147 (12.5%)§ | 95 (14.7%) | 209 (15.2%) | 127 (15.0%) | 177 (15.11)† | 25 (17.1%) | 279 (14.9%)* |
| Family history of CVD | 429 (50.8%) | 619 (52.7%) | 337 (52.1%) | 711 (51.8%) | 434 (51.4%) | 614 (52.3%) | 76 (52.1%) | 972 (51.9%) |
| DM duration, years | 10.6 ± 9.3 | 9.4 ± 8.7* | 10.4 ± 9.5 | 9.6 ± 8.7* | 8.7 ± 9.3 | 10.6 ± 8.7‡ | 10.9 ± 10.8 | 9.8 ± 8.8 |
| Systolic BP, mmHg | 114.0 ± 9.8 | 145.2 ± 17.6§ | 130.5 ± 20.3 | 132.9 ± 21.8* | 131.4 ± 21.2 | 132.7 ± 21.5 | 113.7 ± 9.8 | 133.6 ± 21.4§ |
| Diastolic BP, mmHg | 65.6 ± 7.5 | 81.9 ± 12.7§ | 73.6 ± 13.0 | 75.8 ± 13.6‡ | 74.9 ± 13.5 | 75.2 ± 13.5 | 64.6 ± 8.5 | 75.9 ± 13.5§ |
| LDL-C, mmol/L (mg/dL) | 3.04 ± 0.99 (117.1 ± 37.9) | 3.18 ± 1.00† (122.3 ± 38.5) | 2.09 ± 0.39 (80.5 ± 14.9) | 3.61 ± 0.82§ (138.8 ± 31.1) | 2.98 ± 0.94 (114.4 ± 36.5) | 3.24 ± 1.03§ (124.2 ± 39.1) | 2.05 ± 0.38 (79.2 ± 14.7) | 3.22 ± 0.99§ (123.3 ± 37.7) |
| HDL-C, mmol/L (mg/dL) | 1.14 ± 0.36 (45.7 ± 14.4) | 1.18 ± 0.34* (47.2 ± 13.6) | 1.18 ± 0.38 (47.0 ± 15.1) | 1.16 ± 0.34 (46.4 ± 13.4) | 1.20 ± 0.37 (47.9 ± 14.6) | 1.14.3 ± 0.34§ (45.7 ± 13.5) | 1.15 ± 0.39 (45.9 ± 15.7) | 1.17 ± 0.35 (46.7 ± 13.8) |
| HbA1c, mmol/mol (%) | 59.1 ± 15.9 (7.8 ± 2.1) | 59.1 ± 15.1 (7.8 ± 2.0) | 56.0 ± 12.9 (7.4 ± 1.7) | 60.6 ± 15.9§ (8.0 ± 2.1) | 45.4 ± 3.8 (6.1 ± 0.5) | 68.1 ± 13.6§ (9.0 ± 1.8) | 45.4 ± 3.8 (6.1 ± 0.5) | 59.8 ± 15.1§ (7.9 ± 2.0) |
| BMI, kg/m2 | 30.8 ± 6.4 | 32.5 ± 6.8§ | 31.5 ± 6.7 | 31.9 ± 6.7 | 31.3 ± 6.7 | 32.2 ± 6.7† | 30.5 ± 6.2 | 31.9 ± 6.7* |
| Hypertension medication | 470 (55.7%) | 868 (73.9%)§ | 457 (70.6%) | 881 (64.3%)† | 559 (66.2%) | 779 (66.4%) | 87 (59.6%) | 1,251 (66.8%) |
| Antidiabetes medication | 686 (81.3%) | 934 (79.6%) | 545 (84.2%) | 1,075 (78.4%)‡ | 594 (70.3%) | 1,026 (87.5%)§ | 113 (77.4%) | 1,507 (80.5%) |
| Lipid-lowering medication | 202 (23.9%) | 241 (20.5%) | 225 (34.8%) | 218 (15.9%)§ | 197 (23.3%) | 246 (21.0%) | 54 (37.0%) | 389 (20.8%)§ |
| BP <130/80 mmHg | 844 (100%) | 298 (46.1%) | 546 (39.8%)† | 356 (42.1%) | 488 (41.6%) | 146 (100%) | ||
| LDL-C <2.6 mmol/L (100 mg/dL) | 298 (35.3%) | 349 (29.7%)† | 647 (100%) | 318 (37.6%) | 329 (28.1%)§ | 146 (100%) | ||
| HbA1c <53.0 mmol/mol (7%) | 356 (42.2%) | 489 (41.7%) | 318 (49.2%) | 527 (38.4%)§ | 845 (100%) | 146 (100%) | ||
| HDL-C <1.0 mmol/L (40 mg/dL) (male) or <1.3 mmol/L (50 mg/dL) (female) | 386 (45.7%) | 589 (50.2%)* | 304 (47.0%) | 671 (48.9%) | 449 (53.1%) | 526 (44.8%)‡ | 63 (43.2%) | 912 (48.7%) |
| Obesity (BMI ≥30 kg/m2) | 414 (49.1%) | 729 (62.1%)§ | 368 (56.1%) | 808 (56.6%) | 438 (51.8%) | 705 (60.1%)‡ | 68 (46.6%) | 1,075 (57.4%)* |
Continuous variables are expressed as the mean ± SD, and categorical variables are expressed as frequencies (%). BP, LDL-C, HbA1c, and HDL-C cut points are per previously recommended American Diabetes Association targets. DM duration data were available in 602 ARIC study participants, 457 MESA participants, and 348 JHS participants.
*P < 0.05, †P < 0.01, ‡P < 0.001, §P < 0.0001 compared with those at target.
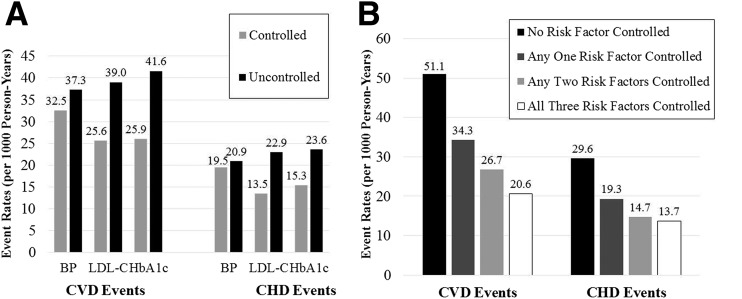
Figure 1 shows the CVD and CHD incidence per 1,000 person-years for individuals who were at versus those who were not at target levels for BP, LDL-C, and HbA1c (Fig. 1A), as well as for the number of targets achieved (BP, LDL-C, and/or HbA1c) (Fig. 1B). For each individual risk factor, individuals at target levels had lower CVD event rates than those who were not at target levels; there were similar findings for CHD events. Incident CVD and CHD risks (per 1,000 person-years) were much greater when no risk factors were at goal compared with when all three targets were achieved (51.1 vs. 20.6 person-years for CVD [P < 0.0001], and 29.6 vs. 13.7 person-years for CHD [P = 0.001]). A test of trend showed the proportions with incident CVD and CHD events decreased with increasing number of risk factors controlled (P < 0.0001).
Figure 1.
Unadjusted CVD and CHD event rates per 1,000 person-years for subjects with DM, by status of being at target level for individual risk factors BP, LDL-C, and HbA1c (A) and by the number of risk factors at target levels (B). BP target <130/80 mmHg; LDL-C target <2.6 mmol/L (100 mg/dL); HbA1c target <53.0 mmol/mol (7%).
Table 3 gives the HRs and 95% CIs for CVD events and CHD events, unadjusted, adjusted for covariates (risk factors plus medication), and adjusted for propensity scores. After adjusting for covariates, individuals at versus not at BP targets had 17% lower risks for both CVD events (P < 0.05) and CHD events (P = NS). For the more contemporary target of <140/80 mmHg, these HRs were 0.90 (95% CI 0.76–1.07, P = 0.23) for CVD and 0.98 (95% CI 0.79–1.21, P = 0.83) for CHD. Those individuals at the LDL-C target had a 33% lower risk for CVD events and a 41% lower risk for CHD events. Those with HbA1c at versus not at target levels had a 37% lower risk for CVD events and a 36% lower risk for CHD events. Compared with those individuals with none of the risk factors (BP, LDL-C, HbA1c) at target levels, those having any one, any two, and all three factors controlled had 36%, 52%, and 62% lower risks for CVD and 41%, 56%, and 60% lower risks for CHD, respectively. Also, in a sensitivity analysis excluding subjects missing certain prior CVD information (n = 121, as noted in research design and methods), results remained virtually identical, indicating the robustness of our findings. Finally, after adjusting by propensity score, very similar HRs were observed for being at target levels for BP and LDL-C and by number of risk factors controlled, but the risk reduction associated with HbA1c being at target level was lower (30% for CVD and 26% for CHD).
Table 3.
Adjusted HRs (95% CI) for CVD and CHD events among subjects with DM by status of individual and composite risk factor targets
| Risk factor comparison | Incident CVD events |
Incident CHD events |
||||
|---|---|---|---|---|---|---|
| HR (95% CI), unadjusted | HR (95% CI), adjusted for covariates | HR (95% CI), adjusted for propensity score | HR (95% CI), unadjusted | HR (95% CI), adjusted for covariates | HR (95% CI), adjusted for propensity score | |
| Individual risk factor controlled | ||||||
| BP <130/80 mmHg vs. BP ≥130/80 mmHg | 0.81 (0.69–0.95)* | 0.83 (0.70–0.98)* | 0.81 (0.68–0.96)* | 0.86 (0.70–1.05) | 0.83 (0.67–1.02) | 0.78 (0.63–0.97)* |
| LDL-C <2.6 mmol/L (100 mg/dL) vs. LDL-C ≥2.6 mmol/L (100 mg/dL) | 0.71 (0.58–0.86)‡ | 0.67 (0.54–0.82)§ | 0.71 (0.58–0.87)‡ | 0.64 (0.49–0.83)‡ | 0.59 (0.45–0.77)‡ | 0.62 (0.48–0.81)‡ |
| HbA1c <53.0 mmol/mol (7%) vs. HbA1c ≥53.0 mmol/mol (7%) | 0.64 (0.54–0.76)§ | 0.63 (0.53–0.76)§ | 0.70 (0.58–0.84)§ | 0.68 (0.54–0.84)‡ | 0.64 (0.51–0.81)‡ | 0.74 (0.59–0.93)* |
| Number of risk factors at target | ||||||
| Any one (BP, LDL-C, HbA1c) at target vs. none at target | 0.67 (0.56–0.80)§ | 0.64 (0.53–0.77)§ | 0.65 (0.54–0.78)§ | 0.65 (0.52–0.82)‡ | 0.59 (0.47–0.75)§ | 0.60 (0.47–0.76)§ |
| Any two (BP, LDL-C, HbA1c) at target vs. none at target | 0.52 (0.41–0.64)§ | 0.48 (0.38–0.61)§ | 0.47 (0.38–0.60)§ | 0.51 (0.38–0.68)§ | 0.44 (0.33–0.59)§ | 0.42 (0.32–0.57)§ |
| All three (BP, LDL-C, HbA1c) at target vs. none at target | 0.46 (0.30–0.69)‡ | 0.38 (0.25–0.58)§ | 0.41 (0.27–0.62)§ | 0.53 (0.32–0.88)* | 0.40 (0.24–0.67)‡ | 0.41 (0.25–0.70)‡ |
Covariates include age, sex, ethnicity, smoking status, HDL-C, BMI, family history of premature CVD, hypertension medication, antidiabetes and lipid-lowering medication (also include LDL-C and HbA1c for BP analysis; systolic/diastolic BP and HbA1c for LDL-C analysis; LDL-C and systolic/diastolic BP for HbA1c analysis).
*P < 0.05, ‡P < 0.001, §P < 0.0001.
In analyses stratified by sex and ethnicity (Supplementary Table 1), there was a tendency for lower adjusted risks associated with BP control for females (HR 0.70, P < 0.01 vs. HR 0.99, P = NS, for CVD in males; HR 0.67, P < 0.05 vs. HR 0.97, P = NS, respectively, for CHD) and African Americans (HR 0.69, P < 0.01 vs. HR 0.97, P = NS for CVD in other races; HR 0.61, P < 0.01 vs. HR 0.95, P = NS, respectively, for CHD), whereas LDL-C control was related to lower risk in males (HR 0.55, P < 0.0001 vs. HR 0.85, P = NS for CVD in females; HR 0.53, P < 0.001 vs. HR 0.70, P = NS, respectively, for CHD) and nonblack subjects (HR 0.63, P < 0.001 vs. HR 0.71, P < 0.05 for CVD in African American subjects; HR 0.50, P < 0.0001 vs. HR 0.74, P = NS, respectively, for CHD). However, interaction terms were nonsignificant (P > 0.10) except for P = 0.03 for LDL-C control by sex for CVD and BP control by race. HbA1c control risks for CVD and CHD were similar by sex and ethnicity. There was a weak trend toward lower risks from all three risk factors controlled for both CVD and CHD in men (HR 0.34 and 0.39, respectively) versus women (HR 0.47 and 0.49) (sex interaction term P = 0.74 for CVD and P = 0.21 for CHD) as well as for African Americans (HR 0.23 and 0.30, respectively) versus other races (HR 0.49 and 0.45, respectively) (ethnicity interaction term P = 0.04 for CVD and P = 0.64 for CHD).
In addition, when results were stratified by DM duration (available in 602 ARIC study participants, 457 MESA participants, and 348 JHS participants), HRs for CVD events in those participants were at an individual target level for BP, LDL-C, and HbA1c were 0.71, 0.72, and 0.64 in those below the mean DM duration and 0.92, 0.72, and 0.72 for those above the mean DM duration; similar HRs were also observed for composite risk factor control in these two subgroups (all interaction tests were nonsignificant). Additionally, the interaction terms of the study cohort with BP, lipid, glucose, or composite control were all nonsignificant (P values of 0.31–0.50 for CHD events and 0.43–0.58 for CVD events), indicating the homogeneity of the effect of risk factor control with outcomes across studies (MESA, JHS, or ARIC study).
Conclusions
In our pooled analysis of subjects with DM in three large-scale U.S. prospective studies, the more factors among HbA1c, BP, and LDL-C that were at goal levels, the lower are the observed CHD and CVD risks (∼60% lower when all three factors were at goal levels compared with none). However, fewer than one-tenth of our subjects were at goal levels for all three factors. These findings underscore the value of achieving target or lower levels of these modifiable risk factors, especially in combination, among persons with DM for the future prevention of CHD and CVD events.
There is a lack of data from population-based cohorts of adults with DM focusing on the impact of having ideal levels of multiple risk factors on future risk of CHD and CVD events, although some clinical trial and observational data exist. Most noteworthy is the Steno-2 clinical trial involving 160 Danish white patients with type 2 DM who were randomized to intensive therapy or conventional therapy for a mean treatment period of 7.8 years focusing on the following targets: HbA1c level <48 mmol/mol (6.5%), fasting total cholesterol level <4.5 mmol/L (175 mg/dL), triglyceride level <2.0 mmol/L (150 mg/dL), and BP <130/80 mmHg. Intensive therapy resulted in a 57% reduction in CVD death and a 59% reduction in CVD events (15), which are nearly identical to our observational study findings. Also, applying UK Prospective Diabetes Study risk engine estimates to combined control of HbA1c, BP, total cholesterol, HDL-C, and smoking among U.S. adults with DM in the National Health and Nutrition Examination Survey, statistically “controlling” all risk factors to goal was projected to prevent an estimated 36–42% of CHD events and, in the case of aggressive control, was projected to prevent 54–60% of CHD events (22). Finally, a recently published 5-year follow-up study (23) of 859,617 adults with DM among 11 U.S. integrated health care organizations showed inadequate risk factor control to be responsible for 11–34% of CVD events. While control of BP, LDL-C, and HbA1c has improved over recent years in U.S. adults with DM, only about one-fourth of such individuals are at control for all three of these factors, according to recent U.S. data (14). Control of risk factors (BP, LDL-C, HbA1c, and smoking cessation) in DM patients with CHD is also suboptimal, with simultaneous control rates varying from 8% to 23% (24). Risk factor control also varies substantially by ethnicity (25), suggesting a need for health care systems to develop approaches to ensure better composite control of risk factors. Meta-analyses of randomized trials (26,27) evaluating quality improvement interventions in adults with type 2 DM have shown modest improvements in HbA1c, BP, and LDL-C with increased use of aspirin and antihypertensive drugs, but not with statin use. In persons with CHD, health care approaches involving physician education, automated reminders, and required performance measures have resulted in improved adherence to recommended therapies and reduced numbers of CHD events and hospitalizations (28,29). Moreover, recently launched is the first real-world global Collaborative Diabetes Registry, an interdisciplinary effort led by the American College of Cardiology in partnership with the American Diabetes Association, the American College of Physicians, the American Association of Clinical Endocrinologists, and the Joslin Diabetes Center (30). These and other approaches are being implemented to improve the quality of care of persons with DM.
Our large representation of African Americans (55% of our study sample), makes our study particularly unique, demonstrating a possibly greater impact of both BP and composite risk factor target attainment on CHD events in African Americans compared with individuals of other ethnicities. In our study, being at the target level for BP tended to be associated with greater relative risk reductions in women and African Americans, which may be influenced by higher uncontrolled baseline factors (e.g., higher systolic BP among African Americans and females). These findings are consistent with those of prior studies showing a high prevalence of hypertension, particularly in older African American women, with control of BP being poor (31). The greater benefit of risk factor control, especially of hypertension, that we observe in African Americans, combined with their current status of poor control of risk factors, suggests an unmet opportunity for improved risk factor control in African Americans with DM.
Our study has several strengths and limitations. An important strength of our study derives from the inclusion of subjects with DM from three large-scale, well-characterized U.S. population–based epidemiologic studies (ARIC study, MESA, and JHS), with standardized evaluation of risk factors and ascertainment of CHD and CVD events that were adjudicated by end points committees. We were also able to exclude any significant bias due to confounding by indication, from adjusting for propensity scores. A potential limitation, however, is the pooling of individuals from cohorts of different time periods, where there may be differences both in control rates and the effects of risk factor control on CVD and CHD event risk. Realizing that the baseline examination data we used for the ARIC study cohort was collected ∼10 years earlier than those of the MESA or the JHS, it is not surprising that the ARIC study had the lowest levels of both LDL-C and HbA1c control, considering that both the guidelines and intensity of treatments available were less stringent. However, interaction terms of this cohort effect with individual and composite risk factor control were all nonsignificant, indicating that the effect of risk factor control on outcomes did not vary by cohort. While our enrolled cohort represents subjects from multiple metropolitan areas around the country, our clinical centers enrolling participants were not entirely representative of the country; it is well recognized that there is significant regional variation in DM care, such as in prescription rates for certain DM medications varying more than twofold between hospital referral regions (32); thus, results may be different had other regions/communities been studied. Although about half of our cohort was African American, Hispanics and Asians were also included in our sample, but the numbers were too small to examine the impact of risk factor target attainment in these groups. More importantly, our report did not investigate the effect of other targets (e.g., nonsmoking status or ideal BMI levels) that are important in DM control because of sample size limitations to look at more than three targets simultaneously. In addition, our determination of being at target for a given factor was based on a single measure. Without having pretreatment levels, we were unable to examine newer targets, such as those based on the percentage of LDL-C level lowering, as specified by more recent guidelines (33). Importantly, during follow-up, new risk factors may have developed in participants or the status of participants may have changed with regard to whether or not they were at target for one or more risk factors, which may have influenced our results. The limited and different reexamination periods for the studies we used precluded us from performing such an evaluation.
Also, while our report used more contemporary risk factor goals that were in effect during the beginning of the MESA and the JHS, but which were stricter than those in effect when the ARIC study cohort was recruited, our intention was to test the effect of specific risk factor targets. For BP, we showed that those subjects at a more current, but less aggressive target level of <140/80 mmHg did not have lower CHD or CVD risks, whereas CVD events were 17% lower in those who were at a target level of <130/80 mmHg. With the recent publication of the Systolic Blood Pressure Intervention Trial (SPRINT) trial (34), subjects randomized to a target systolic BP of <120 mmHg versus <140 mmHg had a 25% lower risk of the development of the primary composite CVD end point. In addition, the recent BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial (35) showed that there was a reduction in CVD secondary to the use of the glucose-lowering agent empagliflozin, but the mechanisms are currently unclear. Finally, although we have chosen specific targets that were based on guidelines for persons with DM free of prior CVD in effect at the time of the conduct of the studies included, an individualized approach for setting targets may be more appropriate. For instance, some higher-risk subjects, such as those with long-standing or more complicated DM, may be suitable for more lenient HbA1c target levels, as recent guidelines (20) have suggested, or for different BP or lipid target levels than we have specified.
Our study of three large prospective U.S. cohorts of persons in whom DM has been diagnosed shows those persons who were at target levels for HbA1c, BP, and LDL-C to have substantially (∼60%) lower risks for CVD and CHD than persons with DM who were not at target levels for such factors. These findings emphasize the importance of composite control of these modifiable risk factors to better address the residual CVD risk seen in persons with DM, the need for the development of health care strategies to better ensure such management, and the need for studies to evaluate and eliminate barriers to risk factor control in persons with DM.
Supplementary Material
Article Information
Acknowledgments. The authors thank the other investigators, the staff, and the participants of the ARIC study, JHS, and MESA for their valuable contributions.
Funding. This study was funded by a contract from Bristol-Myers Squibb with the University of California, Irvine. The ARIC study was performed as a collaborative study supported by National Heart, Lung, and Blood Institute contracts HHSN-268201100005C, HHSN-268201100006C, HHSN-268201100007C, HHSN-268201100008C, HHSN-268201100009C, HHSN-268201100010C, HHSN-268201100011C, and HHSN-268201100012C. The JHS is supported by contracts HHSN-268201300046C, HHSN-268201300047C, HHSN-268201300048C, HHSN-268201300049C, and HHSN-268201300050C from the National Heart, Lung, and Blood Institute, and the National Institute on Minority Health and Health Disparities. The MESA is supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-RR-025005 from the National Center for Research Resources.
Duality of Interest. S.K. was an employee of Bristol-Myers Squibb at the time the study was conducted. J.M. is an employee of Bristol-Myers Squibb. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. N.D.W. initiated the study and contributed to the study design, to data analysis and interpretation, and to the drafting, review, and editing of the manuscript, and in doing so led all of the study efforts. Y.Z., R.P., and C.P. contributed to data analysis and interpretation and to the drafting and review of the manuscript. S.M., A.G.B., A.C., A.R.F., S.K., J.M., H.T., and E.S. contributed to the data interpretation and to the editing and review of the manuscript. N.D.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
References
- 1.American Diabetes Association Cardiovascular disease and risk management. Sec. 8. In Standards of Medical Care in Diabetes—2016. Diabetes Care 2016;39(Suppl. 1):S60–S71 [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Golden SH, Anderson C, et al.; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Quality of Care and Outcomes Research, and the American Diabetes Association . Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2015;132:691–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of medical care for patients with diabetes mellitus. Diabetes Care 2000;23(Suppl. 1):S32–S42 [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 5.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effect of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 7.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 8.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colhoun HM, Betteridge DJ, Durrington PN, et al.; CARDS investigators . Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–696 [DOI] [PubMed] [Google Scholar]
- 10.Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361:2005–2016 [DOI] [PubMed] [Google Scholar]
- 11.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004;291:335–342 [DOI] [PubMed] [Google Scholar]
- 12.Imperatore G, Cadwell BL, Geiss L, et al. Thirty-year trends in cardiovascular risk factor levels among US adults with diabetes: National Health and Nutrition Examination Surveys, 1971-2000. Am J Epidemiol 2004;160:531–539 [DOI] [PubMed] [Google Scholar]
- 13.Malik S, Lopez V, Chen R, Wu W, Wong ND. Undertreatment of cardiovascular risk factors among persons with diabetes in the United States. Diabetes Res Clin Pract 2007;77:126–133 [DOI] [PubMed] [Google Scholar]
- 14.Wong ND, Patao C, Wong K, Malik S, Franklin SS, Iloeje U. Trends in control of cardiovascular risk factors among US adults with type 2 diabetes from 1999 to 2010: comparison by prevalent cardiovascular disease status. Diab Vasc Dis Res 2013;10:505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–591 [DOI] [PubMed] [Google Scholar]
- 16.The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881 [DOI] [PubMed] [Google Scholar]
- 18.Carpenter MA, Crow R, Steffes M, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci 2004;328:131–144 [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Standards of medical care in diabetes—2007. Diabetes Care 2007;30(Suppl. 1):S4–S41 [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Agostino RB., Jr Propensity scores in cardiovascular research. Circulation 2007;115:2340–2343 [DOI] [PubMed] [Google Scholar]
- 22.Wong ND, Patao C, Malik S, Iloeje U. Preventable coronary heart disease events from optimal control of cardiovascular risk factors in US adults with diabetes (projections from utilizing the UKPDS risk engine). Am J Cardiol 2014;113:1356–1361 [DOI] [PubMed] [Google Scholar]
- 23.Vazquez-Benitez G, Desai JR, Xu S, et al. Preventable major cardiovascular events associated with uncontrolled glucose, blood pressure, and lipids and active smoking in adults with diabetes with and without cardiovascular disease: a contemporary analysis. Diabetes Care 2015;38:905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farkouh ME, Boden WE, Bittner V, et al. Risk factor control for coronary artery disease secondary prevention in large randomized trials. J Am Coll Cardiol 2013;61:1607–1615 [DOI] [PubMed] [Google Scholar]
- 25.Holland AT, Zhao B, Wong EC, Choi SE, Wong ND, Palaniappan LP. Racial/ethnic differences in control of cardiovascular risk factors among type 2 diabetes patients in an insured, ambulatory care population. J Diabetes Complications 2013;27:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA 2006;296:427–440 [DOI] [PubMed] [Google Scholar]
- 27.Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet 2012;379:2252–2261 [DOI] [PubMed] [Google Scholar]
- 28.Fonarow GC, Gawlinski A, Moughrabi S, Tillisch JH. Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP). Am J Cardiol 2001;87:819–822 [DOI] [PubMed] [Google Scholar]
- 29.Yusuf S. Two decades of progress in preventing vascular disease. Lancet 2002;360:2–3 [DOI] [PubMed] [Google Scholar]
- 30.Diabetes Collaborative Registry. The Diabetes Collaborative Registry: transforming the future of diabetes care [article online], 2014. Available from https://www.ncdr.com/WebNCDR/Diabetes/publicpage. Accessed 23 February 2016
- 31.Krakoff LR, Gillespie RL, Ferdinand KC, et al. 2014 hypertension recommendations from the Eighth Joint National Committee panel members raise concerns for elderly black and female populations. J Am Coll Cardiol 2014;64:394–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sargen MR, Hoffstad OJ, Wiebe DJ, Margolis DJ. Geographic variation in pharmacotherapy decisions for U.S. Medicare enrollees with diabetes. J Diabetes Complications 2012;26:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone NJ, Robinson JG, Lichtenstein AH, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–2934 [DOI] [PubMed] [Google Scholar]
- 34.Wright JT Jr, Williamson JD, Whelton PK, et al.; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl Med 2015;373:2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.